Metabolic diversity and seasonal variation of soil microbial communities in natural forested wetlands
2021-12-24DiWuQiChiXinSuiMengmengZhangHongbaiJiaGuangyuSun
Di Wu · Qi Chi · Xin Sui · Mengmeng Zhang, ·Hongbai Jia · Guangyu Sun
Abstract This study explores the ef fects of vegetation and season on soil microorganisms and enzymatic activity of dif ferent wetlands in a temperate climate. Microbial carbon metabolism diversity was assessed using community-level physiological prof iles (CLPP) with 31 dif ferent carbon substrates. CLPP indicated that signif icant interactions occur during carbon substrate metabolism of the microorganisms.Furthermore, the dif ferent types of vegetation present in the wetland ecosystem combined with the seasonal ef fects to inf luence microbial carbon metabolism and enzymatic activity. The most signif icant dif ferences occurred to carbohydrates, carboxylic acids, and amino acids. The Mantel test conf irmed positive correlations between soil enzymatic activities and microbial carbon metabolism. Soil microorganisms in Betula ovalifolia and Carex schmidtii wetlands used carbon substrates more ef ficiently in summer than those in other forested wetlands during other periods. Enzymatic activities also showed a similar trend as microbial carbon metabolism. The results demonstrate that microbial carbon metabolism patterns can be used as biological indicators in wetland ecological alterations due to vegetation type or to seasonal factors.
Keywords Forested wetlands · Community-level physiological prof iles (CLPP) · Carbon metabolism diversity · Soil enzymatic activity · Seasonality
Introduction
Wetlands are fragile ecotones between terrestrial and aquatic habitats comprised of open waters, herbaceous vegetation and wetland-adapted trees, yet they have global signif icance and value in the function of ecosystems (Whittaker and Likens 1973; Bergamaschi et al. 2007; Campos et al. 2011).Forested wetlands are situated at the interface between forest and water ecosystems, which are integral components of the existing wetlands (Maltby and Immirzi 1993; Correa-Araneda et al. 2012), and provide a wide range of ecological niches for many organisms (Boyce et al. 2012). Thus, these wetlands signif icantly enrich biodiversity in the region. Forested wetlands are also considered long-term carbon sinks and thus they are crucial in global carbon sequestration(Eswaran et al. 1993; Trettin and Jurgensen 2003).
Plant species regulate a variety of terrestrial ecosystem processes, improve soil carbon storage and stabilization,which are crucial components of the global carbon cycle(Lorenz and Lal 2005; De Deyn et al. 2008). In addition,plants bring about changes that inf luence the diversity of soil microbial communities as well as their enzymatic activities mainly through productivity, community composition,and functional traits (De Deyn et al. 2011; De Vries et al.2012; Legay et al. 2016). Conversely, nutrients required for plant growth depend on the response of microbial communities involved in a wide range of biogeochemical processes such as carbon cycling, nitrogen turnover, and phosphorus conversion (Zelles 1999; Ward 2005; Moseman-Valtierra et al. 2010; Lemanceau et al. 2015; Klimek et al. 2016;Zeng et al. 2016). Previously, most research on forested wetlands have focused primarily on the ef fects of climate(Di Paola et al. 2012; Helbig et al. 2017), hydrology (Watts and Kobziar 2015), tidal inf luences (Anderson and Lockaby 2011), microtopography (Pietrzykowski et al. 2015), and soil greenhouse gas f luxes (Krauss and Whitbeck 2012). Nevertheless, the characteristics and diversity of microbial communities are poorly understood in dif ferent vegetation types of natural forested wetlands, particularly the relationship between diverse microbial communities and plant speciesrich communities.
Global peatlands contain rich carbon reserves and are mainly distributed in temperate, northern region (Watson et al. 2000). In China, forested wetlands are predominant in temperate and cold temperate zones of the northeast (Mu et al. 2013). At present, research on forested wetlands in northeast China is focused on vegetation succession (Mu 2003), carbon storage (Mu et al. 2013), methane f lux (Sun et al. 2011), productivity and biodiversity (Yuan et al. 2006).However, the characteristics and seasonal changes in soil microbial communities within the ecotone of natural forested wetlands are also of equal concern.
