Why stem/progenitor cells lose their regenerative potential
2021-12-24AngelaPicernoAlessandraStasiRossanaFranzinClaudiaCurciIghlidiBariLoretoGesualdoFabioSallustio
Angela Picerno, Alessandra Stasi, Rossana Franzin, Claudia Curci, Ighli di Bari, Loreto Gesualdo, Fabio Sallustio
Angela Picerno, Alessandra Stasi, Rossana Franzin, Claudia Curci, Ighli di Bari, Loreto Gesualdo, Department of Emergency and Organ Transplantation, University of Bari “Aldo Moro”, Bari 70124, Italy
Fabio Sallustio, Department of Interdisciplinary Medicine, University of Bari “Aldo Moro”, Bari 70124, Italy
Abstract Nowadays, it is clear that adult stem cells, also called as tissue stem cells, play a central role to repair and maintain the tissue in which they reside by their selfrenewal ability and capacity of differentiating into distinct and specialized cells. As stem cells age, their renewal ability declines and their capacity to maintain organ homeostasis and regeneration is impaired. From a molecular perspective, these changes in stem cells properties can be due to several types of cell intrinsic injury and DNA aberrant alteration (i.e epigenomic profile) as well as changes in the tissue microenviroment, both into the niche and by systemic circulating factors. Strikingly, it has been suggested that aging-induced deterioration of stem cell functions may play a key role in the pathophysiology of the various agingassociated disorders. Therefore, understanding how resident stem cell age and affects near and distant tissues is fundamental. Here, we examine the current knowledge about aging mechanisms in several kinds of adult stem cells under physiological and pathological conditions and the principal aging-related changes in number, function and phenotype that determine the loss of tissue renewal properties. Furthermore, we examine the possible cell rejuvenation strategies. Stem cell rejuvenation may reverse the aging phenotype and the discovery of effective methods for inducing and differentiating pluripotent stem cells for cell replacement therapies could open up new possibilities for treating age-related diseases.
Key Words: Stem cells; Aging; Self-renewal; Rejuvenation; Aging-associated disorders; Epigenetic changes; Aging environment
INTRODUCTION
During regular physiology or in response to a damage, many tissues expand and regenerate thanks to resident stem cells. Adult stem cells are unusual in that they can self-renew and differentiate into a number of cell types within a tissue. Stem cells were thought to be immortal because they do not undergo replicative senescence, but it is now known that they are vulnerable to damage accumulation. Because of their location at the top of the hierarchy of cellular lineages, their dysfunction may have a greater impact on the of their progeny and they could fail in tissue recovery.
There are two key hypotheses for the etiology of aging. The first is the theory of “antagonistic pleiotropy”, which claims that genes that cause aging are chosen because they offer a gain timely in life[1]. The other is the 'disposable soma' hypothesis, which argues that somatic maintenance is expensive and can only be used as a method to prevent development and reproduction[2].
Predation-prone animals spend extensively in growth and reproduction at the expense of longevity as a result. Many of the processes that promote stem cell aging occur since they provide health and survival advantages during growth or youth, but they are harmful later in life, according to these ideas[3].
Understanding stem cell aging is likely to be important if we consider remarkable regenerative capacity of several tissues such as aging at the organ level of tissues that regenerate continuously. Most mammalian cells undergo a limited number of cell divisions in culture also known as the Hayflick limit[4]. The number of cell divisions allowed in cell culture varies from cell type and species, and only a few types of cells are able to extend this limit.
Embryonic stem cells (ESCs) are unique among all stem cell populations for their virtually infinite capacity to self-renew and pluripotency during embryogenesis. These properties are transferable, and these cells can also reprogram somatic nuclei and presumably confer immortality through somatic cell nuclear transfer (nuclear transferembryonic stem)[5]. ESCs protect themselves from senescence through adaptive mechanisms aimed at maintaining a high genetic stability by efficiently repairing DNA damage and maintenance of epigenetic status[6-10]. They have an intrinsic vigorous barrier to aging and can produce soluble pro-regenerative proteins for rejuvenating processes[11]. It is increasingly evident that adult stem cells (also named tissue stem cells) are rests of embryonic growth, and several of the primary developmental pathways are still active or functional in these cell populations to maintain postnatal organ homeostasis and regeneration.
In the plethora of stem cell classification, mesenchymal stem cells (MSCs) also known as mesenchymal stromal cells can be encountered as less potent stem cells, with more distinct capacity of differentiation. MSCs are multipotent stromal cells that can differentiate into a variety of cell types, including osteoblasts (bone cells), chondrocytes (cartilage cells), myocytes (muscle cells) and adipocytes in a way that is dependent from exposure to the particular soluble factors in their microenvironment[12].
The most common and longest utilized adult source tissues for human MSCs are bone marrow and the adipose tissue stromal vascular fraction, thereby these sources provided the majority of literature data in this field. In the last decade, the possibility to activate and mobilize these cells into site of injury (i.e.for muscle, heart) led researchers and clinicians to optimize their therapeutic use. MSCs treatment has demonstrated to reduce fibrosis, to stimulate of neovascularization, to promote an immunomodulation, and stimulation of endogenous tissue regeneration[13,14].
Compared to MSCs, adult stem cells are cells that reside in specialized niches that help regulate stem cell self-renewal and differentiation. They maintain the ability to differentiate into organ-specific cell types and play a role in regeneration and homeostasis of nearly all tissues during life. Adult stem cell functionality declines with age, and different type of cellular injury as well as changes in the niche and circulating blood factors contribute to this age-related decline[15].
Here, we analyze what is known about aging in several kinds of adult stem cells and consider what changes in stem cell number and function are known to occur with aging, what aspects of stem cell performance make them vulnerable or resilient to aging, and how much stem cell function decline leads to aging. Finally, we examine possible cell rejuvenation strategies.
INFLUENCE OF AGING ON THE REGENERATIVE POTENTIAL
Aging is a phenomenon characterized by the time-dependent functional decline that influences organisms[16]. It is identified by nine hallmarks: genomic instability, telomere attrition, epigenetic alterations, loss of proteostasis, deregulated nutrient sensing, mitochondrial dysfunction, cellular senescence, altered intercellular communication, and stem cell exhaustion[16].
