Priming strategies for controlling stem cell fate: Applications and challenges in dental tissue regeneration
2021-12-24SiYuanZhangJiaYinRenBoYang
Si-Yuan Zhang, Jia-Yin Ren, Bo Yang
Si-Yuan Zhang, Bo Yang, State Key Laboratory of Oral Diseases and National Clinical Research Center for Oral Diseases, West China Hospital of Stomatology, Sichuan University, Chengdu 610041, Sichuan Province, China
Jia-Yin Ren, Department of Oral Radiology, West China Hospital of Stomatology, Sichuan University, Chengdu 610041, Sichuan Province, China
Bo Yang, Department of Oral and Maxillofacial Surgery, West China Hospital of Stomatology, Sichuan University, Chengdu 610041, Sichuan Province, China
Abstract Mesenchymal stromal cells (MSCs) have attracted intense interest in the field of dental tissue regeneration. Dental tissue is a popular source of MSCs because MSCs can be obtained with minimally invasive procedures. MSCs possess distinct inherent properties of self-renewal, immunomodulation, proangiogenic potential, and multilineage potency, as well as being readily available and easy to culture. However, major issues, including poor engraftment and low survival rates in vivo, remain to be resolved before large-scale application is feasible in clinical treatments. Thus, some recent investigations have sought ways to optimize MSC functions in vitro and in vivo. Currently, priming culture conditions, pretreatment with mechanical and physical stimuli, preconditioning with cytokines and growth factors, and genetic modification of MSCs are considered to be the main strategies; all of which could contribute to improving MSC efficacy in dental regenerative medicine. Research in this field has made tremendous progress and continues to gather interest and stimulate innovation. In this review, we summarize the priming approaches for enhancing the intrinsic biological properties of MSCs such as migration, antiapoptotic effect, proangiogenic potential, and regenerative properties. Challenges in current approaches associated with MSC modification and possible future solutions are also indicated. We aim to outline the present understanding of priming approaches to improve the therapeutic effects of MSCs on dental tissue regeneration.
Key Words: Mesenchymal stem cells; Priming; Dental regeneration; Culture conditions; Cytokines; Growth factors; Genetic modification
INTRODUCTION
Research on mesenchymal stromal cell (MSC)-based therapy has made rapid strides over recent decades due to the beneficial biological effects of these cells. MSCs, which are also known as mesenchymal stem cells, are spindle-shaped cells located at perivascular sites in various human tissues and organs, including bone marrow, adipose tissue, umbilical cord, and dental tissue. Each of these MSC sources has its own advantages and disadvantages[1]. In general, MSCs are readily available and easy to culturein vitro, with genetic stability. MSCs can be characterized based on their specific properties: adherence to plastic and a typical immunophenotypic profile (expression of the surface markers CD44, CD73, CD90 and CD105, and a lack of CD34, CD45, CD14 and HLA-DR)[2]. MSCs possess multilineage-differentiation potential into osteoblasts, chondrocytes, adipocytes, and even highly specialized cells, such as myoblasts[3], neurons[4], endothelial cells[5], and hepatocytes[6,7]. In addition, the low immunogenicity and outstanding immunomodulatory properties of MSCs make them ideal therapeutic cell candidates[8]. To date, the experimental and preclinical applications of MSCs span various diseases and conditions, accompanied by promising outcomes.
Over the last few decades, the search for MSC-like cells in specific tissues has led to the discovery of distinct populations of MSCs from various human dental tissues. Dental tissue is a popular MSC source. Compared to cells from other tissues, MSCs from dental tissue can be obtained through minimally invasive procedures. Currently, five main populations of dental tissue-derived MSCs have been successfully isolated and characterized. Postnatal dental pulp stem cells (DPSCs)[9] were the first human dental MSCs identified in pulp tissue. Later, other types of dental tissue-derived MSCs were gradually discovered, including stem cells from human exfoliated deciduous teeth (SHEDs)[10], periodontal ligament stem cells (PDLSCs)[11], dental follicle precursor cells (DFCs)[12] and stem cells from the apical papilla (SCAPs)[13]. In dental tissue, these five types of MSCs are accessible MSC-like populations with superior selfrenewal capacities, immunomodulatory functions, and multilineage-differentiation potential for tissue regeneration.
Nevertheless, the beneficial effects of MSC-based regeneration are not always fulfilled. MSC properties can be influenced byin vitroandin vivobiological, biochemical and biophysical factorsviareciprocal cell-to-cell interactions, the extracellular matrix (ECM), and soluble bioactive factors[14]. MSCsin vivointeract with surrounding cells and tissues in a three-dimensional (3D) microenvironment, producing anti-inflammatory molecules, promoting angiogenesis, preventing cell death, and reconfiguring the ECM[15]. Moreover, MSCs reside in a microenvironment with relatively low oxygen tension (i.e., 1%–5% O2)in vivo, while the O2tensionin vitro(i.e., 20%–21%) is much higher than in the original MSC microenvironment[16]. The different O2tension associated within vitroculture can decrease cell-regenerative capacities, including proliferation, differentiation, and anti-inflammatory responses. MSCs are generally cultured in nutrient- and O2-enriched environmentsin vitro. In contrast, transplanted MSCs are confronted with harsh conditions such as lack of blood supply and inflammation induced by tissue damage; both of which cause apoptosis and senescence, leading to the failure of regeneration. In this context, MSCs have shown promising regenerative properties for tissue repair; however, exploring more effective strategies is still necessary to improve their therapeutic efficacy.
MSC properties cannot be augmented without further enhancement through by priming approaches to protect MSCs against an inhospitable microenvironmentin vivo. To this end, some recent investigations have sought ways to improve MSC functionsin vitroandin vivo. Optimizing culture conditions, preconditioning with cytokines and growth factors, and genetic modifications of MSCs are considered to be the main strategies; all of which contribute to improving MSC transplantation efficiency for tissue regeneration[17]. Several studies have revealed that pretreated MSCs exhibit better cell survival, augmented homing abilities to injury sites, enhanced immunomodulatory properties, optimized proangiogenic abilities, and increased multilineagedifferentiation capabilities[18-20]. Figure 1 shows the six focal improvements in MSCs that contribute to improved therapeutic effects. Research in this field has made tremendous progress and continues to gather interest and spur innovation. In this review, we summarize the approaches proposed to improve dental tissue-derived MSC functions for dental tissue regeneration and divide these approaches into four categories: (1) Culture condition manipulation; (2) Pretreatment with mechanical and physical stimuli; (3) Preconditioning with growth factors and cytokines; and (4) Gene modification. We further discuss these approaches mainly in the context of enhancing the intrinsic biological properties of MSCs such as migration, anti-apoptotic effect, immunomodulation, proangiogenic potential, and regenerative properties.
