Short-term microbial responses to soluble inorganic P input in a tropical lowland rain forest in Amazonia
2021-12-22YuriDESOUSAErikaBUSCARDOCarlosQUESADAHenriqueNASCIMENTOandLaszloNAGY
Yuri W.L.DE SOUSAErika BUSCARDOCarlos A.N.QUESADAHenrique E.M.NASCIMENTO and Laszlo NAGY
1National Research Institute for Amazonia,Manaus 69067-375(Brazil)
2Department of Forestry,Universityof Brasilia,Brasilia 70910-900(Brazil)
3Centre for Functional Ecology,Universityof Coimbra,Coimbra 3000-456(Portugal)
4Department of Animal Biology,Universityof Campinas,Campinas 13083-862(Brazil)
(Received October 10,2018;revised April 16,2019)
ABSTRACT In non-flooded lowland rain forests with low soil phosphorus(P)in parts of Amazonia,P cycling largely occurs via leaf litter recycling by arbuscular mycorrhizal(AM)fungal symbionts.Occasional high input of P into these ecosystems occurs during drought years with increased litterfall.As the length and frequency of drought events are projected to increase in the region,a single-dose nutrient addition experiment was carried out to test how this would impact P cycling.An application rate of 4 kg P ha-1 was used,which corresponds to twice the amount of litter-derived P in an average year.It was hypothesized that i)the added mineral P would be immobilized by soil microorganisms,leading to measurable increase in soil microbial biomass carbon(C)and P and ii)AM colonization rate would be reduced by the pulse in mineral P available for plant uptake.The results did not support either of our hypotheses.The addition of P did not have an effect on AM root colonization,nor was P immobilized by soil microbiota during the experimental period.The lack of a difference between the control and treatment at our study site could be attributed to the relatively low one-offdose of P applied that did not change either the colonization rate of roots by AM fungi or the amount of soil available labile P.To obtain a mechanistic understanding of the availability,capture,and use of P by plant-symbiont associations in tropical rain forest ecosystems,further integrated studies of the soil-plant system combining long-term nutrient manipulations,modeling,and experimental approaches are required.
KeyWords: Amazon basin,arbuscular mycorrhizal fungi,nutrient addition,phosphorus cycling,soil microbial biomass
INTRODUCTION
Mutualistic associations between plants and arbuscular mycorrhizal (AM) fungi are common in most tropical forests(Smith and Read,2008),where they have important roles in nutrient cycling and productivity. The symbiosis helps explore the soil and thus enhances nutrient and water uptake by plant roots(Bolan,1991;Finlay,2004),thereby alleviating potential growth limitation. Mycorrhizal fungi represent a key component in the global carbon(C)cycle(Nottinghamet al.,2013);by obtaining plant photosynthates,they contribute to heterotrophic soil respiration and release substantial quantities of CO2to the atmosphere(Nottinghamet al.,2010).As the C cycle is coupled with those of other elements such as phosphorous (P) and nitrogen (N), the functioning of AM fungal symbiosis directly affects both the P and N cycles(Townsendet al.,2011).
In the tropics,N is mostly limiting in montane forests(Correet al.,2010),while both N and P are in short supply in heath forests on podzolic‘white sand’soil(Mardeganet al.,2009).Low available P in the soils of non-flooded lowland evergreen rain forests in Amazonia,locally known in Brazil as terra firme,results from the predominance of weathered soils,except for those mainly in western Amazonia,which receive inputs from ongoing erosion processes in the Andes(Quesadaet al.,2010).