Aging influences the renewal of stem cells and induces a gradual functional decline of adult tissue-specific stem cells to maintain homeostasis of the tissue in which they reside. Thus, aging-induced deterioration of stem cell functions may play a crucial role in the pathophysiology of the various aging-associated disorders[15].
Some studies have shown that the regenerative potential of MSCs is downregulated with age, which limits their therapeutic use[17]. In fact, MSCs coming from aged donors (> 60 years) displayed an increase in senescent markers compared to cells of young donors, and also reduced proliferation ability and differentiation potential[17].
Senescence also affects the regenerative capacity of human adipose-derived mesenchymal stem cells (hASCs) that play an important role in the treatment of degenerative diseases[18]; hASCs are abundant and easy to obtain from patients during surgery[18,19]. Furthermore, the use of these cells is safe and efficient for regenerative medicine[19]. Aged hASCs have a decreased rate of proliferation and chondrogenic and osteogenic capacity with increased senescence relative to younger cells[18].
The effect of aging is also known on multipotent postnatal stem cells isolated from human periodontal ligaments (PDLs) that are used in periodontal regenerative therapies for reconstruction of tissues destroyed by periodontal diseases[20,21]. Periodontal diseases increase with age, which compromise PDL stem cells (PDLSCs). Studies in aged donors have shown a decreased number of PDLSCs and a reduction in osteogenic and adipogenic activity together with a weakening of the differentiation marker RUNX2[20,21].
Several research groups have studied the impact of aging on bone marrow mesenchymal stem cells (BMSCs), which are essential for promoting hematopoietic cells in addition to contributing to bone formation. The aging of BMSCs and/or their response to age-related changes in environmental stressors, such as the extracellular matrix and circulating metabolites, may prolong aging or age-related pathologies. The results of natural chronological aging of BMSCs are yet unknown, although it appears that with chronological age, BMSCs decrease in frequency and progenitor cell capabilities such as proliferation and differentiation reduce. These functions, however, require more investigation inin vitroandin vivocontexts[22]. Experiments on aged mice show that muscle-derived stem progenitor cells (MDSPCs) have reduced regenerative functions[22,23]. Proliferation and multilineage differentiation are both poor in MDSPCs from elderly and progeroid mice. MDSPCs isolated from young wildtype mice and administered intraperitoneally to progeroid mice, extend their lifespan and improve their health. Moreover, MDSPCs alleviate degenerative alterations and vascularization in areas where donor cells are not detectable, implying that their therapeutic action is mediatedviasecreted factors[23].
Importantly, age has been demonstrated to promote the decline of hematopietic system by multiple molecular and cell-intrinsic mechanisms extensively reviewed by de Haanet al[24].
Hematopoietic stem cells (HSCs) deriving from aging mice and transplanted in younger mice showed lower self-renewal capacity, demonstrating that they are vulnerable to age-related stress and consequently lose self-renewal capacity. This process is influenced by cell-intrinsic and extrinsic factors and can compromise the immune system. Studying the aging process of HSC is important to develop strategies to improve the quality of life in the elderly, as it can make us better understand the mechanisms of age-related immune diseases[24,25]. Aging is responsible also for the progressive neural stem cells (NSCs) loss of function. Studies on adult mice showed that they have a role in maintaining cognitive functions; aging induces the loss of NSC neurogenesis capacity, with consequent brain degeneration. Biological aging of the brain occurs in several neurodegenerative diseases, such as Alzheimer's and Parkinson's, with dysfunction in the NSC compartments[26,27].
Moreover, numerous studies report an active role of Adult Renal Progenitor Cells (ARPCs) in renal repair processes during acute or chronic injuries. It has recently been shown that tubular but not glomerular ARPCs have a regenerative effect on cisplatindamaged proximal renal tubular cells preventing apoptosis and increasing the proliferation of surviving cells particularly through their secretome and the TLR2 engagement revealing a relevant functional role of this receptor in directing the repair by renal progenitors[28]. Additionally, ARPCs play an important role in the prevention of endothelial dysfunction and may be employed in new strategies to protect the endothelial compartment and promote kidney repair[29]. Furthermore, recent studies demonstrated that ARPCs can regulate the immune response by inducing Treg cells of the immune system and modulating double negative T-cells, which are involved in the balance between immune tolerance and autoimmunity[30]. All these regenerative properties of ARPCs can be affectedviarenal senescence, which can affect renal progenitors by both causing renal aging and the inability to repair renal damages.
In fact, increased renal expression of cyclin p16INK4a in the tubular epithelium occurs during aging (and to a lesser extent in glomerular (podocytes and parietal epithelium) and interstitial cells). Alterations in p16INK4a were more noticeable in the cortex compared to the medulla[31-33]. In rodents, the quantity of senescent cells in proximal tubules, but not in the glomeruli, increases with age. Moreover, renal tubular cell senescence correlates with tubular atrophy, interstitial fibrosis, and glomerulosclerosis[31,33]. Instead, the removal of senescent tubular cells leads to decreased glomerulosclerosis[34].
Prolonged or repeated renal injury leads to maladaptive repair leading to chronic kidney disease[35]. A possible explanation lies in the accumulation of senescent cells during aging and post-injury because the senescent cell burden slowly accumulates over time after acute kidney injury (AKI)[35]. Additionally, the level of senescence in graft biopsy before kidney transplantation could predict the outcome in terms of graft function[36] suggesting that targeting senescent cells could be an effective therapeutic intervention in kidney disease.
CD133 is a functional and constitutional marker of renal stem cells. In this context, CD133 expression is fundamental in the regulation of cellular senescence[37]. Indeed, CD133 is implicated in Wnt/b-catenin signaling, and its expression limits cellular senescence. CD133 can act as a permissive factor for Wnt/beta-catenin signaling (plays a role in protecting b-catenin from degradation) and plays a role in tissue repair. Furthermore, its absence altered cell proliferation after injury favoring senescence[38].