DENTAL TISSUE-DERIVED MSCS
Characteristics of dental MSCs
DPSCs are MSC-like cells in the pulp chamber of permanent teeth that originate from the cranial neural crest. Figure 2 shows the different dental regions from which DPSCs originate, together with other dental tissue-derived MSCs. Similar to bone-marrowderived MSCs, DPSCs have enriched expression of the surface markers Stro-1, CD29, CD73, CD90, CD105 and CD166, while they are negative for hematopoietic markers such as CD14, CD45, CD34, CD25 and CD28[9]. DPSCs exhibit rapid proliferation and superior immunosuppressive properties, and they are prone to forming dentin/pulplike complexes. Aside from their odontogenic potential, DPSCs can be reprogrammed into adipocytes, chondrocytes, myocytes and neural cells[21].

Figure 1 Overview of the functional improvements of mesenchymal stromal cell (MSC) properties by priming strategies.
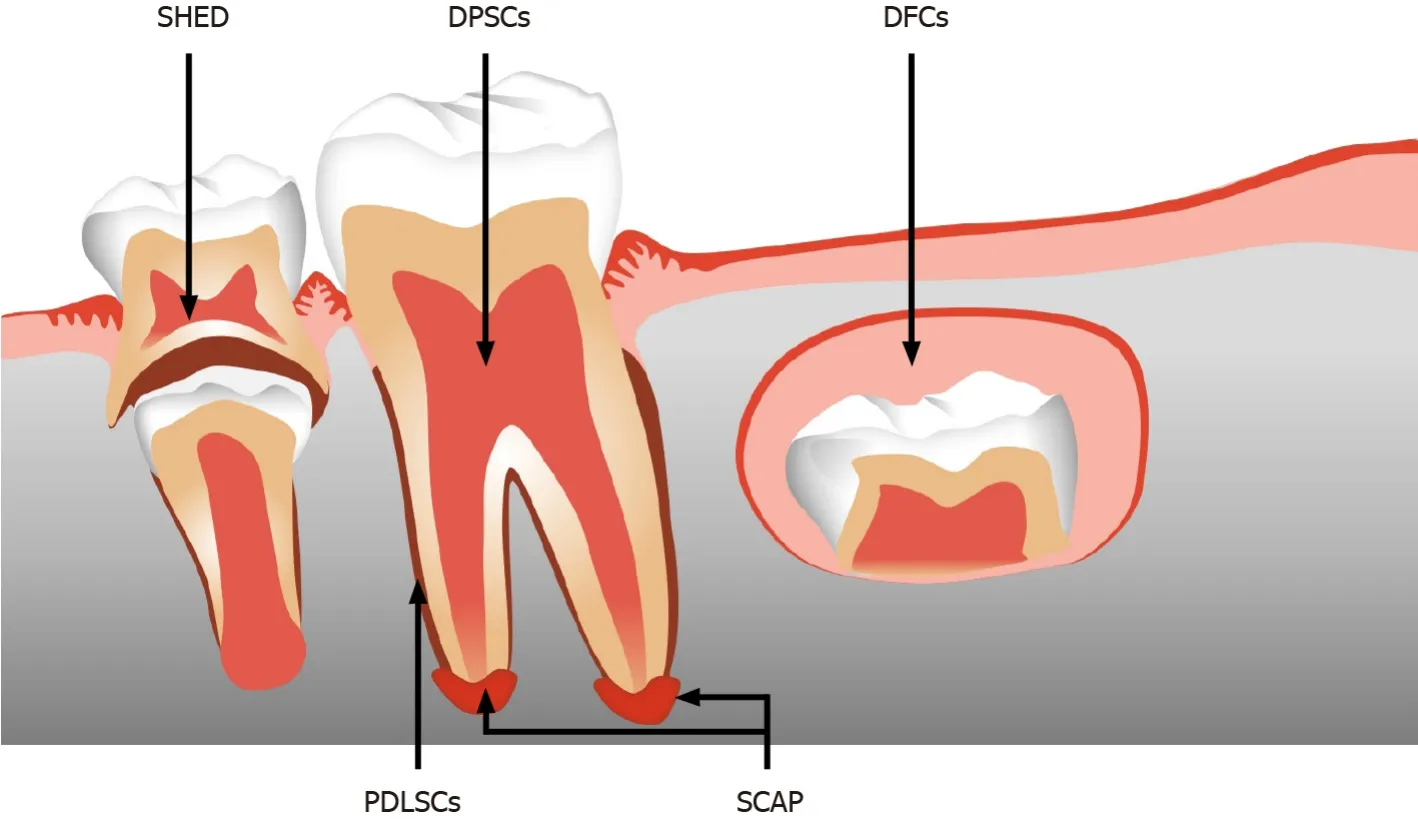
Figure 2 Schematic image of dental mesenchymal stromal cells from different tissue regions
SHEDs were isolated from the dental pulp tissue of exfoliated deciduous teeth. SHEDs share similar MSC regenerative capacities such as self-renewal and multilineage differentiation potential[10]. However, these cells exhibit increased proliferation rates and the spontaneous formation of sphere-like cell clusters. SHEDs are distinct from DPSCs[22]. In addition to the expression of DPSC surface markers, SHEDs also highly express the embryonic stem cell markers Oct4 and Nanog, stagespecific embryonic antigen (SSEA)-3 and SSEA-4, and the neural stem cell marker nestin. After the induction of neurogenesis, SHEDs show higher expression than DPSCs of neuronal and glial cell markers, such as β-III-tubulin, tyrosine hydroxylase, microtubule-associated protein (MAP)2, and nestin[23].
