Effect of biochar applied with plant growth-promoting rhizobacteria(PGPR)on soil microbial community composition and nitrogen utilization in tomato
2021-12-22YuanWANGWenqingLIBinghaiDUandHanhaoLI
Yuan WANGWenqing LIBinghai DU and Hanhao LI
1National Engineering Laboratoryfor Efficient Utilization of Soil and Fertilizer Resources,Tai’an 271018(China)
2College of Resources and Environment,Shandong Agricultural University,Tai’an 271018(China)
3College of Life Sciences,Shandong Agricultural University,Tai’an 271018(China)
(Received December 4,2019;revised March 10,2020)
ABSTRACT Plant growth-promoting rhizobacteria(PGPR)represent an important microbial community group and have beneficial effects on plant growth and development.A pot experiment was conducted to study the effect of biochar applied with PGPR on the soil microbial community composition and nitrogen use efficiency(NUE)of tomato,which could provide a theoretical basis for rational fertilization.Six treatments were designed:no nitrogen(N),PGPR,or biochar control(CK);biochar without N or PGPR(BCK);N without PGPR or biochar(U);N and PGPR without biochar(UP);N and biochar without PGPR(UB);and N,PGPR,and biochar(UBP).The tomato yield in the UP treatment was 9.09%lower than that in the U treatment,whereas that in the UB treatment was 19.93%higher than that in the U treatment.The tomato yield in the UBP treatment was 32.45%,45.69%,and 10.44%higher than those in the U,UP,and UB treatments,respectively.Biochar combined with PGPR increased the relative abundance of Nitrospira and Bradyrhizobium in the soil.At the tomato maturity stage,the soil NO-3 -N content in the UBP treatment was 87.12%,88.12%,and 31.04%higher than those in the U,UP,and UB treatments,respectively.The NUE in the UP treatment was 4.03%lower than that in the U treatment,and that in the UBP treatment was 13.63%,17.66%,and 10.77%higher than those in the U,UP,and UB treatments,respectively.This study showed that biochar combined with PGPR can improve soil microbial community structure and increase the NUE of tomato.
KeyWords: biological fertilizer,microbial community structure,nitrogen cycling,nitrogen use efficiency,tomato yield
INTRODUCTION
Plant growth-promoting rhizobacteria (PGPR) represent an important microbial community group (Kloepper and Schroth, 1981) and have beneficial effects on plant growth and development. Their application in agriculture has increased steadily in recent years,and they are expected to partly replace chemical fertilizers,pesticides,and other growth regulators in the future(Nadeemet al.,2007).They have the ability to fix nitrogen (N), dissolve phosphorus,chelate iron,inhibit root ethylene production pressure,and produce plant growth hormones, antibiotics, and antifungal compounds (Piiet al., 2015). These bacteria play an important role in promoting plant growth and development,and are therefore used as biological fertilizers and biological control agents. Numerous studies have shown that these rhizosphere bacteria can promote plant growth under normal and stress conditions(de Freitas and Germida,1992;Gagnéet al.,1993;Di Salvoet al.,2018).In addition,they play an important role in maintaining soil fertility and improving plant growth and development(Kumariet al.,2019).In a favorable environment,the growth promotion effect of PGPR is obvious;however,under certain conditions,some strains might not play an effective role,because the bacteria may compete or not survive in specific environments(Nadeemet al.,2013).This may be because exogenous microorganisms may not survive under fierce competition by native genera.Therefore, it is necessary to select a carrier of PGPR-like organic materials to protect exogenous microorganisms,for better colonization in soil.The carrier material may improve the success rate of microbial inoculation and render it more conducive to microbial colonization, by providing spatial protection because of its good pore arrangement or improvement of the soil structure(Albaredaet al.,2008;Sahai and Chandra, 2010; Haleet al., 2015). Biochar is a pyrolysis product of organic material formed under high temperature and hypoxic conditions(Lehmann and Joseph,2009).It has a good pore structure,and can effectively improve soil physical and chemical properties and soil fertility(Lairdet al.,2010;Joneset al.,2012).Biochar also plays an important role in mediating soil biological properties.Previous studies have shown that biochar can alter the composition and abundance of soil microbial communities (Elzobair, 2013; Xuet al.,2014; Wuet al.,2019).The application of biochar to soil can cause significant and rapid changes in soil microbial community (Farrellet al., 2013; Tianet al., 2019). This indicates that biochar is beneficial to the growth of indigenous microorganisms in soil.These changes may then affect nutrient cycling or soil structure and subsequently affect plant growth directly or indirectly.However,little research has been done to determine if biochar exerts beneficial effects on exotic microflora,e.g.,promoting their colonization in soil.In this study,we hypothesized that the application of biochar in combination with PGPR would increase microbe colonization,improve soil microbial diversity,promote soil N cycling,and improve crop yield.