Tropical forests stock and sequester large amounts of C,both above-and belowground,and they contribute substantially to the regulation of the global climate system(Fieldet al.,1998).Net primary production in large parts of lowland Amazonia is considered to be related to,and appears to be limited by,the availability of soil P(Vitousek,1984;Quesadaet al.,2012).It has been assumed that in Amazonian terra firme forests,there is a‘tight’P cycling,which relies on the recycling from litter P by AM fungal symbionts(Herreraet al.,1978;Aristizábalet al.,2004);conversely,there appears to be a‘leaky’N cycling,often construed as N excess(Vitousek,1984;Hedinet al.,2009;Brookshireet al.,2012).While the general pattern of AM fungal occurrence and diversity in tropical forests is largely unknown(Alexander and Selosse, 2009), it is widely assumed that the relative availability of C,N,and P determines the symbiotic function of AM fungi(Johnson,2010).Some nutrient addition experiments have explored the nature and extent of nutrient limitations on tropical forest growth(Mirmantoet al.,1999;Wrightet al., 2011) and on compositional and functional aspects of soil microorganisms(Tanneret al.,1992;Faninet al., 2015; Buscardoet al., 2018b) including AM fungi(Kivlinet al.,2011;Liuet al.,2013;Wurzburger and Wright,2015). While it has been suggested that the levels of AM fungal root colonization can be reduced by the addition of P and N(Treseder and Vitousek,2001;Pagano and Scott,2010),the majority of these experiments,with the exception of some studies that looked at the medium- to long-term effects of litter manipulation on soil microbiota(Nemergutet al.,2010;Sheldrakeet al.,2017),have used large and often unrealistic quantities of one or more nutrients,leading to a stoichiometric imbalance of microbial resources(Faninet al.,2017).The input of P in lowland tropical rain forests can potentially increase during occasional severe droughts through a pulse of nutrients associated with an increased litterfall input(Mirmantoet al.,1999;O’Connellet al.,2018).This increase has two components:the higher quantity of litter that reaches the forest floor and its greater P concentration that results from less P resorption before leaf fall than in average climate years(Brandoet al.,2008;Clevelandet al.,2010).
The length of dry season and occurrence of drought periods are relevant in the context of projected climate change impacts(Buscardoet al.,2021).Across Amazonia,profound changes in rainfall quantity,rainfall patterns,and temperature have been projected by recent model experiments, either using dynamic vegetation models that couple atmospheric and soil processes or those that consider land cover/land use change(deforestation;Marengoet al.,2016).According to projected changes,drought frequency and severity could increase and may lead to changes in the characteristics of the ecosystems of the Amazon basin.This would necessarily involve changes in biogeochemical cycles(Buscardoet al.,2021).
To simulate the pulse of P associated with an increased litterfall input during a drought period,a P addition experiment was undertaken in a terra firme forest stand in Amazonia during the dry season.A single-dose of P(4 kg ha-1year-1),corresponding to twice the yearly amount of litter-derived P in terra firme forests(ca.2 kg ha-1year-1)(Buscardoet al.,2016),was applied to 1 m×1 m plots under a variety of tree species.This was comparable with the values expected in large and P-rich litter input during occasional drought years.To test the impact of P addition on soil microorganisms,soil microbial biomass C (MBC) and P (MBP) and AM fungal colonization were repeatedly measured to target the very short-term P effects in a 4-month period that was not coincident with the seasonal pulse-nutrient input.It was hypothesized that i)the added mineral P would be immobilized by soil microorganisms,leading to measurable increase in soil MBC and MBP(H1)and ii)AM colonization rate would be reduced by the pulse in mineral P available for plant uptake(H2).
MATERIALS AND METHODS
Studyarea and experimental design
The experiment was carried out in the Adolfo Ducke Forest Reserve(ADFR),located 26km northeast of Manaus,Brazil.The ADFR covers about 100 km2of natural forest and is characterized by a varied topography with valleys and plateaux, whose elevations range between 40 and 140 m above sea level.The climate is classified as Köppen’s type Af(Kotteket al.,2006),with a period with mild hydrologic deficit(<100 mm month-1)ranging from 1 to 3 months.The annual precipitation is on average 2 100 mm,and mean annual temperature is around 26°C.The precipitation and temperature obtained from the ADFR Meteorological Station in 2014, the year of study, were 2 760 mm and 24.1°C,respectively (Fig. 1). The soil in the well-drained plateau areas is classified as Oxisol (Chauvelet al., 1987), and it supports a tropical lowland evergreen rain forest with an emergent canopy that reaches 30—40 m and an understory characterized by many palms (Ribeiroet al., 1999). The annual litterfall has been estimated at 8 Mg ha-1year-1(Luizão,1989)corresponding to an average P input ofca.3 kg ha-1year-1; the Amazon basin-wide average litterderived P is about 2 kg ha-1year-1(Buscardoet al.,2016).
