Intra- and inter-species variations in carbon content of 14 major tree species in Northeast China
2021-12-24FarisRafAlmayWidagdoFengriLiLongfeiXieLihuDong
Faris Raf i Almay Widagdo · Fengri Li · Longfei Xie ·Lihu Dong
Abstract Reducing greenhouse gas emissions is one of the major challenges in combating global warming. Carbon, including in the form of carbon dioxide (CO 2 ), is considered an essential greenhouse gas under human control to demonstrate success in emission reductions. However,many carbon stock quantif ications in forest ecosystems still rely on the estimated 50% carbon content instead of more precise species-, tissue- and site-specif ic values. Thus, this study aimed to thoroughly measure and analyze the carbon content and variability using the 14 major tree species in Northeast China. Over 600 trees were destructively sampled from three dif ferent major mountainous regions (i.e., the Changbai, Daxing’an, and Xiaoxing’an mountains), and the carbon contents of each species were precisely measured to the sub-tissue level. Carbon contents varied signif icantly between species, with foliage carbon mostly found to be the highest, while root carbon contents were the lowest.Average carbon contents can be ranked as: Ulmus laciniata(43.4%) < Phellodendron amurense (43.5%) < Acer mono(43.8%) < Tilia amurensis (44.2%) < Populus davidiana(44.5%) < Fraxinus mandshurica (44.7%) < Juglans mandshurica (44.9%) < Quercus mongolica (45.3%) < Betulla davurica (45.8%) < Betulla platyphylla (46.7%) < Picea koreansis (46.9%) < Larix gmelinii (47.4%) < Pinus koreansis (48.3%) < Abies nephrolepis (48.3%). Carbon contents were higher in conifers (47.7%) compared to broadleaf species (44.9%). In addition, both tree tissues and growing sites also had a signif icant ef fect on carbon content. At the subtissue level, only stem’s sub-tissues (i.e., bark, heartwood,and sapwood) carbon contents showed signif icant variations.The results suggest that bark should be separated from other stem sub-tissues and considered separately when determining carbon stocks. This research contributes to improving estimates of terrestrial carbon quantif ications, and in particular, the values obtained can be used in China’s National Forest Inventory.
Keywords Carbon concentration · Carbon sequestration ·Hardwoods · Conifers · Temperate forest
Introduction
Increasing atmospheric carbon dioxide (CO2) concentrations has a relatively high contribution to global warming(IPCC 2014). Forest ecosystems have the ability to reduce atmospheric CO2by sequestering carbon in biomass which plays a crucial role in stabilizing the global climate system(Dixon et al. 1994). The United Nations Food and Agricultural Organization (FAO) has estimated that global forests store approximately 296 Gt of carbon in aboveground and belowground biomass, comprising nearly half of a forest’s total carbon stock (FAO 2015). A forest’s capacity to sequester carbon is greater compared with other biomes since the carbon stored within woody tissues is well protected from respiratory release and decomposition (Keenan and Williams 2018), while carbon stored in nonwoody tissues (i.e.,root hairs and foliage) has a more rapid turnover. Thus, forests have a signif icant role in the world’s terrestrial carbon sink (Keenan and Williams 2018), and their biomass has been identif ied by the Intergovernmental Panel on Climate Change (IPCC) as one of the most promising forms to lessen atmospheric CO2(IPCC 2007).
Carbon is among the most plentiful elements in living organisms (Dietze et al. 2014) and is converted from its gaseous form into biological compounds through photosynthesis, contributing to basic metabolic functions and structure of plants (Dietze et al. 2014; Martínez-Vilalta et al. 2016).The carbon stored from this process is determined by multiplying the plant’s dry weight with a carbon conversion factor, the most commonly used factor is 50% for almost all of carbon stock estimations, i.e., for local, regional and global carbon appraisals (Nizami 2012; Guerra-Santos et al. 2014;Tang et al. 2018). The 50% carbon conversion factor was originally determined from the average molecular formula,CH1.44 O0.66, consisting of basic constituents such as 50%carbon, 44% oxygen, 6% hydrogen, and trace quantities of metal ions in live woody tissues (Pettersen 1984; Bert and Danjon 2006; Ma et al. 2018). This particular value has long been recommended as a generic carbon conversion factor by the IPCC to simplify the measurement process and to foster carbon stock quantif ications worldwide (Houghton et al.1990). However, its accuracy is uncertain (Zhang et al. 2009;Jones and O’Hara 2012; Widagdo et al. 2020a).
To-date, various international programs and mechanisms, such as the Reducing Emissions from Deforestation and Forest Degradation (REDD) program under the United Nations Framework Convention on Climate Change (UNFCCC), have been established to f inancially reward projects and countries that contribute to preventing carbon loss from forests (Ebeling and Yasué 2008; Mukama et al. 2012). To support these types of activities, forest carbon stock has to be accurately estimated in order to quantify the carbon benef its and compensation payments (Vieilledent et al. 2012).Moreover, a number of studies have reported that carbon concentrations vary within taxonomic groups (i.e., angiosperms and conifers; Thomas and Martin 2012; Martin et al.2015; Ma et al. 2018), species (Pompa-García et al. 2017;Gillerot et al. 2018; Rodríguez-Soalleiro et al. 2018), origins (Elias and Potvin 2003; Widagdo et al. 2020b), provenances (Wang et al. 2015; Ying et al. 2019), growing sites(Ying et al. 2019; Widagdo et al. 2020a), tissues (Kim et al.2017; Zhou et al. 2019; Dong et al. 2020), and even stem’s sub-tissues such as bark, heartwood, and sapwood (Lamlom and Savidge 2006; Castaño-Santamaría and Bravo 2012;Gao et al. 2016). Thus, investigation of variability in carbon content needs to be continued in order to improve the precision of carbon stock appraisals.