MATERIALS AND METHODS
Experimental design
The experiment was conducted in a greenhouse. The tested soil, classified as brown soil,i.e., a Typic Hapli-Udic Argosol in Chinese Soil Taxonomy, was collected from Tai’an,Shandong,China.The soil properties were as follows: pH, 7.65; organic matter, 13.52 g kg-1; total N,0.68 g kg-1;NO-3-N,64.13 mg kg-1;NH+4-N,23.61 mg kg-1;available phosphorus,26.93 mg kg-1;and available potassium, 80.81 mg kg-1. The variety of tested tomato was Hezela pink fruit(Lycopersicon esculentumMill.).The biochar was made from millet straw.The surface morphology of the biochar was observed using a Tecnai G2 F20 scanning electron microscope(SEM)(FEI Company,USA),while the surface structure was analyzed with a Nicolet 380 Fourier transform infrared (FTIR) spectrometer (Thermo Fisher Scientific,USA).These properties are shown in Fig.1.
In this study,composite strains of PGPR were used,withPaenibacillus polymyxaandBacillus amyloliquefaciensas the effective strains. The number of colony-forming units(CFUs)exceeded 2×108mL-1.The fertilizers applied were urea(46%N),superphosphate(46%P2O5),and potassium chloride (60% K2O). The test containers used for tomato cultivation were pottery pots, with an upper diameter of 40 cm and a height of 35 cm. Each pot was first covered with 5 kg of sand and then filled with 30 kg soil. One tomato seedling was transplanted per plot in April 2018,and harvested in August 2018. The soil moisture content was maintained at 70%±5%of the field water-holding capacity during the experiment.
Six treatments were designed as follows:no N,PGPR,or biochar control(CK);biochar without N or PGPR(BCK);N without PGPR or biochar(U);N and PGPR without biochar(UP); N and biochar without PGPR (UB); and N, PGPR,and biochar(UBP).Each treatment had four replicates.The amount of N applied in the N treatments was 0.34 g kg-1.The dosage of the PGPR agent was 66.7 μL kg-1in the treatments with PGPR.The amount of biochar added was 2%of the soil weight.Phosphorus(0.38 g kg-1P2O5)and potassium (0.47 g kg-1K2O) were applied equally to all treatments.Fertilizers were applied as base fertilizers across all treatments.

Fig. 1 Fourier transform infrared spectrogram showing the functional groups of biochar(a)and scanning electron microscope image showing the microstructure of biochar(b).
Sample collection and determination
Plant and soil samples were collected for the determination of physiological indices and soil physical,chemical,and biochemical properties. Thirty-five days after tomato transplantation,the most recent fully unfolded leaves were collected and transported to the laboratory in an ice box for the determination of photosynthetic pigments and leaf enzyme activities.After the tomato harvest,the aboveground straw and tomato fruits were placed in oven at 105°C for 30 min,and then dried to a constant weight at 65°C.Subsequently,all samples were weighed and ground for further nutrient determination. Soil samples were collected from 0—20 cm depth using a drill(2.0 cm in diameter,100 cm in length)at different stages of plant growth.In each pot,soil samples were collected from two randomly selected points at the same depth,and then mixed into a composite sample.Each treatment had four biological replicates.Soil samples were stored at-20°C prior to soil NO-3-N and NH+4-N determination.The roots of tomato plants were collected,the loose soil was removed from the root surface by kneading and shaking,and the tightly adhered soil(rhizosphere soil)within 2 mm of the root surface was then carefully collected using a sterilized brush.All soil samples were immediately frozen with liquid N and stored at-80°C until microbial determination.
The total N content of plant was determined by digestion with H2SO4and H2O2according to the Kjeldahl method(Mackieet al.,2015).Photosynthetic pigments in tomato leaf were determined by spectrophotometry(Dere,1998).The activities of antioxidant enzymes in plant were determined as follows:the activity of superoxide dismutase(SOD)was determined by the nitroblue tetrazole method,the activity of peroxidase(POD)by the guaiacol method,and the activity of catalase(CAT)by the ultraviolet absorption method(Aebi,1984).For the determination of soil NO-3-N and NH+4-N contents, the fresh soil sample was extracted with 0.01 mol L-1CaCl2,and the extract solution was then analyzed using an auto-analyzer(Model AA3-A001-02E,Bran-Luebbe,Hamburg,Germany).Soil urease(Ure)activity was determined by sodium phenol-sodium hypochlorite colorimetry,and soil CAT activity was determined by the volumetric method(Guan,1986;Trasar-Cepedaet al.,1999).