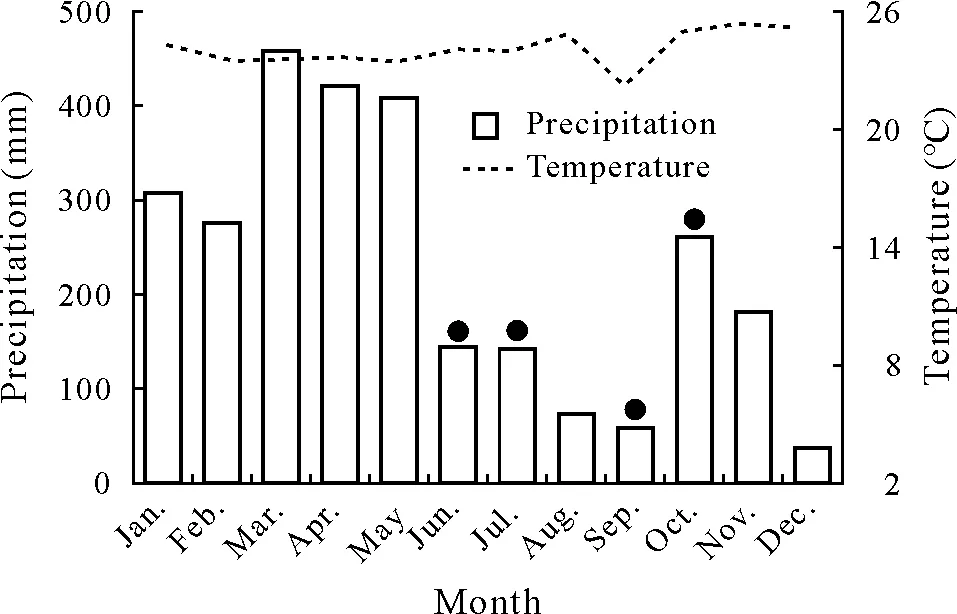
Fig.1 Precipitation and temperature in a lowland evergreen rain forest in the Adolfo Ducke Forest Reserve (ADFR), Manaus, Brazil in 2014.Data were obtained from the Meteorological Station of ADFR.Black dots indicate soil sampling dates.
Ten trees were randomly selected as reference points along a 1 600-m long section of a plateau area, with a minimum inter-tree distance of at least 40 m to avoid spatial autocorrelation of soil variables between plots (Buscardoet al., 2018a). The random variety of target tree species served to represent the condition of high species richness that characterizes these ecosystems.At each point,two 1 m×1 m plots(a control and a treatment)were established at a distance of 1.5 m from the reference tree, in a way that the distance between the plots would be maximized. The treatment plots received an equivalent of 4 kg ha-1year-1P in a single-dose in the form of NH4H2PO4.The use of this compound resulted in concomitant addition of the equivalent of 2 kg ha-1year-1N that could potentially have masked the effects of P. However, the amount of P received from litterfall in Amazonian terra firme forests on Oxisols is about 2 kg ha-1year-1,while litterfall returns,on average,100 kg ha-1year-1N, not counting any potential atmospheric input and biological N2-fixation(Buscardoet al.,2016).In other words,the experimental treatment doubled the annual input of P and received<2%of the annual input of N,the equivalent of about 7 d worth of mean litterfall in a year and thus ecologically negligible.The NH4H2PO4was added in 1 L of aqueous solution on June 16, 2014. Since 1 L of solution represented 0.5%of the previous 30-d average rainfall in the area,the control plots were not watered.To prevent leaching of the applied P by rainfall,fresh litter was carefully removed,in a way so as not to disrupt too much fungal hyphae(Posadaet al.,2012),before the application of the fertilizer and replaced immediately afterwards.
Soil sampling and analyzing
The period of the experiment spanned the transition from the rainy to the dry season until the transition from the dry to the rainy season.This period was chosen so that the pulse of mineral P addition would not coincide with the natural peak of nutrient pulse,after peak litterfall,at the beginning of the rainy season.By this way we expected to observe the effects of the P addition without confounding background effects.Soil samples were taken at the transition period between the rainy and dry seasons (pre-fertilization, day 1), at the beginning of the dry season (day 20), at the peak of the dry season (day 88), and at the transition period between dry and rainy seasons(day 119).Each plot was subdivided into 15 subplots and three were randomly sampled with a volumetric ring(5 cm in diameter and 5 cm in depth)at each sampling time.During the period of the experiment,each subplot was sampled only once.The samples were grouped in a composite soil sample per plot.Soil samples were stored in plastic bags and taken to the laboratory within the same day and processed.
