Response of nitrogen fractions in the rhizosphere and bulk soil to organic mulching in an urban forest plantation
2021-12-24XiaodanSunGangWangYuqianYeQingxuMaQingweiGuanDaveyJones
Xiaodan Sun · Gang Wang · Yuqian Ye ·Qingxu Ma · Qingwei Guan · Davey L. Jones
Abstract Nitrogen is an essential component in forest ecosystem nutrient cycling. Nitrogen fractions, such as dissolved nitrogen, ammonium, nitrate, and microbial biomass nitrogen, are sensitive indicators of soil nitrogen pools which af fect soil fertility and nutrient cycling. However, the responses of nitrogen fractions in forest soils to organic mulching are less well understood. The rhizosphere is an important micro-region that must be considered to better understand element cycling between plants and the soil. A f ield investigation was carried out on the ef fect of mulching soil in a 15-year-old Ligustrum lucidum urban plantation.Changes in total nitrogen and nitrogen fractions in rhizosphere and bulk soil in the topsoil (upper 20 cm) and in the subsoil (20–40 cm) were evaluated following dif ferent levels of mulching, in addition to nitrogen contents in f ine roots,leaves, and organic mulch. The relationships between nitrogen fractions and other measured variables were analysed.Organic mulching had no signif icant ef fect on most nitrogen fractions except for the rhizosphere microbial biomass nitrogen (MBN), and the thinnest (5 cm) mulching layer showed greater ef fects than other treatments. Rhizosphere MBN was more sensitive to mulching compared to bulk soil, and was more af fected by soil environmental changes. Season and soil depth had more pronounced ef fects on nitrogen fractions than mulching. Total nitrogen and dissolved nitrogen were correlated to soil phosphorus, whereas other nitrogen fractions were strongly af fected by soil physical properties(temperature, water content, bulk density). Mulching also decreased leaf nitrogen content, which was more related to soil nitrogen fractions (except for MBN) than nitrogen contents in either f ine roots or organic mulch. Frequent applications of small quantities of organic mulch contribute to nitrogen transformation and utilization in urban forests.
Keywords Rhizosphere · Nitrogen fraction · Organic mulching · Soil–plant interaction · Urban plantation forest
Introduction
Organic mulching plays an important role in urban soil nutrient conservation and restoration. It is an ef fective means to modify the structure of soil ecosystems, which is necessary to ensure that these ecosystems function properly. In addition to its physical ef fects such as water conservation and temperature regulation, organic mulching contributes carbon (C) and nutrients to the soil, with the interaction of multiple factors resulting in complex mechanisms responsible for changes in the soil environment (Kader et al. 2017).Organic mulching improves soil conditions and promotes plant growth (Jiménez et al. 2017; Pal and Mahajan 2017).However, most studies that have evaluated the ef fects of organic mulching on the soil environment have tended to focus on agricultural soils, whereas studies on these ef fects in forest ecosystems, which may be characterised by markedly contrasting dynamics, are rare.
Nitrogen (N) plays a prominent role in controlling the primary production of plants in terrestrial ecosystems (Guignard et al. 2017). Enhanced plant N uptake reduces N leaching loss and increases N availability through root exudates by stimulating the microbial turnover of fast-cycling N pools(Meier et al. 2017). Nevertheless, the accumulation of nitrate as a consequence of continuous N addition tends to cause soil acidif ication (Lucas et al. 2011), which can have inhibitory ef fects on microbial activities related to plant growth and N f ixation (Vitousek et al. 1997). However, there is controversy regarding the response of N fractions to mulching or organic matter addition (Coppens et al. 2006; Rose et al. 2016). The quantity and fractions of N are strongly af fected by plant litter (Zhou et al. 2015). Numerous studies have found that litter is an important C source for soil microorganisms, and litter quality, determined by C:N ratios,phenolic compounds, or soluble C or N content and quantity, can af fect N mineralization and nitrif ication (Templer et al. 2003; Meier and Bowman 2008). At the same time,root exudates and root turnover are also the main factors controlling soil N transformation (Huang et al. 2013). Thus,the underlying mechanisms of N changes are complex and remain to be conf irmed.
Soil total N (TN) is a primary factor determining soil fertility and plant growth in forests, whereas labile N fractions determine the content of soil available N and nutrient cycling processes (Pastor and Post 1986). The available N in soil, in the form of ammonium or nitrates, is strongly correlated with TN (Turner et al. 2017). Dissolved N (DN)undergoes rapid changes in response to environmental variability, and microbial biomass N (MBN) is considered to comprise the largest labile soil organic N fractions, and thus their turnover signif icantly contributes to the soil N cycle(Zhou and Wang 2015; Ren et al. 2016). Investigating these N fractions can contribute to a more comprehensive understanding of how N dynamics and the stability of soil N pools change in response to dif ferent land management practices.
Soil N transformation is regulated by microorganisms(Chen et al. 2018), and microbial sensitivity to seasonal changes can be associated with temperature and N dynamics (Yokobe et al. 2018). Labile N fraction pools and net ammonif ication and nitrif ication rates in forest soils have been found to undergo substantial temporal variations driven by dif ferences in temperature and precipitation over the year (Kaiser et al. 2011), thus the time of year should be considered in studies of soil N dynamics. The spatial variability of soil properties is af fected by multiple factors.Nitrogen transformation rates have been found to decrease with soil depth in response to a reduction in organic matter content (Wild et al. 2015). External anthropogenic interference, such as the application of straw mulch or forage, af fects the topsoil more directly than the subsoil because of the closer contact and nutrient addition, resulting in comparatively higher contents of TN and available N in the topsoil (Fu et al. 2019). Subsoil organic matter is derived from the retention of organic matter that is transported with percolating water (Mikutta et al. 2019), and the changes in subsoil properties (e.g., mineral N content)are similar to or greater than those in the topsoil (Wang et al. 2019). Accordingly, with respect to the stable N pool,there may be equivalent or greater changes in the subsoil compared with those in the topsoil. Thus, if the soil is to be managed sustainably, it is necessary to monitor and predict changes in soil properties throughout the entire soil prof ile (Usowicz and Lipiec 2017).
