Nanofat: A therapeutic paradigm in regenerative medicine
2021-12-24MadhanJeyaramanSathishMuthuShilpaSharmaCharanGantaRajniRanjanSaurabhKumarJha
Madhan Jeyaraman, Sathish Muthu, Shilpa Sharma, Charan Ganta, Rajni Ranjan, Saurabh Kumar Jha
Madhan Jeyaraman, Sathish Muthu, Saurabh Kumar Jha, Department of Biotechnology, School of Engineering and Technology, Sharda University , Greater Noida 201306, Uttar Pradesh, India
Madhan Jeyaraman, Rajni Ranjan, Department of Orthopaedics, School of Medical Sciences and Research, Sharda University, Greater Noida 201306, Uttar Pradesh, India
Madhan Jeyaraman, Sathish Muthu, Shilpa Sharma, Charan Ganta, Indian Stem Cell Study Group, Lucknow 226010, Uttar Pradesh, India
Sathish Muthu, Department of Orthopaedics, Government Medical College and Hospital, Dindigul 624001, Tamil Nadu, India
Shilpa Sharma, Department of Pediatric Surgery, All India Institute of Medical Sciences, New Delhi 110029, New Delhi, India
Charan Ganta, Department of Stem Cells and Regenerative Medicine, Kansas State University, Manhattan, United States 10002, United States
Abstract Adipose tissue is a compact and well-organized tissue containing a heterogeneous cellular population of progenitor cells, including mesenchymal stromal cells. Due to its availability and accessibility, adipose tissue is considered a “stem cell depot.” Adipose tissue products possess anti-inflammatory, anti-fibrotic, antiapoptotic, and immunomodulatory effects. Nanofat, being a compact bundle of stem cells with regenerative and tissue remodeling potential, has potential in translational and regenerative medicine. Considering the wide range of applicability of its reconstructive and regenerative potential, the applications of nanofat can be used in various disciplines. Nanofat behaves on the line of adipose tissuederived mesenchymal stromal cells. At the site of injury, these stromal cells initiate a site-specific reparative response comprised of remodeling of the extracellular matrix, enhanced and sustained angiogenesis, and immune system modulation. These properties of stromal cells provide a platform for the usage of regenerative medicine principles in curbing various diseases. Details about nanofat, including various preparation methods, characterization, delivery methods, evidence on practical applications, and ethical concerns are included in this review. However, appropriate guidelines and preparation protocols for its optimal use in a wide range of clinical applications have yet to be standardized.
Key Words: Adipose tissue; Nanofat; Stem cells; Regenerative medicine; Adipose stem cells
INTRODUCTION
Regenerative medicine encompasses a wide range of approaches, including the use of biologics, stem cell therapy, tissue engineering, cellular reprogramming, and gene therapy to curb various diseases[1,2]. These approaches gave a new dimension to “translational medicine” where the local milieu of the diseased tissue or organs was modulated into a regenerative environment to aid in the healing process[3,4]. Among various available regenerative approaches, stem cell therapy has gained significance. Due to its abundance, availability, and accessibility, the use of adipose tissue and its by-products has sharply increased among varied medical specialties and researchers[5-7].
Adipose tissue biology is complex due to the presence of a heterogeneous cellular population structured in a compact and well-organized manner[8,9]. With the presence of mesenchymal stromal cells as progenitor cells within the organizational structure (Figure 1), the adipose tissue is considered a “stem cell depot”[10]. Compared to embryonic stem cells, adipose stem cells (ASCs) have several advantages: Accessibility, harvesting potential, extraction by a non-terminal procedure, and fewer ethical controversies[11]. Adipose tissue extracts contain various pockets of growth factors, cytokines, adipokines, and transcriptional factors which altogether form secretomes. These acellular secretomes possess more biological activity than whole adipocytes[12]. The products of adipose tissues include microfat, nanofat, microvascular fragments (MVFs), the stromal vascular fraction (SVF), adipose-derived stem cells (ASCs), secretomes, and exosomes, which are obtained by minimal or more than minimal manipulation as per the US-FDA guidelines[13].