At the global scale, temperate mixed forests are largely situated in eastern Asia, northern North America, and northeastern Europe. In China, they are located in the northeast and are crucial for the nation’s carbon budgeting and climatic system (Wang 2006; State Forestry and Grassland Administration 2019). There are 14 major tree species which are mainly found in these temperate forests, namely:Manchurian Elm (Ulmus laciniata),Amur cork (Phellodendron amurense),Mongolian oak (Quercus mongolica),
Manchurian walnut (Juglans mandshurica),Manchurian ash (Fraxinus mandshurica),Amur linden (Tilia amurensis),Maple (Acer mono),Dahurian poplar (Populus davidiana),Dahurian birch (Betula davurica),white birch (Betula platyphylla),Korean spruce (Picea koreansis),larch (Larix gmelinii),Korean pine (Pinus koreansis),and Khingan f ir(Abies nephrolepis). This study aimed to: (1) accurately measure carbon contents of the 14 species to the sub-tissue level; and, (2) analyze both inter- and intra-specif ic carbon content variabilities, one of the key steps in forest carbon assessment.
Materials and methods
Study site
The data were collected from several natural secondary forests within the three major mountainous regions in northeast China (Fig. 1), the Changbai Mountains (CBM), the Daxing’an Mountains (DXM), and the Xiaoxing’an Mountains (XXM). These areas have a continental monsoon climate, and based on the Chinese soil taxonomic system, the soil type is dark brown forest soil (Haplumbrepts or Eutroboralfs). According to the Köppen-Geiger climate classif ication system, these regions areDwa,Dwb, andDwc, indicating dry winters with hot, warm, or cold summers, respectively(Beck et al. 2018). The elevations of these three mountainous regions are highly varied from 300 to 1500 ma.s.l., as well as mean annual temperatures (−4 to 6 °C) and mean annual rainfalls (500–800 mm). In this study, the natural secondary forests were the result of cutovers, and left to be naturally regenerated (Yu et al. 2011). To date, the area of secondary forests in China are managed by the Provincial Forestry and Grassland Unit, which comes under the supervision of the National Forestry and Grassland Department. The dominant species found within these forests are aspen (P. davidianaDode.), Mongolian oak (Q. mongolica),white birch (Betula platyphylla), and Dahurian birch (Betula davurica).
Fig. 1 Distribution of sampling locations in northeast China.CBM Changbai Mountain, DXM Daxing’an Mountain, XXM Xiaoxing’an Mountain
Field sampling and sample preparation
Six hundred and three trees of the 14 most common broadleaf and conifer species were destructively sampled from 18 widespread sites. All had diameters at breast height (DBH)and tree total height (H) of 3.4–47.0 cm and 3.6–27.0 m,respectively. Only healthy trees with a straight bole without visual damage (i.e., major branch loss, fungal infections,or stem abrasions) were selected. In addition, each of the four primary tissues were divided into three sub-tissues in order to thoroughly analyze carbon concentration variability.Belowground biomass (roots) was separated based on its diameter (d); namely, RS—small (d< 2 cm), RM—medium(2
Basic inventory data were collected before felling, e.g.,DBH, H, and length and width of crowns. Once felled, the aboveground section was separated into stem and crown at the point of the f irst live branch. The stem was then partitioned into one-meter sections and a 2-cm thick disc was taken from each portion. The crown section was divided equally into lower, middle, and upper layers, and 3–5 fresh branches and foliage samples were taken from each layer.All samples were carefully chosen, extracting only lignif ied green branches as samples. Roots were excavated within a 3-m circle. Apart from f ine roots (<2 mm), approximately 100–200 g of each root class was sampled, weighed,recorded, and transferred to the laboratory. DBH, H and carbon concentrations for all samples are shown in Fig. 2.
Carbon concentration measurements

Fig. 2 Species-specif ic carbon concentrations for the 14 species;abbreviations on the left refer to species and numbers on the right indicate the total number of samples for each species. D DBH, H total height, ULC Ulmus laciniata, PAR Phellodendron amurense, AMN Acer mono, TAS Tilia amurensis, PDV Populus davidiana, FMS Fraxinus mandshurica, JMS Juglans mandshurica, QMC Quercus mongolica, BDV Betulla davurica, BPL Betulla platyphylla, PCK Picea koreansis, LGM Larix gmelinii, PKS Pinus koreansis, ANP Abies nephrolepis
All samples were oven dried at 80 °C to constant weight.Each of the ±5 mg f inely ground samples were put into a container for further weighing using an MS304S Mettler Toledo analytical balance (accuracy to 0.0001 g). Prior to measurements, all containers were washed with distilled water along with analytical grade acetone, vacuum desiccated overnight until dry (Lamlom and Savidge 2003).For determining carbon concentrations, each sample was burned at 1200 °C in a vial containing pure oxygen. The carbon concentration was then determined via a non-dispersion infrared ray (NDIR) analyzer (Multi N/C 3000 analyzer with 1500 Solids Module; Analytik Jena AG, Germany).The analyzer was calibrated and stabilized between runs using a 12% standard concentration of CaCO3(standard curve,r2> 99.99%), in which standards were included after every 15–20 samples to ensure that the readings were reliable. Volatile carbon was not measured by this method; thus,there might be a possible loss in the volatile carbon fraction during the measurements (Thomas and Malczewski 2007).