Bussolati’s group demonstrated the role of CD133 expressed by tubular cells during injury and its role in the repairing process. Furthermore, CD133 favored cell proliferation in the regenerative phase and limited cell senescence. In fact, the CD133 expression in ARPCs was reduced by cisplatin, but its expression was re-acquired one week after the cisplatin damage. Instead, CD133-knockdown ARPCs (CD133-Kd) displayed a significantly lower proliferative ability during the recovery phase compared to the normal CD133 ARPCs. Furthermore, the expression of the bgalactosidase senescence marker was significantly higher in the CD133-Kd cells compared to normal ARPCs demonstrating the role of CD133 in preventing senescence[38]. Therefore, aging clearly influences the regenerative ability of ARPCs.
HOW STEM CELLS AGE
Stem cell exhaustion is the result of multiple types of aging-associated damages and it is one of the phenomena responsible for tissue and organismal aging[16]. Many mammalian tissue-resident stem cells display a substantial decline in replicative function as they mature. The renewal ability of human tissues declines with aging of stem cells altering their capacity to differentiate in different types of cells[15]. Moreover, age-related loss of self-renewal in stem cells leads to a reduction in stem cell number[39]. Nevertheless, it may be possible to generate therapeutic approaches to age-related diseases based on interventions to delay, prevent, or even reverse stem cell aging[39].
Understanding how stem cells age may help understanding the normal aging process at the organ level, specifically in tissues with continuous regeneration[3]. These processes are influenced by various cell-intrinsic and cell-extrinsic pathways[40].Indeed, recent discoveries have revealed a complex interaction among cellintrinsic, environmental, and systemic signals linked to stem cell function loss during aging[40].
Researchers have worked to understand the main mechanisms within vitroandin vivoexperiments. The principal causes of stem cells aging are accumulation of toxic metabolites, DNA damage, proteostasis, mitochondrial dysfunction, proliferative exhaustion, extracellular signaling, epigenetic remodeling, and loss of quiescence[40-42]. Many of these aging mechanisms are in common with differentiated cells but stem cell exhaustion, or the quantitative and qualitative loss in stem cell function with time, has a more important impact on tissue aging compared to differentiated cells and has been postulated as one of the aging causes. Adult stem cells perform a critical function in tissue homeostasis by repairing and regenerating tissues throughout life. They maintain practically all tissues and organs, including the forebrain, bone, and muscle, and stem cell exhaustion, defined as a drop in stem cell number and function, is documented in essentially all tissues and organs maintained by adult stem cells. Furthermore, age-related alterations in hematopoietic stem cell HSC differentiation result in fewer adaptive immune cells being produced[43].
In addition, a decline in protein homeostasis or proteostasis occurs in aging cells with consequent accumulation of damaged and misfolded proteins[44]. This is critical especially for the degenerative disease onset. In addition, a reduced capacity of proteostasis can trigger a condition of endoplasmic reticulum stress that contributes to a loss of regenerative potential of aged HSCs[45]. Since HSCs have an age-dependent decrease in nutrient uptake, it is possible that aging of stem cells may be related to nutrient metabolism as well[40].
The extracellular signals and the microenvironment can affect the stem cells senescence. Stem cells reside in specialized microenvironments called niches, which promote their maintenance and regulate their functions[46]. The aging of niche cells and age-dependent alterations in the acellular components of stem cell niches can cause irreversible or detrimental changes in stem cell function[40].
Age causes a decrease in the number of cap cells and hub cells, which act as support cells for germline stem cells (GSCs) in the testes and ovaries, according to studies in Drosophila melanogaster. The disruption of the stem cell niche disrupts BMP signaling, which is required for GSC maintenance, resulting in lower E-cadherin levels and a weakening of the link between GSCs and cap or hub cells. The GSC niche ages as a result of this mechanism. Overexpression of the BMP receptor rescues the agedependent decline of GSC[47].
AGING ENVIRONMENT
In 1978, Schofield proposed the ‘niche’ hypothesis to describe the physiologically specialized microenvironment able to maintain the stem cells phenotype and regulate their functions[48-50].
In the recent years, advancements in our comprehension of organ aging revealed that systemic and niche microenvironment, by the release of soluble factors, can deeply influence the stem cells activity in different tissues ranging from hematopoietic, brain, skeletal muscle or hair follicle[51-54] (Figure 1). From seminal studies, more than twenty years ago, emerged that aged muscle successfully regenerates when transplanted in a young host, and from the other side, young muscle displays impaired regeneration when grafted into an aged host[3]. This heterochronic (i.efrom individuals of different age) tissue transplant studies, revealed that the age of the host animal was a key determinant factor of the regenerative success of the transplant in muscle, since strictly linked to the decline in stem cells reserve function[55].