Soil microbial communities are sensitive ecological indicators for evaluating soil quality using diverse metabolic processes to mediate soil function (Chodak et al. 2015;Prasse et al. 2015; Kumar et al. 2017). Microbial communities coexisting in wetland soils are susceptible to environmental changes, and their structure and diversity play a crucial role in wetland ecological functions (Yu et al. 2012;Sims et al. 2013; Chou et al. 2017). The Biolog microplate technique is very sensitive and can accurately ref lect changes in soil microbial communities (Qian et al. 2014).The technique contains 31 environmentally relevant carbon sources and is widely used to study the functional diversity of microbial communities by evaluating the catabolic potential and activity of specif ic carbon sources (Bissegger et al. 2014; Pierce et al. 2014). Although only living microorganisms are considered in community-level physiological prof iles (CLPPs), carbon utilization patterns can still be used as indicators to evaluate structure and functional potential of microbial communities (Barreiro et al. 2015). Furthermore, soil enzymes are sensitive bio-indicators involved in biochemical functions of soil and in catalyzing several vital reactions (Dick et al. 1996; Bergstrom et al. 1998).
The Wuyiling Forested Wetland, located in the Xiao Xing’an Mountains in northeast China, is a well-preserved wetland at a high latitude. There are numerous kinds of natural forested wetlands along the transition zone from water to forest. In this study, aBetula platyphylla-Larix gmeliniiwetland (forested wetland), anAlnus sibiricawetland (forested wetland), aBetula ovalifoliawetland (shrub wetland), andCarex schmidtiiwetland (herbaceous vegetation wetland),typical successive forested wetland ecotones in this area,were selected. Understanding the link between seasonal factors, wetland vegetation types, and soil microbial diversity is an important predictor of the response by wetland ecosystems to environmental changes and could be used as a basis for stabilizing and restoring wetlands. The specif ic aims of this study were: (1) to assess the seasonal distribution and functional characteristics of soil microbial communities in a forested wetland ecosystem; (2) to determine the ef fects of ecotone vegetation on soil microbes and enzymatic activities; and, (3) to demonstrate the relationships between soil enzymatic activities and soil microbial communities over dif ferent seasons.
Materials and methods
Study site and sampling
The study is located in Wuyiling Nature Reserve in the eastern Xiaoxing’an Mountains of northeast China(48°33ʹ–48°50ʹN, 129°00ʹ–129°30ʹE). They are well-preserved wetlands at a high latitude, at an altitude of 350–550 m a.s.l. and experiences a temperate continental climate.Mean annual temperature and precipitation are − 1.1 °C and 584 mm, respectively. The frost-free and freezing periods are approximately 97 days and 180 days, respectively. The soil is a dark brown soil, while azonal soils, ones without zonal organization or structure, include meadow soil, swamp soil,and peat soil (Cai et al. 2010).
Four wetlands, typical successive ecotones in Wuyiling Nature Reserve were selected. These includedB. platyphylla-L. gmeliniiwetland (BLW),A. sibiricawetland(ASW),B. ovalifoliawetland (BOW), andC. schmidtiiwetland (CSW). To determine the ef fect of season on the functional characteristics of soil microbial communities, soil samples were collected in winter (December 2015), spring(May 2016), summer (August 2016), and autumn (October 2016). Three 20 m × 20 m plots for each wetland were established and a 10-spot sampling method used to collect and mix soil samples from each plot. The litter layer in each plot was removed and soil samples in the upper 10-cm layer were collected. The samples were sieved for determination of microbial community-level physiological prof iles and enzyme activity analyses.
Determination of community-level physiological prof iles(CLPP)
Community-level physiological prof iles (CLPP) were conducted using the Biolog™ EcoPlate (Biolog Inc., Hayward,CA, USA) according to Classen et al. ( 2003) and Li et al.( 2012). The plates were incubated at 25 °C for 10 days and the color development in each well was recorded with a Sunrise Microplate Reader (Tecan Co. Ltd., Austria) at an optical density (OD) of 590 nm at the following times; zero hours, 24, 48, 72, 96, 120, 144, 168, 192, 216, and 240 h,respectively.