Statistical analysis
To assess the carbon content’s inter- and intra-species variability, PROC GLM was used with the statistical analysis software SAS version 9.3. The ef fect of seven variables on carbon concentration were determined; namely, DBH,H, growing regions (CBM, DXM, and XXM), taxonomic group (conifers and broadleaves), species, tissues and subtissues. Some variables are inherently nested within others,such as species within a taxonomic group and sub-tissues within a tissue. Thus, a partially nested analysis of variance(ANOVA) through a general linear model was used. The interaction of species and growing regions was intentionally excluded since not all species were present in all regions. To further analyze the ef fect of region and tree tissues on carbon contents within each species, a least signif icance dif ference(LSD) test was conducted.
Results
Overall variations in carbon concentrations
Apart from DBH and H, all variables (i.e., taxonomic group,species, region, and tissues) had signif icant variations in carbon contents (p< 0.05, Table 1). This indicates that, at least in our dataset, the dif ferences in DBH and H did not signif icantly af fect carbon contents. Average (±SE) carbon concentration across all taxonomic groups, species, regions,tissues, and sub-tissues was 45.7 ± 0.04%. A mean scatter diagram of the multiple comparison test (using the Tukey-Kramer adjustment method) was used to visualize the ef fect of the three growing regions (CBM, XXM, and DXM) and the four primary tissues—roots, stem, branches, and foliage,on carbon concentration values. The results show that both growing sites and tissue types signif icantly af fect carbon concentrations (Fig. 3).

Table 1 Ef fects on carbon contents across the 14 species
Inter-species variation in carbon concentration
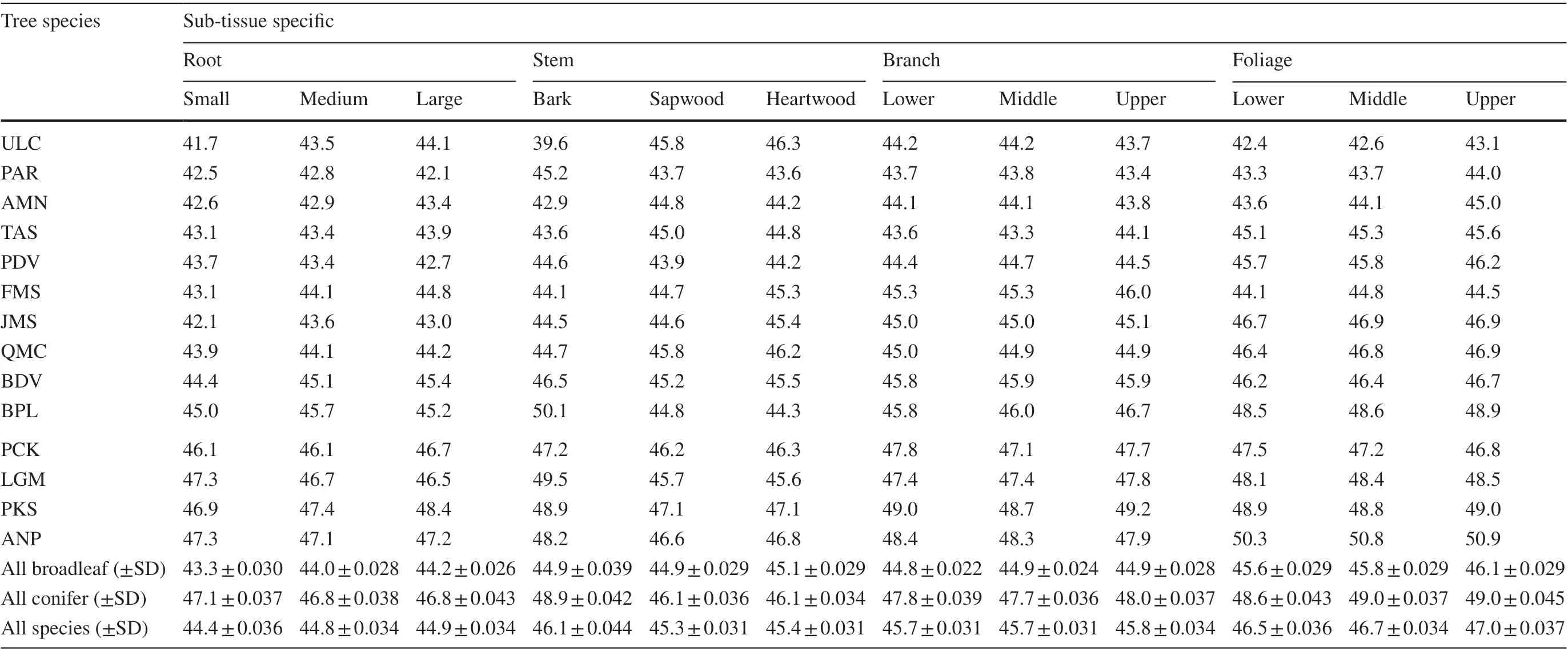
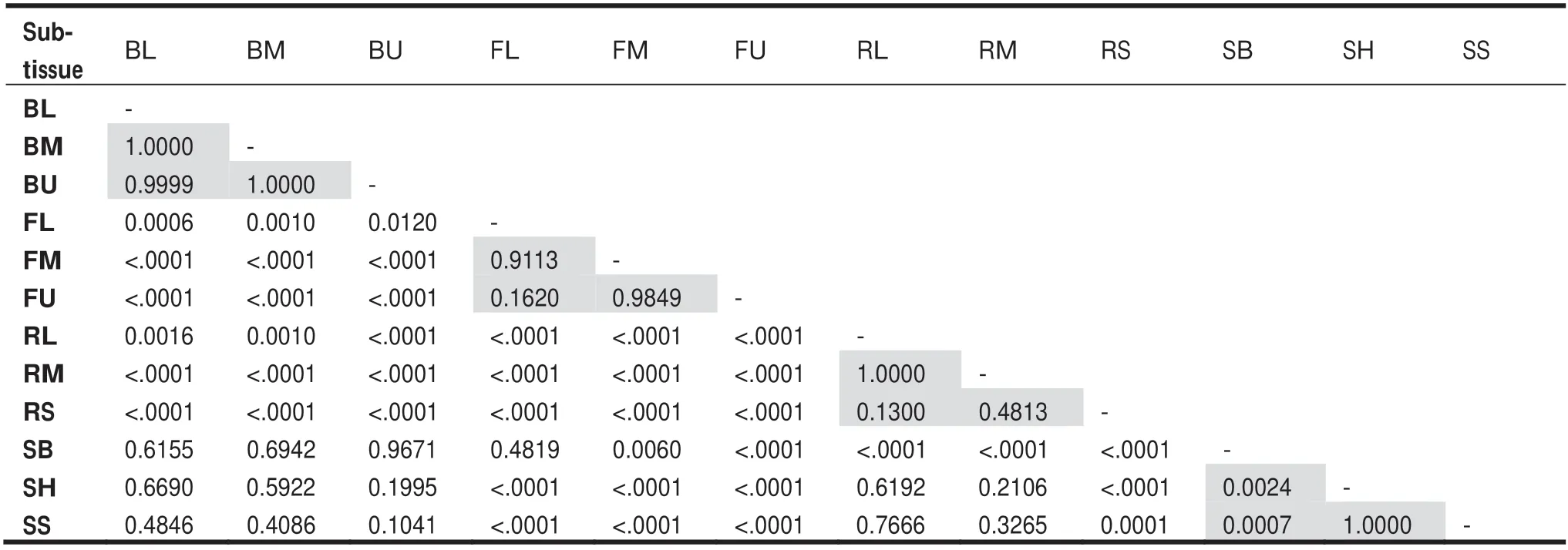
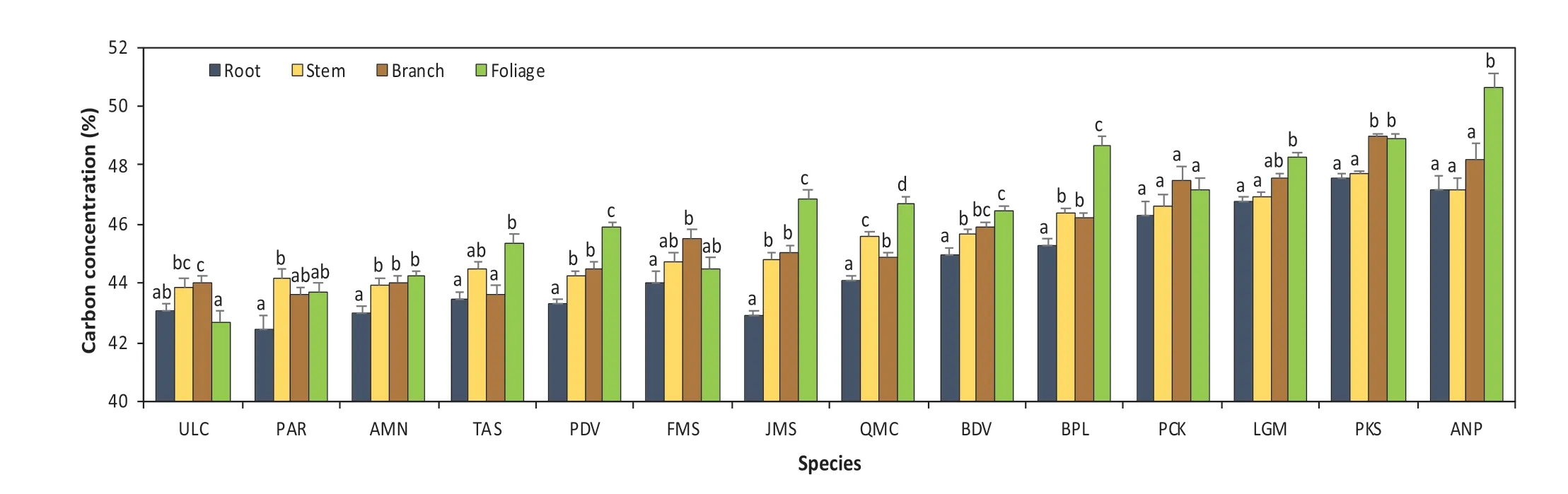
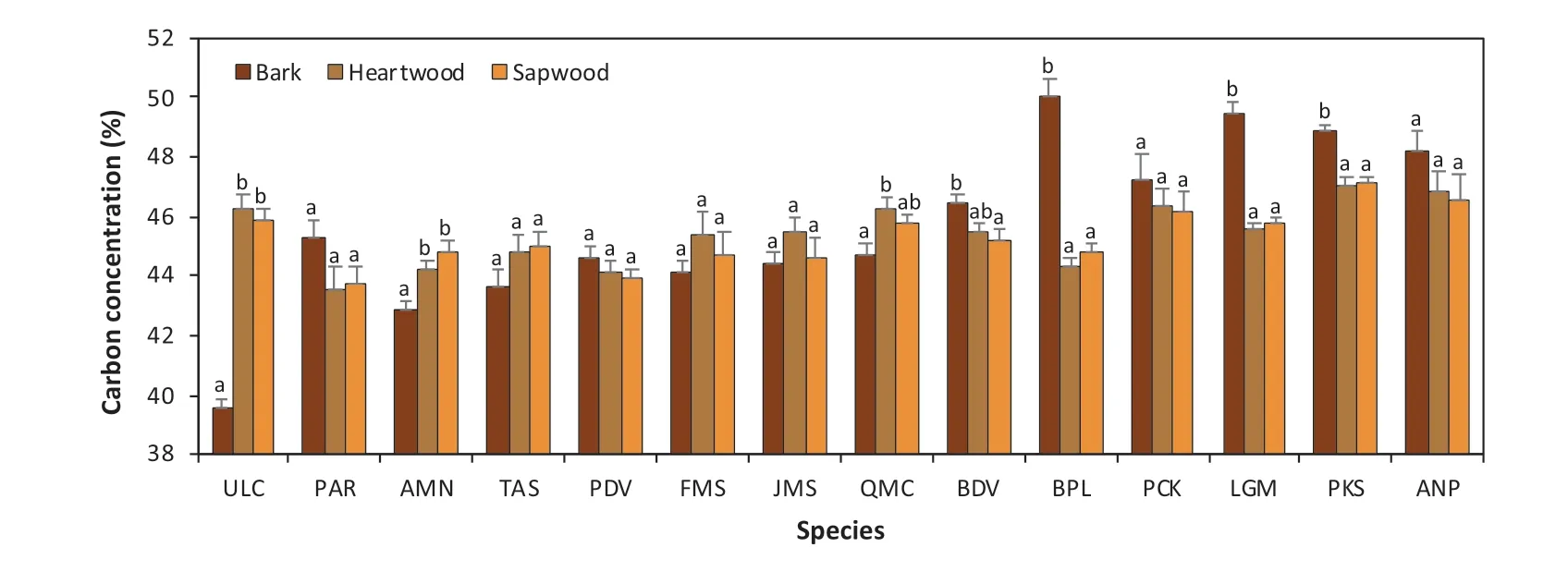
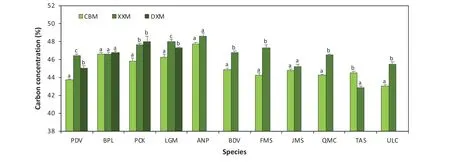
In this research, carbon concentrations of the 14 species were measured to the sub-tissue level. Carbon concentration values for each species over nine sub-tissues are shown in Table 2 and varied signif icantly across species and sub-tissues (Tables 1 and 2), ranging from 39.6%within the bark ofU. laciniatato 50.9% within the upper foliage ofA. nephrolepis. Among all species,U. laciniataandA. nephrolepisconsistently exhibited the lowest and highest average carbon concentration values over the nine sub-tissues at 43.4% and 48.3%, respectively. Mean carbon contents followedU. laciniata Fig. 3 Carbon concentration mean-mean scatter diagrams within the three growing sites and four primary tree tissues across the 14 species Carbon concentrations dif fered