SCAPs and DFCs are MSCs that are derived only from developing permanent teeth. SCAPs are found at the apices of growing teeth, and DFCs are located in connective tissue sacs surrounding the enamel organ[13,24]. These two types of dental MSCs can form adherent clonogenic clusters and differentiate into adipocytes, odontoblasts/osteoblasts, cementoblasts, and periodontal ligament. SCAPs have been reported to possess greater potential to form dentin than DPSCs, due to their higher proliferation capacity and greater telomerase activity. DFCs are regarded as the parent cells of periodontal tissue and can form periodontal tissues, including alveolar bone, periodontal ligament, and cementum.
PDLSCs are derived from the human periodontal ligament, which is a connective tissue that lies between the cementum and the alveolar bone socket. PDLSCs have been demonstrated to be a reliable source of periodontal tissue regeneration. Compared to DPSCs, PDLSCs exhibit higher expression of scleraxis, a tendon-specific transcription factor[11]. These cells can be readily expandedin vitroand generate cementum/periodontal ligament-like complexesin vivo. In addition, the osteogenic differentiation capability of PDLSCs was demonstrated by the formation of calcified nodules and the expression of alkaline phosphatase (ALP), matrix extracellular protein (MEPE), bone sialoprotein (BSP), osteocalcin (OCN), and transforming growth factor (TGF)-β receptor I.
Dental MSC applications and limitations
Dental MSCs have been extensively investigated in preclinical studies. Moreover, several clinical trials have been reported in recent years[25-27]. Some reviews have already summarized the benefits of dental MSCs in regenerative medicine[28-30]. In short, dental MSCs have been reported to promote the regeneration of dental tissues, bone, cartilage, muscle, and nerves[31-34]. Moreover, dental MSCs have also been implicated in the treatment of various diseases, such as brain ischemia[35], liver fibrosis[36], diabetes[37], rheumatoid arthritis[38], and Alzheimer’s disease[39]. However, dental MSCs have thus far exhibited only moderate benefits in clinical studies, and researchers are still struggling to move forward to advanced phases (III and IV) of clinical trials. To secure efficient and successful translation of clinical procedure for dental MSCs, substantial approaches concerning functional improvements must be established.
CELL FUNCTION OPTIMIZATION STRATEGIES
Culture condition manipulation
In general, MSCs reside in a confined microenvironment called the stem cell nichein vivo, which includes not only MSCs themselves but also other supporting cells and ECM. The stem cell niche is the basis of tissue homeostasis. Dental MSCs are isolated from individuals; these cells must be cultured and expandedin vitroto obtain a sufficient number of cells before transplantation. However,in vitroculture does not entirely replicatein vivocell behavior, and cells may lose their tissue-specific functions. Cell culture conditions, such as O2tension and 3D culture, influence cell behavior. Recreating the physical and mechanical microenvironment experienced by MSCsin vivois important in reproducing the stem cell niche. Several studies have reported that manipulation of conventional culture conditions could enhance the regenerative efficacy of MSCs. Here, we introduce two widely used alternative culture methods: hypoxic preconditioning and 3D spheroid culture. The enhancement of intrinsic biological properties of MSCs by culture condition manipulation and other priming strategies are summarized in Table 1.
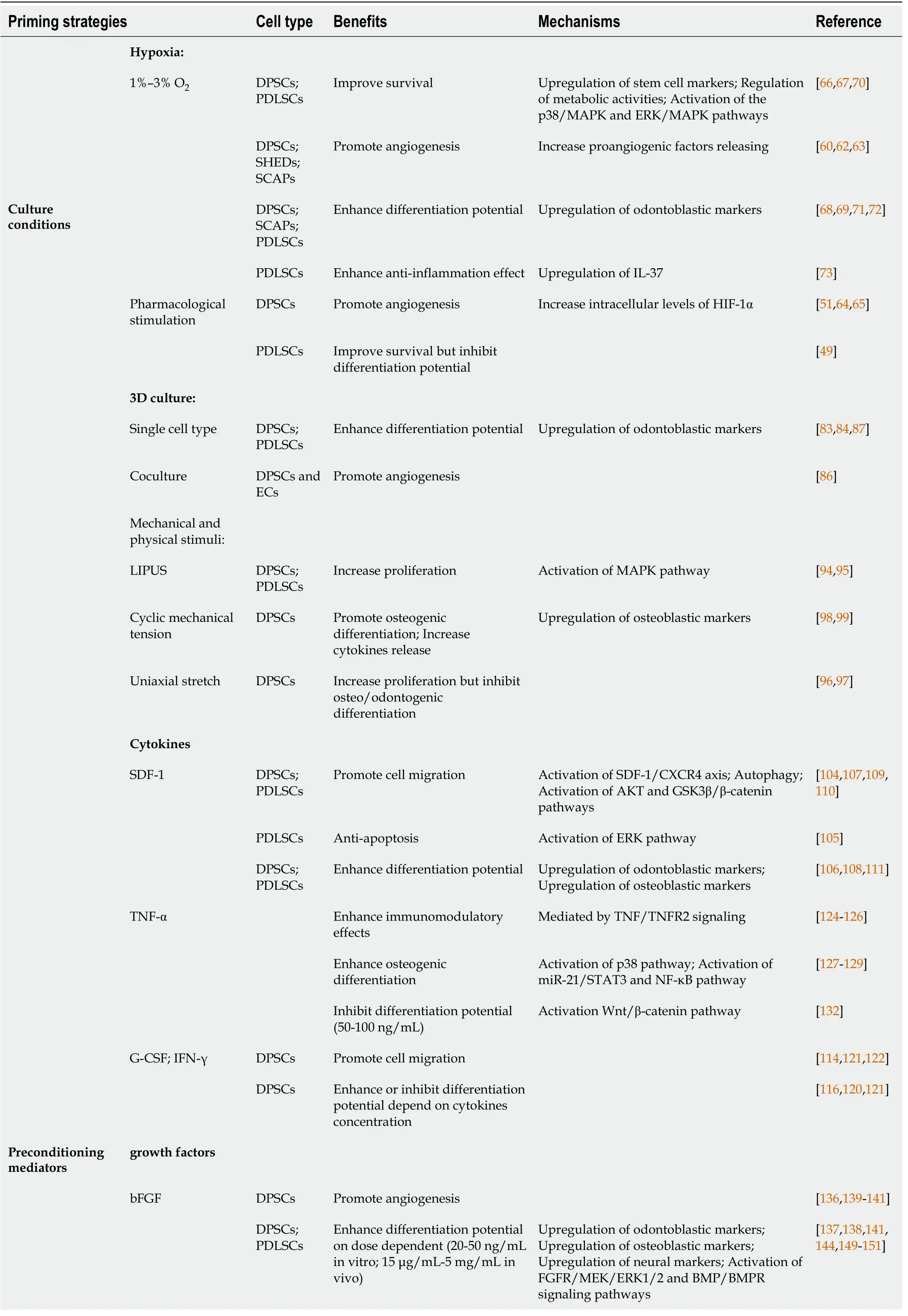
Table 1 Priming strategies for controlling mesenchymal stromal cell fate
Hypoxic preconditioning
O2is one of the critical factors associated with cellular homeostasis, as a lack of O2could result in disease pathogenesis. O2tension varies from 1% to 12% in the physiological state in adult organs and tissue, depending on the degree of tissue vascularization and metabolic activities[16,40]. MSCs residing in the general dental microenvironment are exposed to low O2tension (i.e., 3%–6% O2)[41], while MSCsin vitroare typically exposed to higher O2concentrations (i.e., 20%–21% O2). The negative impact of ambient O2tension on MSCs culturedin vitro, such as decreased proliferative capacity, DNA damage, and senescence, was reported by a number of studies[42-46]. When MSCs are expanded in normoxia and then transplanted into injured tissue, they face hypoxic conditions and undergo apoptosis. Hypoxic preconditioning of MSCs is regarded as a better way to mimic the naïve MSC niche and improve their therapeutic potential than normoxic culture conditions[47].
