Impacts of silver nanoparticles on enzymatic activities,nitrifying bacteria,and nitrogen transformation in soil amended with ammonium and nitrate
2021-12-22XiaohongLIUJuanWANGLingliWULiZHANGandYoubinSI
Xiaohong LIU,Juan WANG,Lingli WU,Li ZHANG and Youbin SI
Anhui Province KeyLaboratoryof Farmland Ecological Conservation and Pollution Prevention,School of Resources and Environment,Anhui Agricultural University,Hefei(China)
(Received November 7,2019;revised January 1,2020)
ABSTRACT Silver nanoparticles(AgNPs)are effective antimicrobial compounds that are used in a myriad of applications.Soil microorganisms play crucial roles in nitrogen cycling,but there is a lack of comprehensive understanding of the effects of AgNPs on enzymatic activity in the nitrogen cycle,nitrifying bacteria,and nitrogen transformation in soil.Herein,enzyme activities were determined following the addition of different forms of nitrogen,ammonium nitrogen((NH4)2SO4),nitrate nitrogen(KNO3),and amide nitrogen(urea,CO(NH2)2)at 200 mg N kg-1,into the soil amended with AgNPs at 0,10,50,and 100 mg kg-1.After 7 d of incubation with 10 mg kg-1 AgNPs,the activities of urease,nitrite reductase(NiR),nitrate reductase(NaR),and hydroxylamine reductase(HyR)were reduced by 12.5%,25.0%,12.2%,and 24.2%,respectively.Of particular note,more than 53.5%,61.7%,and 34.7%of NaR,NiR,and HyR activities,respectively,were inhibited at 100 mg kg-1AgNPs.The abundance(most probable number)of ammonia-and nitrite-oxidizing bacteria(AOB and NOB,respectively)was measured using real-time quantitative polymerase chain reaction(qPCR)and the Cochran method.The abundance of AOB and NOB decreased when AgNPs were present in the soil.The NH4NO3 amendment increased copy numbers of bacterial and archaeal amoA nitrification functional genes,by 38.3%and 12.4%,respectively,but AgNPs at 50 mg kg-1 decreased these values by 70%and 56.4%,respectively.The results of 15N enrichment(atom%excess)of NH+4 and NO-3 experiments illustrated the influence of AgNPs on soil nitrogen transformation.According to the 15N atom%excess detected,the conversion of 15N-labeled NH+4 to NO-3 was significantly inhibited by the different levels of AgNPs in soil.The reduced gross nitrification rate further confirmed this finding.Overall,this study revealed considerable evidence that AgNPs inhibited nitrogen cycle enzyme activity,the number of nitrifying bacteria,the abundance of the amoA gene,and the gross nitrification rate.Silver nanoparticles inhibited nitrogen transformation,and the rate of nitrification was also negatively correlated with AgNP levels.
KeyWords: amoA,gross N transformation,nitrification gene,nitrogen cycling,nitrogen isotope fractionation,soil enzyme activities
INTRODUCTION
Silver nanoparticles(AgNPs)have become increasingly prevalent in the plastics,health,textiles,and paint industries(Leeet al.,2003;Wanget al.,2006).The widespread use of AgNPs has the potential to result in environmental release into sewage systems when disposed of unsafely,which could influence microbial ecosystem services(Yanget al.,2013).The possible environmental effects of AgNPs have drawn considerable attention due to their wide range of impacts on microorganisms(Kimet al.,2009;Raiet al.,2009).Previous studies have demonstrated a safe and controlled method to release nanoparticles into the environment (Kaegiet al.,2008),with a particular emphasis on the contamination of soil and water resources and the associated risks to global microorganism communities(Navarroet al.,2008).Wastewater treatment plants are the largest AgNP sinks,while soils are also a potential sink due to the agricultural application of biosolids containing Ag levels as high as 856 mg kg-1(McClellan and Halden,2010).
The dissolution of AgNPs increases antibacterial activity by releasing Ag+ions that act through multiple mechanisms(Xiuet al.,2012).For instance,they have been shown to interact with protein portion that contain phosphorus,nitrogen,or sulfur atoms(Fenget al.,2000).Silver nanoparticles attach to bacterial cells and increase microbial cell permeability as well as disrupt respiration(Dasgupta and Ramalingam,2016).Silver nanoparticles exhibit a greater impact on soil bacteria and enzymatic activities than AgNO3at the same Ag level(Rahmatpouret al.,2017).In our previous study,Ag+released from AgNPs severely damaged the cell membrane ofNitrosomonas europaea(Wanget al.,2017).Some recent studies on the microbial toxicity of AgNPs suggest that these biomolecules potentially play a role in their ecotoxicity and bioavailability(Choi and Hu,2009;Fabregaet al.,2011).
Soil enzymes are derived from the metabolic activities of soil microorganisms,active substances released during the decomposition of biological debris,and plant root exudates from soil microbial activity(Sunet al.,2018).Silver nanoparticles have previously been shown to be the most toxic to the phosphomonesterase,arylsulfatase,leucine-aminopeptidase,andβ-D-glucosidase enzymes at low Ag dosages(Peyrotet al.,2014).The activities of these soil enzymes are sensitive to the strengths and outcomes of biochemical processes occurring within the soil system.Therefore,an examination of soil enzymes is crucial in all soil quality monitoring experiments.There is increasing concern regarding the impact of nanomaterials on soil enzymes,due to a greater understanding of their environmental effects observedviaindicators measured(Eivaziet al.,2018).
Nitrogen cycle is essential for energy conversion and balance in the ecosystem, and is critical for agricultural productivity.Nitrification is important in the nitrogen cycle;it is carried out by ammonia-oxidizing archaea(AOA)and ammonia-oxidizing bacteria (AOB) that convert NH+4to NO-2, followed by nitrite-oxidizing bacteria (NOB) that convert NO-2to NO-3(Plazaet al.,2001).The expression of nitrification genes such asamoA is correlated with AOB and AOA abundance in nitrification processes,and the expression of these genes can be affected by AgNPs(Liet al.,2017).