Organic mulching has the notable ef fect of enhancing plant growth as a consequence of promoting an increase in the absorption of nutrients, which in turn can lead to a modif ication in the soil environment via the secretion of root exudates synthesised in response to the subsequent changes in plant nutrient cycling (Kuzyakov and Xu 2013). The environmental complexity driven by plant–soil interactions increases after mulching. Although the individual ef fects of relatively few plant factors have been observed, they have been found to show interactive ef fects with soil factors (Chen et al. 2016b); however, such plant–soil interactions are rarely considered. As an important micro-region of chemical exchange and energy f low between plants and the soil, the rhizosphere constitutes an important zone for plant–microbial interactions. Examining this micro-region can also provide valuable insights into N cycling in soil and plant ecosystems (Kuzyakov and Xu 2013). However, to date, there has been no study investigating changes in the rhizosphere environment in response to organic mulching. Accordingly, the response of N fractions to organic mulching in rhizosphere and bulk soil were determined to further understand the N cycle between plants and soil, and to f ind a suitable level of mulching for urban forest management. We hypothesised that organic mulching would signif icantly stimulate N fractions, with the ef fects being dependent on the amount of applied mulch; that MBN would be the most sensitive N fraction responding to organic mulching; that N fractions in the rhizosphere would be more sensitive than those in the bulk soil; and that more organic mulch would have a pronounced ef fect on soil N transformation because of the more nutrient input.
Materials and methods
Study site
The experimental plot was located in Xiaolingwei,Xuanwu District, Nanjing, China (32°02ʹ37″–32°02′39″N,118°49ʹ41″–118°49ʹ43″E, 37 m a.s.l, f lat terrain) (Fig. 1).The study site lies within the northern subtropical monsoon climate zone which is characterised by distinct seasons, with an annual precipitation of 1000–1050 mm, an annual average temperature of 15.4 °C, and an annual average 2213 h of sunshine. According to the historical records of Zhongshan Cemetery in Nanjing, buildings were originally located on the area; however, these were later demolished and the region was subsequently covered with forest plantations established on 50–60 cm of soil. For the purposes of the present study, a 15-year-old pureLigustrum lucidumW. T. Aiton (broad-leaf privet; family, Oleaceae)plantation was selected in which trees were >2 m apart and with a canopy density of approximately 85%. The average tree height was 7.5 m, and the average diameter at breast height 10.9 cm. The basic physical and chemical properties of the soil at the site are shown in Table 1.
Experimental design
Four adjacent trees were randomly selected to establish one experimental plot (Fig. 2), and 32 such plots were established in total. According to the “Technical specif ication for the application of organic mulch on urban and rural greening” of Shanghai, China, and related literature (Dietrich et al. 2019; Zhang et al. 2020), we applied three treatments:35, 70, and 140 kg of mulch/tree, along with a crosscheck control (CK) without mulch. The organic mulch extended evenly 80 cm away from the trunk, allowing for a buf fer(>0.5 m) between the trees, and was carefully mounded around each tree as uniformly as possible to a height of 5,10, or 20 cm above the ground (OM5, 35 kg mulch; OM10,70 kg mulch; OM20, 140 kg mulch). Treatment was completed by the end of November 2017. The organic mulch used was urban garden litter that had been semi-decomposed(Shanghai Moqi Garden Co., Ltd., Shanghai, China), the basic physical and chemical properties of which are shown in Table 2.
Data collection

Fig. 1 Diagrammatic sketch of the geographical location of the study site

Table 1 Physical and chemical properties of the soil at experimental site
Soil and plant samples were collected 6, 9, and 12 months after mulching (i.e., May, August, and November 2018),with f ive randomly selected plots (i.e., f ive replicates, one plot per replicate) for each treatment at each time point.Each experimental plot was only used once during the entire experiment, and 20 trees were used for one sample.Soil prof iles were obtained from pits dug 50 cm away from the trunk of the tree, and each prof ile was divided into two sections (0–20 cm and 20–40 cm below the mulch layer). Soil blocks 20 × 20 × 20 cm3were removed from each depth, and the rhizosphere soil was collected by gently shaking of f soil that had adhered to the roots, whereas the remainder of the soil was considered as bulk soil. In addition, in each plot, f ine roots (<2 mm in diameter) and leaves from the sampled trees were collected, as well as samples of organic mulch. All soil and plant samples were placed in self-sealing bags and taken to the laboratory for analysis. Soil samples were also obtained using cylindrical steel cores (100 cm3) for the determination of bulk density(BD) of each soil prof ile. Soil temperatures at each depth were recorded in situ using Thermochron temperature loggers (DS1923Hygrochron, OnSolution Pty Ltd, Baulkham Hills, NSW, Australia).
Estimates of soil pH were obtained from 1:2.5 soil–water mixtures using a PHS-3C glass electrode pH meter (Leici Inc., Shanghai, China). After air-drying, the samples were sifted (0.15 mm mesh), and soil organic carbon (SOC) and TN calculated using an Elemental Vario EL element analyser (Elementar, Langenselbold, Germany).Dissolved nitrogen (DN) was extracted with 0.5 M K2 SO4and determined using a TOC-VCPH + TNM-1 organic carbon analyser (Shimazu Inc., Kyoto, Japan). Ammonium and nitrate were both identif ied colorimetrically in 1 M KCl extracts (1:5 w/v) using a San++ continuous f low analyser (Skalar, Breda, Netherlands). Microbial biomass N (MBN; KN= 0.54) was obtained using chloroform fumigation–K2 SO4extraction methods and calculated using the aforementioned organic carbon analyser. Total phosphorus(TP) was determined by digestion with H2 SO4 –HClO4and quantif ied based on molybdenum blue colorimetry. Available phosphorus (extracted with 0.03 M NH4 F-0.02 M HCl)was identif ied by molybdenum blue colorimetry. Total potassium was determined by inductively coupled plasmaatomic emission spectroscopy ICP-AES (ICAP6300,Thermo Fisher Scientific, Waltham, MA, USA) after wet digestion with HClO4 –HF. Available potassium was extracted with 1 M CH3 COONH4(pH = 7.0) solution and measured by ICP-AES after f iltering. Soil organic matter was determined after digestion by the K2 Cr2 O7titration method, and soil moisture content by oven drying samples at 105 °C. Collected f ine roots and leaves were washed and oven- dried. The organic mulch samples were air-dried,ground, and sifted (0.15 mm mesh), and the N content determined using the aforementioned element analyser.

Fig. 2 Diagrammatic sketch of experimental plots

Table 2 Physicochemical properties of the organic mulch used in the study
Statistical analysis
All statistical analyses were conducted using R v. 3.5.3(R Development Core Team 2018). To evaluate the differences in soil N fractions among treatments, time after mulching, soil depth, and their interactions, linear mixed effects models were calculated using the R package “lme4” (Bates et al. 2015). Treatment (four levels),soil depth (two levels), time after organic mulching(season, three levels), and their interactions (three-way)were treated as fixed factors, and the sampling plots were treated as random factors. Comparisons of the N fractions and N content in fine roots, leaves, and organic mulch between treatments were carried out using two-way analyses of variance (ANOVA) and Tukey’s pair-wise comparison tests. Figure preparation and redundancy analysis(RDA) were conducted using the ‘ggplot2′ and ‘vegan’(Oksanen et al. 2019) packages in R, respectively. Comparisons of soil N fractions between the rhizosphere and bulk soils were performed usingt-tests. Differences were considered to be statistically significant at theP< 0.05 level.