The physicochemical properties of the soil under each reference tree were determined.Particle size analysis was conducted with 10 g of air-dried soil,following the pipette method(Gee and Bauder,1986).Exchangeable cations were determined by extraction from 5 g of air-dried soil,using the silver-thiourea method(Pleysier and Juo,1980),followed by atomic absorption spectrophotometry(iCE3000 Series AA spectrometer,Thermo Scientific,USA).Calcium(Ca2+)and magnesium(Mg2+)were determined in lanthanum solution,while sodium (Na+) and potassium (K+) dilutions were made in cesium chlorite solution. For the quantification of aluminum (Al3+), the method of Embrapa (2009) was followed. Briefly, soil extraction was made using 5 g of air-dried soil in 1 mol L-1KCl solution and Al3+was then titrated with 0.025 mol L-1NaOH solution.
For the determination of soil density,soil samples(0—5 cm)were collected with a volumetric ring outside of,but close to the edge of the plots and dried in an oven at 105°C for 48 h (or until constant weight). Soil density (D, g cm-3) was determined by the formula:D= mass of dry soil/ring volume.For the determination of soil volumetric water content(θ,cm3cm-3),a fresh sub-sample(5 g)was brought to a temperature of 105°C for 24 h immediately after collection.Theθvalue was calculated using the apparent soil density and the weight difference between fresh and ovendried soil.Soil pH was measured with a pH meter(mPA210,MS Tecnopon,Brazil)after shaking 10 g of air-dried soil for 1 h in 25 mL of water.
Soil total C(TC)and N(TN)were determined for each plot with a Vario Max CN analyzer(Elementar Analysensysteme GmbH,Germany)using 1 g of milled soil.Due to insufficient amounts of samples,this analysis could not be made for two of the samples collected on day 119.
Soil MBC was determined using the chloroform fumigation method(Brookeset al.,1985;Vanceet al.,1987)and quantified as the difference between the C concentrations of a sample of 25 g of soil extracted in 1 mol L-1K2SO4and another one following fumigation with alcohol-free chloroform and incubation for 24 h at room temperature.
The determination of soil P fractions was made using the method proposed by Hedleyet al.(1982)and modified by Carter (1993). In our experiment, the labile P fraction(Plabile),readily available for plant uptake,was extracted with bicarbonate. The determination of Plabilewas made spectrophotometrically(UV 1240,Shimadzu,Japan),following the method of Murphy and Riley(1962).For determining soil total P(TP),an aliquot from the bicarbonate extract was digested with ammonium persulphate and the P content was determined spectrophotometrically.The organic P fraction,i.e., the P immobilized in soil microbial biomass (MBP),was determined by calculating the difference between the TP and Plabilerecovered with and without fumigation. At day 88,the calculation for determining organic P resulted in negative data for two samples;these data were excluded from the statistical analyses.
Root colonization byAM fungi
The colonization rate of roots by AM fungi was determined using the modified method of Schenck(1982).About 50 segments of fine roots of 1 cm were selected per soil sample.The roots were washed with water to remove soil and organic matter particles and then heated for 1 h in a 100 g L-1KOH solution to remove cytoplasmic content.The roots were subsequently cleaned in 10%H2O2for 30 min,followed by a period of 5 min of acidification in a 3.5%HCl solution.After that,the roots were washed with abundant distilled water.Subsequently,they were colored with a heated solution of trypan blue in lactoglycerol for 1 h.The colonization rate was determined by using a stereoscopic microscope(40×).Segments of roots were classified according to the presence or absence of fungal structures.The colonization rate of root(defined as the percent of root fragments with AM colonization present)was calculated as the proportion of colonized root segments in relation to the total root segments per sample.
Statistical analyses
Treatment(P addition)effects on the analyzed parameters were tested with a mixed effect linear model in the R environment(R Development Core Team,2016),using the following model:model<-lme(variable~day+treatment,random=~1|plot).The temporal dynamics were analyzed by pairedt-test, using the difference between the values of the variables of interest in fertilized and control plots.Prior to statistical analyses,data on AM colonization rate,soil C and N, and C:N ratio were transformed by using ar csin((datum/100)), and P data by sin(log10) to meet normality and homoscedasticity assumptions.
RESULTS
Soil pH and water content
Soils were clayey in texture(clay=73%±1%(mean±standard error),silt=16%±1%,and sand=11%±0.5%)and acidic(pH=4.0±0.05)(Table SI,see Supplementary Material for Table SI).Water content and pH varied over the duration of the experiment,indicating the effects of seasonality(Fig.2,Table SII,see Supplementary Material for Table SII);however,there were no significant differences between the control and treatment plots either at the beginning of the experiment(i.e.,day 1)or following P addition.The values ofθdecreased gradually between day 1(transition between rainy and dry seasons,θ=0.22 cm3cm-3)and day 88(peak of the dry season,θ=0.19 cm3cm-3),and increased again at the transition between dry and rainy seasons (day 119,θ=0.20 cm3cm-3)(Fig.2).The values of pH were higher at the beginning and at the end of the experimental period(pH=4.1),while significantly lower values were detected at the beginning(day 20,pH=3.5)and during the peak of the dry season(day 88,pH=3.7)(Table SII).