Soil microbes that drive nitrogen cycling influence the mineralization of inorganic N,but AgNP toxicity can inhibit the activity of these bacteria(Luoet al.,2015).The impact of AgNPs on nitrifying bacteria has been extensively examined(Arnaout and Gunsch,2012;Yuanet al.,2013),but extrapolating such toxicological data to an ecosystem scale can be difficult.Ag+/AgNPs at 1 mg L-1can significantly inhibit nitrification in a water treatment process,and AgNPs were found to be more detrimental than an equivalent exposure of Ag ions to nitrifying bacteria(Choi and Hu,2009).Soil nitrification processes are mediated by nitrifying bacteria that are essential for the nitrogen cycle in agricultural soils(Masrahiet al.,2014).However,microbial toxicity of Ag-NPs can indirectly disrupt soil nitrogen transformation,soil quality,and agricultural production(Wanet al.,2014).
Previous studies have demonstrated that AgNPs can damage nitrifying bacteriaviamembrane disruption and reactive oxygen species generation(Luoet al.,2015;Dasgupta and Ramalingam, 2016). However, the influence of AgNPs on the transformation of nitrogen fertilizer added to agriculture soil has not yet been adequately explored.Herein,we investigated the toxic impact of AgNPs on soil enzymatic activities,nitrifying bacteria,andamoA gene copy numbers.The stable isotope15N has been widely used recently in research on nitrogen cycle as it yields accurate results, is safe,and does not interfere with natural processes in the ecosystem(Shindo and Nishio,2005).In this study,the isotope15N can accurately reflect the state of nitrogen conversion before and after nanosilver exposure;thus,15N-labeledand NO-3were used to study the effect of AgNPs on N transformation and to assess the dose-response relationship.These will contribute to our understanding of how AgNPs affect nitrogen cycling in soil and the environmental risks associated with AgNP release.
MATERIALS AND METHODS
Materials
Silver nanoparticles were purchased from Nanjing Emperor Nano Material Co.(Nanjing,China).The suspensions of AgNPs at 1 000 mg L-1were stored in the dark at 4°C before use.The morphology of the AgNPs were observed using transmission electron microscopy(TEM,Model H-7650, Hitachi, Japan). As shown in Fig. 1, AgNPs were 50-nm spherical particles and showed slight agglomeration.
Topsoil(20 cm depth)was collected from the Anhui Agricultural University farm(China).It contained 19.06 g kg-1organic carbon,and 1.77 g kg-1total nitrogen,28.9%clay,with a cationic exchangeable capacity(CEC)of 12.40 cmol kg-1and a pH of 6.70,and was AgNP free.
Soil incubation experiment
The soil samples were air dried,homogenized,and then passed through a 5-mm sieve. The AgNP contents in the soil samples of 10, 50, and 100 mg kg-1were achieved after amendment with AgNPs,and AgNP-free soil was set as the control(Tsyuskoet al.,2012).All soil samples were incubated at 30°C for 7 d with a final moisture content of 60%water-holding capacity(WHC).Afterward,the soil samples were observed for enzyme activities,the number of cultivable AOB and NOB,and the abundances of nitrification genes.
To determine the effects of different AgNP doses on enzyme activity in soil amended with different forms of nitrogen,solutions of ammonium nitrogen((NH4)2SO4),nitrate nitrogen(KNO3),and amide nitrogen(urea,CO(NH2)2)were each added at 200 mg N kg-1to soil amended with AgNPs. The mixture was then incubated under the same conditions as described above,and each treatment had three replicates.
Enzyme activityassayand chemical analysis
The activity of urease was quantified by mixing amended soil(5 g of dry soil)with 10 mL urea solution(10%),1 mL methylbenzene,and 20 mL citrate buffer(pH 6.7),followed by incubation at 37°C for 24 h.The indophenol colorimetric method was used to quantify the resulting NH+4from ureasemediated urea hydrolysis,and calorimetric measurements were determined at 578 nm, with the enzymatic activity described using mg NH+4-N kg-1h-1(Huet al.,2014).
To determine the activities of nitrate reductase(NaR),nitrite reductase(NiR),and hydroxylamine reductase(HyR),0.5%NaNO2solution,0.5%NH2OH,and 1.0 mL volumes of 1% KNO3solution, 0.5% NaNO2solution, and 0.5%NH2OH were added to 1.0 g soil samples, respectively.The mixtures were incubated at 30°C for 24 h, and the consumption rates of NO-3-N,NO-2-N,and NH2OH were measured to determine the activities of NaR,NiR,and HyR,respectively(Huet al.,2014).
Soil NH+4-N and NO-3-N were extracted using 2 mol L-1KCl solution and measured using a continuous-flow analyzer(FLAStar 5000,Foss,Germany).
Determination of AOB and NOB and quantification of amoA gene
The most probable number(MPN)of AOB and NOB was determined using six-fold dilutions from 10-2to 10-7of Stephenson medium according to the Cochran method(Cochran,1950).Cultures were incubated at 28°C for 14 d.The MPN of AOB and NOB was determined using Stephenson A and B media prepared according to instructions by Wanget al.(2017).The quantity of AOB and NOB were determined using NO-2-N and NO-3-N production as indicators,respectively;these were measured using Griess’s reagent and diphenylamine.The MPN of nitrifying microorganisms was estimated using the Cochran method(Cochran,1950).