In general, hypoxic conditions require a reliable experimental device to maintain stable O2tension for cell culture. Commonly used CO2incubators have difficulty producing low O2levels. Currently, there are several approaches to achieve hypoxic conditions for cultured cells[48]. One approach involves using a specialized hypoxic chamber inside a standard CO2incubator. This is a convenient and low-cost method. However, the major drawback is gas leakage that may disrupt the experimental processes and cause fluctuations in O2concentrations in the incubator. Moreover, O2concentrations can be temporarily disturbed by every time incubator doors are opened and take time to stabilize. Hypoxic culture can also be performed in a tri-gas incubator, which is an effective device to generate stable experimental O2concentrations. CO2and nitrogen (N2) are supplied to reduce the O2levels in the incubator. Because of ability to control the gas mixture (CO2, O2and N2), the tri-gas incubator is currently considered to be a practical approach to provide the closest conditions to those in the body. A third method is pharmacological agents that stimulate hypoxia. Cobalt chloride (CoCl2)[49,50] and deferoxamine (DFO)[51] are well-known hypoxia mimetics that act by inducing the expression of hypoxia inducible factors (HIFs), which play vital roles in the hypoxia signaling pathway and guide the cellular response to hypoxia. Stabilization of HIF-1 can also be achieved by propyl hydroxylase inhibitors (PHDs), which block enzymatic activity to inhibit HIF-1 degradation during hypoxia[52].
DPSCs: Dental pulp stem cells; PDLSCs: Periodontal ligament stem cells; SHED: Stem cells from human exfoliated deciduous teeth; SCAP: Stem cells from the apical papilla; LIPUS: Low-intensity pulsed ultrasound; SDF-1: Stromal cell-derived factor-1; TNF-α: Tumor necrosis factor-α; G-CSF: Granulocytecolony stimulating factor; IFN-γ: Interferon-γ; bFGF: Basic fibroblast growth factor; IGF-1: Insulin-like growth factor-1; Bcl-2: B-cell lymphoma 2.
Hypoxic preconditioning was reported to have positive impacts on the survival and proangiogenic properties of MSCs. When cultured in low-serum medium, 1% O2pretreated MSCs can prevent damage through increased paracrine secretion of proangiogenic factors, such as vascular endothelial growth factor (VEGF) and basic fibroblast growth factor (bFGF)[53]. Low O2concentrations also increased metabolic activity and decreased caspase-3/7 activity, thus reducing the sensitivity of MSCs to the ischemic microenvironment[54]. MSCs induced by 2% O2showed decreased tumorigenic potential, as indicated by significantly reduced transformation into tumor-associated fibroblasts[55]. Compared to normoxic conditions at 20% O2,hypoxic conditions at 3% O2improved genetic and chromosomal stability, ensuring the safety of MSCs[56]. Regarding MSCs derived from dental tissue, hypoxic preconditioning has been evaluated in DPSCs[57,58], SHEDs[59], SCAPs[60] and PDLSCs[61].
DPSCs, SHEDs and SCAPs are involved in pulp regeneration. These cells have been demonstrated to support the process of pulp revascularization under hypoxia by releasing proangiogenic molecules[60,62,63]. DFO, CoCl2, and PHD inhibitors induce hypoxia-stimulated VEGF production in explanted dental pulp by increasing intracellular levels of HIF-1α[51,64,65]. The mesenchymal stem cell marker STRO-1 is reported to be increased in dental pulp cells under hypoxic conditions[66]. Fukuyamaet al[67] revealed that the proliferation of dental pulp cells was initially suppressed under hypoxia but increased afterward because of activation of the metabolism-related enzyme AMP-activated protein kinase. Hypoxic conditions can modulate the mineralization potential of DPSCs. Higher expression of odontoblastic markers such as OCN, dentin sialophosphoprotein (DSPP), and dentin matrix acidic phosphoprotein-1 (DMP1) was observed under hypoxia[68]. SCAPs preconditioned with 1% O2exhibited upregulated osteogenic and neuronal differentiation as well as angiogenesis[69].
Hypoxia promoted PDLSC clone formation and proliferationviathe p38/MAPK and ERK/MAPK signaling pathways[70]. A study on PDLSCs under hypoxia showed enhanced osteogenic differentiation bothin vitroandin vivo[71]. This effect could be the reason that hypoxia mediates the expression of RUNX2 in PDLSCsviaHIF-1αinduced VEGF[72]. However, hypoxia induced by CoCl2maintained the stemness of PDLSCs while inhibiting the osteoblastic differentiation of PDLSCs[49]. The secretome of hypoxia-preconditioned PDLSCs showed an augmented anti-inflammatory effect mediated by interleukin (IL)-37 expression[73]. After culture in 1% O2for 24 h, the proteomic profile of PDLSCs, mainly proteins related to energy metabolism, autophagy, and stimuli-responsive proteins, was changed[74].