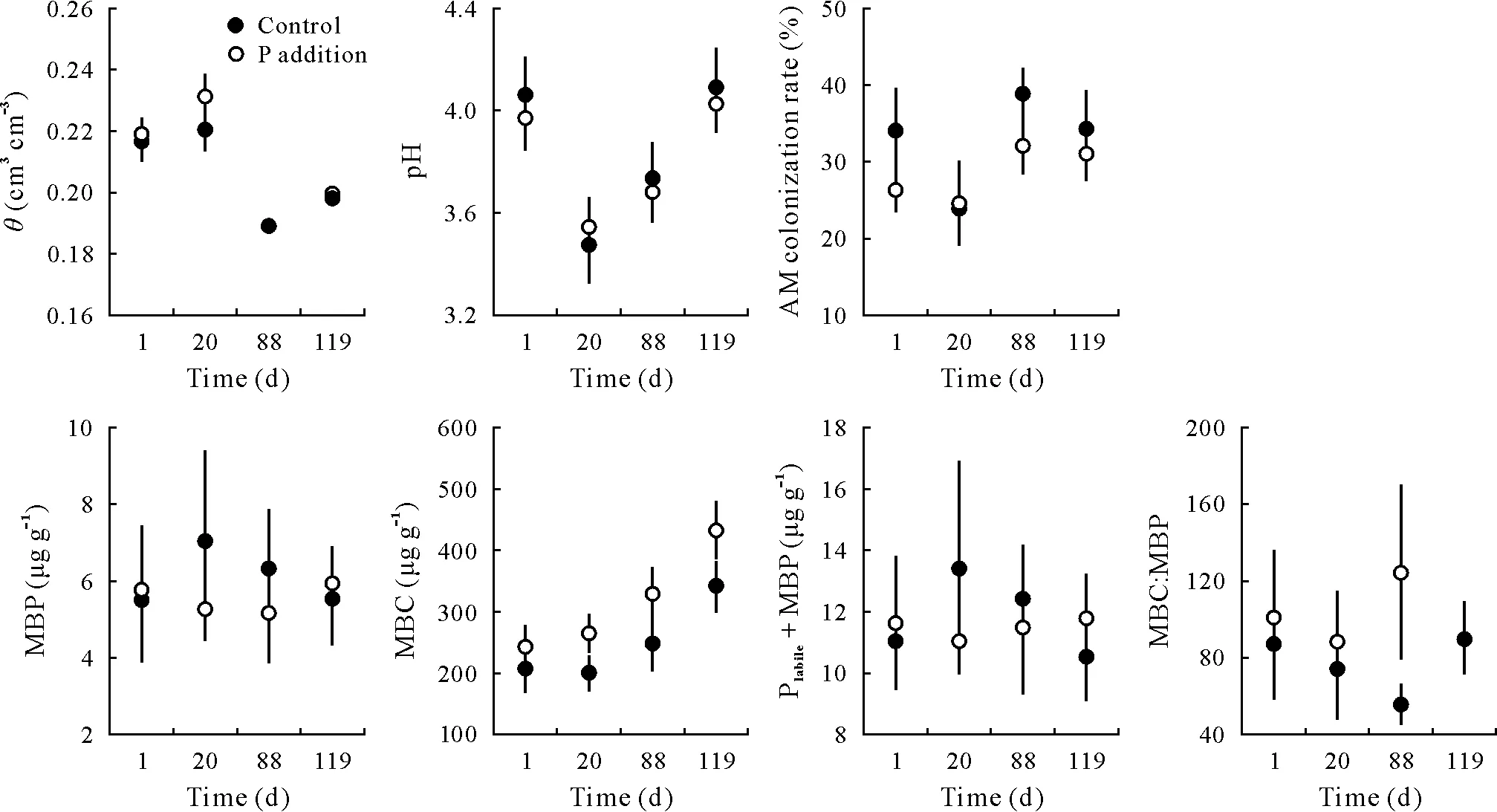
Fig.2 Soil physicochemical properties and microbial composition in the top 5 cm layer in response to P addition in a tropical lowland rain forest in Amazonia during the experimental period(day 1,pre-treatment;day 20,beginning of dry season;day 88,peak of dry season;and day 119,transition between dry and rainy seasons).Vertical bars indicate standard errors of the means(n=10).θ =volumetric water content;AM=arbuscular mycorrhizal;MBP=microbial biomass P;MBC=microbial biomass C;Plabile =labile P.