Microbial activity in each microplate was expressed as average well color development (AWCD);Shannonindex,Simpsonindex,McIntoshindex, andRichnessindex were calculated (Rogers and Tate III 2001; Wang et al. 2016) a follows:

AWCD represents the average catabolic activity of the microbial community;AiandAare the absorbencies for each carbon source well and the control well, respectively.

McIntosh(U) index represents the evenness of species;niis the relative absorbency of theith well.
Richness(S) index indicates species diversity of the microbial community;Sis the well number with an (A i − A)value above 0.25.
Determination of enzyme activity
The activities of soil enzymes, including urease (URE), acid phosphatase (ACP), peroxidase (POD), and invertase (INV),were determined using a soil enzyme activity kit (Suzhou Comin Biotechnology Co., Ltd., Suzhou, China) according to the manufacturer’s protocol.
Data analysis
The similarity percentage (SIMPER) procedure was used to determine which carbon source categories and substrates provided the largest contribution to the average dissimilarity of CLPPs among the wetlands using PAST2 software(Clarke 1993). Principal component analysis (PCA) was performed to analyze the carbon substrate utilization of the microbial community for each wetland according to season using SPSS version 16.0 for Windows (SPSS Inc., Chicago,IL, USA).
One-way analyses of variance (ANOVA) with Tukey’s post-hoc test at a signif icance level of 0.05 were used to test the ef fect of wetland types and seasons on soil bacterial-CLPP, and soil enzymatic activity using SPSS version 16.0(SPSS Inc., Chicago, IL, USA). The interactive ef fects of type of wetland and season on soil bacterial-CLPP and soil enzymatic activity were analyzed with a two-way repeated measures ANOVA. A redundancy analysis (RDA) was performed using CANOCO software (Canoco for Windows 4.5,Microcomputer Power Inc., Willis, TX, USA) to reveal relationships among bacterial-CLPP and soil enzymatic activity(Wollenberg 1977). A Mantel test analyzed the relationship between soil enzymatic activity and bacterial substrate utilization according to the matrix of Spearman, Euclidean similarities, and 999 permutations with the R software package.
Results
Soil biological and metabolic activity
A slight increase in the average well color development(AWCD) was observed in all soil samples at an early stage of the incubation period, which had gradually diminished by later stages (Fig. S1). The AWCD of the soil microbial community was lower during winter and spring, and increased rapidly during summer, indicating an improvement in soil microbial metabolic activity.
A signif icant increase in the AWCD (except for theA.sibiricawetland) in summer was observed, compared to the other three seasons. AWCD increases for the four wetlands followed the order:C. schmidtiiwetland >B. ovalifoliawetland >A. sibiricawetland >B. platyphylla-L. gmeliniiwetland. Signif icant dif ferences in AWCD at 96 h were observed among the four wetlands during four seasons via ANOVA analysis (Fig. S2 and Table S1). Two-way repeated measures ANOVA on AWCD at 96 h was carried out and a signif icant seasonal (P< 0.001) and wetland type (P= 0.001) ef fect was detected (Table S2).
Substrate utilization was used to evaluate the diversity,dominance, evenness, and richness of the microbial populations present in the dif ferent wetlands via theShannonindex,Simpsonindex,Mclntoshindex, andRichnessindex, respectively (Fig. 1). There were signif icant dif ferences between the dif ferent wetlands during the same season (Table S3).Signif icantly higherShannondiversity index values were observed in theB. platyphylla–L. gmeliniiwetland andA.sibiricawetland in spring; however, maximum values were found inC. schmidtiiwetland in summer (Table S3). TheMclntoshindex revealed that most of the wetlands in summer andA. sibiricawetland in spring produced higher functional evenness, indicating that community evenness was inf luenced by season.
The four indices showed signif icant changes over the four seasons in each of the wetlands (Table S3), indicating that the soil microbial community could possess a variable catabolic capacity. The four indices were lower in winter or spring (except for theA. sibiricawetland in spring), indicating the utilization of a lower number of substrates. The results show that most of the indices were higher in summer than in the other seasons, showing a similar trend to the AWCD. Moreover, the interaction between wetland type and season had a signif icant ef fect on the four indices (Table S4).