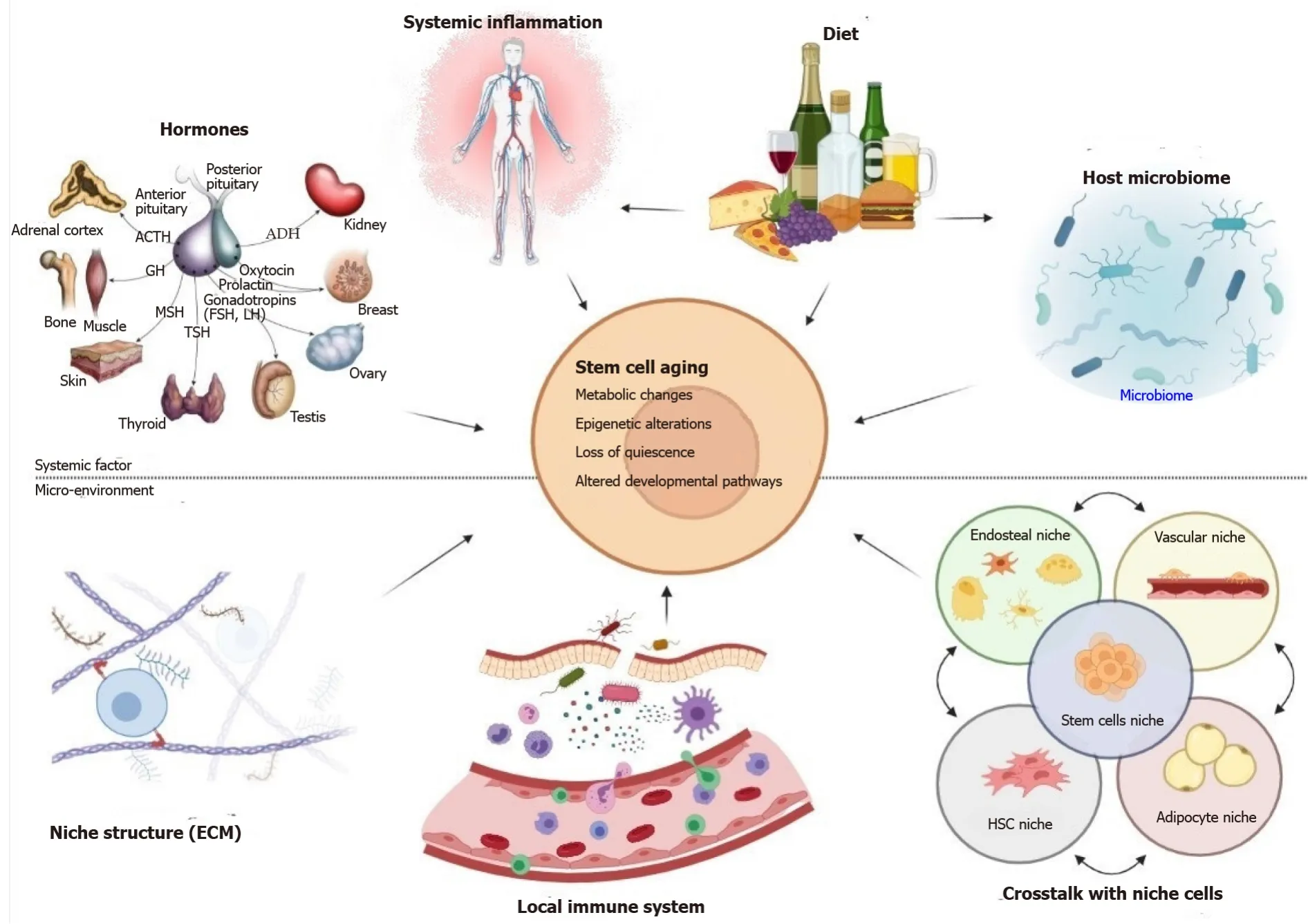
Figure 1 During the aging process, systemic influences and changes in the local microenvironment have an effect on stem cell activity.
Several authors postulated that systemic factors could boost tissue regeneration in young animals while inhibiting regeneration in old animals, and that these factors can regulate the main biochemical pathways that control progenitor cell regenerative characteristics. To test this hypothesis, a new experimental model of heterochronic parabiosis was performed by surgical fusion of the circulatory systems of two mice from different ages, allowing the sharing of circulatory system, thus the exposition of the two animals to the same circulating factors[56].
Parabiosis with young mice (2–3 mo) greatly improved muscle regeneration in the older partner (19–26 mo). Importantly, the activation of resident, aged progenitor cells, rather than the engraftment of circulating progenitor cells from the young partner, was nearly entirely responsible for the improved regeneration of aged muscle. These results indicated that the impaired regenerative potential of aged satellite cells can be improved by means of an increase of positive factors in young mouse serum, a decrease or dilution of inhibitory factors present in old mouse serum, or both. Similar results were also found in the liver from aged mice subjected to heterochronic parabiosis with a young partner. In the context of skeletal muscle stem cells aging (satellite cells), the impairment of Notch signalling leads to diminished regeneration of aged muscle (10). Interestingly, the heterochronic parabiosis restored Notch signalling in aged satellite cells. These findings imply that systemic variables that alter with age can influence the age-related drop in progenitor cell activity. These data have been later confirmed by several groups who performed heterochronic transplantation and parabiosis experiments in several model using aged-satellite cells[3,57,58], neural stem cells, and germline stem cells[3].
It should be noted that these experimental findings could be translated also in clinical settings. In renal transplantation contexts, premature renal aging was found to be modulated by soluble factors. Liuet al[59] showed that blood from young mouse was able to reduce acute kidney injury in older mouse, thus a youthful systemic milieu was able to attenuate inflammation, oxidative stress, and apoptosis after renal damage. In addition, transplantation of young bone marrow can rejuvenate the hematopoietic system and preserved cognitive function in old recipient mice[59-62].
NICHE MICROENVIROMENT
The aging microenvironment could be induced by extrinsic inflammatory soluble factors or by a dysbalanced release of intrinsic stem cells protective mediators. In the latter situation, elderly niche cells may be unable to deliver appropriate signals to stem cells, such as morphogen and growth factor signaling, influencing cell destiny decisions (Figure 1).
In mouse muscle's elderly satellite cell niche, the elevated levels of Fgf2 harmfully influence self-renewal[58]. Other circulating factors, such as insulin and IGF-1, that have been correlated to a youthful microenvironment were associated with caloric restriction, as recently demonstrated in growth hormone receptor knockout mice[63].
The extrinsic soluble factors are correlated to the establishment, with the accumulation of senescent cells in aging tissues, of persistent, low-grade inflammatory state called inflammaging, frequently observed in the elderly. Senescent cells secrete inflammatory factors, growth regulators, proteases and other signalling molecules, affecting neighbouring cells in the local environment and promoting senescence and inflammation. The production of a complex mixture of secreted factors is called senescenceassociated secretory phenotype (SASP) and includes several cytokines as IL-6, IL-8, CXCXL1, TNF- α, TGF-β, GROa. NF-κB appeared as the central molecular regulator of SASP phenotype. Elevated levels of TGF-β that increase with aging, accumulated in aged muscle of old mice hampering the regeneration and the satellite cell proliferation[64].