signif icantly between the biomass tissues across all species (F-values= 129.16,p< 0.0001). Carbon concentrations of all species are as follows: foliage (46.7%) > branch (45.7%) > stem (45.6%) > root(44.7%). Both foliage and roots consistently showed the highest and lowest carbon concentrations for all four conifers(48.9% and 46.9%) and all ten broadleaf (45.9% and 43.9%)species, respectively. Average carbon content values for all species varied from 42.5% for roots ofP. amurenseto 50.7%for the foliage ofA. nephrolepis. Apart fromU. laciniata,the lowest carbon concentrations were always found in the roots, ranging from 42.5% to 47.6% (Fig. 4). The tissue with the highest carbon concentrations varied according to the species, in which foliage generally had the highest carboncontent (9 out of 14 species, Fig. 4). The highest carbon concentrations forU. laciniata, F. mandshurica, P. koreansis,andP. koreansiswere in the branches, while forP. amurense,it was within the stem. However, for four of these f ive species (excludingP. koraensis), most of the tissues with the highest carbon concentrations only had a signif icant dif ference with the roots (Fig. 4).sapwood, or even both, for seven of the 14 species. Among these, bark had the lowest carbon concentrations forU.laciniata, A. mono,andQ. mongolica, whereas the highest was found inB. davurica, B. platyphylla, L. gmelinii,and Table 2 Carbon concentration based on specifi c sub-tissues for the 14 species. Dashed line separates the broadleaves and conifers Table 3 Mean comparison ( p value) among each sub-tissue, pooling all species; grey cells represent the sub-tissue mean comparisons within the same tissue Fig. 4 Comparisons of both species- and tissue-specif ic carbon concentrations for the 14 species; bars denote the standard error, letters represent the groups’ signif icant dif ference according to LSD test at 5% signif icance level. ULC Ulmus laciniata, PAR Phellodendron amurense, AMN Acer mono, TAS Tilia amurensis, PDV Populus davidiana, FMS Fraxinus mandshurica, JMS Juglans mandshurica,QMC Quercus mongolica, BDV Betulla davurica, BPL Betulla platyphylla, PCK Picea koreansis, LGM Larix gmelinii, PKS Pinus koreansis, ANP Abies nephrolepis Between the stem’s sub-tissues across all species, the average carbon content of the bark is the highest (46.1%),sapwood the lowest (45.3%), and heartwood slightly higher(45.4%). The dif ference in carbon contents between the stem sub-tissues varied with species (Fig. 5). The dif ference between heartwood and sapwood carbon concentrations was insignif icant for all species. Carbon contents of the bark were signif icantly dif ferent from either heartwood orP. koreansis(Fig. 5). There was no signif icant dif ference in carbon content of lower, middle, and