Results
Ef fects of organic mulching on soil N fractions in rhizosphere and bulk soils
Time after mulching and soil depth had signif icant ef fects on N fractions, whereas the ef fects of dif ferent mulching treatments were detected only with respect to rhizosphere MBN(Table 3). Rhizosphere MBN with OM5 was signif icantly higher than those of the other two treatments and CK, mainly 6 months after organic mulching (Fig. 3). The interactions of time, depth, and mulching had a signif icant ef fect on TN in bulk soil and DN and nitrate in the rhizosphere. Seasonal variation in the N fractions in rhizosphere and bulk soil were consistent at dif ferent soil depths, although changes in the rhizosphere were more pronounced than those in bulk soil, particularly for MBN. With an increase in time after mulching, there was a gradual decrease in MBN, the seasonal variation of which was similar to that observed for TN. Ammonium content was highest 12 months after mulching, whereas values of DN and nitrate content were found to be highest 9 months after mulching. Organic mulching hadgreater ef fects on the N fractions in the subsoil (20–40 cm)than that in the topsoil (0–20 cm), especially for DN and MBN. The OM5 treatment had the highest ef fects on MBN as well as on DN in the subsoil.
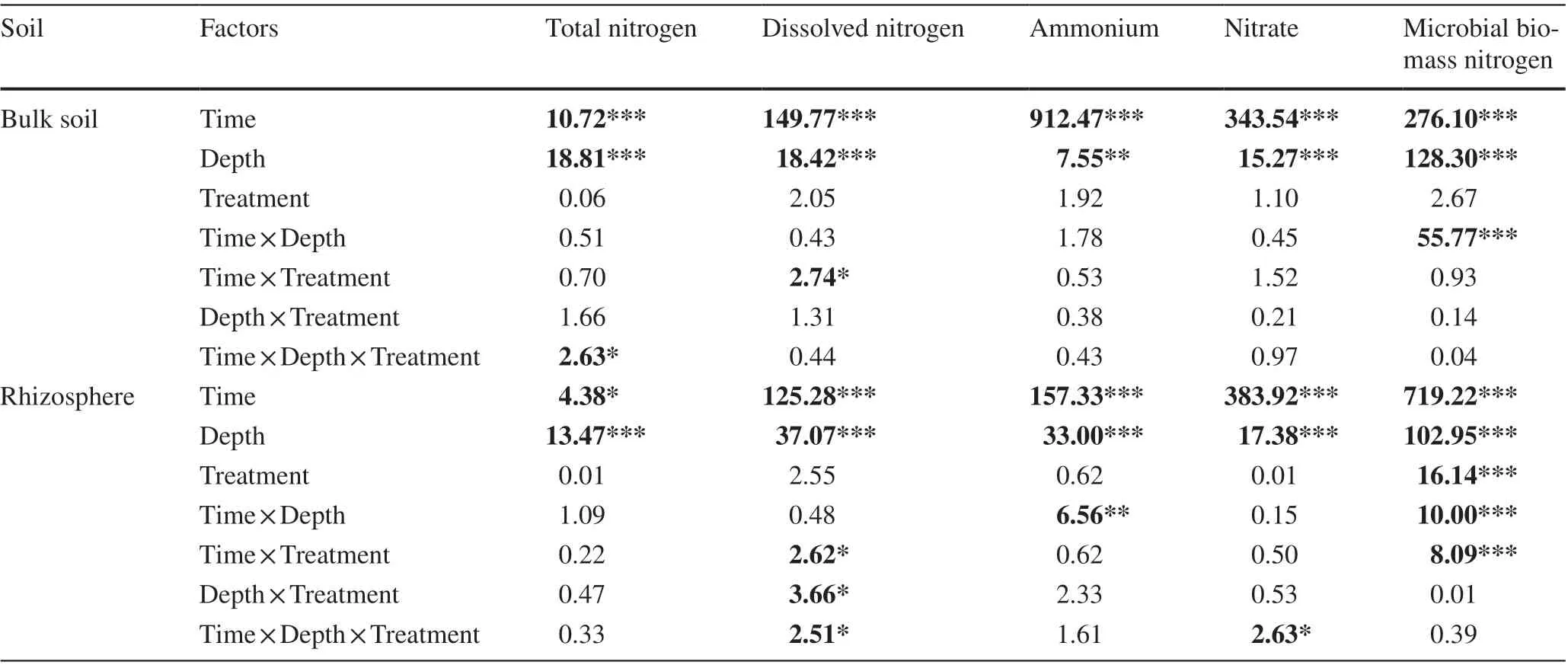
Table 3 Analysis of variance of the ef fects of dif ferent treatments, depths, and time on soil nitrogen fractions
Changes in the N contents of f ine roots, leaves,and organic mulch
Nitrogen levels in f ine roots increased and in organic mulch decreased gradually over time, but the dif ference of N in f ine roots and organic mulch between treatments was not signif icant (Fig. 4). Leaf N content decreased 6 months after mulching, later increased, and subsequently decreased again in the dormant season. OM10 and OM20 had signif icant ef fects on leaf N 6 months and 12 months after mulching,respectively, whereas it was 9 months after mulching for OM5 and OM20 compared with other treatments.
Association of N fractions with soil and plant properties
Redundancy analysis indicated a signif icant positive correlation between each N fraction in the rhizosphere and the corresponding fraction in bulk soil (Fig. 5 and Table S1 in Supplementary information). Total N was mainly af fected by the ratio of N to phosphorus (N:P), for which we detected a signif icant positive correlation (Fig. 5 and Table S2 in Supplementary information). In contrast, dissolved N was negatively correlated with the TP and N contents of leaves.In addition, ammonium, nitrate, and MBN appeared to be mainly related to soil physical properties (i.e., water content, temperature, and BD), and nitrate was negatively correlated with leaf N content. Nitrogen levels in f ine roots were positively correlated with rhizosphere TN and DN but negatively correlated with rhizosphere MBN. Moreover, leaf N was mainly negatively correlated with DN and nitrate and positively correlated with ammonium, whereas mulch N levels were positively correlated with MBN and negatively with ammonium.