These findings highlight the advantages of hypoxic pretreatment in dental MSC culture. However, to enable the practical use of hypoxia-treated dental MSCs, some issues should be considered. In experimental settings, a wide range of O2concentrations (from 5% to < 1%) were used in different studies, and each concentration may stimulate different properties of dental MSCs. This issue was indicated in adiposederived MSCs (ASCs). Choiet al[75] reported that the differentiation potential of ASCs was improved significantly by 2%–5% O2, while a lower O2level reduced the effect. Moreover, MSCs of different dental origins require distinct O2tensions to meet tissuespecific demands. For instance, the O2concentration required by DPSCs for pulp regeneration could differ from that of PDLSCs for periodontal tissue regeneration. Therefore, the optimal O2level for maximizing the capacity of each type of dental MSCs should be determined before clinical application.
3D spheroid culture
In traditional two-dimensional (2D) culture, cells attach to a plastic surface and grow as a monolayer in a culture flask. However, this is a highly artificial environment that fails to recapitulate cell-to-cell or cell-to-ECM interactions, stimulating dedifferentiation capacity and decreasing the therapeutic performance of MSCs[76]. 3D spheroid culture techniques have been applied to overcome this tissue. 3D spherical cell aggregates are multicellular structures that consist of cells, ECM, and paracrine factors, mimicking the spontaneous metabolic microenvironment in both nutrient and O2concentrations and providing superiorin vivomodels compared to monolayer culture systems[77]. Several studies have compared the therapeutic effects of 2D- and 3Dcultured MSCs and shown the improved regenerative capacity of spheroid-cultured MSCs[78]. It has been reported that spheroid culture potentiates the proliferative and differentiative capacity of MSCs[79]. MSCs cultured as spheroids showed altered adhesion molecule gene expression patterns and enhanced immunomodulatory capacity[80]. In addition, the augmented migration and homing efficiency of MSCs to the damaged site were found to promote engraftment afterin vivotransplantation[81].
Based on these findings, the regenerative potential of 3D spheroid cultured dental MSCs was evaluated in various preclinical studies. Xiaoet al[82] found that DPSCs were able to form large aggregates on Matrigel under osteogenic induction by undergoing a process of central cell death, cavitation, and spontaneous multilineage differentiation. Yamamotoet al[83] fabricated 3D DPSC aggregates through a lowattachment method. The DPSC spheroids exhibited improved odonto/osteoblastic differentiation abilityin vitrothat was mediated by integrin signaling. Leeet al[84] then demonstrated that 3D DPSC spheres exhibited enhanced odontogenic differentiationin vivo. In addition, the spheres were enriched in pluripotency transcription factors, such as Sox2, Lin28, Esrrb and Klf4. 2D- and 3D-cultured DPSCs were further compared by microarray analysis, and the expression of genes related to the ECM, cell differentiation, cell-to-cell/cell-to-matrix, and osteoblast differentiation was promoted[85]. Dissanayakaet al[86] fabricated microtissue spheroids by coculturing DPSCs and human umbilical vein endothelial cells in an agarose 3D Petri dish. Upon subcutaneous implantationin vivo, effective pulp-like tissue formation and capillarylike structures were successfully anastomosed with the host vasculature. PDLSC spheroids were also examined and showed enhanced osteogenic differentiation regulated by SFRP3-mediated ALP activation[87]. Collectively, DPSC or PDLSC spheroids could maintain their enhanced therapeutic functions bothin vitroandin vivo.
Although there is a general consensus that 3D spheroid culture exhibits therapeutic advantages over monolayer culture, several technical points still need to be considered to optimize 3D culture methods. Currently, hanging drops and low attachment surfaces are the two primary methods of spheroid fabrication, but both methods are inefficient and have low yields in the laboratory. Automated 3D-bioreactor systems have been designed to minimize labor-intensive, time-consuming procedures and improve the yield of 3D-cultured MSCs[76,77,88]. The size and cell number of each spheroid and the culture period are other important factors to consider. Since the core area of spheroids is often hypoxic and lacks nutrients, excessively large spheroids could lead to cell death or dysfunction[89]. In addition, the culture duration (shortterm or long-term) tends to affect the density of spheroids, which influences the cell gene expression profiles and other secretory factors[81]. Therefore, researchers need to determine an effective culture protocol to obtain a sufficient number of homogeneous spheroids with stable therapeutic efficacy.
Mechanical and physical stimuli
Dental MSCs are mechanosensitive cells that can recognize and transform mechanical changes into cellular responses[90,91]. Sübayet al[92] found that the application of orthodontic extrusive forces to teeth had no significant adverse effect on human pulp tissue. Since then, several studies have shown that mechanical stimuli such as lowintensity pulsed ultrasound (LIPUS), uniaxial mechanical stretch, cyclic mechanical tension and cyclic uniaxial compressive stress are able to induce the proliferation of dental MSCs. In addition, physical stimuli including surface topographies, dynamic hydrostatic pressure and pulsating fluid flow have been reported to promote the differentiation of dental MSCs[93].
The positive effects of mechanical and physical stimuli on the biological behavior of dental MSCs have been well described. Gaoet al[94] showed that LIPUS, which is applied clinically to promote healing, can promote DPSC, PDLSC and BMMSC proliferation in an intensity- and cell-specific dependent mannerviaactivation of distinct mitogen-activated protein kinase (MAPK) pathways. The author further demonstrated that Piezo1 and Piezo2, two mechanosensitive membrane ion channels, contributed to transducing ultrasound-associated mechanical signals and activating downstream MAPK signaling processes in dental MSCs[95]. Two other studies noted that uniaxial stretch increased the proliferation of DPSCs but inhibited their osteo/odontogenic differentiation potential, indicating that additional studies are required to clarify the intra- and intercellular mechanisms associated with mechanical stress[96,97]. To better understand the effect of mechanical stress on dental MSC differentiation, Hanet al[98] observed increased proliferation and mRNA expression levels of osteogenic markers under cyclic mechanical tension. These findings suggest that the mechanical cyclic tension is a potent positive modulator of osteogenic differentiation in DPSCs. Yanget al[99] demonstrated that compressive stress can induce cell morphology changes and odontogenic differentiation in DPSCs. The study showed increased expression of ALP, DMP1, BMP2, DSPP and collagen (COL) type I under compressive stress, indicating that mechanical stimuli could initiate repair mechanisms in the dentin-pulp complex. It should be noted that Leeet al[100] showed that mechanical stimuli increased the release of proinflammatory cytokines and antioxidant defense enzymes. This study demonstrated that proinflammatory cytokines and reactive oxygen species produced in response to mechanical strain might play a key role in activating the early cellular signals involved in Nrf2-ARE-mediated gene transcription, providing a guidance for cellular protection or suppressing harmful side effects.