Genomic DNA was extracted directly from soil samples using PowerSoil®DNA isolation kits(MoBio Laboratories,USA), according to the manufacturer’s instructions. For bacterial identification, V3—V4 hypervariable regions of the bacterial 16S rRNA gene were amplified using primers 338F(5′-ACTCCTACGGAGGCAGCAGCA-3′)and 806R(5′-GGACTACHGGGGTWTCTAAT-3′).Sequencing was performed using an Illumina Miseq PE300 platform at the Personalbio Company (Shanghai, China), following the manufacturer’s instructions.The original sequencing data were screened for quality and paired using the Flash software(Magoč and Salzberg,2011).
The paired sequences were identified and assigned to the corresponding samples to obtain the valid sequences of each sample.The QIIME software was used to identify interrogative sequences,check and eliminate chimeric sequences,and count high-quality sequences(DeSantiset al.,2006).The UCLUST sequence alignment tool was used to merge and classify the high-quality sequences,based on 97%sequence similarity.From the results of OTU classification and identification,the specific composition of each sample at each classification level was obtained.The composition and abundance distribution tables of each sample at five levels,i.e.,phylum,class,order,family,and genus,were obtained using the QIIME software, and the phylum level analysis results were presented as a histogram.Key biomarkers(i.e.,key community members) were screened using the linear discriminant analysis effect size(LEfSe)method(Segataetal.,2011).Multivariate linear regression was used to fit the data of bacterial community structure with a given influential factor,and the displacement test was used to determine if the factor had a significant effect on the structure of the bacterial community. The relative abundance matrix at the generic level was analyzed by redundancy analysis (RDA) using the R software(McArdle and Anderson,2001).Statistical significance was determined by a permutation test,Monte Carlo permutation procedure(1 000 times).A sorting map of three elements(sample-taxon-influential factor)was generated.Sequences have been deposited in the National Center for Biotechnology Information’s GenBank Sequence Read Archive database under BioProject No.PRJNA541127.
Data analysis
Nitrogen use efficiency(NUE,%)was calculated using the following formula(Devkotaet al.,2013).
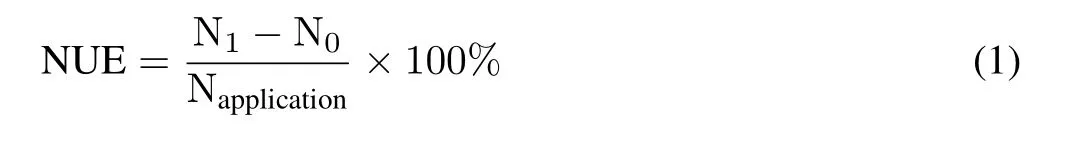
where N1is the N accumulation in each N application treatment and N0is the N accumulation in CK.
The differences in soil chemical properties, bacterial diversity and abundance,tomato yield,and other measured parameters in the different treatments were tested using oneway analysis of variance followed by the least significant difference test using the SAS 8.0 software package. The significance level was set atP <0.05.
RESULTS
Effect of biochar applied with PGPR on tomato yield
The application of PGPR in combination with biochar resulted in a higher tomato yield than the application of PGPR or biochar alone(Table I).The tomato yield in the UBP treatment was 4 940.9 g pot-1, which was 45.69%,10.44%,and 32.45%higher than those in the UP,UB,and U treatments,respectively.The yield in the UB treatment was 19.93%higher than that in the U treatment,while that in the UP treatment was 9.09%lower than that in the U treatment.In terms of yield components,biochar combined with PGPR significantly increased the single fruit weight of tomato,but had no significant effect on the number of tomato fruits.The single fruit weight in the UBP treatment was 26.31%,36.97%,and 11.71%higher than those in the U,UP,and UB treatments,respectively.The single fruit weight in the UB treatment was 19.93%higher than that in the U treatment,while that in the UP treatment was 7.78%lower than that in the U treatment.
Effects of different treatments on microbial community structure in tomato rhizosphere soil
Taxonomic composition analysis at the phylum level.In all the soil samples from different treatments,Cyanobacteria (26.50%), Proteobacteria (23.40%), Actinobacteria(20.30%),Chloroflexi(10.30%),Acidobacteria(6.00%), and Gemmatimonadetes (5.80%) were the main bacterial communities at the phylum level,accounting for 92.3%of the total sequences.Bacteroidetes(2.7%),Saccharibacteria (1.3%), Nitrospirae (0.8%), Firmicutes (0.6%),Planctomycetes(0.4%),and Verrucomicrobia(0.3%)were not abundant, although they were detected in all samples(relative abundance<5%but>0.1%)(Fig.2).
Differences in the taxonomic composition.Based on multiple comparisons,LEfSe detected more bacterial taxa(86evolutionary branches) in the UBP treatment than in other treatments(Fig.S1,see Supplementary Material for Fig. S1).Nitrospira, a group of Gram-negative bacteria,act as nitrifiers and can oxidize nitrite to nitrate.Bradyrhizobiumcan fix N. Thus, we further analyzed the relative abundance distribution ofNitrospiraandBradyrhizobiumin the different treatments(Fig.3).The relative abundance ofNitrospiraandBradyrhizobiumwas significantly increased(P= 0.008) by the combined application of PGPR and biochar,compared with their sole application.The relative abundance ofNitrospirain the UBP treatment was 54.05%,185.00%,and 72.72%higher than those in the U,UP,and UB treatments,respectively.The relative abundance ofBradyrhizobiumin the UBP treatment was 184.62%, 85.00%, and 117.65%higher than those in the U,UP,and UB treatments,respectively.