Soil microbial DNA was extracted from 0.5 g soil samples using the FastDNA SPIN kit for soil(MP Biomedicals,Cleveland,USA),following the manufacturer’s instructions with some minor modifications. The concentration of the DNA extracted was determined using a NanoDrop ND-1000 UV-Vis spectrophotometer(NanoDrop Technologies,Wilmington,USA)and verified by 1.0%agarose gel electrophoresis. The soil DNA extract was stored at-20°C.Extractions were performed in triplicate after 7 d incubation, and the obtained DNA was pooled according to the procedure outlined by Smallaet al(2001)to get an average representation of the microbiota.
The abundance of the nitrification functional geneamoA was measured with real-time quantitative polymerase chain reaction(qPCR)using an ABI 7500 RT-PCR system(Thermo Fisher Scientific,USA).The primer pairsamoA-1F/amoA-2R(Rotthauweet al.,1997)and Arch-amoAF/Arch-amoAR(Franciset al., 2005) were used to amplify bacterial and archaealamoA gene fragments, respectively. The assays were set up using the SYBR Premix Ex Taq kit(TaKaRa,Dalian,China)with 20 μL reactions containing 10 μL SYBR Premix Ex TaqTM(2×), 0.8 μL forward primer, 0.8 μL reverse primer, 0.4 μL ROX Reference Dye II (50×), 2.0 μL of the extracted DNA,and 6.0 μL double-distilled H2O.Product specificity was examined using a melting curve analysis(65—95°C,0.5°C per read,with a hold time of 5 s)at the end of each PCR run.Independent triplicates of each assay were taken for all soil samples.
15N tracing experiment
The15N tracing experiment was conducted by adding either15N-labeled NH+4or NO-3to the soil. Six replicate samples(100 g dry soil)from soil amended with different doses of AgNPs (0, 10, 50, and 100 mg kg-1) were put in 500 mL containers and incubated overnight at 28°C.The following day, three replicate samples for each dose of AgNPs were15N-labeled using 2 mL of a15N-labeled solution containing a 10 atom%excess of15NO3,while the remaining three replicate samples were processed using 2 mL15N-labeled solution containing a 10 atom%excess of14NO3,and water was added to ensure a final moisture content of 60%WHC(Masseet al.,2016).The15N-labeled solutions were injected drop by drop using a transfer pipette and gently homogenized with the soil in jars. At 2 h, 1,3, 5, 7, 15, and 30 d after incubation, 10 g of soil (dry weight) was removed, and 50 mL of 2 mol L-1KCl (1:5 weight/volume)was used to extract inorganic nitrogen from the soil.After extraction,NH+4-N and NO-3-N concentrations were measured using a continuous-flow analyzer(FlAstar 5000, Foss, Sweden). Samples remaining after extraction were stored at 4°C in specimen bottles and analyzed by a MAT-253 isotopic mass spectrometer (Thermo Finnigan,USA)to detect the diffusion of15NO-3and15NH+4.
A moderate amount of soil extract solution(ensuring a NO-3concentration of about 50 μg)from every sample was pipetted separately into bottles,and 2.5 mL of 0.2 mol L-1sulfamic acid was added.The mixture was shaken for 5 min to ensure that NO-2-N was converted entirely into N2.The sample bottles were then opened,and 0.05 g Cd-Cu reactor,5 mL of 1 mol L-1sodium acetate,and an acetic acid buffer solution were added before the bottles were recapped and incubated in an orbital incubator at 120 r min-1at 20°C for 24 h(Butterbach-Bahlet al.,2013).Headspace samples were then transferred to 100 mL headspace bottles using a 20 mL gas-tight syringe,and the15N concentration of NO-3taken as N2O was determined along with the15N enrichment.
The15N measurements for NH+4were separated from those of NO-3using MgO on a steam distillation system.A 0.02-mol L-1H2SO4solution was added to trap nitrogen converted to(NH4)2SO4.The resulting H2SO4solution that contained NH+4was further dried at 65°C, and then was used for measuring the15N enrichment level with a C/N analyzer isotope ratio mass spectrometer(Europa Scientific Integra,UK)(Zhanget al.,2011).
Calculation of gross nitrogen transformation rates
Gross ammonification rate(GAR,mg NH+4-N kg-1dry soil d-1)and nitrification rate(GNR,mg NO-3-N kg-1dry soil d-1), NH+4consumption rate (Ca, mg NH+4-N kg-1dry soil d-1),and NO-3consumption rate(Cn,mg NO-3-N kg-1dry soil d-1)were calculated according to the methods described previously by Hart(2006):
where[NH+4]0and[NH+4]t(mg kg-1)are the total NH+4-N concentration in soil at time 0 and timet(d),respectively,APE0and APEtare the atom%15N excess of NH+4at time 0 and timet,respectively,[NO-3]0and[NO-3]t(mg kg-1)are the total NO-3-N concentration in soil at time 0 and timet,respectively,and NPE0and NPEtare the atom%15N excess of NO-3at time 0 and timet,respectively.
Statistical analysis
The measurement error was defined as the standard deviation of the means of the three samples. Statistical analyses were conducted using Excel 2013 and origin 9.0.A Student’st-test was used to evaluate the significance of the results, and all statistical tests were performed with a significance level ofP <0.05.
RESULTS
Effects of AgNPs on enzyme activities in soil treated with different forms of nitrogen
Enzymatic activity responded differently to the different forms of nitrogen added to the soil. Urease, NiR, NaR,and HyR activities were significantly influenced by the type of inorganic nitrogen added to the soil that had been amended with different doses of AgNPs (Fig. 2); urease activity increased with adding NH+4-N and inhibited with adding NO-3-N(Fig.2a).Meanwhile,the addition of NO-3-N increased NiR and NaR activities(Fig.2b and c)in soil samples without AgNPs.