Moreover, the pro-fibrotic TGF-β impaired the function of neural stem cells[65], whereas the factor GDF11 showed beneficial effect on the stemness potential of satellite and neuronal stem cells[3].
Taken together, these studies suggest that there are both extrinsic systemic factors and intrinsic niche mediators that can accelerate or delay the aging of stem cells in the niche microenvironment[3,49,50]. A youthful environment can support effective tissue regeneration, whereas an older environment either does not promote or actively hinders it. It will be of great interest to characterize the factors that can modulate the tissue stem cell potential.
It's worth noting that the loss of tissue regeneration potential with age is not irreversible and can be slowed down by controlling systemic variables. These findings show that tissue-specific stem and progenitor cells retain much of their inherent proliferative capability even as they age, but that age-related alterations in the systemic environment and niche in which progenitor cells reside prevent these cells from fully activating for productive tissue regeneration.
MOLECULAR MECHANISMS IMPACTING STEM CELL MARKERS AND PROPERTIES
Aging of adult stem cells is mediated by several molecular mechanisms that are the same involved in the progression of somatic cells aging[41]. This process is the result of multiple mechanisms that act together to induce a progressive decline of stem cell functions, such as regenerative power and in some cases a strong decrease in cell number[41]. Among the principal mechanisms, recent studies reported mitochondrial dysfunction, the release of reactive oxygen species (ROS), DNA damage and telomere shortening, epigenetic modifications and mitochondrial DNA[40,66] (Figure 2).
The decline of stem cell function observed in many tissues during aging is accompanied by complex changes of the chromatin structure including changes in histone modifications and DNA methylation which both affect the transcription of tissue-specific genes[67] (Figure 3).
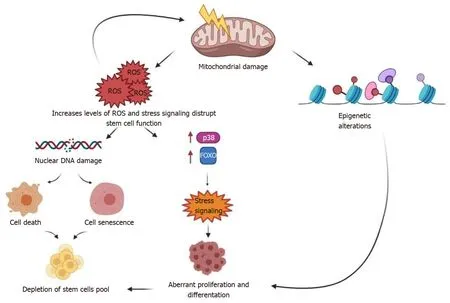
Figure 2 Molecular damage in stem cells as they age.
Mouse embryonic stem cells (mESCs) have higher acetylation and lower methylation levels than differentiated cells, and the chromatin landscape of pluripotent cells has been extensively examined. Increased transcriptional activity and hyperdynamic behavior of chromatin-associated factors in ESCs are consistent with the signatures of a more “active” chromatin conformation.
A co-localization of active and repressive chromatin marks at promoters and enhancers of developmentally regulated genes occurs in mESCs in addition to the surprisingly high dynamics of stem cell chromatin. The H3K4me3 and H3K27me3 chromatin signatures are thought to label genes that are repressed in ESCs but are poised to allow for alternative fates. Mutations in either H3K27 or H3K4 methyltransferase result in severe defects in ESC growth[68].
Current data support the concept that epigenetic regulation erodes in aging stem cells. InCaenorhabditis elegans, loss of function of a gene namedWdr5extended the life span by about 30% decreasing the levels of a histone methyltransferase that leads to trimethylation of lysine 4 on histone 3. It is unclear why the reduction of H3K4me3 is correlated with longer life span. In contrast, in yeast lower levels of H3K36me3 were found to reduce replicative life span while ablating genes that diminish the mark increased the yeast life span[69,70].
Besides, aged murine HSCs are characterized by an increase in global DNA methylation levels[71]. In line with the findings inCaenorhabditis elegans, H3K4me3 Levels tend to rise in aging HSCs particularly on genes involved in maintaining HSC identity. The repressive H3K27me3 mark increased with age also in skeletal muscle stem cells (MuSCs). In particular, this increase was associated with repression of genes that regulate specific differentiation programs in HSCs while it was associated with repression of genes encoding histone genes themselves in MuSCs. Moreover, MSCs from aged individuals have a decline of histone 3 lysine 9 trimethylation—a mark associated with proper maintenance of heterochromatin. However, this is a characteristic of aging of several human adult stem cells[43] (Figure 4).
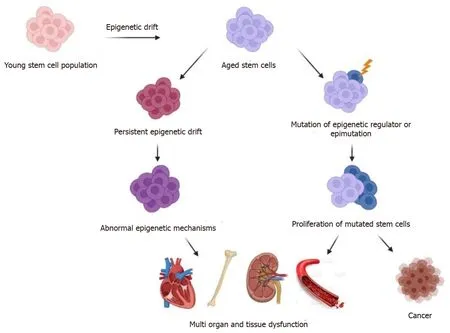
Figure 3 Adult stem cells are altered by epigenetic drift and clonal expansion as they age.
Another important role in histone methylation of aging stem cells is played by KDM5B. It is a key epigenetic regulator of the H3K4 methylation during cell differentiation, and it acts to reset the epigenetic landscape during differentiation by demethylating H3K4 at the level of self-renewal genes in trophoblast stem cells[72]. H3K4 is implicated in self-renewal activity in HSCs. Moreover, this mark increases with age and covers broader regions.
Studies on epigenetic changes during stem cell aging have been boosted by multiomic technologies, and these innovative studies in different stem cell types have revealed keys underlying the hypothesis of these age-related epigenetic erosions. Locus-specific alterations in DNA methylation show hypermethylation at promoters of polycomb group target genes and hypomethylation at repeat regions. Analysis of DNA methylome and transcriptome shows an increase in DNA methylation at promoters of genes associated with differentiation and a reduction at genes associated with HSC maintenance[73].
DNMT1 is the principal DNA methyltransferase in mammalian cells. It is a large and a highly dynamic enzyme with multiple regulatory features that can control DNA methylation in cells[74]. DNMT1 and DNMT3 are essential for SC self-renewal. In human ESCs, deletion of DNMT1 results in rapid cell death[75,76] However, the deletion of DNMT3 promotes HSC self- renewal and impaired differentiation[73,77].