TABLE I Tomato yields and yield components in different treatments
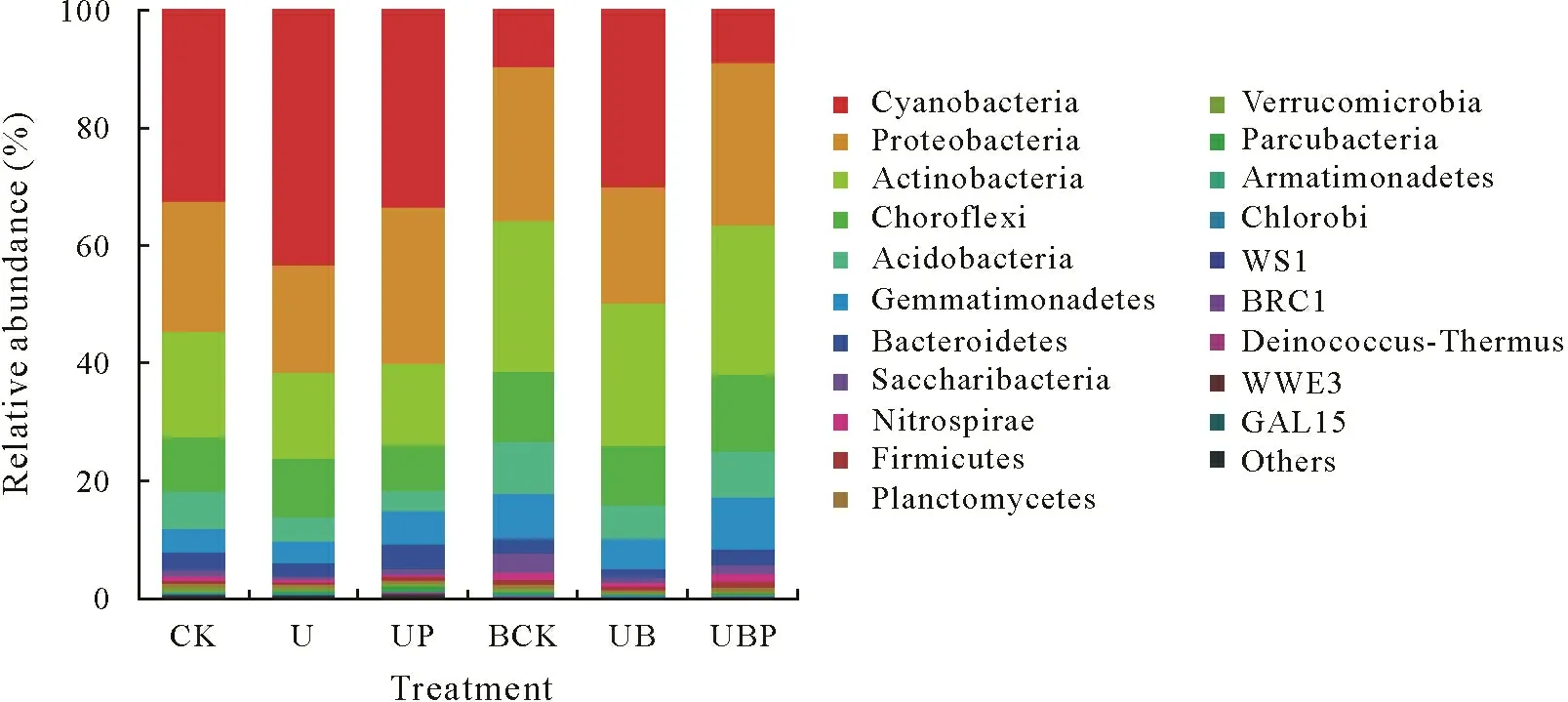
Fig.2 Bacterial abundance distribution at the phylum level in the rhizosphere soil of tomato under different treatments.Groups with relative abundance higher than 1%are shown in the figure.Groups with relative abundance less than 1%were integrated into“others”.See Table I for the detailed descriptions of the treatments CK,U,UP,BCK,UB,and UBP.

Fig.3 Relative abundance distribution of Nitrospira and Bradyrhizobium in the rhizosphere soil of tomato under different treatments.Vertical bars represent the standard deviations of means(n =3).Bars with the same letter(s)for each bacterium are not significantly different at P <0.05.See Table I for the detailed descriptions of the treatments CK,BCK,U,UP,UB,and UBP.