After 7 d of incubation with AgNPs at 10 mg kg-1and without adding nitrogen,activities of urease,NaR,NiR,and HyR were reduced by 12.5%, 12.2%, 25.0%, and 24.2%,respectively(Fig.2).Furthermore,after 7 d of incubation with the highest concentration of AgNPs at 100 mg kg-1,NaR,NiR,and HyR activities were reduced by more than 53.5%,61.7%,and 34.7%,respectively.Silver nanoparticles were therefore toxic to soil bacteria due to their effect on the activity of functional enzymes,which suggests that AgNPs may inhibit nitrogen transformation in soil.
The NH+4-N increased urease activity by 15.4%when AgNPs were present at 10 mg kg-1.The addition of NO-3-N markedly increased soil enzyme activity as compared with the control samples without nitrogen amendment when the AgNP content was 50 mg kg-1;NaR,NiR,and HyR activities increased by 45.3%,13.8%,and 31.6%,respectively.It was speculated that the addition of inorganic forms of nitrogen masked the inhibition of enzyme activity by AgNPs.
Effects of AgNPs on abundances of AOB,NOB,and amoA gene in soil amended with NH4NO3
The MPN estimates of soil AOB and NOB increased after incubation with NH4NO3amendment at 0 mg kg-1AgNPs(Fig.3).However,amendment with AgNPs inhibited bacteria growth;exposure to AgNPs at 10 mg kg-1resulted in MPN values of 2.36× 105colony forming units (cfu)g-1dry soil for AOB and 1.94× 105cfu g-1dry soil for NOB, which were both lower than the values of the controls.Populations of bacteria decreased with an increase of AgNPs,with NOB showing a more substantial reduction;this suggests that the NOB could be more sensitive to AgNPs than AOB.
Furthermore,functional gene copy number was strongly associated with nitrification,which was negatively correlated with AgNP content(Fig.3).Following exposure to AgNPs at 0,10,50,and 100 mg kg-1,bacterialamoA gene copy numbers were 6.07× 106, 4.03× 106, 1.83× 106, and 0.52×106copies g-1dry soil, and archaealamoA gene copy numbers were 8.34×107, 7.86×107, 3.64×107,and 6.36×106copies g-1dry soil,respectively.In short,AgNP concentration was negatively correlated with bacterial or archaealamoA gene copy number.
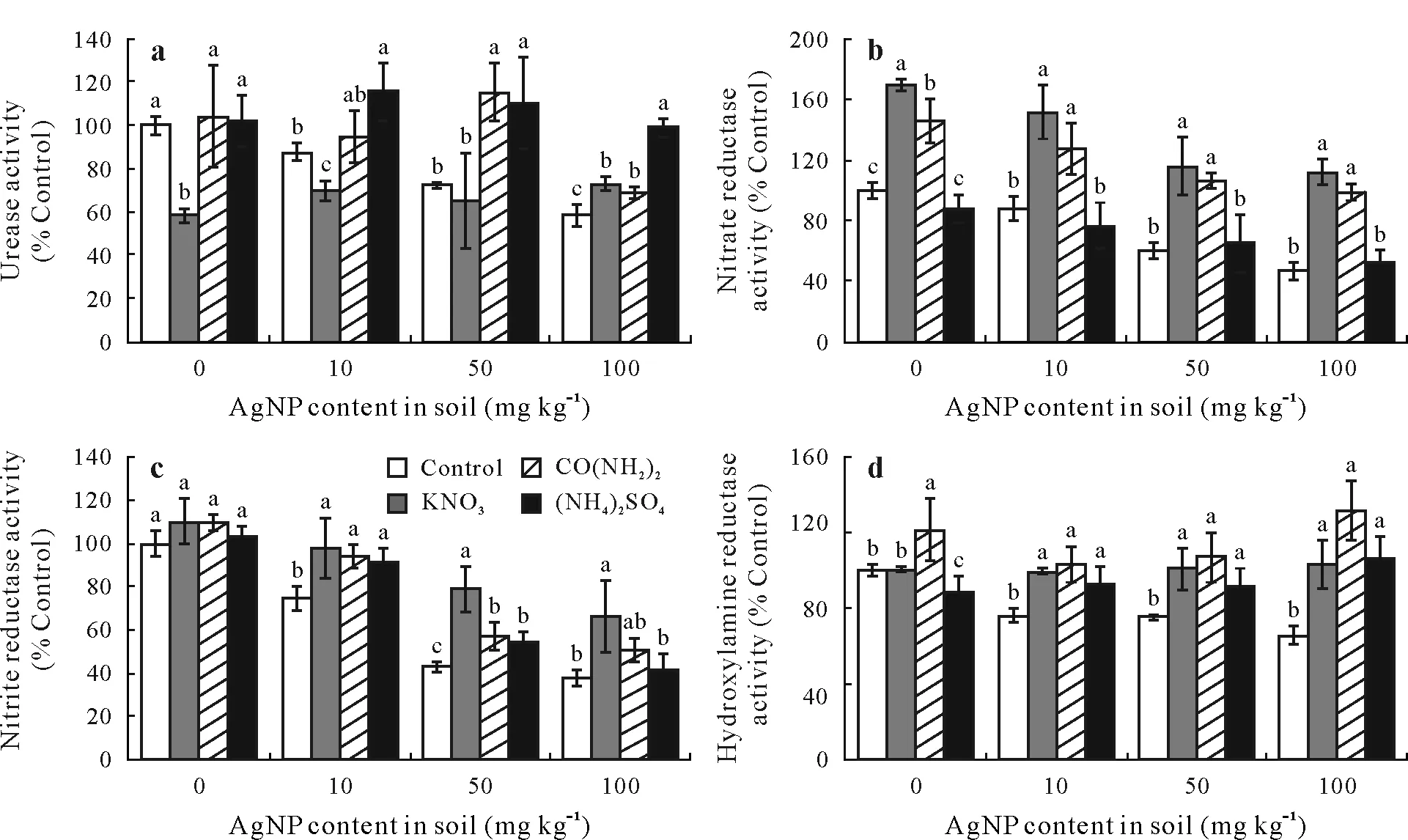
Fig.2 Effects of silver nanoparticle(AgNP)contents(0,10,50,and 100 mg kg-1)on activities of urease(a),nitrate reductase(b),nitrite reductase(c),and hydroxylamine reductase(d)in soil treated with different forms of nitrogen(200 mg N kg-1),KNO3,CO(NH2)2(urea),and NH4)2SO4.AgNP-free soil was set as the control.Conrol=the controls without nitrogen amendment.Error bars indicate standard deviations of means(n=3);means with different letters are significantly different between treatments at each AgNP level.