Utilizing the paracrine effects of the ASCs, the progenitors at the site of interest are stimulated to evoke a clinical response. Upon the addition of peptides, specific growth factors, and cytokines to help in the transfer of secretome molecules containing mRNA and signaling factors to the site of action, their regenerative potential is exponentially increased[14,15].
Out of all the adipose tissue-derived products, researchers are more interested in nanofat and stromal vascular fraction in clinical practices and research since their preparation involves concentration techniques which result in an increased quantity of ASCs[16,17]. Nanofat is one of the richest sources of adipose-derived stem cells and other progenitor cells[16,18-20]. The product “nanofat” is highly attractive in terms of compact pockets of stem cells and biological peptides[16]. There is evidence of the regenerative and tissue remodeling potential of nanofat in dermatological disorders such as scars, wrinkles, pigmentation, chronic wounds, small joints, and certain ligament-tendon targets[21]. Hence, nanofat is a potential adipose tissue product in translational and regenerative medicine.
NANOFAT
In 2013, Tonnardet al[16] developed an injectable byproduct of adipose tissue called “nanofat”, which was obtained by emulsification and filtration of the lipoaspirates[22-24]. The mechanical disintegration of adipose tissue is to reduce the particle size and to obtain an injectable product[25]. Such adipose-derived particles called nanofat render stromal cell populations to retain vasculature and naïve cellular matrix[26]. Nanofat is an ultra-purified adipose tissue-derived product that is devoid of mature adipocytes but contains CD34+ rich ASCs, microvascular fragments [fragments of arterioles, venules, and capillaries as they are identified by immunohistochemical staining for CD31 and α-SMA], growth factors [vascular endothelial growth factor (VEGF), platelet-derived growth factor (PDGF), hepatocyte growth factor (HGF), transforming growth factor-beta (TGF-β), basic fibroblast growth factor (bFGF), insulin-like growth factor 1 (IGF-1), and granulocyte-macrophage colony-stimulating factor (GM-CSF)], biological peptides [lipoxins, resolvins, protectins, neurotrophic factors, angiogenin, matrix metalloproteinase 9, leukemia inhibitory