Cytokine and growth factor priming to enhance MSC functions
It is generally accepted that cytokine and growth factor priming may influence host tissuesviaparacrine effects. The interaction between growth factors and their receptors on cell surfaces activates downstream signal transduction for cell survival, migration and differentiation. Moreover, MSCs have inherent immunomodulatory characteristics to inhibit T cell and B cell activation and dendritic cell differentiation, impair the cytolytic potential of natural killer (NK) cells, and promote regulatory T cell (Treg) differentiation[101]. However, the immunomodulatory effect of MSCs is not always achieved but requires stimulation by inflammatory factors, such as interferon (IFN)-γ, tumor necrosis factor (TNF)-α and IL-1β[102]. Therefore, priming MSCs with cytokines and growth factors in this context is suggested to be a supplemental molecular strategy to foster the therapeutic potential of MSCs and contribute to establishing a hospitable microenvironment for dental tissue repair.
Cytokine priming
Stromal cell-derived factor-1 priming:Stromal cell-derived factor (SDF)-1, also known as chemokine CXC ligand (CXCL)12, plays a major role in cell trafficking and homing. SDF-1 has been shown to bind to the G-protein coupled receptor CXC receptor (CXCR)4 to induce SDF-1/CXCR4 signaling[103]. SDF-1 pretreatment enhanced proliferation, migration and differentiation, and inhibited apoptosis in DPSCs and PDLSCs[104-107]. Stimulating CD105+DPSCs with SDF-1 was shown to significantly improve the therapeutic effects in a canine pulpectomy model[108]. SDF-1-induced migration was reported to be mediated by the AKT and GSK3β/β-catenin pathways[109]. Autophagy is also involved in SDF-1-mediated DPSC migration during pulp regeneration[110]. In periodontal regeneration, Lianget al[111] demonstrated that cotreatment with SDF-1/exendin-4 facilitated the proliferation, migration, and osteogenic differentiation of PDLSCs and promoted periodontal bone regeneration. Similar effects on periodontal regeneration were also shown in studies of SDF-1 cotransplantion with bFGF[112] or parathyroid hormone (PTH)[113].
Granulocyte-colony stimulating factor priming:Granulocyte-colony stimulating factor (G-CSF) is a cytokine that stimulates the bone marrow to produce and release neutrophils into the bloodstream. G-CSF is frequently used to mobilize hematopoietic stem cells from the bone marrow to the systemic circulation[114]. G-CSF has a migratory effect on DPSCs similar to that of SDF-1, suggesting a potential alternative to SDF-1. Stimulation of MSC migration by G-CSF was demonstrated to strictly depend on the expression of G-CSF receptor (G-CSFR)[114]. It was revealed that 44%–56% of G-CSF-mobilized DPSCs were G-CSFR-positive cells[115]. G-CSFmobilized DPSCs showed higher regenerative potential than untreated DPSCs[116].
Proinflammatory cytokine priming
IFN-γ priming:IFN-γ is a well-known proinflammatory cytokine secreted by activated T and NK cells. IFN-γ stimulates 2,3-indolamine dioxygenase (IDO) expression in MSCs to enhance immunosuppressive properties[117]. Wadaet al[118] revealed that DPSCs, PDLSCs and gingival mesenchymal stem cells have immunosuppressive properties that are mediated partly by IFN-γ produced by activated peripheral blood mononuclear cells (PBMCs). Another study further demonstrated that the immunosuppressive effect of DPSCs on PBMC proliferation and B cell immunoglobulin production was significantly enhanced by IFN-γ and mediated by TGF-β[119]. Sonodaet al[120] found that DPSCs isolated from diseased teeth with pulpitis had impaired immunosuppressive abilities, but these abilities could be restored by IFN-γ treatment. This study revealed that IFN-γ improved dentin formation and T cell suppression of pulpitis-derived DPSCs by enhancing telomerase activity. In addition, other studies reported that healthy DPSCs exposed to IFN-γ exhibited increased proliferation and migration but impaired odonto/osteogenic differentiation, which may be regulated by the nuclear factor (NF)-κB and MAPK signaling pathways[121]. Increased release of CXCL6 and CXCL12 by IFN-γ-primed DPSCs may contribute to the homing of MSCs for pulp repair[122].
TNF-α priming: TNF-α is a pleiotropic cytokine produced predominantly by macrophages in response to bacterial endotoxin. TNF-α priming has a similar effect as that of IFN-γ priming and upregulates the expression of immunoregulatory factors, such as prostaglandin E2, IDO and hepatocyte growth factor (HGF)[123]. TNF signaling plays dual roles that are likely to be transduced through its two distinct receptors, TNFR1 and TNFR2. The interaction of TNF-α with TNFR1 mediates proinflammatory effects and cell death, while the interaction with TNFR2 mediates anti-inflammatory effects and cell survival. Recent studies have revealed that TNF–TNFR2 signaling but not TNF–TNFR1 signaling is a crucial mediator that regulates the regenerative and immunomodulatory effects of MSCs[124]. TNFR2 expression is corelated with NF-κB, which could be a possible explanation for the effect of TNF priming. The researchers confirmed the results by investigating the role of TNFR2 in proangiogenic functions, the suppression of T cells, the induction of Tregs, and alternations in T cell cytokine secretion pattern. Moreover, it was also mentioned by the authors that TNFR2 expression was essential after TNF pretreatment[125,126]. Paula-Silvaet al[127] showed that 10 ng/mL TNF-α stimulated the differentiation of DPSCs toward an odontoblastic phenotypeviathe p38 signaling pathway while downregulating matrix metalloproteinase (MMP)-1 expression. The miR-21/STAT3 and NF-κB signaling pathways are reported to be involved during osteogenic differentiation[128,129]. Liuet al[130] revealed continuous transition in transcriptome changes during TNF-α mediated osteogenic differentiation. The TGF-β and PI3K/Akt pathways are sequentially activated. TNF-α (50 ng/mL) stimulated DPSC migration through upregulation of integrin α6[131]. However, the osteogenic differentiation of DPSCs was suppressed by high dose TNF-α (50–100 ng/mL) by activating Wnt/β-catenin signaling[132]. In addition to odontoblastic differentiation, TNF-α increased the angiogenic potential of cells in a coculture model of DPSCs and endothelial cells[133]. TNF-α in combination with lipopolysaccharide promoted angiogenesisviaVEGF and sirtuin 1 signaling in DPSCs[134].