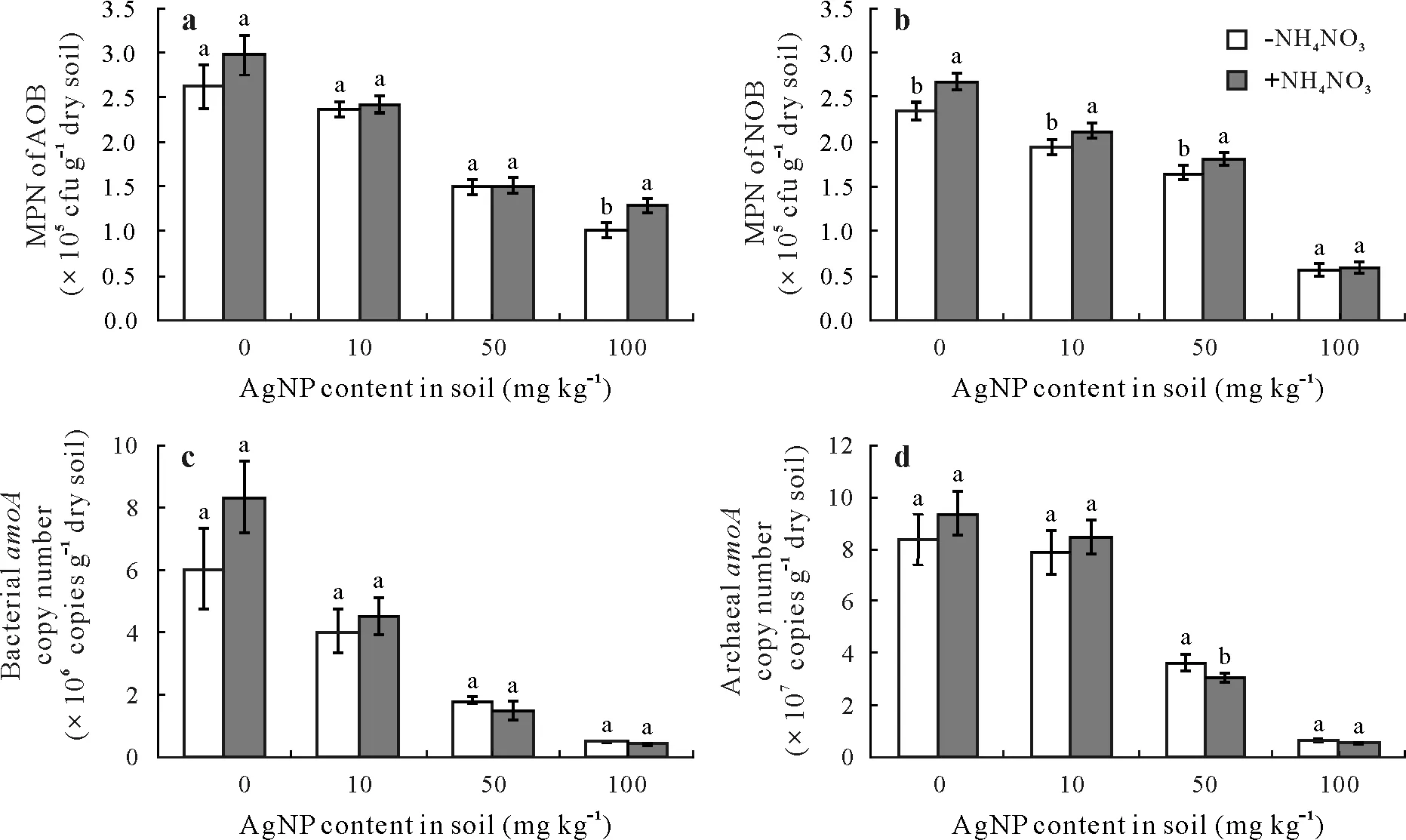
Fig. 3 Effects of silver nanoparticle (AgNP) contents (0, 10, 50, and 100 mg kg-1) on the abundances of ammonia-oxidizing bacteria (AOB, a),nitrite-oxidizing bacteria(NOB,b),bacterial amoA gene(c)and archaeal amoA gene(d)in soil with(+)and without(-)NH4NO3 amendment.Error bars indicate standard deviations of means(n=3);means with different letters are significantly different between treatments at each AgNP level.MPN=most probable number;cfu=colony forming units.
ArchaealamoA copy numbers were higher than those of bacterialamoA in soil amended with the same amount of AgNPs and NH4NO3.Silver nanoparticles inhibited the predominant group of nitrifiers,but the abundance ofamoA gene increased in soil controls treated with NH4NO3without AgNPs. When the soils were amended with high AgNP contents of 50 and 100 mg kg-1, the copy numbers of bacterial and archaealamoAgenes decreased to low levels in soil in both the presence and absence of NH4NO3.