Discussion
Response of soil N fractions to organic mulching
Contrary to our hypothesis, the OM5 treatment had signif icant ef fects on N fractions compared to those of the other two treatments, especially for rhizosphere MBN. The application of smaller amounts of organic mulch could contribute to enhancing relative decomposition and nutrient utilization by soil microorganisms because of the signif icant increase of MBN. Meanwhile, N immobilization could be increased when a large amount of mulch is added (Cao et al.2018). Thick mulch prevents water from entering the soil and reduces the ability of plants to absorb water accompanied by low nitrate movement and accumulation toward the root surface, i.e., rhizosphere (Guo et al. 2002; Gorska et al.2008). The C/N ratio in the original soil was low (Table 1),and the decomposition of mulch consumed N, which further enhanced the N limitation of the soil and thus did not signif icantly accelerate the element cycling in the soil with a large amount of mulch (Valenzuela-Solano and Crohn 2006). In addition, a large amount of organic mulch may restrict its own decomposition (N contents in OM10 and OM20 were higher than in OM5), preventing it from producing more available N. Accordingly, we speculate that frequent applications of small quantities of organic mulch might represent a benef icial strategy that favours N transformation and utilization in urban forests.
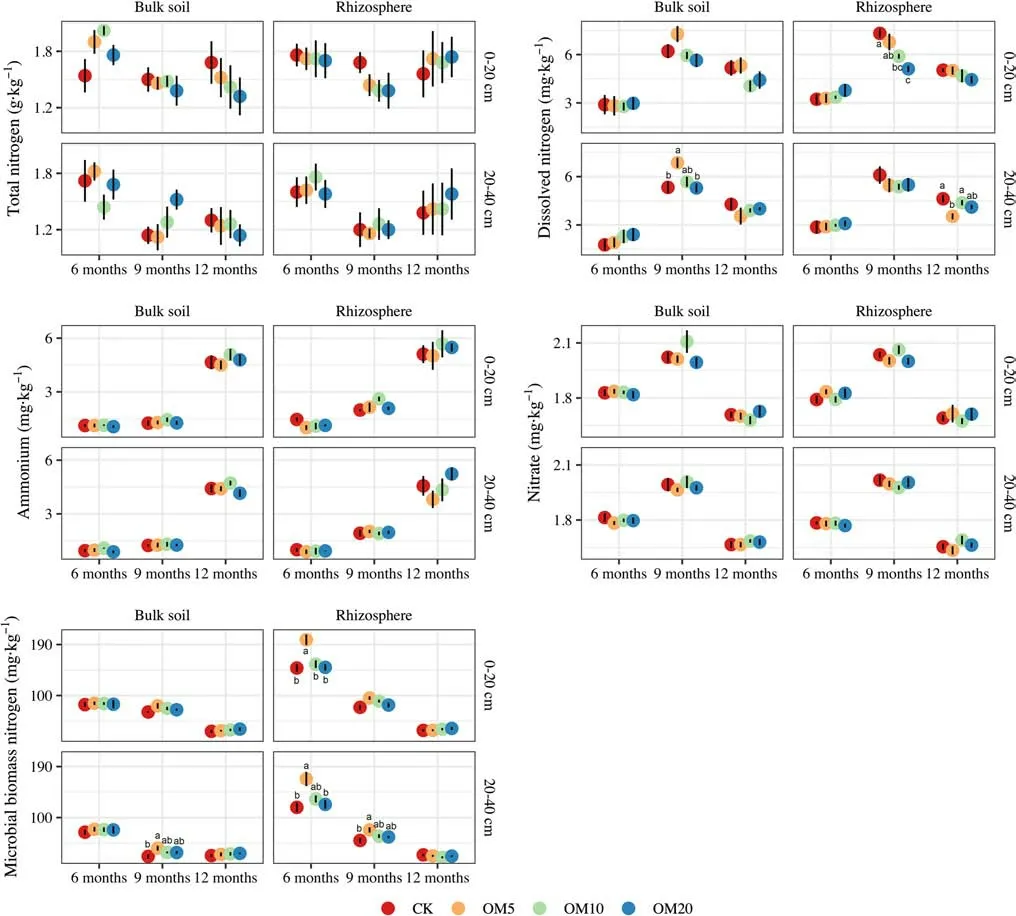
Fig. 3 Ef fects of organic mulching on soil nitrogen fractions in rhizosphere and bulk soils over time; error bars represent standard error of the mean (SE), values are means ± SE ( n = 5). CK control(no mulch), OM5 5 cm (35 kg) mulch, OM10 10 cm (70 kg) mulch,OM20 20 cm (140 kg) mulch. Dif ferent letters represent statistical dif ferences among treatments at dif ferent soil depths and time after mulching according to Tukey’s test ( P < 0.05)
From the results, MBN is more sensitive than other N fractions to organic mulching, and N immobilization increased after mulching. Soil MBN is readily decomposed, which can be directly transformed into ammonium and nitrate through mineralization (Turner et al. 2017). Our results also revealed that MBN was correlated with DN,ammonium, and nitrate. In addition, MBN was signif icantly correlated with soil physical properties (i.e., temperature,water content, and bulk density), which can be directly af fected by mulching. Increases in MBN and the MBN: TN ratio were detected (Fig. 3, Fig. S1 and Table S4 in Supplementary information). Organic mulch-derived N assimilated by microorganisms, to a certain extent, may have contributed to reducing N loss via mineral N leaching. Moreover, the pH of bulk soil was correlated with MBN, although not as strongly as temperature, water content, or BD; pH was also an indicator of small changes in our study. In this regard,it has been found that pH, owing to its prominent ef fects on soil microorganisms, is an important factor af fecting N mineralization and nitrif ication (Kader et al. 2013; Yao et al.2018), and thus to a certain extent, may have an indirect inf luence on N fractions.

Fig. 4 Nitrogen content in f ine roots, organic mulch and leaves over time after organic mulching (X axis). Error bars represent standard error of the mean (SE), values are means ± SE ( n = 5). Dif ferent letters represent statistical dif ferences among treatments at dif ferent soil depths and time after organic mulching according to Tukey’s test( P < 0.05)
The results of the RDA revealed strong correlations between the N fractions and soil physical properties (temperature, water content, and BD), whereas TN was af fected to a greater extent by soil elements (i.e., carbon and phosphorus), indicating that N fractions are more susceptible to environmental factors, whereas TN tends to be more stable.The initial increase observed for TN in the topsoil can be attributed to the direct addition of organic mulch. Total N showed a signif icant positive correlation with the soil N:P ratio (Fig. 5, Table 3), and the addition of organic much resulted in a higher N: P ratio (Table 1 and Table 2), which in turn increased TN. However, TN began to decrease at the end of the growing season (9 months after mulching), which could be ascribed to changes in the N fractions (Fig. 3) and the ratios of these fractions to TN (Fig. S1, Table S4 in Supplementary information) during the growing season. Given that ammonium increased continuously, whereas nitrate initially increased and subsequently decreased, a greater quantity of nitrate underwent gaseous conversion via denitrif ication or was leached into the soil water at the end of the growing season (Smolander et al. 2019), with the latter ef fect being enhanced by a signif icant increase in rainfall during the summer. Meanwhile, nitrate may also have been taken up more by plants after organic mulching in the growing season. However, we found that rhizosphere TN showed the opposite trend to that of bulk soil, indicating that larger quantities of potentially labile N fractions were stimulated in the rhizosphere in response to organic mulching, which may be associated with N cycling, and that TN accumulated during the non-growing season (12 months after mulching).