factor, and macrophage migration inhibitory factor], and cytokines [BMP-2 and -4, IL-1RA, -4, -8, -10, -11, and -13] as shown in Figure 2[16,23]. It is a liquefied, autologous injectable product with the property of biological integration with adjacent cells and tissues due to its homogenous consistency. The size of nanofat components is approximately 400 to 600 μm[27].
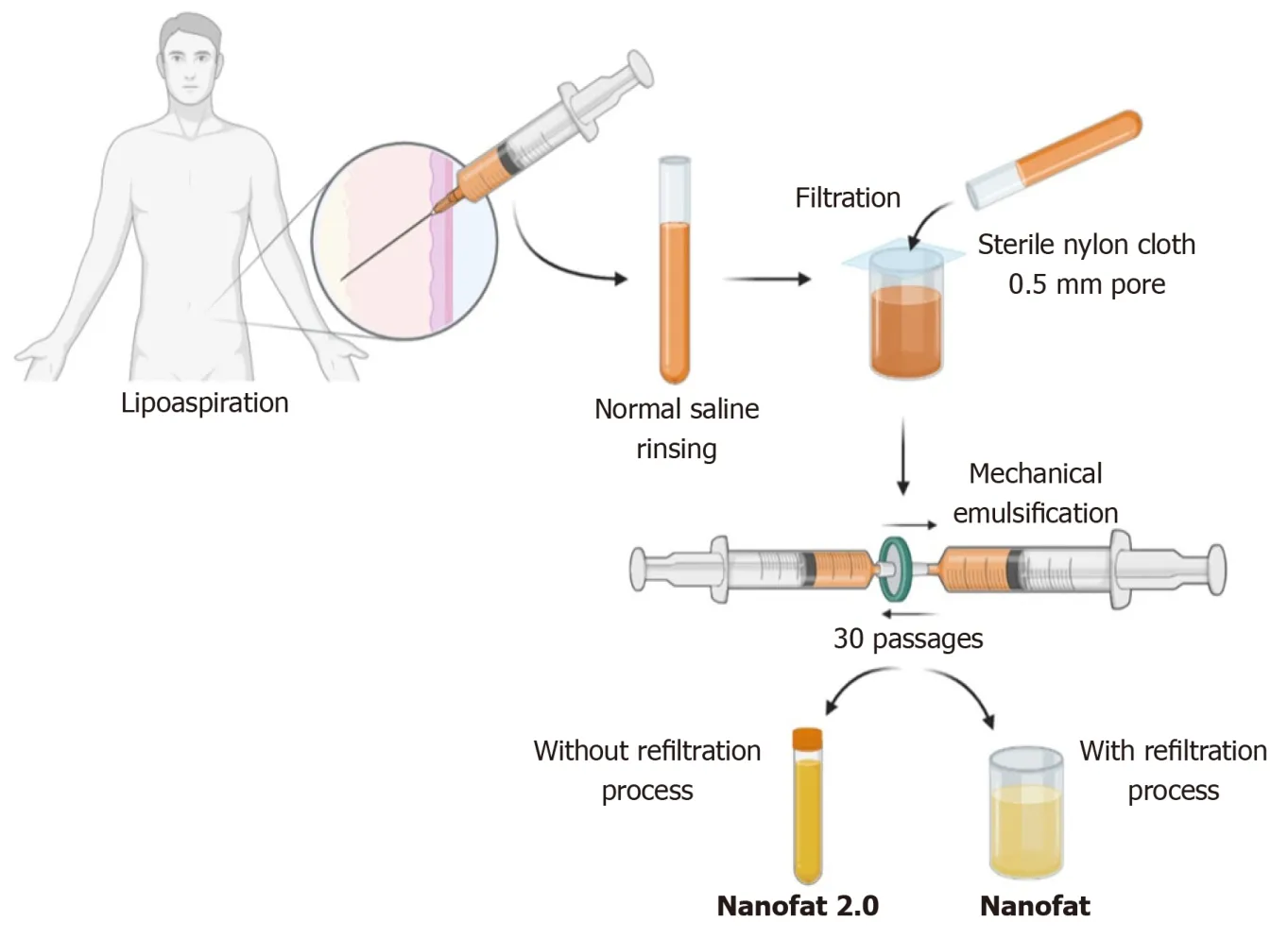
Figure 3 Schematic representation of nanofat preparation.
Nanofat behaves on the line of adipose tissue-derived mesenchymal stromal cells[28]. At the site of injury, these stromal cells initiate a site-specific reparative response comprised of remodeling of extracellular matrix (ECM), enhanced and sustained angiogenesis, immune system modulation, and cellular turnover[28]. These properties of stromal cells provide a platform for the usage of cellular therapy in various diseases. In 2016, Tamburinoet alobserved the better chances of cellular engraftment of nanofat in various diseases with better functional outcomes[29]. No observation of volume loss, contour irregularities, and liponecrosis was made by grafting nanofat.
ISOLATION OF NANOFAT
In 2013, Tonnard described a preparation protocol (Figure 3) for harvesting nanofat[16]. After the infiltration of modified Klein solution (lidocaine 800 mg/L and adrenaline 1:1000000) in the lower abdomen, adipose tissue harvesting was performed. To obtain “nanofat”, the adipose tissue should be harvested with a multiport 3 mm cannula with sharp side holes of 1 mm in diameter. Then the harvested adipose tissue is rinsed with normal saline, followed by filtration through a sterile nylon cloth (0.5-mm pore size) mounted over a sterile canister. Mechanical emulsification of adipose tissue is done by passing the adipose tissue between two syringes connected by a Luer-Lock connector for a minimum of 30 passages, where the size of the adipose tissue is stepped down with every passage and finally converted into an emulsion. The emulsified adipose tissue appears whitish. The emulsified fatty liquid was again filtered over the sterile nylon cloth and the effluent was collected in a sterile container which is named “nanofat”[16]. Nanofat preparation reduces the processing time, cost, and regulatory constraints compared to the enzymatic digestion of the adipose tissue[30,31].