upper canopy positions for both branches and foliage, as well as among small,medium, and large roots (Table 3). Fig. 5 Comparisons of the stem tissue carbon concentration for the 14 species; bars denote the standard error, letters represent the groups’ signif icant dif ference according to LSD test at 5% signif icance level. ULC Ulmus laciniata, PAR Phellodendron amurense,AMN Acer mono, TAS Tilia amurensis, PDV Populus davidiana, FMS Fraxinus mandshurica, JMS Juglans mandshurica, QMC Quercus mongolica, BDV Betulla davurica, BPL Betulla platyphylla, PCK Picea koreansis, LGM Larix gmelinii, PKS Pinus koreansis, ANP Abies nephrolepis Fig. 6 Comparisons of carbon concentrations on the three sites for the 14 species; bars denote standard error, letters represent the groups’ signif icant dif ference according to LSD test at 5% signif icance level. PDV Populus davidiana, BPL Betulla platyphylla, PCK Picea koreansis, LGM Larix gmelinii, ANP Abies nephrolepis, BDV Betulla davurica, FMS Fraxinus mandshurica, JMS Juglans mandshurica, QMC Quercus mongolica, TAS Tilia amurensis, ULC Ulmus laciniata The ef fects of the three sites on carbon concentrations were also analyzed for each species (Fig. 6). Four species were present on all three sites (CBM, DXM, and XXM),seven species on two sites (CBM and XXM), and the balance from one site (CBM). For eight of the 11 species sampled from either three or two sites, ANOVA revealed a signif icant dif ference between these regions (Fig. 6). Apart fromT. amurensis, all species sampled from the CBM range consistently had the lowest carbon concentrations, ranging from 43.8% forU. laciniatato 47.8% forA. nephrolepis.The highest carbon concentrations varied with species, in which eight of 11 species were from the XXM range (Fig. 6).B.platyphyllaandP. koreansiscarbon concentrations were highest in the DXM,whileT. amurensiscarbon was highest in the CBM.Average carbon content of all species from the XXM site is the highest (47.0%) along with the DXM(47.0%), and the lowest in the CBM site (44.7%). Chemical traits of both foliage and roots have received considerable attention in comparative studies of ecosystem function and ecology (Reich et al. 1997; Wright et al.2004; Tsunoda and van Dam 2017). However, many measurements of carbon contents are only on stems or boles(Martin and Thomas 2011; Castaño-Santamaría and Bravo 2012; Azeem et al. 2019). This is perhaps because woody stems account for the largest biomass component in a tree(Laiho and Laine 1997) and net primary production at the stand level (Gower et al. 2001; Zhang et al. 2009). Hence,tree stems are regarded as a major carbon sinks contributing to mitigating the ef fects of climate change. Our results show that the average stem carbon concentrations