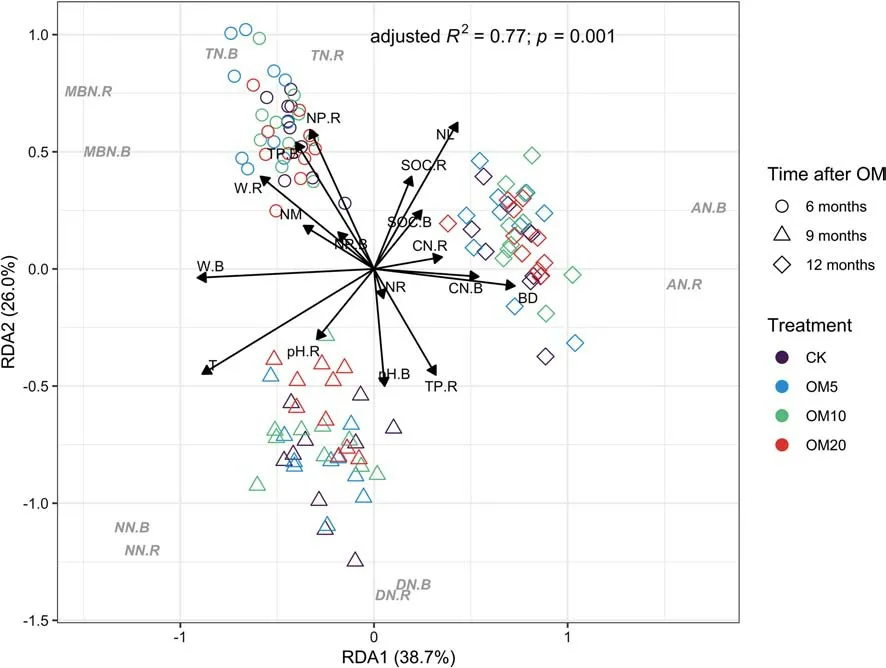
Fig. 5 Redundancy analysis (RDA) ordination plot of nitrogen fractions constrained by environmental variables that signif icantly explain variation. TN total nitrogen, DN dissolved nitrogen, AN ammonium,NN nitrate, MBN microbial biomass nitrogen, BD bulk density, T temperature, W water content, SOC soil organic carbon, CN ratio of carbon to nitrogen, TP total phosphorus, NP ratio of nitrogen to phosphorus, NR nitrogen content in f ine roots, NL nitrogen content in leaves, NM nitrogen content in organic mulch, B in bulk soil, R in rhizosphere, CK control (no mulch), OM5 5 cm (35 kg) mulch, OM10 10 cm (70 kg) mulch, OM20 20 cm (140 kg) mulch, OM organic mulching
Organic mulching has the effect of stimulating the available N in soil, and in response to this treatment, a greater quantity of available N is assimilated into the soil organic N pool by microbial activity (Mooshammer et al. 2014), given that DN in the topsoil increases during the early growth stage, whereas there is a corresponding decrease in the DN: TN ratio (Fig. S1, Table S4 in Supplementary information). The formation of DN is dependent on the rate of exchangeable N decomposition(Kalbitz et al. 2000), and in the present study, soil DN and ammonium increased at the beginning of the growing season (6 months after mulching), thereby indicating that the addition of organic mulch provides a greater quantity of available N for the soil. This may have the ef fect of enhancing nutrient exchange and increasing the generation of nitrate via nitrif ication. Dissolved nitrogen content is mainly related to TP, and the addition of organic mulch is more conducive to the promotion of N mineralization in the rhizosphere when levels of soil phosphorus are low(Chen et al. 2016a). DN is also generally correlated with pH, and given that the pH of our plot soils tended to be neutral (Table 1), we suspect that this provided conditions conducive to microbial activity and N transformation, thereby favouring an increase in soil DN (Cheng et al. 2013). A substantial quantity of DN was utilized during the growing season and large amounts of nitrate were absorbed by the plants. Meanwhile, the DN: TN ratio in the subsoil increased after mulching (Fig. S1, Table S4 in Supplementary information), which is assumed can be ascribed to an increase in rainfall during the early period of the growing season. This resulted in a large quantity of available N leaching to the deeper soil layer (DN in the subsoil increased signif icantly after 9 months mulching; Fig. 3), thereby contributing to an increase in the N content of f ine roots in the subsoil (DN correlated positively with f ine root N content; Fig. 5).
On the basis of our results, it is speculated that organic mulching initially improves the soil physical environment and provides a direct source of available N which is assimilated into the soil N pool via microbial activity, resulting in increases in TN and N fractions. With time, copious rainfall during the growing season promoted the availability of TN in the topsoil, whereas a certain portion of the N fractions leached into deeper soil, and larger amounts of nitrate underwent gaseous conversion via denitrif ication. Although plants absorb large quantities of nutrients from the soil, the distribution of absorbed N across increasing plant biomass results in decreasing levels in both leaves and topsoil f ine roots. Finally, during the dormant season, there were reductions in TN and DN, which could be attributed to the dry and cold conditions during winter, whereas rhizosphere TN and ammonium increased due to root outputs and direct changes in nutrient accumulation by plants. A large amount of organic mulch could improve the immobilization of inorganic N by microbes in the short term. In addition, the dif ferences in correlations between the nitrogen levels of leaves,f ine roots, and N fractions may be attributable to dif ferences in the N utilization of leaves and f ine roots (Ma et al. 2017).