CHARACTERIZATION OF NANOFAT
Nanofat has gained significant importance in biocellular regenerative medicine[21]. The characterization of nanofat components is based on cellular composition, stromal cell concentrations, and total nuclear counts after membrane lysis. Nanofat is the product of volumetric reduction of mature adipocytes by 95% and contains a concentrated and compact volume of a heterogeneous group of cells equivalent to SVF[21,32]. However, nanofat may be a superior cell source compared to SVF. Seséet al[26] estimated the total cellular load in the mechanically prepared nanofat to 6.63 million cells/g lipoaspirates whereas in enzymatically disintegrated SVF it was 0.68 million cells/g lipoaspirates. The nucleated cellular count in nanofat was 70% and in SVF was 7.3%. The cellular burden in nanofat contains the stromal cellular population which was equivalent to the lipoaspirate[26]. The low yield of cell counts in SVF was shown to be due to inefficient enzymatic digestion and the vast majority of the cells remained within the extracellular matrix[26].
Nanofat provides a higher concentration of bioactive micromolecules at the target or recipient site, which acts as a bridge to enhance the site-secreted chemotactic agents[26]. The cellular components present in nanofat are multi- and pluripotent and have the potential to differentiate into various cell lineages such as adipose tissue or connective tissue. The extracellular matrix present in nanofat likely caters to the cellular components to sustain the survival of progenitor cells in its composition. Hence, nanofat can be extrapolated for pre-clinical and translational research in tissue engineering[33]. Various pre-clinical and clinical experiments have shown that nanofat grafting helped in angiogenesis, immunomodulation, enhancing collagen deposition, and preventing fibrosis[34,35].
The theories of adipocyte survival after fat grafting include the “graft survival theory” (some transplanted viable adipocytes survive and remain alive for a longer period) and “fat regeneration theory” (under ischaemic conditions, adipose-derived progenitor cells get activated and undergo regeneration)[36,37]. Zhenget al[38] demonstrated that fat extract co-transplantation with nanofat enhances fat integrity, survival, and activation of more adipocyte precursors with a higher number of CD31 positive blood vessels, and more Ki67 positive proliferating cells. Under ischaemic conditions, nanofat co-transplantation with fat extract demonstrated proangiogenic and anti-apoptotic effects with multi-differentiation potential[38].
CELLULAR AND MOLECULAR CHARACTERISTICS OF NANOFAT
The components of nanofat are the derivatives from adipose tissue; hence, nanofat behaves on the line of adipose tissue-derived mesenchymal stem cells (MSCs) at the cellular and molecular levels. Nanofat contains the lowest number of SVF cells. Hence, nanofat is considered the poorest form of SVF.Mohamed-Ahmedet alexhibited higher expression of CD34 and CD49d in ASCs; they also showed that CD34 expression helps in long-term MSC proliferation[39]. ASCs express Runx-1 and ALP after 14 d of passage, which resulted in the extended proliferation, maturation, and differentiation of AD-MSCs. The osteogenic capacity of AD-MSCs was induced by mechanical stimulation of culture along with the addition of vitamin D3, PDGF, and BMP-2[40,41]. ASCs activate adipogenesis by induction of adiponectin, leptin, LPL, perilipin, and fatty acid-binding protein-1 through PPAR-γ and increased lipid vesicle formation compared to BM-MSCs[42]. The decreased potential for ASC-based chondrogenesis was due to the decreased expression of TGF-β-R1 and BMP-2 and -4[43,44]. The chondrogenic activity of ASCs is identified by the expression of types 2 and 10 collagen, biglycan, aggrecan, and decorin genes in the differentiated cells[45]. ASCs possess a higher potential for adipogenic differentiation than for osteogenic and chondrogenic differentiation when compared with BM-MSCs[39,46,47].