A particular role in the epigenetic regulation of stem cell senescence is played by sirtuin proteins—a class of histone deacetylase enzymes (class III HDACs). SIRT1 plays a role in several stem cell lines, in stem cell differentiation, and regulation of quiescence. This leads to phenotypes typical of aging and premature differentiation[42,78]. In humans, SIRT6-SIRT7 regulated MSC senescence by modulating a heterochromatin-LINE1-cGAS-STING axis indicating that condensed heterochromatin is needed to safeguard genomic integrity[79].
A further key-point proteins for aging regulation is Tet methylcytosine dioxygenase 2 (TET2) a member of the ten-eleven translocation enzyme family that converts 5mC to 5hmC and modifies DNA methylation status[80]. TET2 is downregulated in aged NSCs although this can be reversed with parabiosis. Dietary restriction, such as daily or intermittent caloric restriction (CR), affects the transcription of the methylcytosine dioxygenases TET1 and TET3, which are involved in DNA demethylation. Moreover, CR increases the SIRT1-7 enzymatic activity[81]. Some diets could induce epigenetic changes. Interestingly, extra-virgin olive oil can affect histone acetylation processes inducing hyperacetylation of histone H3 in cell cultures[82] (Figure 1).
PIWI proteins can regulate epigenetic mechanisms in some animal models. PIWI proteins play key functions in biological and developmental processesviathe regulation of cellular mRNAs in addition to their role in transposable element repression[83]. Moreover, they bind small noncoding RNAs called piRNAs (Piwiassociated RNA) and the Piwi-piRNA complex leads to epigenetic regulation[84]. In drosophila, the Piwi are key factors limiting aging-related changes in intestinal stem cells[85]. The DNA damage repair system also regulates stem and progenitor cell functions and is affected by aging.
More generally, there is a growing body of evidence that accumulating mutations at stem and progenitor cell level contribute to aging related defects in organ maintenance and lead to cancer development[86].
Therefore, once again, it is clear how a strong connection emerges between epigenetic profiles, genetic elements, and genomic stability in the self-renewal potential of the stem cell. We need to continue to investigate more thoroughly to study sophisticated mechanisms that can regulate these features as well as role that additional methylation/acetylation mechanisms and genetic factors could have in the self-renewal activity and in diseases related to aging. At the same time, it is necessary finalize all of these aspects for the identification of new and more sensible aging markers and therapeutic targets.
In general, stem cells are considered an immortal reserve for tissue regeneration, but several evidences demonstrated that these cells are susceptible to advanced age[66]. Although these cells develop different protective mechanisms to counteract agingrelated injury and maintain their self-renew property, their functions started to decline with aging[87,88].
Oxidative stress is still recognized as one of the principal triggers in aging process, determining the impairment of antioxidant pathway and subsequent accumulation of cytoplasmic toxic debris that lead to apoptosis, necrosis or autophagic processes[89]. Within aged tissue, stem cells lose their antioxidant defense mechanisms and can show reduced capacity to regenerate and counteract stress oxidative injury. Several studies found that human MSCs increased ROS levels during progressive replications and became susceptible to oxidative damage activating several pathways and genes involved in aging process such as p53, FOXO1, Nrf2, micro RNAs and long noncoding RNAs[90].
Dysfunctional mitochondria also play a central role in aging process independently of ROS release and accumulation. In DNA Polymerase gamma deficient mice, mutations in mtDNA increased with aging and were correlated to muscle loss and sarcopenia condition[91]. Accordingly, another study demonstrated that mtDNA mutations in DNA Pol G deficient mice, induced respiratory chain deficiency and premature aging phenotype[92].
In addition, also mtDNA, as nuclear DNA, is exposed to mutations contributing to the development of aging. This process is strongly intensified by the oxidative stress injury and is aggravated by the decline of mtDNA reparative mechanisms in senescent cells[93] (Figure 4).
The mtDNA mutations can be sequenced from induced pluripotent stem cell (iPSC) lines from human skin or blood samples[94]. In this way, it would be easier to analyze and screen mutated genes in mtDNA of iPSC and directly obtaining information of mutated mtDNA of adult cells. Furthermore, it may be possible to find the mtDNA genes involved in several disorders associated with aging and to discover new therapeutic targets[95].
Aging process also alters mitochondrial biogenesis, reducing the number of functional mitochondria and the energy needed for cellular functions. Therefore, the combination of mitochondrial impairment and the decrease of biogenesis leads to aggravation of the aging process[96].
Nuclear DNA damage, induced by several external factors such as radiations, toxins and endogenous mediators like ROS and error in DNA replication mechanism, is associated with accelerated aging. Interestingly, defects in DNA repair processes have been found not just in aging but also in various human progeroid syndromes, which are relatively rare genetic disorders with clinical signs that resemble physiological age, such as hair loss, short stature, skin tightness, cardiovascular disease, and osteoporosis: the Werner syndrome, Bloom syndrome, xeroderma pigmentosum, trichothiodystrophy, Cockayne syndrome, or Seckel syndrome[97]. The role of Nrf2 in cell fate determination and cellular ROS control of HSCs and human airway basal stem cells was later discovered in studies on Keap1-knockout mice[98,99]. Nrf2 is involved in stem cell aging and in HSC homeostasis. Partially through direct association between Nrf2 and CXCR4, Nrf2 deficiency induces cell-intrinsic hyperproliferation and impaired HSC migration and retention in the bone marrow niche[98,99].
Another important key player in aging process is the telomere shortening, widely observed in human and mice studies[90]. In contrast to somatic cells, both embryonic and adult stem cells express telomerase, a reverse transcriptase enzyme (TER), and telomerase RNA component (TERC) which induce the extension of telomeric sequences and reduce the telomere shortening process[90]. TERC provide the template sequence for reverse transcription and help to assemble the ribonucleoprotein complex during maturation process. The interaction between TER and the protein component telomerase reverse transcriptase determines the catalytic activity, processivity, and telomere-binding ability of telomerase[100]. When defective, they can induce premature aging. Several studies showed the importance of telomerase enzyme activity to extend lifespan, reduce aging process[90] and avoid cancer development[86] .Emerging evidences underlined the involvement of several miRNA in stem cells functions such as potency, differentiation and self-renewal[101,102]. In addition, each type of stem cell contains a specific miRNA profile. Interestingly, some miRNA confers to stem cells the capacity to respond to several injury and to prevent the development of aging[101,102]. Thus, miRNA could be used in rejuvenate therapies, in order to counteract several diseases associated to aging, like myocardial infarction, neurodegenerative diseases, blood diseases, and muscle[101,102].