Growth factor priming
bFGF priming:bFGF, also known as FGF-2, is considered to be an important growth factor that assists tissue regeneration[135]. bFGF plays a potential role in the multipotent differentiation of DPSCs. Studies have reported that bFGF stimulates DPSC proliferation, angiogenesis, odontoblastic differentiation, and neuronal differentiationin vitroat concentrations of 20–50 ng/mL[136-138].In vivostudies showed that bFGF at concentrations of 15 μg/mL–5 mg/mL significantly promoted angiogenesis[139,140] and odontoblastic differentiation[141,142] for pulp regeneration. Gorinet al[143] found that bFGF-treated DPSCs increased angiogenesis through HGF and VEGF secretion. bFGF priming augmented proangiogenic properties compared to the effect of hypoxia. Sagomonyantset al[138] reported that bFGF-induced mineralization of DPSCs was mediated by activation of the FGFR/MEK/ERK1/2 and BMP/BMPR signaling pathways. Zhanget al[144] revealed that bFGF induced neural differentiation in DPSCs by upregulation of nestin, MAP-2, β3-tubulin, glial intermediate filament protein (GFAP), and silent information regulator protein 1 expression through the ERK and AKT signaling pathways. bFGF assists DPSC differentiation by increasing the gene expression of MEPE, DSPP, DMP-1 and OCN[138]. bFGF is also involved in the production of hyaluronic acid (HA), which is a key component of the ECM. Tooth development and odontoblastic differentiation require HA synthesis[145]. It is suggested that bFGF-primed DPSCs promote anti-inflammatory effects and odontoblastic differentiation through increased HA secretion[146,147]. Furthermore, bFGFprimed DPSCs exhibit altered expression of cytokines, including IL-6, IL-8, monocyte chemoattractant protein-1, macrophage inflammatory protein-1α, and CC chemokine ligand 20[148]. The PKC/PI3K–AKT/MAPK signaling pathways have been shown to contribute to cytokine upregulation[148]. Consistent with pulp regeneration studies, bFGF priming for periodontal tissue regeneration has shown similar results, including enhanced PDLSC migration, proliferation, osteogenesis and neurogenesisin vitroandin vivo[149-152].
However, some studies showed that bFGF did not benefit cell differentiation[142,153-155]. Heet al[153] and Kimet al[142] showed that DPSCs preconditioned with 20 ng/mL bFGF exhibited increased cell proliferation, but inhibited cell differentiation. Likewise, Takeuchiet al[154] reported that bFGF promoted cell proliferation at a concentration of 50 ng/mL but inhibited cell differentiation at 100 ng/mL. Odontoblastic differentiation markers such as COLI[156], ALP activity[155], and calcium deposition were reported in some studies to have no changes[157]. Although there was a contradictory effect of bFGF on cell behaviors, most previous studies confirmed the potential effect of bFGF on cell proliferation. The effect of bFGF on cell differentiation was suggested to depend on the spatially and temporally controlled priming of MSCs by bFGF[158,159]. bFGF induced odontoblastic differentiation in dental pulp at the early stage (days 3–7)[138] but inhibited odontoblastic differentiation during late exposure (days 7–21)[158,159]. These findings suggested that the concentration of bFGF and the release duration should be accurately controlled.
Insulin-like growth factor-1 priming:Insulin-like growth factor (IGF)-1, a member of the insulin-like peptide family, has been shown to play an essential role in the growth and differentiation of various tissues, including teeth. Tooth germ explants treated with IGF-1 showed increased formation of dentin and enamel[160]. IGF-1 promoted DPSC proliferation and osteogenic differentiation by activating the mammalian target of rapamycin (mTOR) signaling pathway[161]. IGF-1 was also found to target EphrinB1 to regulate tertiary dentin formation[162]. Maet al[163] further revealed the role of the IGF-1/IGF-1R/hsa-let-7c axis in regulating the committed differentiation of SCAPs. Yanet al[164] reported that IGF-1 rescued the adverse effects of high glucose concentration on DPSCs and protected against apoptosis. Regarding periodontal ligament regeneration, IGF-1 was shown to enhance the survival of PDLSCs[165]. Yuet al[166] described the beneficial effect of IGF-1 on PDLSC proliferation and osteogenesisviathe ERK and JNK MAPK pathways.
Preconditioning with cytokines and growth factors is a promising way to improve the therapeutic efficacy of cells for dental tissue regeneration. However, these treatments synergistically or antagonistically influence MSC properties. Further and intensive studies need to be conducted to clarify the optimal concentration or combination of these factors and verify the detailed mechanisms according to their chemical characteristics.
Genetic modification of MSCs is an experimental technique that introduces exogenous DNA into MSCs to produce or overexpress specific factors[167]. This technique can enhance MSC survival and functions after transplantation, particularly in a hostile environment. To date, genes involved in survival, migration and regenerative properties have been mainly targeted in MSCs for dental tissue regeneration. Genetically modified dental MSCs were found to be more effective than wild-type cells. Here, we introduce several reports on enhancing the function and therapeutic effects of dental MSCs through gene modification.
Genetic modification to enhance retention and migration
It is generally accepted that transplanted MSCs are vulnerable to the harsh microenvironmentin vivo; most cells can be cleared or become dysfunctional within a short time. This situation hinders the migration of transplanted MSCs to the target site to exert their effects. Therefore, enhancing cell retention and migration capabilities is crucial in improving the therapeutic efficacy of transplanted MSCs. The overexpression of genes related to apoptosis inhibition and self-renewal can be an effective method to achieve this goal. Factors secreted by genetically modified MSCs may exert therapeutic effectsviaparacrine actions.