There were 31 dif ferent carbon sources in the Biolog EcoPlate which were divided into six categories. The characteristics of microbial carbon utilization for the six categories were recorded at 96 h incubation (Fig. 2). Soil microbial communities sampled in the four wetlands reacted with amino acids, carboxylic acids and the carbohydrates as the most commonly utilized substrates, followed by polymers,amines, and miscellaneous. Signif icant dif ferences were observed in carbon source utilization between the dif ferent wetlands in spring and summer (except for theA. sibiricawetland in spring) (Table S5). However, the f ive carbon categories (amines, amino acids, miscellaneous, polymers and carbohydrates) were more intensively utilized by soil microorganisms in theC. schmidtiiandB. ovalifoliawetlands in summer than those in the other wetlands. Consumption of the above carbon categories was also higher than in the other three seasons, indicating that microbial metabolic capacity had signif icantly increased during summer.
Principal component analysis of carbon substrate utilization

Fig. 1 Functional diversity indices for carbon utilization by soil microbial communities after 96 h incubation in dif ferent seasons; lower case letters indicate signif icant dif ferences between dif ferent wetlands in the same season; upper case letters indicate signif icant dif ferences between dif ferent seasons for each wetland (Tukey’s multiple range test)

Fig. 2 Characteristics of microbial carbon utilization ( a:amines, b: amino acids, c: carboxylic acids, d: miscellaneous,e: polymers, f: carbohydrates)after 96 h of incubation; lower case letters indicate signif icant dif ferences between dif ferent wetlands in the same season,upper case letters indicate signif icant dif ferences between the seasons for each wetland type(Tukey’s multiple range test)
Principal component analysis (PCA) was performed to further investigate the possible dif ferences in carbon source utilization patterns within the different seasons. The two-dimensional PCAs explained most of the total variance (Fig. 3). Scatter plots showed that utilization capacity was markedly dif ferent in the four wetlands according to the season. These results indicate that microbial substrate utilization in summer was signif icantly dif ferent from the other seasons, with the exception of theB. platyphylla–L.gmeliniiwetland, and values in winter were similar to those of autumn.
The correlation coef ficients in PCA ref lected the discrepancy of carbon utilization in the dif ferent soils. The higher the absolute value, the greater the inf luence of the substrate,which created a signif icant dif ferentiation in the carbon source. Dif ferentiation of PC1 primarily resulted from the 25 carbon substrates from the four wetlands, including three amines/amides, two polymers, seven carbohydrates, seven carboxylic acids, four amino acids, and two miscellaneouscarbon sources (Table S6). These 25 carbon sources were positively correlated with PC1. PC2 was dependent on the utilization of the following substrates: three amino acids,f ive carboxylic acids, one polymer and one miscellaneous carbon source. Only 2-hydroxy benzoic acid, L-phenylalanine, and tween 40 were negatively correlated with PC2. The number of high utilization substrates increased inA. sibiricaandC. schmidtiiwetlands than in the other two wetland.

Fig. 3 Principal component analysis of microbial community carbon source utilization patterns in a B. platyphylla— L.gmelinii wetland, b A. sibirica wetland, c B.ovalifolia wetland,and d C. schmidtii wetland
The similarity percentage (SIMPER) analysis revealed that the contribution to the average dissimilarity was from the utilization of dif ferent carbon sources (Table 1).These dif ferences in CLPPs were observed at the carbon source category level, mostly due to the variation in the utilization of carbohydrate (30.3%), carboxylic acid (22.6%), and amino acids (18.6%). The most signif icant contribution to the average dissimilarity was from the utilization of carbohydrate, which was highest in theA. sibericawetland in spring and lowest in theC. schmidtiiwetland in winter. The dif ferences among the wetlands at the substrate level were primarily caused by the diversif ication in the utilization of 16 substrates (Table S7). Thus, the greatest individual substrate contribution (> 4%) was from the utilization of two carboxylic acids (itaconic acid and D-galacturonic acid),four carbohydrates (N-acetyl- D-glucosamine,β-methyl- Dglucoside, D-cellobiose, and D-mannitol), and three amino acids ( L-asparagine, L-arginie, and L-serine).