Effects of AgNPs on soil nitrogen transformation
Concentrations of NO-3-N in soil samples increased when KNO3and(NH4)2SO4were present as compared with the controls without nitrogen amendment(Fig.4a).When exposed to AgNPs,soil amended with KNO3,CO(NH2)2(urea),and(NH4)2SO4registered NO-3-N concentrations of 72.36—92.42,43.76—56.28,and 37.38—40.03 mg kg-1,respectively.In the CO(NH2)2treatments with AgNPs amendment,the NO-3-N concentration decreased with increasing AgNP contents.Furthermore,the application of mineral nitrogen fertilizer increased the total mineral nitrogen(NH+4-N and NO-3-N)concentration when compared with the controls without nitrogen amendement.
The applications of CO(NH2)2and (NH4)2SO4increased the NH+4-N concentration in the soil when compared with the controls without nitrogen amendement. In the controls without nitrogen amendement,the NH+4-N concentration increased with increasing AgNP content after short-term exposure(Fig.4b).The NH+4-N concentrations in soil samples were significantly different from those of the controls without nitrogen when mineral nitrogen fertilizers were added to the soil,suggesting that the type of fertilizer influenced the treatment outcomes. At the highest AgNP content of 100 mg kg-1, the applications of CO(NH2)2and(NH4)2SO4resulted in NH+4-N concentrations of 70.4 and 83.98 mg kg-1,respectively,while the control NH+4-N concentration was 12.98 mg kg-1. The average NH+4-N concentration increased with an increase of AgNPs for all forms of mineral nitrogen fertilizer added to the soil.
Effects of AgNPs on soil nitrogen concentrations and 15N enrichments of NH+4 and NO-3
The soils were amended with different contents of Ag-NPs(0,10,50,or 100 mg kg-1),and either15NH4NO3orNO3was added to each treatment.Soil nitrogen concentrations and15N enrichment of NH+4and NO-3are shown in Fig.5.At the preliminary stage of incubation(0—7 d),the soil NH+4-N concentration gradually decreased(Fig.5a),while the NO-3-N concentration gradually increased(Fig.5b).After 7 d of incubation,the soil nitrogen concentration did not significantly change.However,amendment with AgNPs led to an increase in soil NH+4-N concentration and a decrease in NO-3-N concentration; soils amended with high AgNP contents contained more NH+4-N but less NO-3-N during the incubation period.Nitrifying bacteria in soil presumably transformed NH+4into NO-2before oxidizing it to NO-3.As AgNPs inhibited the activity of nitrifying bacteria,this decreased the transformation rate of NH+4and disrupted nitrification. Thus, the increase in NO-3-N concentration was mainly owing to nitrification,and the inhibition of nitrification led to a lower NO-3-N concentration in the soil supplemented with AgNPs than without AgNPs.
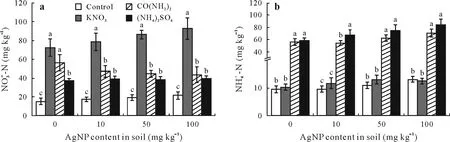
Fig.4 Effects of silver nanoparticle(AgNP)contents(0,10,50,and 100 mg kg-1)on concentrations of NO-3 -N(a)and NH+4 -N(b)in soil treated with different forms of nitrogen(200 mg N kg-1),KNO3,CO(NH2)2 (urea),and NH4)2SO4.Error bars indicate standard deviations of means(n=3);means with different letters are significantly different between treatments at each AgNP level.Control=the controls without nitrogen amendment.
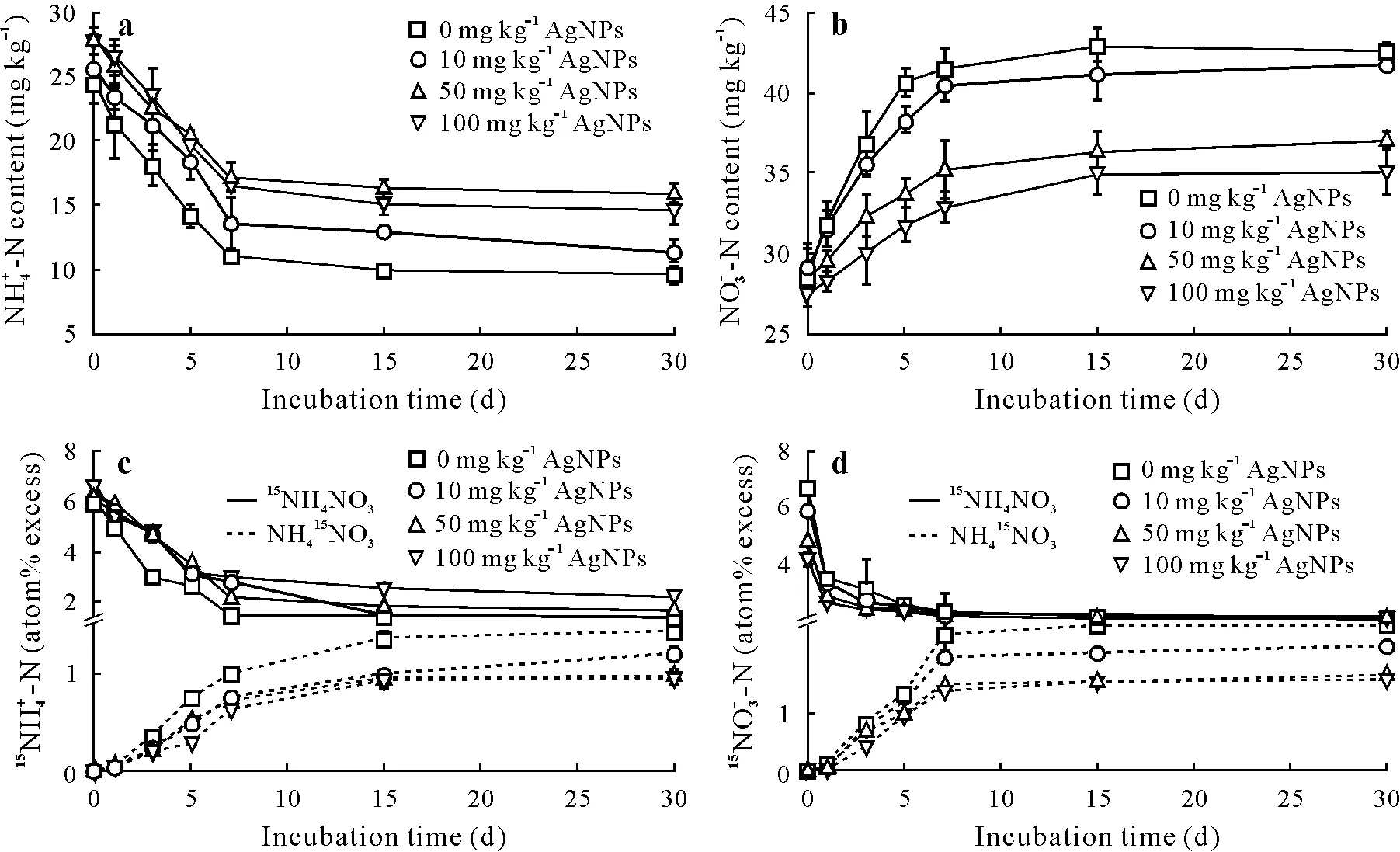
Fig.5 Effects of different contents of silver nanoparticles(AgNPs,0,10,50,and 100 mg kg-1)on nitrogen concentrations(a and b)and 15N enrichments(c and d)of NH+4 (a and c)or NO-3 (b and d)in soil with 15NH4NO3 or NH154 NO3 applied.Error bars indicate standard deviations of means(n=3).