Although several molecules and pathways were widely described in aging process to determine and monitor senescent cells, specific and univocal markers are still missing.
The principal features to identify senescent cells include changes in cellular morphology, increased SA-β-gal activity, alterations in chromatin state, modification of gene expression of important kinases involved in cell cycle (p16,p21,p53), telomere shortening and the acquirement of SASP phenotype[103].
Considering that senescence can modify cellular functions, the percentage of senescent stem cells is evaluated by monitoring stem cell state and functions.
Recent studies discovered new senescence markers that could help to better characterize senescent stem cells. Among them, TRAIL (TNF-related apoptosis-inducing ligand) receptor CD264 that has been proposed as a marker of bone marrow-MSC cellular age and it was significantly associated with increasedp21expression profile and negatively correlated with proliferation[104].
Also, CD143 was found to be expressed in senescent MSC[105]. Therefore, highthroughput immunophenotypic analysis could be an advantageous method to discover senescent cells and in same time characterize their identity[105]. In addition, the rapid turnover of cytoskeleton filament actin in senescent cells could be studied by real-time labelling with a fluorogenic probe and is strongly associated to aged MSCinvitrosystem[106].
Together these approaches could be useful to identify senescent adult stem cells and to discover new therapeutic strategies to overcome physiological and pathological aging.
STEM CELL AGING UNDER PHYSIOLOGICAL AND PATHOLOGICAL CONDITIONS: DIFFERENCES
Adult stem cells undergo aging process both in physiological and pathological conditions. Senescent MSCs play a key role in many diseases especially in ageassociated diseases. In osteoarthritis, a small population of MSCs participate in increasing articular cartilage degradation and bone sclerosis[107]. These cells become dysfunctional and senescent thus enhancing cartilage hypertrophy and osteodegeneration[107].
Idiopathic pulmonary fibrosis is characterized by an irreversible loss of lung function. Here, lung fibroblasts acquire senescent phenotype and modify the microenvironment thus influencing the MSC behavior to sustain the inflamed microenvironment and influence the surrounding cells[108].
Interestingly, cardiac progenitor cells of patients with cardiovascular diseases expressed higher levels of senescent markers and presented reduced self-renewal, differentiation, and regenerative potential[109]. Therefore, these cells negatively impact cardiac-tissue regeneration.
In addition, neural progenitor cells may play important roles in neuro-degenerative diseases including Alzheimer’s Disease and Parkinson’s[110]. In primary progressive multiple sclerosis, NSC expressed senescent markers and secreted inflammatory mediators like HMGB-1 that negatively influence the microenvironment impairing maturation of oligodendrocyte progenitor cells. Therefore, senescent NSC could contribute to induce aberrant neural aging in several neurological disorders[111].
Considering these studies, we see that cellular senescence is a beneficial compensatory mechanism to avoid accumulation of damaged cells. This mechanism could induce deleterious consequences in stem cells population in older subjects or in the presence of pathological conditions inducing the loss of regenerative capacity. Therefore, there is an increasing need to find therapeutic strategies to promote senescence in tumor cells on one side and avoid this process in stem cells on the other.
CELL REJUVENATION STRATEGIES
One of the main aims of regenerative medicine is the capacity to rejuvenate tissues. This could occurviaendogenous stem cells or exogenous replacement cells derived from stem or progenitor cells to restore or rejuvenate tissues[112]. Recent discoveries also established that aging is not "irreversible" implying that aging of cells, tissues, and organisms can be "rejuvenated" rather than merely delayed[113]. Recent developments in our knowledge of tissue regeneration as well as the discovery of effective methods for inducing and differentiating pluripotent stem cells for cell replacement therapies promise to open up new possibilities for treating age-related diseases[112]. Reduced ROS levels can be employed to reverse aging phenotypes produced by uncontrolled accumulation of ROS, allowing aged stem cells to reactivate[40].
Treatment with antioxidants such as N-acetylcystein (NAC) and targeting toxic metabolites can considerably improve survival and tissue repair ability of stem cells[114]. NAC treatment enhances the survival of a distinct population of myogenic stem cells in skeletal muscle bothin vitroandin vivoand restores their quiescence and reconstitution capability[40]. In FoxO-deficient mice, therapy with NAC may improve defects in HSC quiescence, survival, and repopulating ability[40].
Furthermore, increasing the activity of DNA repair mechanisms may help stem cells avoid developing age-related abnormalities. Studies in mice show that late-life reactivation of the telomerase RNA component mTERC can reverse degenerative phenotypes in elderly animals that are genetically weak in telomerase activity (due to inactivation of the telomerase RNA component mTERC)[115]. In contrast, increasing telomerase activity may induce malignance tumor.
There are a limited number of studies on rejuvenation of aged stem cells targeting mitochondrial functions[116]. It has recently been demonstrated that PPAR agonists improve the role of hematopoietic stem cells by enhancing fatty acid oxidation[116]. Targeting sirtuins, AMPK, mTOR, NAD+ metabolism, nuclear receptors (such as PPARs and estrogen-related receptors), transcriptional factors/co-factors (such as PPARGC1, FOXO, NCORs), as well as activators of UPRmt, and mitochondrial fusion/fission or mitophagy, are some of the other strategies for improving mitochondrial functions[116]. These approaches, however, must maintain the balance of stem cell self-renewal, proliferation, and differentiation.
Another important treatment was investigated in aged mice with either recombinant GDF11 or oxytocin that reverse the dysfunction of aged satellite cells and restore vigorous regenerative function in aged mice to show that regeneration in aged mice is reversible suggesting that young blood contains humoral “rejuvenating” factors that can restore regenerative function[117].