of the 14 species (45.6%) was less than the 50% generic conversion factor. Even after calculating average stem carbon contents based on taxonomic group (47.0% for conifers and 45.0%for broadleaf species), the values are still below the widely used carbon conversion factor. It is worth noting that the oven-dried method adopted in this study might result in a loss in the volatile carbon fraction, possibly contributing to underestimating carbon contents. Previous studies show that volatile losses vary among tree species and oven temperatures. Lamlom and Savidge ( 2003), Thomas and Malczewski( 2007), and Martin and Thomas ( 2011) demonstrated that volatile carbon loses were ~ 2.0%, ~2.2% and ~ 2.5% when samples were oven-dried at 93 °C, 105 °C, and 110 °C,respectively, for 14 temperate species in China, 41 temperate species in Canada, and 59 tropical species in Panama.If the carbon losses occur linearly with oven temperature,the carbon contents obtained in this study would have been 1 or 2% higher if the kiln- or freeze-dried method was used instead (oven temperature in this study was 80 °C). However,a direct cross-study comparison is dif ficult and unreliable due to discrepancies in species, methods, sample treatments,and laboratory equipment. All samples were oven-dried to the same 80 °C temperature, and thus volatile losses could be considered as systematic and common to all data. Several studies have reported that the largest biomass(≈60%) within an individual tree are found in the stems (Zhu et al. 2013; Ozdemir et al. 2019; Widagdo et al. 2020b).However, to precisely measure the carbon stock of an individual tree, a measure of each tree tissue’s carbon content is needed since type of tissue signif icantly af fects carbon concentrations (Table 1, Figs. 3 and 4). This result also corroborates the work of Rodríguez-Soalleiro et al. ( 2018),Zhou et al. ( 2019), and Dong et al. ( 2020). In this study,root carbon concentrations across all species (44.7%) was always the lowest compared to other tree tissues, while foliage had the highest carbon concentrations (46.7%), in line with previous studies (Zhang et al. 2009; Ma et al. 2018).Variations between sub-tissues were also analyzed. The stem’s sub-tissues (bark, heartwood, and sapwood) carbon contents varied signif icantly, while dif ferences between root’s, branch’s, and foliage’s sub-tissues were insignif icant(Table 3). Research by Bert and Danjon ( 2006) has shown that some changes in carbon concentration were related with the size of the tree tissue or its position within the tree. However, dif ferent results were obtained in this study; diameter and height did not have any signif icant ef fect on the carbon concentrations. Moreover, Table 3 shows that root size and both branch and