The results of the15N enrichment of NH+4and NO-3also illustrated the influence of AgNP amendment on soil nitrogen transformation.The increased15NO-3-N content in the15NH4NO3treatment indicated that NH+4oxidation and mineralization occurred simultaneously.The primary input for the15NO-3pool was nitrification, which transformed NH+4to NO-3,resulting in an increase in the15NO-3atom%excess.The reaction was inhibited in the soil with AgNPs amendment; the15NO-3enrichment in the conversion of labeled NH+4to NO-3significantly decreased,and the15N atom%excess was reduced by different AgNP contents in soil(Fig.5c and d).During the incubation period,15NH+4enrichment gradually decreased at 0 mg kg-1AgNPs with the15N-labeled NH+4pool,but it significantly increased with a treatment of 100 mg kg-1AgNPs.The change of NH+4concentration was influenced by amendment with AgNPs,but did not show a remarkable dose-response relationship.Nevertheless,lower NO-3concentrations were still observed in response to high AgNPs contents in soil.
Effects of AgNPs on gross nitrogen transformation rates
The actual rate of conversion of NH+4to NO-3was measured by the15N isotopic dilution method to calculate gross nitrification.The relationships between gross nitrogen transformation rates and AgNP content in soil are shown in Fig.6.
Gross ammonification and NH+4consumption rates were higher in the control group than in the group treated with AgNPs.When the contents of AgNPs in the soil were 10,50,and 100 mg kg-1,the gross ammonification rates were 2.34,3.12,and 2.52 mg NH+4-N kg-1dry soil d-1,and the NH+4consumption rates were 4.07, 4.85, and 4.14 mg NH+4-N kg-1dry soil-1,respectively.As shown in Fig.6,AgNPs reduced the gross ammonification and NH+4consumption rates to some extent; however, the content of AgNPs did not have a significant correlation coefficient(r2=0.016 99,r2=0.015 58).
Gross nitrification and NO-3consumption rates were negatively correlated with the contents of AgNPs in soil,and the highest rates observed were in the controls without AgNPs. Moreover, there were strong linear correlations between AgNP content and both the gross nitrification rate and NO-3consumption rate(r2=0.968 42,r2=0.566 36)(Fig.6),suggesting that the amount of AgNPs in soil greatly influenced the process of nitrification mediated by nitrifying bacteria.
DISCUSSION
Soil enzyme activity,especially of highly sensitive enzymes such as urease,can be negatively impacted by AgNPs(Shinet al.,2012).A study of the influence of exogenous nitrogen on extracellular enzymatic activity in soil found that the highest enzyme activities were observed following organic nitrogen treatment(Guoet al.,2011;Let al.,2013).Urease activity and its urea-type substrates are crucial in the transformation of urea-containing fertilizers (Songet al.,2014).Urease activity was inhibited by the addition of KNO3in this study;however,the activities of NiR,NaR,and HyR,the three major enzymes involved in nitrogen cycle,increased.High NiR activity was noted in plots treated with urea alone,which is in line with findings from a previous report(Liet al.,2008).
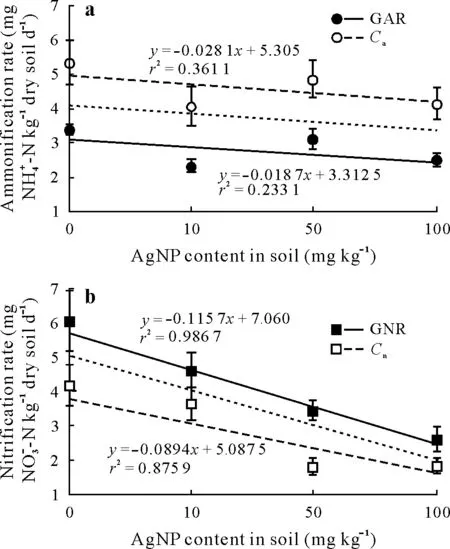
Fig.6 Relationships between gross ammonification rate(GAR),NH+4 consumption rate(Ca),gross nitrification rate(GNR),and NO-3 consumption rate(Cn)with silver nanoparticle(AgNP)content in soil using the15N isotopic dilution method.Dotted lines represent relationships for all rates with AgNP content in soil.Error bars indicate standard deviations of means(n=3).