Soil enzyme activities
To explain the impact of season and wetland types, activities of four soil enzymes were determined (Fig. 4). Activities varied by wetland type. As shown in Fig. 4 a, invertase activity varied signif icantly with season, higher activities appeared in summer in most of the wetlands except for theA. sibiricawetland, and an increase was observed in theC.schmidtiiwetland while lower values occurred in winter.Similar to invertase, urease activity exhibited signif icant dif ferences in winter and summer for the dif ferent wetland types (Fig. 4 b, Table S8); the highest activity was found in theC. schmidtiiwetland in summer.

Table 1 SIMPER analysis of dissimilarity in CLPPs at the carbon source category level among dif ferent wetlands
Acid phosphatase activities varied among the four wetlands in the dif ferent seasons, suggesting that this enzyme’s activities were sensitive to season and wetland type. The highest activity was found inC. schmidtiiwetland in summer (22.44 ± 0.53 μmol/day/g), signif icantly higher than in the other wetlands. There was no signif icant dif ference in acid phosphatase activity among the dif ferent wetlands in spring or autumn (Table S8). The ef fect of wetland types was more pronounced on peroxidase activities. Its activity in theA. sibiricaandB. ovalifoliawetlands was higher in spring compared to the other wetlands but was signif icantly lower in summer and autumn.
Two-way repeated measures ANOVA on soil enzyme activities showed a signif icant seasonal ef fect (P< 0.001)(Table S9). The dif ferent wetland types also had a signif icant ef fect on the activities of urease (P= 0.003), acid phosphatase (P= 0.008), and peroxidase (P= 0.001),whereas no signif icant dif ferences were found for invertase(P= 0.082). This indicated that urease, acid phosphatase,and peroxidase were more sensitive than invertase for different wetland type. Moreover, soil enzyme activities were signif icantly af fected by the interaction between season and wetland type (Table S9).
Relationships between CLPP and soil enzymatic activities
The f irst two RDA axis were calculated for all the wetland samples with 15.7% and 5.1% for the variance, respectively(Fig. 5). The RDA showed a positive relationship between the utilization of the microbial carbon substrates and soil enzymatic activities. Two distinct groups were identif ied along the RDA1 axis in the ordination diagram. Soil samples in group 1 included the most wetland samples in summer and autumn, and were distributed along the forward direction of RDA1 with higher enzyme activity and stronger utilization of the carbon substrate than that of soil samples in group 2. These samples in group 2 were from mostly samples in winter and spring, and exhibited less enzyme activity and carbon substrate utilization and were spread along the negative direction of RDA1.
According to the Mantel test, positive correlations were found between soil enzyme activities and the CLPPs-based microbial carbon metabolism in the wetland soils (Table 2).The results show that invertase (r= 0.186,P= 0.005) and peroxidase (r= 0.237,P= 0.002) were signif icantly positively related to the CLPP-based carbon metabolism, whichindicates that there were stronger correlations between either invertase or peroxidase and microbial carbon metabolism compared to other soil enzyme activities.
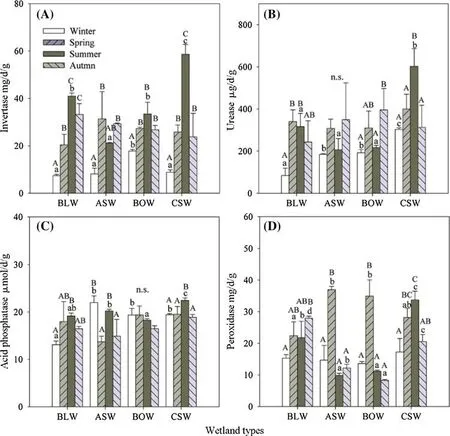
Fig. 4 Activities of soil enzymes a invertase (INV), b urease (URE), c acid phosphatase (ACP) and d peroxidase(POD) in dif ferent wetlands.Lower case letters indicate signif icant dif ferences between wetland types in the same season; upper case letters indicate signif icant dif ferences between seasons for each wetland type(Tukey’s multiple range test)
Discussion
In this study, there was a distinct seasonal shift in soil microbial communities in four dif ferent wetlands. Utilization of the six carbon sources was evaluated using Biolog EcoPlates and varied signif icantly with the season. Utilization of carbon sources by soil microorganisms was higher in spring and/or summer than in winter. Similarly, an increase in the diversity indices and AWCD was observed from winter to spring or to summer; this could be attributed to the fact that cold or frozen soils have limited microbial growth(Table S10). Limited availability of substrates also reduces microbial abundance while a release of nutrients increased microbial diversity and activity in the thawing (Sharma et al. 2006; Jef feries et al. 2010; Zhang et al. 2017). Vanhala ( 2002) reported that there were readily available carbon compounds in soil following a thaw. Seasonal variation trends exhibited dif ferences in metabolic function observed among the dif ferent wetlands on the PCA plot. The PCA results showed that the points in summer exhibited a positive shift toward PC1 or PC2 compared with the other seasons. Overall, seasons played an important role in adjusting the distribution of soil microbial communities in dif ferent wetlands.