Rhizosphere MBN are more sensitive to organic mulching
Microbial biomass nitrogen in the rhizosphere was more sensitive to mulching than bulk soil and more strongly af fected by soil environmental changes. The rhizosphere is directly inf luenced by plant roots, and its associated nutrient composition, soluble organic matter content, enzyme activity,and microbial diversity are richer than those of bulk soil.Thus, it is associated with more rapid nutrient cycling and energy conversion and also makes a substantial contribution to the distribution of N in soil (Kuzyakov and Xu 2013). The results of the RDA indicated a better correlation between N content in f ine roots, leaves, organic mulch, and most N fractions in the rhizosphere compared with those of bulk soil. Previous studies have consistently demonstrated a more prominent ef fect of fertilization on the rhizosphere than on bulk soil, thereby indicating that rhizosphere secretions play an important role in this process (Ai et al. 2012). Biochemical parameters in the rhizosphere explain better the changes in soil respiration and its components after nutrient addition,and compared with the bulk soil environment, available N in the rhizosphere is more closely correlated with soil respiration and its components (Liang et al. 2019). However, a limitation of this study is that neither root secretion nor soil respiration was examined to further understand the mechanisms of N transformation between plants and soil.
Time and soil depth are important factors af fecting soil N fractions
The length of time after mulching (season) and soil depth were found to have a signif icant ef fect on N fractions studied,as well as soil physical and chemical properties (Table S3 in Supplementary information), and these two factors were more important than organic mulching itself in af fecting the soil environment. Liu et al. ( 2018) suggested that soil N levels and cycling are more sensitive to temporal variations,while Wang et al. ( 2016) indicated that soil depth, rather than the sampling time or system of fertilization, has a more pronounced ef fect on N f ixation. Our observations indicated that time makes a greater contribution to the N fractions than soil depth, whereas soil depth has a more prominent ef fect on total nitrogen than time. This also demonstrates that the labile N fractions are more af fected by temporal variations that control soil moisture and temperature, whereas TN is more af fected by accumulation processes regulated by soil nutrients, as discussed above. Furthermore, the results of these N fraction changes could be dif ferent as the time after mulching increases. For example, Kitou and Yoshida ( 1994)showed that mulching with plant residues increased N f ixation in the early growth stage of soybean (Glycine max.L.)and improved soil fertility at later stages. Soil total organic N at a 0–20 cm depth increased with increasing time after gravel-sand mulching up until 11 years, and then decreased signif icantly between 11 and 16 years (Qiu et al. 2015). As this study only took samples in one year, this could be a limitation. However, interactions among time, soil depth,and treatment appeared to have no signif icant ef fects on most N fractions, presumably due to the complexities of the soil environment wherein they interact. Accordingly, more dynamic and long-term studies on the ef fects of organic mulching on the soil environment in forest ecosystems should be done to gain a better understanding of the interaction mechanisms.
Conclusions
Our results suggest that microbial biomass nitrogen is more sensitive than other nitrogen fractions to organic mulching,and MBN in rhizosphere was more sensitive than in bulk soil, and strongly af fected by changes in the soil environmental. Total nitrogen and dissolved nitrogen were more susceptible to soil phosphorus limitations, whereas ammonium, nitrate, and MBN were strongly af fected by physical factors of the soil environment such as temperature, water content, and bulk density. Time after mulching (season) and soil depth are important factors that need to be considered in research relating to soil nitrogen dynamics. Small quantities of organic mulch contribute to nitrogen decomposition and utilization, whereas a large amount of organic mulch improves nitrogen immobilization.
AcknowledgementsWe gratefully acknowledge the administration of the Dr. Sun Yat-sen Mausoleum for providing the experimental area and for the labour support at the site.
Authors’ contributionsQ Guan and X Sun conceived and designed the experiments, DL Jones developed methodology. X Sun and G Wang performed the experiments. X Sun and Y Ye analysed the data. X Sun wrote the manuscript; Q Ma provided editorial advice.
Open AccessThis article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing,adaptation, distribution and reproduction in any medium or format,as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. References
Ai C, Liang G, Sun J, Wang X, Zhou W (2012) Responses of extracellular enzyme activities and microbial community in both the rhizosphere and bulk soil to long-term fertilization practices in a f luvo-aquic soil. Geoderma. https:// doi. org/ 10. 1016/j. geode rma.2011. 07. 020
Bates D, Mächler M, Bolker BM, Walker SC (2015) Fitting linear mixed-ef fects models using lme4. J Stat Softw 1406(1):133–199.https:// doi. org/ 10. 18637/ jss. v067. i01
Cao Y, Sun H, Zhang J, Chen G, Zhu H, Zhou S, Xiao H (2018) Ef fects of wheat straw addition on dynamics and fate of nitrogen applied to paddy soils. Soil Tillage Res. https:// doi. org/ 10. 1016/j. still.2017. 12. 023
Chen Y, Sun TT, Qian HY, Fan JB, He YQ, Sun B (2016a) Nitrogen mineralization as a result of phosphorus supplementation in longterm phosphate def icient soil. Appl Soil Ecol. https:// doi. org/ 10.1016/j. apsoil. 2016. 04. 019
Chen YL, Ding JZ, Peng YF, Li F, Yang GB, Liu L, Qin SQ, FangK YYH (2016b) Patterns and drivers of soil microbial communities in Tibetan alpine and global terrestrial ecosystems. J Biogeogr 43(10):1–13. https:// doi. org/ 10. 1111/ jbi. 12806
Chen YM, Xu X, Jiao XG, Sui YY, Liu XB, Zhang JY, Zhou K, Zhang JM (2018) Responses of labile organic nitrogen fractions and enzyme activities in eroded mollisols after 8-year manure amendment. Sci Rep. https:// doi. org/ 10. 1038/ s41598- 018- 32649-y
Cheng Y, Wang J, Mary B, Zhang JB, Cai ZC, Chang SX (2013) Soil pH has contrasting ef fects on gross and net nitrogen mineralizations in adjacent forest and grassland soils in central Alberta,Canada. Soil Biol Biochem 57:848–857. https:// doi. org/ 10.1 016/j.soilb io. 2012. 08. 021