foliage position within the crown did not have any signif icant ef fect on carbon contents. These discrepancies might be related to environmental variations and experimental dif ferences. Tree bark has long been recognized to have chemical divergences from other tree tissues (Srivastava 1964; Vidensek et al. 1990). In spite of several general chemical constituents (polyphenols, fats, sterols, hemicellulose, cellulose), bark has also developed unique polymeric materials (Bert and Danjon 2006) and contains half of the cellulose of the trunk (Labosky 1979; Vázquez et al. 1987). In this study, bark carbon concentrations were always higher than that of the heartwood and sapwood for all conifers.Among the 10 broadleaf species, bark carbon concentrations of six species (U. laciniata, Q. monglica, J. mandshurica, F. mandshurica, T. amurensis, and A. mono) were the lowest compared to the other two sub-tissues. This is consistent with the literature which showed that greater carbon contents in the bark might be caused by higher levels of tannins, suberin, lignin, and extractives (Hergert 1960; Porter 1974; Bert and Danjon 2006), particularly for conifer species (Srivastava 1964; Vidensek et al. 1990;Nemli et al. 2006). From an ecological perspective, this pattern is related with bark functions in controlling water def iciency and protecting the tree from f ire (Hengst and Dawson 1994) and insects (Franceschi et al. 2005). The current study also noted that the dif ference between carbon contents of the heartwood and sapwood were insignif icant for all species (Fig. 5). In the present research, average carbon concentrations of the four conifers were higher than those of the 10 broadleaf species, both on average (47.7% cf. 44.9%) and separately across the roots (46.9% cf. 43.8%), stems (47.0% cf.45.0%), branches (47.8% cf. 44.9%), and foliage (48.9%cf. 45.8%). The reasons for this is because conifers have approximately 10% higher lignin content than broadleaf species (Savidge 2000). Of all the macromolecules within a tree’s woody tissues, lignin has the highest carbon percent (Savidge 2000; Lamlom and Savidge 2003). We also found that the carbon concentrations varied across the species and the growing regions, as conf irmed by a number of studies (Elias and Potvin 2003; Azeem et al. 2019; Ying et al. 2019). Kozlowski ( 1992) reported that individual trees with dif ferent growth and metabolism characteristics had various dif ferences in carbon compounds; hence,intra- and inter-specif ic variations in carbon concentrations would be inf luenced by silvicultural practices, stand characteristics (i.e., age of tree and position within the crown), and growing conditions. Using species-, tissue-,and site-specif ic carbon content instead of the common 