Fig. 5 RDA analysis showing the relationship between carbon substrates and soil enzyme activity among the dif ferent wetlands; red lines represent soil enzyme activities, black circles dif ferent wetlands,blue diamonds the dif ferent carbon substrates; W, winter; SP, spring;SU, summer; A, autumn
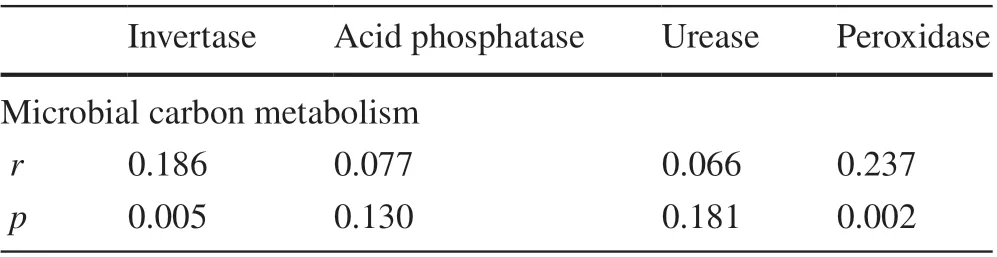
Table 2 Mantel test between microbial carbon metabolism and soil enzyme activities
Variations in microbial communities due to dif ferent types of vegetation in the wetland environment remain less understood. The surrounding vegetation is a chief source of carbon and energy for microbes and help preserve soil fertility (Bastida et al. 2008; Chodak et al. 2016). Microbial richness, evenness, and diversity indices were consistent with average well colour development in theB. ovalifoliaandC.schmidtiiwetlands and showed a signif icant increase in summer, indicating that soil microbial community had higher metabolic activity during this period. Microbial functional indices fromB. platyphylla-L. gmeliniiandA. sibiricawetlands were dominant in winter and spring. Previous research has considered that soil microbial communities are inf luenced by the dif ferent quality and quantity of litter input,root exudates, and substrate availability due to changes in plant species and life cycle (Brimecombe et al. 2007; Andruschkewitsch et al. 2014; Siles et al. 2016; Zeng et al. 2016).Metabolic preferences of the soil microbial communities based on the utilization of carbon substrates could possibly ref lect the taxonomic composition and dif ferent organic compounds present in the soil from their specif ic wetland environment (Banning et al. 2012; Chodak et al. 2016). In this study, a distinct shift in microbial carbon metabolism indicated that the four wetlands produced dif ferent ef fects on the microbial communities in their soils. Amino acids,carboxylic acids, and carbohydrates were the main carbon sources available to the soil microorganisms, suggesting that the soil microorganisms in the wetlands could be capable of metabolizing readily available carbon compounds that exist in soil. The apparent dissimilarities were due to increased use and ef ficiency of these three carbon groups by the microbial communities in the summer compared with other seasons. The results of the SIMPER analysis verif ied that amino acids, carboxylic acids, and carbohydrates were the main contributors found in the carbon source substrates, and most of these carbon sources contributed more in summer than in the other seasons. The decomposition products from herbaceous and woody materials were primarily utilized (except for summer) by the soil microbial communities in theB.platyphylla–L. gmeliniiandA. sibiricawetlands. Moreover,root exudates, as another type of carbon substrates, were mainly metabolized by the microbial communities in these wetlands (Salomo et al. 2009), implying that these wetland vegetations released more exudates and imported more litter during the seasons other than summer.