One strategy to enhance the survivability of grafted DPSCs is to overexpressBcl-2, an antiapoptotic gene that is important for maintaining cell viability. Several studies have demonstrated the effects ofBcl-2overexpression on proliferation, antiapoptosis, and osteo/odontogenic differentiation[168,169]. The forced expression of stemnessrated genes such asSox2,Oct4andNanog, which contribute to the maintenance of pluripotency in embryonic stem cells, is reported to improve proliferation and prevent senescence in MSCs. Huangel al[170] showed that DPSCs overexpressingOct4andNanogexhibited enhanced proliferation, as well as osteogenic/chondrogenic/ adipogenic differentiation.Sox2-overexpressing DPSCs showed beneficial effects on proliferation, migration and adhesion capability[171]. In general, MSCs transduced with pluripotent genes show remarkable benefits in their proliferation. However, conflicting results regarding differentiation potential and possible adverse effects such as tumor formation should be considered in the context of clinical applications[172,173].
Forkhead box protein (Fox)O1 is a master regulator that mediates glucose metabolism, tumorigenesis, oxidative stress and bone formation[174]. Huanget al[175] investigated the role of FoxO1-transfected PDLSCs in regulating oxidative stress resistance and osteogenesis. The authors found that FoxO1 overexpression protected PDLSCs against oxidative damage and promoted ECM mineralization by increasing the expression of the osteogenic markers Runt-related transcription factor (Runx)2 and SP7 in an inflammatory environment. This study demonstrates the promising antiinflammatory role of FoxO1 in periodontium regeneration for periodontitis treatment.
Genetic modification to modulate osteo/odontogenic differentiation
Lineage differentiation can be achievedin vitroby priming MSCs with extrinsic signaling molecules or by modifying culture conditions. Genetic modification of MSCs may be an alternative way to induce stable and effective lineage transdifferentiation[176]. BMP family members are regarded as crucial factors that initiate and maintain osteo/odontogenesis. Taşlıet al[177] carried out genetic modification of BMP2 and BMP7 in human tooth germ stem cells (hTGSCs). The researchers found that overexpression of BMP2 and BMP7 in hTGSCs led to enhanced expression of early markers of osteo/odontogenic differentiation, such as DSPP, OCN and COL1A. Yanget al[178-180] reported that DPSCs transfected with BMP2 showed increased expression of ALP, OCN, COL1A, BSP, DSPP and DMP1, indicating stimulation of osteo/odontogenic differentiation.In vitrotests showed that transfected DPSCs differentiated into odontoblast-like cells without osteogenic induction[180]. Zhanget al[181] investigated BMP2-transfected SCAPs. The modified SCAPs underwent cell differentiation toward the odontogenic lineage by upregulating theALP,OCN,DSPPandDMP1genes. Another promising inducer of osteo/odontogenesis is growth/differentiation factor (GDF)11, also known as BMP11. Nakashimaet al[182] reported that DPSCs overexpressing GDF11 exhibited induced expression of dentin sialoprotein and the formation of large amounts of reparative dentin in canine teeth. In addition, the authors demonstrated that a GDF11-transfected cell mass stimulated reparative dentin formation on the amputated pulp[183]. These results revealed the feasibility of using BMPs in gene-modified MSC applications for endodontic regeneration.
Runx2 is a crucial factor for bone formation and tooth development. Panet al[184] demonstrated that Runx2-overexpressing DFCs upregulated osteoblast/cementoblastrated genes and enhanced osteogenic differentiationin vitro. The authors also investigated the effects of mutant Runx2 without the VWRPY motif, which is responsible for suppressing transcriptional activation by Runx2. Overexpression of mutant Runx2 compared with full-length Runx2 led to higher expression levels of OPN, COLI and CP23 in DFSCs.
CONCLUSION
MSCs are increasingly being investigated as promising cell materials for tissue regeneration therapies due to their multilineage differentiation capabilities. MSCs derived from different dental tissues can be used as alternatives to bone marrow- and adipose tissue-derived MSCs. Numerous studies have demonstrated the high therapeutic potential of dental MSCs in various diseases, such as pulpitis, periapical, coronary artery, and neurodegenerative diseases.
Prior to clinical application, many efforts are still needed to focus on innovative strategies to maximize the therapeutic potential of MSCs. As summarized in this review, state-of-the-art technologies, including advanced culture systems, priming with cytokines and growth factors, and genetic editing, combined with understanding the therapeutic mechanism of dental MSCs, would significantly improve efficacy of MSC treatment in dental tissue regeneration. Overall, modified MSCs exhibit better therapeutic effects with high specificity on targets than ordinary cells. More studies should be carried out to explore the therapeutic impact of the combined application of different modification or priming techniques to ensure improved outcomes and novel discoveries and further enhance therapeutic goals.
Researchers should also consider that MSC priming approaches must meet proper criteria and specific quality for clinical applications. The multilineage differentiation potential of MSCs is the required minimal criterion since it is a liability for many clinical applications. However, if MSCs are applied to treat neurodegenerative disorders, such as Parkinson’s disease, then their osteogenic and adipogenic potentials are hazards. Thus, to utilize MSC products for specific applications, it is sometimes possible to lose certain MSC-defining characteristics through appropriate priming approaches[185]. Rather than focusing on the minimal criteria, as primed MSCs get closer to clinical application, they should meet the criteria that correlate with the safety and efficacy of the MSC product for treating specific diseases.
However, in preclinical trials, priming approaches for MSCs still have many limitations, including high costs, immunogenicity, donor-to-donor variability, variable source-dependent effects, and lack of good manufacturing practice (GMP) grade certification for clinical applications. Further studies are currently needed to evaluate the long-termin vivotumorigenic potential of primed MSCs and the efficacy of each priming method for different clinical applications. Primed MSCs should meet qualified cell therapy standards and allow for GMP grade production while not compromise the quality attributes of the cells or be overly expensive. Ultimately, in conjunction with rigorous preclinical and clinical trials, primed MSCs can have enormous potential for wider applications in clinical settings in the future.
杂志排行
World Journal of Stem Cells的其它文章
- Epigenetic regulation of dental pulp stem cells and its potential in regenerative endodontics
- Effects of immune cells on mesenchymal stem cells during fracture healing
- Regulation of the mesenchymal stem cell fate by interleukin-17:Implications in osteogenic differentiation
- Why stem/progenitor cells lose their regenerative potential
- Nanofat: A therapeutic paradigm in regenerative medicine
- Application of adipose-derived stem cells in treating fibrosis