As a result, in addition to systemic influences, targeting senescent cells and their secretome in aged tissues may help restore stem cell activity[40]. Clearing senescent cells from progeroid mouse tissues through ablation of p16Ink4a-expressing cells delays the onset of diseases in many aging organs, including the fat, muscle, and eye, was done using an inducible genetic model for senescent cell ablation. Senescent cell clearance at the end of life did not boost age-related pathologies, but it did slow their development.
Furthermore, senolytics, a new class of drugs that selectively kill senescent cells, represent a great potential for improving health span[118]. They could be beneficial in a variety of age-related pathologies, such as sarcopenia and metabolic disorders[119].
Drugs such as rapamycin can be used to rejuvenate aging cardiac stem cells[118] through the inhibition of mTOR—the major downstream component in the PI3K senescence pathway. This action leads cells from a senescent to a quiescent stage[118].
The WNT/β-catenin pathway is another potential target for rejuvenation of hMSCs used in stem cell therapy for cardiac repair[118]. The WNT/β-catenin pathway is related to stem cell renewal and differentiation through regulation of CTNNB1, which plays a crucial function in cardiogenic development. The age is connected with reduction of MSC proliferation and differentiation and WNT/β-catenin signaling. Lithium therapy increases β-catenin availability to boost myogenic differentiation and can revive some functions of MSCs from aged people[118,120].
However, epigenetic rejuvenation has been proposed to be the safest and most successful form of regenerative medicine. It can delay aging and the onset of ageassociated decline and diseases to extend health span and lifespan[81,121]. Different methods can induce epigenetic reprogramming. For example, metabolic manipulation like caloric restriction influences DNA methylation and histone modifications. Another method could be plasma exchange to obtain the same effects of heterochronic parabiosis. Here, the circulatory systems of young and old animals are surgically linked allowing immune cells and secreted factors in the blood to swap. It has rejuvenating effects in old animals, reducing age-related dysfunction in a variety of tissues. Finally, pharmaceutical administration and senescent cells ablation can be useful to alter gene expression and reprogramming aged cells to a younger state[81]. Dasatinib, for example, destroys senescent fat cell progenitors while quercetin kills senescent human endothelial cells and mouse bone marrow stem cells. Quercetin is a flavonoid found all over nature and regulates the function of DNMTs, HDACs, and histone methyltransferases. Quercetin acts as a geroprotector by enhancing selfrenewal and restoring heterochromatin architecture in aged MSCs[81].
As a consequence, aging phenotypes may be reversed in these rejuvenation procedures, restoring the regenerative activity of stem cells with therapies that are promise for the treatment of a variety of disorders, including sarcopenia, heart failure, acute kidney damage, and neurodegeneration. Even while geroprotective chemicals have been linked to a "younger" chromatin architecture, further research is needed to understand how these longevity-promoting medications interact with epigenetic networks to halt the aging process.
CONCLUSION
During the aging process, stem cells in various tissues develop defects that prevent them from performing critical functions such as restoring tissue damage and preserving tissue homeostasis. A decline in the maintenance of a healthy proteome, metabolic changes, alterations in intrinsic and extrinsic signaling pathways, DNA damage, and epigenetic changes are all examples of these defects.
Understanding how stem cell aging affects distant tissues and overall health span is just the tip of the iceberg. This line of research is important because it lays the groundwork for stem cell-based treatments to help people live longer lives. Rejuvenating intervention may restore stem cells function and the possibility to use these cells for therapy.
We highlighted several interventions in this review that have shown or may show tremendous promise in increasing the function of aged stem cells in a range of scenarios. Unfortunately, many of these therapies are not acceptable or unlikely to be clinically applicable (such as transgenic partial reprogramming or heterochronic parabiosis). Small chemical, diet-based, and some microenvironment modification techniques, on the other hand, have shown to be more clinically effective. While dietbased treatments have showed some promise in increasing the function of aged stem cells, they have yet to demonstrate the potential to restore lost function in a person who is already old. Many of the present solutions need more research. The effects of most therapy options addressed have not been tested on every stem cell compartment, leaving gaps in our understanding of their systemic impacts. Furthermore, more longitudinal studies are needed to fully understand the effectiveness of these therapies and to investigate any potential detrimental side effects. Most of the research merely observe the animals for a few weeks before sacrificing them for examination. However, it is unknown whether advances in certain stem cell compartments will produce toxicity elsewhere in the body or if they would lead to long-term stem cell depletion, senescence, or malfunction more quickly with most treatments. Furthermore, much research has concentrated on stem cells derived from disease models, which may not be applicable to aging.
While there has been fascinating research into the secretome’s regenerative potential, clinical translation of a secretomic strategy will most certainly be hampered by manufacturing issues and batch-to-batch variability, which reduces consistency. Identifying the most potent secreted factors, or the most effective mixture of secreted components, may be more advantageous. To achieve optimal efficacy, these elements can be manufactured individually and blended in specified ratios. Moreover, Centenarian studies may provide unique insights into the relationship between stem cell aging and longevity[122]. Finally, since epigenome changes are theoretically reversible and there is evidence that epigenome reprogramming can improve tissue maintenance, regenerative ability, and health, the idea of epigenetic incorporation of damage signals as a cause of stem cell and organism aging holds new promise for translational approaches.
ACKNOWLEDGEMENTS
We thank Francesca Giannuzzi for the assistance in illustrations.
杂志排行
World Journal of Stem Cells的其它文章
- Priming strategies for controlling stem cell fate: Applications and challenges in dental tissue regeneration
- Epigenetic regulation of dental pulp stem cells and its potential in regenerative endodontics
- Effects of immune cells on mesenchymal stem cells during fracture healing
- Regulation of the mesenchymal stem cell fate by interleukin-17:Implications in osteogenic differentiation
- Nanofat: A therapeutic paradigm in regenerative medicine
- Application of adipose-derived stem cells in treating fibrosis