TYPES OF NANOFAT
Nanofat 2.0
The unfiltered adipose tissue was initially called adult staminal cells by Lombardoet al[48] since they had higher proliferation capacity than the filtered cells. In 2017, Lo Furnoet al[49] modified the method described by Tonnardet al[16] for nanofat, omitting the final filtration and squeezing the emulsified adipose sample through nylon cloth. Lo Furnoet al[49] named this product “nanofat 2.0”, which was highly rich in the stromal cell population and possessed an exponential proliferation capacity. Lo Furnoet al[49] have demonstrated faster epithelization of the wound gap within 8 d by placing nanofat 2.0.
After harvesting the adipose tissue from the abdominal region under low negative pressure through a multiport 3 mm cannula with a hole diameter of 1 mm, the resultant lipoaspirate must be subjected to rinsing, filtration, and mechanical emulsification through serial passages between two 10-cc syringes connected by a Luer Lock connector. The resultant by-product after 30 passages is called “nanofat 2.0” (Figure 3)[49].
Nanofat 2.0 components stained highly positively for CD44, CD90, and CD105, which are the most specific immunohistochemical markers for mesenchymal stromal cells[27,49]. Moreover, they stained weakly positively for CD14, CD34, and CD45, which are the lineage markers for hematopoietic stem cells. Histological studies showed the loss of tissue integrity in nanofat 2.0 but revealed huge numbers of adipose-derived stromal cells and cellular debris[49]. Due to the availability of stromal cells and endothelial progenitor cells, nanofat 2.0 resulted in the healing of wounds and long-standing non-healing ulcers where a large volume of soft tissue augmentation was needed[48]. Lo Furnoet al[49] demonstrated that nanofat 2.0 possessed increased stromal cell and endothelial precursor density and higher proliferative capacity than nanofat. Since nanofat 2.0 is subjected to less mechanical stress in preparation, the viability of the cellular content of the product could be enhanced compared to nanofat[48-51]. The modified nanofats are described in Table 1.
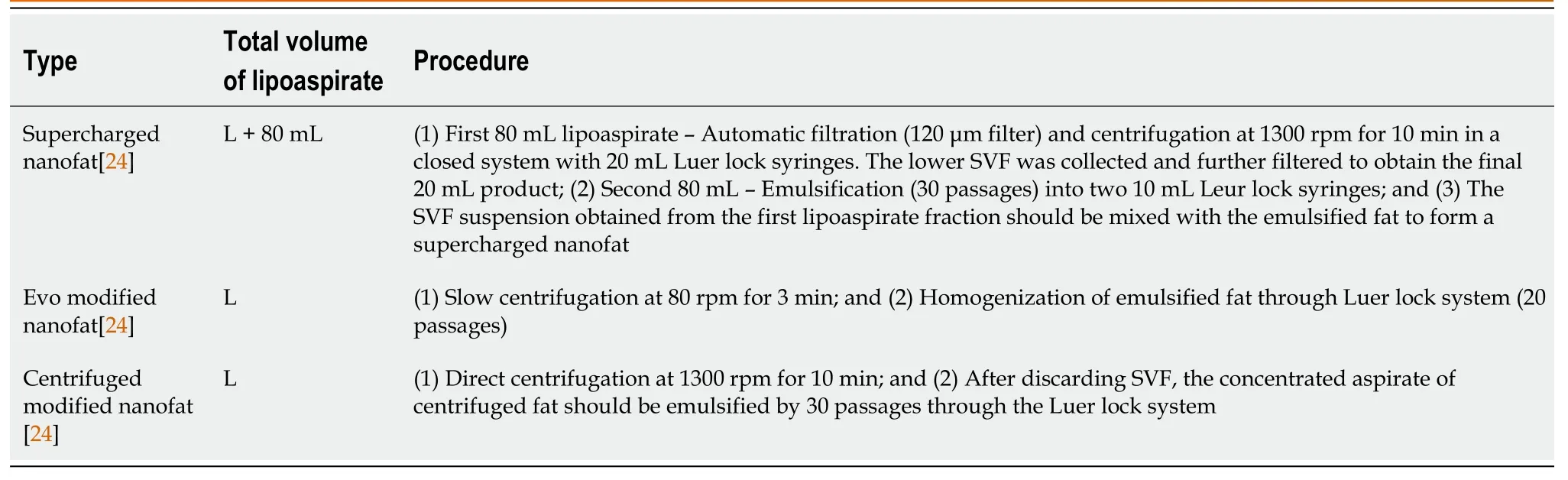
Table 1 Modified nanofats
Vivo nanofat
In 2018, Biet al[52] formulated the preparation of nanofat with a combination of enzymatic disintegration and mechanical emulsification of adipose tissue and named this technique “Vivo nanofat”. The harvested lipoaspirate is rinsed with 1 mL of 0.2 mg/mL of collagenase I enzyme and the final volume is incubated at 37 °C for 15 min. The final concentrate is centrifuged at 300 G for 7 min and the supernatant fraction is filtered through a 0.6 mm sized cell strainer. The final effluent obtained is called Vivo nanofat. The cellular viability of adipocytes and stromal stem cells has been preserved to a great extent in Vivo nanofat[52]. Although the authors claim that the concentration of collagenase used (0.075%) was less than the amount used for adipose stromal cell separation, the effects of their concentration in the final derivative need further exploration.