Soil TC and TN
Soil TC(46.2vs.50.3 g kg-1),TN(3.5vs.3.7 g kg-1)and TC:TN ratio(13.2vs.13.7)did not significantly differ between the control and fertilized plots(Fig.2,Table SIII,see Supplementary Material for Table SIII).However,these elements showed significant seasonal variations during the experimental period(Table SII).The TC value was significantly higher on day 88 compared with those on days 1 and 20 and decreased again on day 119.A similar trend was observed for TN with the highest value at the peak of the dry season and for TC:TN ratio that showed the highest values on days 88 and 119.
Soil MBC,Plabile and MBP
Soil MBC did not differ significantly between the control and fertilized plots (Fig. 2, Table SIII). The last day of collection(day 119),corresponding to the transition period between dry and rainy seasons, was characterized by the highest MBC values both in fertilized (342 μg g-1) and control plots(433 μg g-1).The sum of Plabileand MBP(soil available P following fumigation)did not significantly differ between the control and fertilized plots at any sampling point(Fig.2,Table SIII).No significant temporal changes were detected in Plabileplus MBP during the experimental period(Fig.2,Table SII).The MBP fraction did not change significantly between the control (6.13±0.90 μg g-1)and fertilized plots (5.58±0.62 μg g-1) and between different sampling points during the experimental period.The MBC:MBP ratio was also not significantly affected by fertilization (77±12vs. 100±16) and did not follow a temporal pattern(Fig.2,Table SII).
Root colonization byAM fungi
Root colonization by AM fungi was on average 32.8%in the control and 28.5% in the P-treated plots, with no significant differences between them at any sampling point(Fig.2,Table SIII).However,significant differences were detected in the colonization rate in the control plots between days 1 and 20, with lower colonization rate values at the beginning of the dry season and between days 20 and 88,with an increase in the colonization rate during the peak of the dry season(day 88)(Fig.2,Table SIII).The highest root colonization rate was recorded on day 88 for both control and fertilized plots.
DISCUSSION
Doubling the input of annual P that reaches the forest floor of an Amazonian terra firme forest through litterfall(4 kg ha-1year-1) had no effect on either soil labile P,MBC and MBP,or the colonization rate of root by AM fungi during the 4-month period that followed the P addition.
Contrary to our first hypothesis(H1),microbial biomass(both C and P)did not increase after the application of P.Soil microbiota in tropical rain forests appears to be limited by P(Clevelandet al.,2002)and generally responds positively by increasing its biomass after P application (Turner and Wright,2014;Faninet al.,2015;Zhuet al.,2015),even if,in some cases,the effects are transient(Liuet al.,2013)or no changes are detected due to P saturation(Chenet al.,2014).However, most of the cited fertilization experiments have used high doses of P(50—150 kg ha-1year-1),making it difficult to make comparisons with the results of the current experiment,in which a more realistic but much lower dose of inorganic P was applied.A litter manipulation experiment conducted in tropical lowland forest in Costa Rica showed that doubling the amount of litter during a 2-year period could increase microbial biomass,both C and N(Nemergutet al.,2010),while there was no P effect in a similar experiment after 9 years in a terra firme forest in Panama(Sheldrakeet al.,2017).However,in these cases,it has to be considered that the extra amount of added litter represents a much more complex C and nutrient input compared with the mere addition of inorganic P.Recent fertilization experiments have suggested that microbial communities in tropical forests could be co-limited by C and P(Barantalet al.,2012;Faninet al., 2012) and may respond, with greater biomass and activity, to the increased availability of a labile C source(Camenzindet al.,2018).