50% conversion factor will provide more accurate results on terrestrial carbon stock estimations, as has been demonstrated by previous studies (Zhang et al. 2009; Martin and Thomas 2011; Dong et al. 2016; Widagdo et al. 2020a).Thus, the continuity of research on carbon contents of species, tissues of species (stem, branches, roots, foliage),and regional-specif ic carbon contents needs to continue in order to increase the accuracy of carbon stock estimations. The data obtained in this research are useful for accurate carbon stock estimates of the following species,particularly for those in northeastern China:U. laciniata,P. amurense, Q. mongolica, J. mandshurica, F. mandshurica, T. amurensis, A. mono, P. davidiana, B. davurica, B.platyphylla, P. koreansis, L. gmelinii, P. koreansis,andA.nephrolepis. This research highlights the importance of considering intra- and inter-species variation in carbon contents,which has broad impacts for increasing the accuracy of global carbon quantif ications. Carbon contents varied signif icantly across the growing regions, taxonomic groups,species, and tissues. Both DBH and tree total height did not have any ef fect on carbon contents. Among the nine sub-tissues analyzed, carbon contents of the stem’s subtissues (bark, heartwood, and sapwood) dif fered signif icantly, while carbon contents of the root’s, branch’s, and foliage’s sub-tissues were insignif icant. Based on these results, it is recommended that bark should be separated from heartwood and sapwood and considered separately when measuring carbon stock of an individual tree. More attention is required to improve the estimates of forest carbon inventories. AcknowledgementsThe researchers thank the faculty and students of the Department of Forest Management, Northeast Forestry University (NEFU), P. R. China, who provided and collected the data for this study. We also highly appreciate the help of Nathan J. Roberts (NEFU)for f inal polishing of the English text.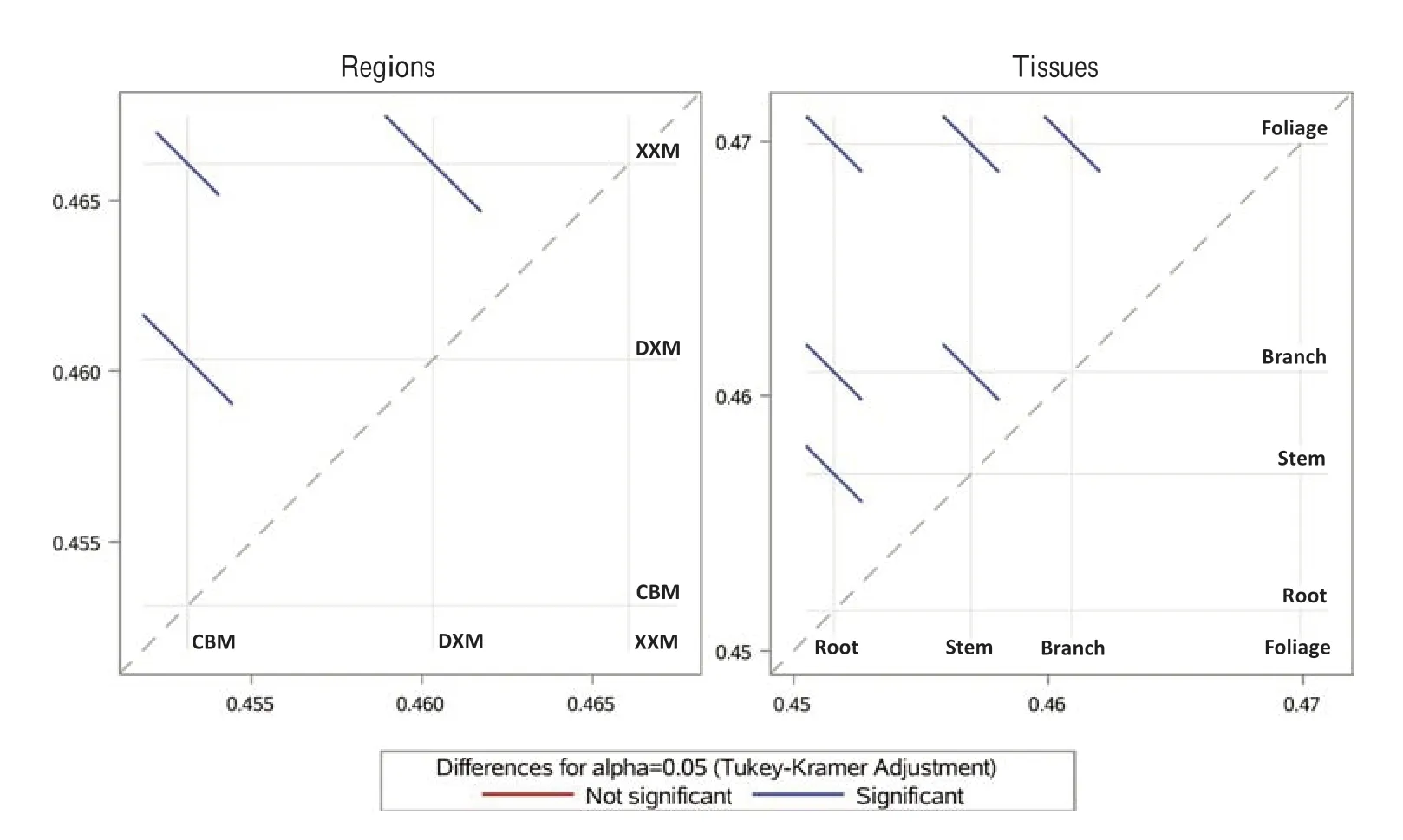
Intra-species variation in carbon concentrations
Discussion
Conclusions
杂志排行
Journal of Forestry Research的其它文章
- Genome-wide identif ication and cold stress-induced expression analysis of the CBF gene family in Liriodendron chinense
- Decay rate of Larix gmelinii coarse woody debris on burned patches in the Greater Khingan Mountains
- Characterizing conservative and protective needs of the aridland forests of Sudan
- Point-cloud segmentation of individual trees in complex natural forest scenes based on a trunk-growth method
- Accuracy of common stem volume formulae using terrestrial photogrammetric point clouds: a case study with savanna trees in Benin
- Appropriate search techniques to estimate Weibull function parameters in a Pinus spp. plantation