In addition, the utilization of the carbon substrates was signif icantly inf luenced by interactions between the wetland types and seasons. Carbon substrate utilization by soil microorganisms in theC. schmidtiiwetland increased considerably in summer, which indicated that the microbial communities might be subject to seasonal vegetation changes (Siles et al. 2016). Furthermore, theC. schmidtiiwetland had a high evenness index, further explaining seasonal dependence of microbial functional diversity (Andruschkewitsch et al. 2014). Wittebolle et al. ( 2009) reported that, due to the dif ferent weights between substrate richness and evenness, the evenness in biodiversity was more adaptive to a rapid response from the natural community to selective stress, compared to richness. This study supported the f inding that microbial functional diversity from semi-natural grasslands was higher than from agricultural or forest soils(Andruschkewitsch et al. 2014). While carbon is not the only factor regulating the composition of the soil microbial community (Allison et al. 2007), the diverse substrate patterns in the soil supplied a more heterogeneous resource to be utilized by the microorganisms (Andruschkewitsch et al. 2014).
The dif ferences observed in the CLPPs among the different wetlands were further ref lected in soil enzyme activities. Soil enzymes are essential for producing the crucial reactions involved in soil ecological processes and the interactions related to substrate ef fectiveness, microbial activity, nutrient dynamics, and environmental conditions (Sinsabaugh et al. 2008; Chavarría et al. 2016; Luna et al. 2016). Soil enzyme activities of the wetlands in this study were primarily divided into two categories: hydrolytic (invertase, acid phosphatase, and urease) and oxidative (peroxidase) enzymes. Enzyme activities signif icantly varied by season and vegetation types (Fig. 4). There was a general trend towards an increase in enzyme activities,particularly for invertase and acid phosphatase in summer compared to the other three seasons, and an obvious seasonal dif ference existed (P< 0.001) (Table S4). The reaction substrate invertase is an organic substance composed of carbon, nitrogen and other elements. The decomposition rate of carbon and nitrogen compounds increased as summer soil temperatures rose, which might have resulted in the peak in invertase activity which usually occurs in summer (Fan et al. 2009). Recent studies have found that soil hydrolytic enzyme activity in several forests increased with temperature(Baldrian et al. 2013), and this could be attributed to higher temperatures increasing the rate of enzyme reactions (Siles et al. 2016). Chavarría et al. ( 2016) also demonstrated that seasonal enzymatic activity were associated with temperature and moisture variations. Soil moisture actively controls enzyme activities by impacting the dif fusion of enzymes and substrates; however, temperature played the principal role if soil moisture was not restricted (Steinweg et al. 2012).Zhang et al. ( 2018b) found that seasonality was the critical factor that inf luenced enzyme activity and microbial biomass due to the impact on the rate of enzyme metabolism caused by temperature (Bergstrom et al. 1998). In addition, seasonal variations in urease, acid phosphatase, and peroxidase activities in dif ferent wetlands were similar to invertase. Peak activity of soil enzymes occurred primarily in spring and/or summer. Plants need to absorb large amounts of nutrients via stimulating the secretions of enzymes during the growing period; nevertheless, soil enzyme activities often declined even when the soil had substantial nutrients available (Stark et al. 2014; Pan et al. 2018). In this study, soil enzyme activity showed signif icant seasonal dif ferences, however, the seasons were not the only factor inf luencing enzyme activity.