Effects of different treatments on enzyme activities in tomato rhizosphere soil
The determination and analysis of soil enzyme activity in the tomato rhizosphere revealed that biochar combined with PGPR could effectively improve Ure activity in the rhizosphere soil;however,soil CAT activity was significantly decreased(Fig.4).The Ure activity in the rhizosphere soil in the UB treatment was significantly higher(by 18.20%)than that in the U treatment.There was no significant difference in soil Ure activity between the UP and U treatments.Soil Ure activity in the UBP treatment was 34.20%,44.78%,and 13.51%higher than those in the U,UP,and UB treatments,respectively.
There was no significant difference in soil CAT activity between the UP and U treatments.The CAT activity in the rhizosphere soil of the UB treatment was significantly lower(16.00%) than that in the U treatment. Soil CAT activity in the UPB treatment was 25.21%and 31.18%,lower than those in the U and UP treatments,respectively.There was no significant difference between the UPB and UB treatments in terms of the CAT activity.
Effect of biochar combined with PGPR on soil NO-3 -N and NH+4 -N
Compared with the UB treatment,the combined application of biochar and PGPR (UBP treatment) effectively increased soil NO-3-N content at the fruit bearing and mature stages of tomato, as well as the soil NH+4-N content at the fruit setting stage of tomato.The UP treatment had no significant effect on soil NH+4-N and NO-3-N contents when compared with the U treatment(Table II).Soil NO-3-N contents in the UB and UBP treatments at the maturity stage of tomato were 43.79% and 87.12% higher, respectively,than that in the U treatment,and met the nutrient requirement of tomato in the later growth stage.Soil NH+4-N contents in the UB and UBP treatments were 82.07%and 136.83%higher,respectively,than that in the U treatment at the fruit setting stage.Soil NO-3-N content in the UBP treatment was on average 15.97% higher than that in the UB treatment during the whole growth period of tomato.
Effect of biochar applied with PGPR on NUEand N distribution in tomato organs
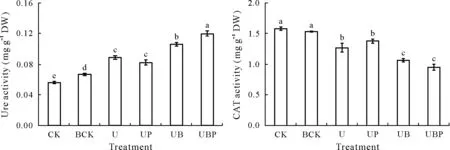
Fig.4 Enzyme activities in the rhizosphere soil of tomato under different treatments.Vertical bars represent the standard deviations of means(n=4).Bars with the same letter are not significantly different at P <0.05.See Table I for the detailed descriptions of the treatments CK,BCK,U,UP,UB,and UBP.Ure=urease;CAT=catalase;DW=dry weight.
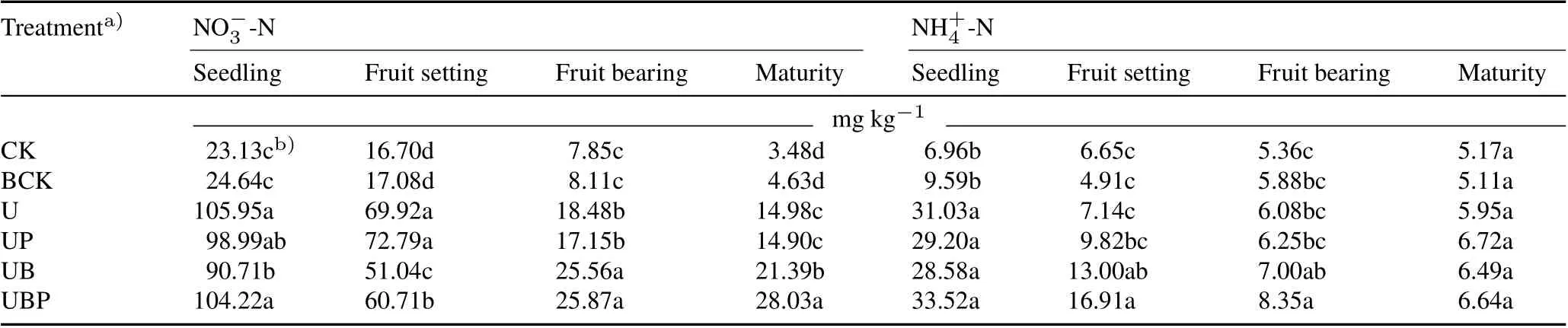
TABLE II Soil NO-3 -N and NH+4 -N contents at different growth stages of tomato under different treatments
The combined application of biochar and PGPR significantly increased the utilization rate of N fertilizer, as compared to the individual application of either biochar or PGPR(Table III).The NUE in the UB treatment was 2.86%higher than that in the U treatment,while that in the UP treatment was 4.03%lower than that in the U treatment.The NUE in the UBP treatment was 13.63%,17.66%,and 10.77%higher than those in the U,UP,and UB treatments,respectively.The addition of PGPR alone resulted in a decrease in the NUE;however,when PGPR was used in combination with biochar,the NUE was further improved relative to the UB treatment, indicating that biochar had a positive effect on PGPR.