DELIVERY OF NANOFAT
The application and delivery of fat grafting to the recipient site are based on optimal vascularity for adipocyte survival. Nanofat can be delivered through micro-needling, intradermal, subcutaneous, and local infiltration depending on the need of the individual and the disease per se[53-55].Delivering nanofat through small gauge cannulas reduces the recipient site morbidity, risk of bleeding, and poor graft uptake[56]. In fat grafting, the revascularization starts from the peripheral zones; hence, the center of the graft experiences a longer ischaemic time. Moreover, compared to a single injection, experts resort to repeated doses of fat grafting for enhanced benefits[56]. The fat grafting must be applied to the recipient site by withdrawing the cannula in a “fanning out” pattern.
The size of the cannula is the most important criterion to determine the fat application, graft uptake, and survival in the recipient site. However, there is a lack of consensus among studies on the ideal size to be utilized. While the conventional recommendation is to use a cannula less than 2.5 mm diameter to enhance the vitality of fat graft, but Erdimet al[59] did not note similar findings in their study on cell viability with differing needle gauge sizes[57-60].
APPLICATIONS OF NANOFAT
Stem cells are an important component of regenerative medicine with increased significance and use in clinical applications. The newer concept of “Regenerative Surgery” has a great scope in augmenting and managing soft tissue defects and reconstructive procedures[61,62], of which adipose tissue-derived nanofat is gaining rapid attention.
From the early 20th century, autologous fat grafting has gained much attention in the field of biocellular regenerative medicine and tissue engineering. Autologous fat grafting and the products of adipose tissue fragmentation have been used to restore the volume of soft tissue defects in the field of plastic surgery and soft tissue reconstruction. Considering the regenerative potential of adipose tissue, researchers are exploring to identify the key element responsible for its function. The adipose cells were considered the storehouse of progenitor cells and bioactive micromolecules[30]. By concentrating the progenitor cells within the adipose tissue complex, the regenerative capacity of the adipose-based products is enhanced to aid in their applications[21].
Nanofat grafting enhances neoangiogenesis without producing any visible scars and provides a favorable outcome in aesthetic medicine for breast, buttocks, and genital augmentation, facial rejuvenation, and facial volume augmentation[63-66]. The preclinical and clinical studies with the usage of nanofat have demonstrated the regenerative capacity of nanofat.
Plastic surgery
Autologous fat transplantation or lipofillers remain the most suitable management modality available for breast reconstruction. Adipose tissue-derived nanofat can maintain natural breast shape and conceal the underlying prosthesis while augmenting breast size[67,68]. In gluteal augmentation, fat grafting can replace implant-based gluteal augmentation if the patient has adequate and available fat stores[69,70].