Soil enzyme activity of the wetlands was also af fected by the vegetation. Signif icant dif ferences were observed in different wetland soils. Dif ferent plant species provide varying qualities and quantities of residues and root exudates which are used as substrates to increase the number of microorganisms, and are thereby indirectly involved in the regulation of soil enzyme activities (Nayak et al. 2007; Mayor et al. 2016).However, vegetation also af fects the characteristics of soil enzymes by changing soil structure, altering the diversity of root symbiotic microbes (He et al. 2002), and by dif ferences under the vegetation ref lecting soil used to participate in biochemical processes and various nutrient cycles (Zhang et al. 2018a). In this study, theC. schmidtiiwetland soil had signif icantly higher invertase, urease, and acid phosphatase activities than soils in the other three wetlands, indicating a competitive advantage for this wetland due to enrich soil enzymes, especially in summer. Recent studies have suggested that invertase activity inf luences soil biological activity (Bandick and Dick 1999), directly af fecting soil carbon cycle and soil nutrient supplement f low (Zhang et al. 2018b).Phosphatase changed the conversion capacity of phosphorus to a higher level which is conducive to most plants and soil microorganisms (Maharjan et al. 2017; Xie et al. 2017). Urease activity indicates nutrient recycling and could be used to characterize the prof it and loss of nitrogen within soil ecosystems (Baddam et al. 2016; Michele et al. 2017; Xie et al. 2017). Urease activity of theC.schmidtiiwetland in summer was signif icantly lower than in the other wetlands,indicating that low N availability activated urease synthesis through a feedback mechanism (Yin et al. 2014). Similarly,phosphatase activity was negatively correlated with its availability in natural nutrient gradients (Brockett et al. 2012;Godin et al. 2015). Conversely, limited nutrient resources can also induce microbes to increase secretion of enzymes to obtain more nutrients (Allison and Vitousek 2005). Thus an increase in soil enzyme activity could withstand a decline in nutrient cycling from either a disturbance in the type of vegetation or a def iciency in nutrients.
Redundancy analysis showed a positive relationship between soil enzyme activities and the utilization of the microbial substrates. The Mantel test also conf irmed these positive relationships for all of the enzymatic activities that occurred during microbial utilization of the carbon substrate.In addition, there was a highly signif icant positive correlation between enzyme activities (invertase and peroxidase)and availability of carbon substrates for the growth of soil microbes. Higher enzyme activities are an indicator of the presence of complex substrates (German et al. 2011). Cai et al. ( 2018) showed that acid phosphatase activity was positively correlated with microbial biomass phosphorous, urease activities were positively correlated with microbial biomass nitrogen and water soluble organic nitrogen. This study found signif icantly higher enzyme activity and stronger carbon utilization during the summer months, which could be attributed to higher temperatures inducing microorganisms to enhance metabolism and stimulate the secretion of enzymes (Sardans et al. 2006). This variation in microbial activity and function could af fect enzyme activities (Yang et al. 2012; Kader et al. 2017). Soil enzyme activity is one of the most sensitive indicators of soil nutrient status and fertility (Moghimian et al. 2017), and was greatly af fected by the dif ferent wetlands. Furthermore, plant residue input and root exudates from the dif ferent types of vegetation utilized by the microorganisms as substrates enhanced enzyme production (Nayak et al. 2007). Thus, changes in microbial activity, composition, and/or vegetation inf luenced soil enzyme activities (Yang et al. 2012; Kader et al. 2017).
Conclusions
This study found that metabolic activity of soil microorganisms along a forest to grassland gradient ref lected vegetation-specif ic and season-specif ic features. Soil enzyme activity was positively correlated with carbon metabolism of the microbial communities. This indicated a possible relationship between soil enzyme activities and CLPP-based microbial carbon metabolism. Microbial utilization of carbon substrates ref lected the metabolic and community diversity of soil microorganisms, and suggested the diversif ication of available soil carbon resources. Therefore, microbial carbon metabolism characteristics could act as biological indicators of ecological changes occurring within forested wetland ecotones.
杂志排行
Journal of Forestry Research的其它文章
- Genome-wide identif ication and cold stress-induced expression analysis of the CBF gene family in Liriodendron chinense
- Decay rate of Larix gmelinii coarse woody debris on burned patches in the Greater Khingan Mountains
- Characterizing conservative and protective needs of the aridland forests of Sudan
- Point-cloud segmentation of individual trees in complex natural forest scenes based on a trunk-growth method
- Accuracy of common stem volume formulae using terrestrial photogrammetric point clouds: a case study with savanna trees in Benin
- Appropriate search techniques to estimate Weibull function parameters in a Pinus spp. plantation