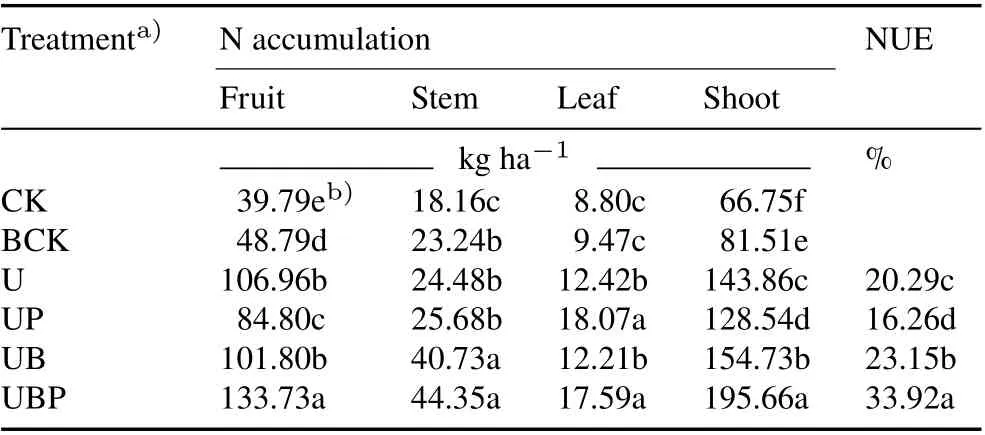
TABLE III Nitrogen (N) distribution in tomato organs and nitrogen use efficiency(NUE,calculated using Eq.1)in different treatments
Effects of different treatments on photosynthetic pigments in tomato leaf
The chlorophyll and carotenoid contents in tomato leaf were significantly increased by the combined application of PGPR and biochar (Table IV). The carotenoid content in the UB treatment was 15.63% higher than that in the U treatment; however, the total chlorophyll content was not significantly different between the two treatments.Thecarotenoid content in the UP treatment was 15.63%higher,while the total chlorophyll content was 16.02%lower than that in the U treatment, and these differences were significant. The carotenoid content in the UBP treatment was 18.75%higher than that in the U treatment,and there was no significant difference between the UBP and the UP and UB treatments.The total chlorophyll content in the UBP treatment was higher than those in the U,UP,and UB treatments,by 18.78%,41.45%,and 22.86%,respectively,and all the differences were significant.

TABLE IV Photosynthetic pigment contents in tomato leaf under different treatments
Effects of different treatments on enzyme activities in tomato leaf
The antioxidant enzyme activities in tomato leaf were effectively improved by the combined application of PGPR and biochar,whereas the addition of PGPR or biochar alone had no significant effect(Fig.5).The SOD activity in tomato leaf in the UBP treatment was 11.04%and 15.64%higher than those in the U and UP treatments, respectively, and there was no significant difference between the UB and UBP treatments. The POD activity in tomato leaf in the UBP treatment was 9.40%and 13.24%higher than those in the U and UP treatments,respectively;however,there was no significant difference between the UB and UBP treatments.The CAT activity in tomato leaf in the UBP treatment was significantly higher than those in the U, UP, and UB treatments,by 47.84%,29.63%,and 25.27%,respectively.

Fig.5 Enzyme activities in tomato leaf under different treatments.Vertical bars represent the standard deviations of means(n=4).Bars with the same letter(s)are not significantly different at P <0.05.See Table I for the detailed descriptions of the treatments CK,BCK,U,UP,UB,and UBP.SOD=superoxide dismutase;POD=peroxidase;CAT=catalase;U=unit;FW=fresh weight.
DISCUSSION
The results showed that biochar combined with PGPR significantly increased tomato yield,and tomato yield in the UBP treatment was 45.69%, 10.44%, and 32.45% higher than those in the UP, UB, and U treatments, respectively.We attempted to determine the mechanism of the increase in yield in response to the combined application of biochar and PGPR,i.e.,if it was due to the effect of biochar or PGPR acting alone or a combination of the two.The results showed that when biochar was applied alone(UB),the tomato yield was 19.93%higher than that under conventional fertilization(U),indicating that biochar had a positive effect on tomato growth.This result is consistent with those of other studies,which demonstrated that biochar application could improve the growth of many plants,including maize(Zea maysL.)and wheat(Triticum aestivumL.)(Uzomaet al.,2011;Zhaoet al.,2013;Rafaelet al.,2019).However,the application of PGPR alone did not have any positive impact on the tomato yield; on the contrary, it led to a significant reduction in tomato yield when compared to the conventional fertilization treatment. This may be due to inherent differences in the plant species,competition by indigenous soil bacteria,and unfavorable soil environmental factors,which might interfere with the reproduction and activity of the introduced PGPR,rendering their effect unstable. Many studies have shown that PGPR,which promote plant growth and enhance plant resistance to various diseases and abiotic stresses in the laboratory,are less effective under field conditions,which is usually due to inadequate colonization of microbes in the rhizosphere soil or the endoderm of plants (Kent and Triplett,2002).Therefore,it is necessary to explore methods to promote the colonization of PGPR in soil.