Nanofat injections can reduce the atrophic scars due to the presence of adipose tissue-derived stromal cells and avoid the need for surgical procedures[32]. The underlying mechanisms for scar retraction by nanofat are uncertain. Nanofat components can regenerate dermis and subcutaneous fatty tissues and enhance the dermo-epidermal junction. They regenerate by laying down adipose tissue-derived ECM, collagen deposition, and neoangiogenesis[71,72].
Zhanget al[73], in a preclinical study, emphasized the scar reduction in rabbit ears by decreasing the α-SMA and collagen type Ι gene expression and enhancing collagen deposition with the usage of adipose-derived MSCs. Adipose tissue-derived MSCs restore collagen fibrillary organizations and downregulate the fibrosis of the scar tissue. Klingeret al[74] described that autologous fat grafting allows the skin to rejuvenate more softly and flexibly, and matches the color of neighboring skin which could be utilized to rejuvenate the texture and color of the skin of the scars present in joints, eyelids, face, and mouth.
Burns:With the advancements in tissue engineering, it is now possible to regenerate the burnt and scarred tissues with minimal scarring and donor site morbidity. Nanofat grafting beneath and within the substance of the scar improves the quality, integrity, and texture of the scar[75]. The histological evidence of fat grafting to scar demonstrates the hyperplasia of dermis and epidermis, vasculogenesis, and collagen deposition. Clinically, the fat grafted scar shows improved scar tone, texture, thickness, elasticity, flexibility, and color of the scar along with reduced scar size[76,77].
Dermatology and aesthetic surgery
The most common procedure for managing facial aesthetics is autologous fat transplantation. Though the transplanted adipose tissue gets absorbed easily, a few progenitor cells stimulate the process of regeneration. The cells present in nanofat in combination with platelet-rich fibrin (PRF) enhances the proliferation and adipogenic lineage differentiation.
Due to this combination treatment with nanofat and PRF, a trend towards the disappearance of wrinkles and improved facial contour and skin rejuvenation have been observed attributable to the autocrine and paracrine effects of stromal cells in nanofat and anti-aging properties of PRF[44,69,70]. This combination treatment enhances the long-term benefits and is being increasingly utilized in the restoration of facial contouring in the field of aesthetic and cosmetic medicine. The skin texture, elasticity, and moisture, and facial rejuvenation can be achieved with nanofat admixed with PRF[78].
In a pre-clinical trial, nanofat injection improved the thickness of the dermal layer and promoted angiogenesis in the photoaged skin of a nude mouse[79,80]. A wide range of improvements were seen in wrinkles, discolorations, and scars due to burns with nanofat applications[81-83]. Lianget al[84] emphasized that the combination treatment of nanofat and PRF improves facial depression when compared with hyaluronate filler.
Aesthetically, nanofat grafting is used for the correction of dark circles[85,86], malar bags[56], hollow eyes[86], and blepharoplasty[87]. Due to fat atrophy in the aging process, nanofat has emerged as a plausible technique for facial rejuvenation[88-90].
Apart from being a primary essential tool in revision rhinoplasty, nanofat is being increasingly used in primary rhinoplasty procedures also[91]. Nanofat is being used to correct slight skin irregularities which do not require cartilage grafting. Moreover, considering the cost of the revision rhinoplasty, nanofat grading is being employed frequently as a cost-effective procedure[92,93].
Orthopedics
Due to the wide range of reconstructive and regenerative potentials of nanofat, the applications of nanofat can be extrapolated to orthopedic surgery. The mechanically emulsified adipose tissue can regenerate the degenerated and diseased tendon, ligaments, and articular cartilage[28].
Segretoet al[94] evaluated the role of a combination of nanofat grafting with autologous PRP in non-healing infected wounds. The application of nanofat with the micro-needling technique improved the delivery of cellular components into fibrosclerotic tissues and enhanced the regeneration of soft tissue in chronic non-healing wounds. The addition of autologous PRP along with nanofat enhances the proliferative capacity and motility of adipose tissue-derived stromal cells[95,96].
Due to the multi-differentiation potential of adipose tissue, which is a component in nanofat grafting, it could be extrapolated for utilization in avascular necrosis of the femoral head, mild to moderate grades of osteoarthritis of knees, tendinopathies, and non-union of fractures.