Coppens F, Garnier P, De Gryze S, Merckx R, Recous S (2006) Soil moisture, carbon and nitrogen dynamics following incorporation and surface application of labelled crop residues in soil columns.Eur J Soil Sci 57(6):894–905. https:// doi. org/ 10. 1111/j. 1365-2389. 2006. 00783.x
Dietrich G, Recous S, Pinheiro PL, Weiler DA, Schu AL, Rambo MRL, Giacomini SJ (2019) Gradient of decomposition in sugarcane mulches of various thicknesses. Soil Tillage Res 192:66–75.https:// doi. org/ 10. 1016/j. still. 2019. 04. 022
Fu X, Wang J, Sainju UM, Liu W (2019) Aggregate size distribution and associated carbon and nitrogen in mulched winter wheat and spring corn. Can J Soil Sci 99(4):1–13. https:// doi. org/ 10. 1139/cjss- 2019- 0015
Gorska A, Ye Q, Holbrook NM, Zwieniecki MA (2008) Nitrate control of root hydraulic properties in plants: translating local information to whole plant response. Plant Physiol 148(2):1159–1167. https://doi. org/ 10. 1104/ pp. 108. 122499
Guignard MS, Leitch AR, Acquisti C, Eizaguirre C, Elser JJ, Hessen DO, Jeyasingh PD, Neiman M, Richardson AE, Soltis PS, Soltis DE, Stevens CJ, Trimmer M, Weider LJ, Woodward G, Leitch IJ (2017) Impacts of nitrogen and phosphorus: From genomes to natural ecosystems and agriculture. Front Ecol Evol 5(5):70.https:// doi. org/ 10. 3389/ fevo. 2017. 00070
Guo S, Brück H, Sattelmacher B (2002) Ef fects of supplied nitrogen form on growth and water uptake of French bean (Phaseolus vulgarisL.) plants: nitrogen form and water uptake. Plant Soil 239(2):267–275. https:// doi. org/ 10. 1023/A: 10150 14417 018
Huang Z, Wan X, He Z, Yu Z, Wang M, Hu Z, Yang Y (2013) Soil microbial biomass, community composition and soil nitrogen cycling in relation to tree species in subtropical China. Soil Biol Biochem 62:68–75. https://d oi.o rg/ 10. 1016/j.s oilb io. 2013. 03. 008
Jiménez MN, Pinto JR, Ripoll MA, Sánchez-Miranda A, Navarro FB(2017) Impact of straw and rock-fragment mulches on soil moisture and early growth of holm oaks in a semiarid area. CATENA 152:198–206. https:// doi. org/ 10. 1016/j. catena. 2017. 01. 021
Kader MA, Sleutel S, Begum SA, Moslehuddin AZM, De neve S(2013) Nitrogen mineralization in sub-tropical paddy soils in relation to soil mineralogy, management, pH, carbon, nitrogen and iron contents. Eur J Soil Sci 64(1):47–57. https:// doi. org/ 10.1111/ ejss. 12005
Kader MA, Senge M, Mojid MA, Ito K (2017) Recent advances in mulching materials and methods for modifying soil environment. Soil Tillage Res 168:155–166. https:// doi. org/ 10. 1016/j.still. 2017. 01. 001
Kaiser C, Fuchslueger L, Koranda M, Gorfer M, Stange CF, Kitzler B, Rasche F, Strauss J, Sessitsch A, Zechmeister-Boltenstern S, Richter A (2011) Plants control the seasonal dynamics of microbial N cycling in a beech forest soil by belowground C allocation. Ecology 92(5):1036–1051. https:// doi. org/ 10. 1890/10- 1011.1
Kalbitz K, Solinger S, Park J-H, Michalzik B, Matzner E (2000)Controls on the dynamics of dissolved organic matter in soils:a revies. Soil Sci 165(4):277–304. https:// doi. org/ 10. 1097/ 00010 694- 20000 4000- 00001
Kitou M, Yoshida S (1994) Mulching ef fect of plant residues on soybean growth and soil chemical properties. Soil Sci Plant Nutr 40(2):211–220. https:// doi. org/1 0. 1080/0 0380 768. 1994. 10413 295
Kuzyakov Y, Xu X (2013) Competition between roots and microorganisms for nitrogen: Mechanisms and ecological relevance. New Phytol 198(3):656–669. https:// doi. org/ 10. 1111/ npy. 12235
Liang G, Cai A, Wu H, Houssou AA, Ren C, Wang Z, Gao L, Wang B, Li S, Song X, Cai D (2019) Soil biochemical parameters in the rhizosphere contribute more to changes in soil respiration and its components than those in the bulk soil under nitrogen application in croplands. Plant Soil 435:111–125. https:// doi.o rg/ 10. 1007/s11104- 018- 3886-0
Liu D, Huang Y, Yan H, Jiang Y, Zhao T, An S (2018) Dynamics of soil nitrogen fractions and their relationship with soil microbial communities in two forest species of northern China. PLoS ONE 13(5):e0196567. https:// doi. org/ 10. 1371/ journ al. pone. 01965 67
Lucas RW, Klaminder J, Futter MN, Bishop KH, Egnell G, Laudon H,Högberg P (2011) A meta-analysis of the ef fects of nitrogen additions on base cations: implications for plants, soils, and streams.For Ecol Manag 262(2):95–104. https:// doi. org/ 10. 1016/j. foreco.2011. 03. 018
Ma Q, Cao X, Xie Y, Xiao H, Tan X, Wu L (2017) Ef fects of glucose on the uptake and metabolism of glycine in pakchoi (Brassica chinensisL.) exposed to various nitrogen sources. BMC Plant Biol 17(5968):1008–1010. https:// doi. org/ 10. 1186/ s12870- 017- 1006-6
Meier CL, Bowman WD (2008) Links between plant litter chemistry,species diversity, and below-ground ecosystem function. Proc Natl Acad Sci 105(50):19780–19785. https:// doi. org/ 10. 1073/pnas. 08056 00105
Meier IC, Finzi AC, Phillips RP (2017) Root exudates increase N availability by stimulating microbial turnover of fast-cycling N pools.Soil Biol Biochem 106:119–128. https:// doi. org/ 10. 1016/j. soilb io. 2016. 12. 004
Mikutta R, Turner S, Schippers A, Gentsch N, Meyer-Stüve S, Condron LM, Peltzer DA, Richardson SJ, Eger A, Hempel G, Kaiser K,Klotzbücher T, Guggenberger G (2019) Microbial and abiotic controls on mineral-associated organic matter in soil prof lies along an ecosystem gradient. Sci Rep 9(1):20194. https:// doi. org/ 10. 1038/s41598- 019- 46501-4
Mooshammer M, Wanek W, Hämmerle I, Fuchslueger L, Hofhansl F,Knoltsch A, Schnecker J, Takriti M, Watzka M, Wild B, Keiblinger KM, Zechmeister-Boltenstern S, Richter A (2014) Adjustment of microbial nitrogen use ef ficiency to carbon: nitrogen imbalances regulates soil nitrogen cycling. Nat Commun 5:3694.https:// doi. org/ 10. 1038/ ncomm s4694
Oksanen J, Blanchet FG, Friendly M, Kindt R, Legendre P, Mcglinn D, Minchin PR, O’hara RB, Simpson GL, Solymos P, Henry M,Stevens H, Szoecs E, Maintainer HW (2019) vegan: Community Ecology Package. R package version 2.5–5. https:// CRAN.R- proje ct. org/ packa ge= vegan. Community Ecol Packag
Pal PK, Mahajan M (2017) Tillage system and organic mulch inf luence leaf biomass, steviol glycoside yield and soil health under subtemperate conditions. Ind Crops Prod 104:33–44. https:// doi. org/10. 1016/j. indcr op. 2017. 04. 012
Pastor J, Post WM (1986) Inf luence of climate, soil moisture, and succession on forest carbon and nitrogen cycles. Biogeochemistry 2(1):3–27. https:// doi. org/ 10. 1007/ BF021 86962
Qiu Y, Xie ZK, Wang YJ, Malhi SS, Ren JL (2015) Long-term ef fects of gravel—sand mulch on soil organic carbon and nitrogen in the Loess Plateau of northwestern China. J Arid Land 1:46–53.https:// doi. org/ 10. 1007/ s40333- 014- 0076-7
R Development Core Team (2018) R: A language and environment for statistical computing. Vienna, Austria. ISBN 3-900051-07-0.http:// www.R- proje ct. org.