The tomato yield in the treatment with combined application of biochar and PGPR(UBP)was 10.44%higher than that in the treatment with biochar alone (UB). This yield increase could be attributed to the effect of PGPR,suggesting that the combined application of biochar and PGPR had a superimposed effect.Although the application of PGPR alone had a negative effect on tomato yield, its combination with biochar had a positive effect,which not only reversed the negative effect of the sole application of PGPR,but also promoted the role of PGPR in soil.Biochar is a porous material and its abundant pores can provide a habitat for microorganisms and a shelter against other biological invasion(Lehmann and Joseph, 2015). Scanning electron microscopy(SEM)images revealed abundant pore spaces for the tested biochar(Fig.1).Biochar can further improve soil porosity and aeration by improving soil structure,and can provide a good niche for PGPR colonization in soil.In addition,the high pH of biochar can regulate the pH of acidic soil,which may be one of the important reasons for the promotion of the colonization of beneficial bacterial in soil by the combined application of biochar and PGPR.The RDA ordination at the species level showed that soil microbial properties were largely related to soil physical and chemical properties(RDA model,P=0.001),among which pH had a strong effect on bacterial community composition and was significantly correlated with the bacterial community(Fig.6).
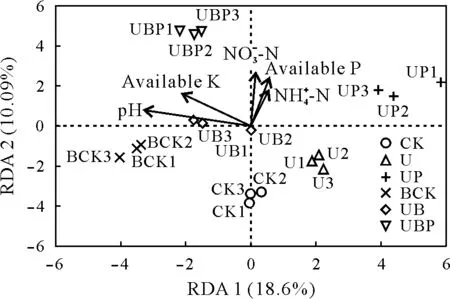
Fig.6 Redundancy analysis(RDA)of the relative abundance of bacterial community in the rhizosphere soil of tomato under different treatments.Each point represents a sample.The closer the distance between the two points,the higher the similarity of community structure between the two samples.Arrows represent different influential factors.When the angle between the influential factors is acute,the two factors are positively correlated;when the angle is obtuse, the correlation is negative. The longer the ray, the greater the effect of the factor.The position of the projection point on the arrow represents the approximate value of the factor in the corresponding sample.See Table I for the detailed descriptions of the treatments CK,U,UP,BCK,UB,and UBP.
Further study revealed that PGPR combined with biochar significantly altered the dominant microbial community in soil.The relative abundances ofNitrospiraandBradyrhizobiumwere significantly higher under combined application of PGPR and biochar than in the treatments with biochar or PGPR alone.Many members ofNitrospiraparticipate in nitrite oxidation in the process of nitrification(Daimset al.,2015;Hanet al.,2018).Bradyrhizobiumforms nodules in the roots of host plants,and can fix atmospheric N,transforming it into a form available to plants(ammonia)(Mylonaet al.,1995). The relative abundance ofNitrospirain the UBP treatment was 54.05%,185.00%,and 72.72%higher than those in the U, UP, and UB treatments, respectively. The relative abundance ofBradyrhizobiumin the UBP treatment was 184.62%,85.00%,and 117.65%higher than those in the U,UP,and UB treatments,respectively.This indicated that the combined application of biochar and PGPR increased the relative abundance of microbial groups with nitrification and N fixation abilities in the tomato rhizosphere soil,which might affect the transformation and cycling of N in soil.
The soil enzymatic system represents the metabolic power of soil organisms, which can catalyze biochemical processes in the soil ecosystem, and can also be used to characterize soil ecological function and the intensity and direction of soil nutrient transformation processes.Changes in soil microbial community also affect soil enzyme activities.Some soil enzymes come from the secretion of microorganisms.Soil Ure activity is usually related to the number of soil microorganisms(Paulson and Kurtz,1969),and it increases with an increase in the organic matter content(Roscoeet al.,2000).Compared to the conventional fertilization treatment(U), the application of biochar alone (UB) increased soil Urea activity by 18.20% and by 31.71% when combined with PGPR(UBP).The result shows that the application of biochar alone can improve soil microbial community and enzyme activity,and the combined application of biochar with PGPR can have a greater synergistic effect.