The concentrations of NH+4-N and NO-3-N in soil amended with mineral nitrogen fertilizer were significantly affected by the presence of AgNPs (Fig. 4), as compared to soil not amended with nitrogen fertilizer(Vallejoet al.,2001;Zerullaet al.,2001).Nitrification inhibition could also be due to the presence of Ag+ions, not just the nano-Ag form(Yuanet al.,2013).Thus,AgNPs may change ecosystem productivity and biogeochemistry due to its negative impact on the soil-plant system,consequently causing vital ecosystem damages (Klaineet al., 2008; Navarroet al.,2008).Additionally,the presence of AgNPs may increase the nitrification and ammoxidation of inorganic N fertilizer in agricultural fields.On the other hand,studies on the impact of AgNPs on enzymatic activity, nitrifying bacteria, and nitrogen transformation in soil amended with ammonium or nitrate have been short-term experiments.Assays spread over a longer period of several years could be devised to assess the tolerance of microbial and nitrogen transformation rates to AgNP in the long term.
Nitrification is important for nitrogen cycling mediated by nitrifying bacteria, and the abundance of ammoniaoxidizing bacteria can be estimated by measuringamoA gene copy numbers (Vallejoet al., 2001; Myroldet al.,2014). The results showed that the archaealamoA gene copy number was higher than that of bacterialamoA in soils with added nitrogen,which was similar to previous findings(Zerullaet al., 2001; Leiningeret al., 2006; Yuanet al.,2013;Ouyanget al.,2016).An increase in the archaealamoA gene copy number was reported in response to the addition of ammonia or composted manure as a nitrogen source,resulting largely from the dominantamoA gene-containing AOA speciesNitrososphaera,although pure strains have not yet been cultured successfully(Yuanet al.,2013;Ouyanget al., 2016). In this study, the addition of NH4NO3to the soil increased bacterialamoA and archaealamoA gene copies in the controls and the low-dose AgNP treatments,but NH4NO3did not stimulate ammonia-oxidizing bacteria under exposure to high doses of AgNPs.In other words,the effect of NH4NO3onamoA copy number was dependent on the AgNP dose.In previous studies,a high ammonium concentration increased the copy number of the bacterialamoA gene but not that of the archaealamoA(Klaineet al.,2008;Tayloret al.,2012).Nevertheless,NH4NO3stimulated both bacterialamoA and archaealamoA genes in this study,while AgNPs were shown to inhibit these genes in this study.Considering that the copy numbers of bacterialamoA and archaealamoA genes were strongly correlated with the oxidation of ammonia,AgNPs might inhibite the nitrification process.
Increased gross N transformation rates suggest that Ag-NPs play a significant role in microbial nitrogen cycling activity.Thus,these measurements can be used to assess the effect of AgNPs on nitrogen cycling in soil.The relationship between the gross nitrogen transformation rate and the AgNP content can be investigated using the15N pool dilution method(Hart,2006).Ammonia oxidizing bacteria are the major contributors to nitrification activity, and account for 76%of the conversion of 100 mg kg-1NH+4-N in agricultural soil(Navarroet al.,2008;Xiaet al.,2011).To investigate the influence of AgNPs on nitrogen transformation,the15Nlabelling of NH+4and NO-3was used in combination with different treatments of AgNPs.The primary input for15NH+4is the mineral nitrogen pool in natural soils,and15NH+4is also the substrate for nitrification;hence,the inhibition of gross ammonification rate by AgNPs was not significant.The main output for the15NO-3pool was denitrification,which reduced the15NO3atom% excess. When NH154NO3was applied to soil,the difference in15NO-3atom%excess was only significant in the controls,which was consistent with a previous study,which showed that AgNPs had no significant effect on denitrification(Myroldet al.,2014;VandeVoortet al.,2014).
CONCLUSIONS
Silver nanoparticles inhibited soil enzyme activity,population of nitrifying bacteria, abundance of the functional geneamoA,and gross nitrification rate.Silver nanoparticles inhibited nitrogen transformation,and the dose of AgNPs linearly correlated with the nitrification rate but not the ammonification rate.Nitrifying bacteria were likely more sensitive to the toxicity of AgNPs than other bacteria. In this case,the dose-response effects of AgNPs on nitrification should be more significant than their effects on other reactions in the nitrogen cycle.However,the mechanisms controlling how nitrogen transformation responds to AgNPs in soil require further research,including the impact this can have on gas(N2O and N2)emissions.
CONTRIBUTION OFAUTHORS
Xiaohong LIU and Juan WANG contributed equally to this work.
ACKNOWLEDGEMENT
This study was supported by the National Natural Science Foundation of China(No.41430752).
杂志排行
Pedosphere的其它文章
- Letter to the Editor Molecular characterization of an extensively drug-resistant Acinetobacter baumannii isolated from a corn culture soil
- Reclamation of oil-induced soil hydrophobicity in the hyper-arid Evrona Nature Reserve,southern Israel
- Impacts of land use and salinization on soil inorganic and organic carbon in the middle-lower Yellow River Delta
- Rice(Oryza sativa L.)seedlings enriched with zinc or manganese:Their impacts on cadmium accumulation and expression of related genes
- Responses of the methanogenic pathway and fraction of CH4 oxidization in a flooded paddy soil to rice planting
- Effect of biochar applied with plant growth-promoting rhizobacteria(PGPR)on soil microbial community composition and nitrogen utilization in tomato