Interestingly,the average value of the MBC on the last day of collection in our experiment(at the transition between dry and rainy seasons) was higher than that at any of the other sampling points, with a more visible trend in the fertilized plots. Generally, the values of MBC tend to be smaller in evergreen tropical rain forests during the dry season compared with those in the rainy season(Turner and Wright, 2014). The increase in precipitation frequency in concomitance with our last soil sampling could,therefore,explain the observed increase in MBC,which was limited during the dry season by water availability.However,other concomitant factors may have influenced the increase of MBC at the transition between the dry and rainy seasons.Higher values of soil TC and TN were observed at the peak of the dry season when compared with those at the other sampling points.This could be explained by the fact that,during the driest period of the year,litter accumulates on the forest floor and that the start of the rainy season promotes the movement of readily decomposable dissolved organic matter from the litter layer(Clevelandet al.,2006),which becomes available to the soil microbiota(Nottinghamet al.,2012).In the present study,the experimental period was chosen to avoid an overlap between the pulse of mineral P addition and the natural peak of nutrient pulse and to be able,therefore,to observe the effects of the P addition without confounding background effects.However,our results indicate that soil microbes could potentially benefit from an extra P supply exactly after the litterfall peak at the beginning of the rainy season,when microbial activities tend to increase.
The colonization rate of root by AM fungi observed at our study site(on average 31%)is comparable to that estimated by Treseder and Cross(2006)for tropical forests(ca.36%).Contrary to our second hypothesis (H2), the amount of P applied to our experimental plots did not change the colonization rate of root by AM fungi.A study conducted in four Neotropical rain forests over large geographic separation distances and with differing soil fertility and above-ground biomass,has shown that,while both root biomass and length were negatively correlated with soil N and P, AM hyphal length,which together with the fungal colonization rate of roots represents another way to measure the intensity of AM symbiosis,was not related to nutrients,root properties,or above-ground biomass (Powerset al., 2005). Similarly, a recent study conducted in a lowland tropical forest in Panama to investigate the consequences of long-term litter addition on the AM fungal communities has shown no significant effect of litter manipulation on the proportion of root length colonized by AM hyphae, arbuscules, or vesicles and on extra-radical AM fungal biomass(Sheldrakeet al.,2017).Previous experiments on P nutrient addition conducted in Neotropical forests have used large quantities of inorganic P.The results from these experiments were conflicting:some(100 kg ha-1year-1for 7—9 years)have shown no significant alteration in the rate of AM fungal colonization by hyphae and other structures(Treseder and Allen,2002),others(100 kg ha-1year-1for 2 years)a decrease(Treseder and Vitousek,2001),and yet others(50 kg ha-1year-1for 14 years)an increase(Wurzburger and Wright,2015).However,Treseder and Allen(2002)have reported differences in AM hyphal biomass between the soils with previous low P concentration(then fertilized for 9 years) and with initial higher levels of P (then fertilized for 7 years). Phosphorous addition increased the AM hyphal biomass in the first case, while an opposite effect was observed in the second case.While the authors suggested that these results could be related,to a certain extent,to soil fertility prior to P addition,they also pointed out that the role played by mycorrhizal fungi in forest ecosystems goes beyond being a structure that facilitates plant nutrient absorption.Results obtained from other experiments,conducted in controlled conditions,have shown that P addition was not the main factor regulating the mycorrhizal symbiosis(Gamageet al., 2004; Patreze and Cordeiro,2004),indicating that carbohydrates transferred from the host tree may be more important for AM fungi than soil P.An additional consideration is that added P may have resulted in root growth that outpaced AM colonization,which may have led to relatively lower observed colonization rates(D.Janos,pers.comm.).On the other hand,the observed differences in AM colonization rates over time,i.e.,as soil moisture reduced,might indicate a slowing of root growth and thus,higher apparent colonization rates.Alternatively,the lack of a difference between the control and P-treated plots at our study site could be attributed to the one-offrelatively low dose of P applied that did not change either the colonization rate of roots by AM fungi or the amount of soil available P.
We can offer some possible explanations in relation to the fate of the applied P.Dukeet al.(1994)have suggested that soil nutrient pulses may cause fine root proliferation and that this could represent a more important mechanism adopted by plants for the capture of nutrients.Fine root proliferation could therefore represent an alternative response mechanism to the application of inorganic P at our study site.While it is known that litter can be colonized by mycorrhizal fungi that directly transfer P to plants(Herreraet al.,1978),rapid pulses of mineral nutrients can promote the fast growth of roots at the expense of mycorrhizal symbiosis (Dukeet al., 1994).This fact could help understand why we did not observe alteration in mycorrhizal association over the experiment.However,since fine root growth was not assessed in the present study,and the mycorrhizal colonization peak at day 88 suggests slow root growth over the dry season,as confirmed by previous research in the area and in other lowland Neotropical rain forests(Luizãoet al.,1992;Malhiet al.,2014;Buscardoet al.,2016,2018a),further investigation should be carried out to test this possibility.