The increase in soil Ure activity led to the transfer of urea and thus an increase in soil NH+4-N content,which regulated the supply of soil N.The contents of soil NH+4-N in the UBP and UB treatments were 82.07%and 136.83%,respectively,higher than that in the U treatment at the fruit bearing stage of tomato.Because the application of biochar with PGPR increased the relative abundance ofNitrospirain soil,the high relative abundance of the spiral nitrite sequence cluster 3b community responded quickly to the high ammonia input,thus oxidizing ammonium to nitrite,promoting nitrification,and increasing the content of NO-3-N in the soil(Ward and Bouskill, 2011; Posmaniket al., 2014). The soil NO-3-N content in the UBP treatment was 87.12%, 88.12%, and 31.04%higher than those in the U,UP,and UB treatments,respectively. Fertilizer use efficiency depends mainly on the crop nutrient absorption ability and soil and fertilizer nutrient supplying ability(Chienet al., 2009). Combined application of biochar and PGPR increased the relative abundance of microorganisms and soil Ure activity related to N transformation in tomato rhizosphere soil, promoted the transformation and circulation of N, improved soil N availability,and promoted N absorption by the tomato plants,thereby improving the NUE by plant.The NUE in the UBP treatment was 13.63%, 17.66%, and 10.77% higher than those in the U,UP,and UB treatments,respectively.
Photosynthetic pigments participate in the absorption and transmission of light energy and the initiation of primary photochemical reactions during photosynthesis,and are key indicators of the photosynthetic capacity of crops (Sosik and Mitchell,1995).The antioxidant enzymes SOD,POD,and CAT can form a complete antioxidant chain and clear excessive superoxide radicals in the plant,so as to protect the plant from oxidative damage,improve plant resistance,and promote dry matter accumulation (Daset al., 2015).Nitrogen is an important component of chlorophyll and antioxidant enzymes, and its content is closely related to the leaf chlorophyll content and plant antioxidant enzyme activity(Hokmalipour and Darbandi,2012;Kapooret al.,2019). The combined application of biochar and PGPR,with rational N supply ability, had a beneficial effect on the chlorophyll content and enzyme activity in tomato leaf.The chlorophyll content in the UBP treatment was 18.78%,41.45%, and 22.86% higher than those in the U, UP, and UB treatments,respectively.The increase in the chlorophyll content increased the photosynthetic capacity and dry matter accumulation in tomato plants,thereby increasing the tomato yield.Previous studies have shown that the addition of N can increase the activities of SOD,POD,and CAT in maize under water stress and that the antioxidant enzyme activities(SOD,CAT,and ascorbate peroxidase)of okra plants increase when inoculated with PGPR under salinity stress (Zhanget al.,2007;Habibet al.,2016).In the present study,the activities of plant resistance-related enzymes,such as SOD,POD,and CAT,were increased in tomato leaf,and plant resistance was enhanced in the UBP treatment,which might be partly due to the improvement of soil nutritional status and partly due to the introduction of beneficial microbes.
Our results suggest that PGPR combined with biochar can promote PGPR colonization in the soil,improve the activities of related enzymes, and enhance the transformation of N and other soil nutrients,thus ensuring nutrient uptake and the normal process of photosynthesis in tomato plants and thereby promoting the accumulation of photosynthates and increasing tomato yield.However,there are some limitations associated with pot experiments,and the results further need to be verified by long-term field experiments.
CONCLUSIONS
Biochar combined with PGPR had positive effects on soil microbial community structure, soil enzyme activity,and soil N transformation.The tomato yield in the treatment with biochar combined with PGPR (UBP) was 32.45%,45.69%, and 10.44% higher than those in the U, UP, and UB treatments,respectively.The NUE in the UBP treatment was 13.63%,17.66%,and 10.77%higher than those in the U,UP,and UB treatments,respectively.Our results suggest that biochar combined with PGPR significantly enhanced the microbial community diversity and enzyme activity in tomato rhizosphere soil,improved the availability of soil N and crop N uptake,and increased dry matter accumulation.
ACKNOWLEDGEMENT
This research was funded by the National Key R&D Program of China(No.2017YFD0200804),the Shandong Provincial Key R&D Program of China(No.2017CXGC-0306),and the Shandong Provincial Development Plan of China(No.2013GNC11309).
SUPPLEMENTARY MATERIAL
Supplementary material for this article can be found in the online version.
杂志排行
Pedosphere的其它文章
- Letter to the Editor Molecular characterization of an extensively drug-resistant Acinetobacter baumannii isolated from a corn culture soil
- Soil organic matter content and chemical composition under two rotation management systems in a Mediterranean climate
- Impacts of land use and salinization on soil inorganic and organic carbon in the middle-lower Yellow River Delta
- Rice(Oryza sativa L.)seedlings enriched with zinc or manganese:Their impacts on cadmium accumulation and expression of related genes
- Responses of the methanogenic pathway and fraction of CH4 oxidization in a flooded paddy soil to rice planting
- Soil chronosequence and biosequence on old lake sediments of the Burdur Lake in Turkey