Ren C, Sun P, Kang D, Zhao F, Feng Y, Ren G, Han X, Yang G (2016)Responsiveness of soil nitrogen fractions and bacterial communities to af forestation in the Loess Hilly Region (LHR) of China. Sci Rep 6:28469. https:// doi. org/ 10. 1038/ srep2 8469
Rose TJ, Keen B, Morris SG, Quin P, Rust J, Kearney L, Kimber S, Van Zwieten L (2016) Application of woody biochar and woody mulch to mitigate nitrous oxide emissions from a poultry litter-amended soil in the subtropics. Agric Ecosyst Environ 228:1–8. https:// doi.org/ 10. 1016/j. agee. 2016. 05. 004
Smolander A, Törmänen T, Kitunen V, Lindroos AJ (2019) Dynamics of soil nitrogen cycling and losses under Norway spruce logging residues on a clear-cut. For Ecol Manage 449:117444. https:// doi.org/ 10. 1016/j. foreco. 2019. 06. 041
Templer P, Findlay S, Lovett G (2003) Soil microbial biomass and nitrogen transformations among f ive tree species of the Catskill Mountains, New York, USA. Soil Biol Biochem 35(4):607–613.https:// doi. org/ 10. 1016/ S0038- 0717(03) 00006-3
Turner S, Meyer-Stüve S, Schippers A, Guggenberger G, Schaarschmidt F, Wild B, Richter A, Dohrmann R, Mikutta R (2017)Microbial utilization of mineral-associated nitrogen in soils. Soil Biol Biochem 104:185–196. https:// doi. org/ 10. 1016/j. soilb io.2016. 10. 010
Usowicz B, Lipiec J (2017) Spatial variability of soil properties and cereal yield in a cultivated f ield on sandy soil. Soil Tillage Res 174:241–250. https:// doi. org/ 10. 1016/j. still. 2017. 07. 015
Valenzuela-Solano C, Crohn DM (2006) Are decomposition and N release from organic mulches determined mainly by their chemical composition? Soil Biol Biochem 38(2):377–384. https:// doi. org/10. 1016/j. soilb io. 2005. 06. 002
Vitousek PM, Aber JD, Howarth RW, Likens GE, Matson PA, Schindler DW, Schlesinger WH, Tilman DG (1997) Human alteration of the global nitrogen cycle: Sources and consequences. Ecol Appl 7(3):737–750. https://d oi.o rg/1 0. 1890/ 1051- 0761(1997) 007[0737:HAOTGN] 2.0. CO;2
Wang J, Zhang D, Zhang L, Li J, Raza W, Huang Q, Shen Q (2016)Temporal variation of diazotrophic community abundance and structure in surface and subsoil under four fertilization regimes during a wheat growing season. Agric Ecosyst Environ 216:116–124. https:// doi. org/ 10. 1016/j. agee. 2015. 09. 039
Wang F, Wang J, Wang Y (2019) Using multi-fractal and joint multifractal methods to characterize spatial variability of reconstructed soil properties in an opencast coal-mine dump in the Loess area of China. CATENA 182:104111. https:// doi. org/ 10. 1016/j. catena.2019. 104111
Wild B, Schnecker J, Knoltsch A, Mooshammer M, Gentsch N,Mikutta R, Alves RJE, Gittel A, Lashchinskiy N, Richter A (2015)Microbial nitrogen dynamics in organic and mineral soil horizons along a latitudinal transect in western Siberia. Global Biogeochem Cycles 29(5):567–582. https:// doi. org/ 10. 1002/ 2015G B0050 84
Yao X, Zhang N, Zeng H, Wang W (2018) Ef fects of soil depth and plant–soil interaction on microbial community in temperate grasslands of northern China. Sci Total Environ 630:96–102. https://doi. org/ 10. 1016/j. scito tenv. 2018. 02. 155
Yokobe T, Hyodo F, Tokuchi N (2018) Seasonal ef fects on microbial community structure and nitrogen dynamics in temperate forest soil. Forests 52(3):470–479. https:// doi. org/ 10. 3390/ f9030 153
Zhang H, Pang H, Zhao Y, Lu C, Liu N, Zhang X, Li Y (2020) Water and salt exchange f lux and mechanism in a dry saline soil amended with buried straw of varying thicknesses. Geoderma 365:114213.https:// doi. org/ 10. 1016/j. geode rma. 2020. 114213
Zhou ZH, Wang CK (2015) Soil resources and climate jointly drive variations in microbial biomass carbon and nitrogen in China’s forest ecosystems. Biogeosciences Discuss 12(14):6751–6760.https:// doi. org/ 10. 5194/ bgd- 12- 11191- 2015
Zhou WJ, Sha LQ, Schaefer DA, Zhang YP, Song QH, Tan ZH, Deng Y, Deng XB, Guan HL (2015) Direct ef fects of litter decomposition on soil dissolved organic carbon and nitrogen in a tropical rainforest. Soil Biol Biochem 81:255–258. https:// doi. org/ 10.1016/j. soilb io. 2014. 11. 019
杂志排行
Journal of Forestry Research的其它文章
- Genome-wide identif ication and cold stress-induced expression analysis of the CBF gene family in Liriodendron chinense
- Decay rate of Larix gmelinii coarse woody debris on burned patches in the Greater Khingan Mountains
- Characterizing conservative and protective needs of the aridland forests of Sudan
- Point-cloud segmentation of individual trees in complex natural forest scenes based on a trunk-growth method
- Accuracy of common stem volume formulae using terrestrial photogrammetric point clouds: a case study with savanna trees in Benin
- Appropriate search techniques to estimate Weibull function parameters in a Pinus spp. plantation