Most P in tropical forests resides in either organic forms(i.e.,organic matter,microbial biomass,stable organic P),or in geochemically protected forms (i.e., ‘sorbed’ onto mineral surfaces or protected within mineral matrices),the so-called occluded P fraction,which is relatively unavailable for biological uptake(Reedet al.,2011).Sayeret al.(2012)reported that the addition of nutrients, including P, in the form of litter facilitates the absorption of greater proportions of nutrients by plants compared with that applied in an inorganic form.This fact has been related to the rapid cycling of organic matter and colonization of the litter by fine roots,suggesting that the majority of P used by plants is derived from internal cycling rather than from mineral P.On adding inorganic P,it enters the labile P pool,however temporarily.Using the extractable(resin plus bicarbonate)P values as a guide,it appears that our P addition did not increase the available fraction of inorganic P.As there were no increases in microbial P either,the added inorganic P may have been absorbed on soil material (entered the stable inorganic P pool),or taken up by plant roots.Rapid adsorption by the predominant Al and Fe oxides (along with kaolinite clay minerals)is likely(Barber,1984).
There are many open questions that remain with regard to P and plant-soil interactions in tropical forest ecosystems.For example,some recent studies may call into question the‘tight’ litter P recycling for the P cycle. The results of an experimental litter removal have shown that litter removal did not reduce plant productivity and leaf litter P during the first 6 experimental years,indicating that trees were able to access P from alternative sources to maintain productivity(Sayeret al.,2012).A previous study conducted at the same study site had shown that,while the overall soil inorganic P pool remained unchanged 3 years after litter removal,the stable organic P pool in the upper 2 cm layer was reduced by 23%(Vincentet al., 2010). These studies, while interesting and by far more realistic than high-dose agricultural fertilizer addition experiments,fail to propose an underlying mechanism that could explain a natural phenomenon that would produce long-term doubling of litter production in tropical forest ecosystems. On the other hand, periodic drought-induced peaks do occur and can substantially impact nutrient cycling in the short term(O’Connellet al.,2018).The results of our short duration experimental study,conducted 4 years after the last drought year in Amazonia,did not indicate comparable impacts.We agree with Moriet al.(2018)that we are yet to understand some fundamental biological processes and soil chemical properties and their interactions on P dynamics of soil microbiota in tropical forests before we can realistically tackle P limitationper se,for example,by being able to test P effects by establishinga prioriecologically significant expected differences.
CONCLUSIONS
The addition of P in our study did not appear to have caused significant changes in the levels of organic and inorganic forms of labile P in the soil surface layer.The lack of effects of P on the colonization of roots by AM fungi suggests that inorganic P is not the main factor that regulates the intensity of AM symbiosis.Similarly,the fact that soil microbial biomass was not altered following P addition suggests that P, applied in relatively small doses, did not stimulate soil microbiota during the study experimental period.We suggest that further careful experimental evidence is needed to disentangle the roles of biological processes and soil chemical properties in the dynamics of P in tropical forests.
ACKNOWLEDGEMENTS
Y.W.L.S.held a M.Sc.grant from the Brazilian Coordination for the Improvement of Higher Education Personnel(CAPES); E.B. held a post-doctoral grant from the Portuguese Foundation for Science and Technology(No.SFRH/BPD/77795/2011). We thank Prof. David Janos from the University of Miami,USA for his helpful and constructive comments on a previous version of the manuscript.We are also grateful to the Associate Editor,two anonymous reviewers,and Dr.Tessa Camenzind from the Freie Universität Berlin, Germany for their comments and suggestions that improved the quality of the manuscript.
SUPPLEMENTARY MATERIAL
Supplementary material for this article can be found in the online version.
杂志排行
Pedosphere的其它文章
- Letter to the Editor Molecular characterization of an extensively drug-resistant Acinetobacter baumannii isolated from a corn culture soil
- Distribution characteristics and diversities of cbb and coxL genes in paddy soil profiles from southern China
- Assessment of compost and three biochars associated with Ailanthus altissima(Miller)Swingle for lead and arsenic stabilization in a post-mining Technosol
- Synthesis of an eco-friendly nanocomposite fertilizer for common bean based on carbon nanoparticles from agricultural waste biochar
- Letter to the Editor Soil carbon availability affects nitrogen transformation under irrigated lucerne
- Impacts of silver nanoparticles on enzymatic activities,nitrifying bacteria,and nitrogen transformation in soil amended with ammonium and nitrate