COMPLICATIONS OF NANOFAT GRAFTING
The lesser the fat graft is manipulated and the sooner it is injected, the higher the chances of its survival in the target site[97]. Minor complications related to the harvesting are due to the liposuction technique. The possible complications range from bruising, hematoma formation, donor-site pain, infection, contour irregularities, and damage to the underlying structures when the aspiration cannula enters peritoneal or muscular territories[98-103]. Breast augmentation with lipofilling was associated with complications such as fat necrosis, oil cyst formation, and calcifications when performed in large volumes into poorly vascularized areas. Cellulitis at the donor site[104], transient digital numbness[105], infections at both the recipient and harvest sites[106], and cyst formation[106,107] in 10% of hand rejuvenation patients, along with the common complications of fat grafting such as temporary dysaesthesia[106], fat necrosis[106,108], and reabsorption of the grafted fat[107] were also reported.
Facial rejuvenation by lipofilling involves complications related to the fat graft injections in "dangerous" areas of the face such as the glabella and nasolabial folds[88,109]. Accidental intra-arterial injections may result in cerebral or ocular artery thrombosis resulting from the reflux of fat into the ophthalmic artery and the internal carotid artery[88,109]. To prevent such devastating complications, confirmation of the absence of blood reflux into the syringe before injecting the graft is a necessary routine, along with a slow pace of injection at low pressure, and the use of a blunttip cannula[88,109].
ETHICAL CONCERNS WITH NANOFAT GRAFTING
Therapeutic use of cellular products, including human cells, tissues, and tissue-based products, comes under the regulation of the Food and Drug Administration (FDA) in the United States and the European Medicines Agency in the European Union[110-112]. For a cellular product to be approved by the regulatory authorities, it should be minimally manipulated and intended for homologous use. Moreover, the entire procedure must be performed on the same day[112]. The main concern with this clause is to clarify the applications which account for the “homologous” use.
The FDA, while formulating these guidelines regarding fat-based therapeutic products, has considered only the adipocyte, not taking into account the potential constituents of the extracellular matrix such as multipotent stromal cells, pericytes, and endothelial precursor cells and restricted their approved usage only to the spectrum of disorders homologous to the utility of only the adipose lineage cellular component of the tissue complex. Appropriate homologous use of these heterogeneous populations with undesignated cellular capabilities needs to be clarified. Since nanofat is processed in a non-enzymatic method, it comes under the minimal manipulation norms of the FDA guidelines. Moreover, it is possible to procure, process, and place the cells in the target environment in a single surgical procedure, thereby reducing the need for additional procedures and the risk of contamination or genomic instability. The functional properties of extracellular matrix fragments, cellular debris, and blood cells in the heterogeneous composition of nanofat need to be defined. Consequently, problems of reproducibility and standardization methods may arise considering the subjectivity involved in the preparation process[113]. Hence, it is challenging to compare the efficacy of product protocols even when they are used for similar scenarios[114]. Therefore, increased efforts to optimize the preparation protocols with standardized methods of tissue manipulation for clinical purposes and analysis of grafting are needed.
CONCLUSION
Nanofat, being a compact bundle of stem cells with regenerative and tissue remodeling potential, is a potential adipose tissue product in translational and regenerative medicine. Considering its wide reconstructive and regenerative potential, the applications of nanofat can be extrapolated to various disciplines. However, appropriate guidelines and preparation protocols for its optimal use have yet to be standardized for its vast range of clinical applications.
杂志排行
World Journal of Stem Cells的其它文章
- Priming strategies for controlling stem cell fate: Applications and challenges in dental tissue regeneration
- Epigenetic regulation of dental pulp stem cells and its potential in regenerative endodontics
- Effects of immune cells on mesenchymal stem cells during fracture healing
- Regulation of the mesenchymal stem cell fate by interleukin-17:Implications in osteogenic differentiation
- Why stem/progenitor cells lose their regenerative potential
- Application of adipose-derived stem cells in treating fibrosis
