Rice(Oryza sativa L.)seedlings enriched with zinc or manganese:Their impacts on cadmium accumulation and expression of related genes
2021-12-22GaoxiangHUANGChangfengDINGYibingMAYurongWANGZhigaoZHOUShunanZHENGandXingxiangWANG
Gaoxiang HUANGChangfeng DINGYibing MAYurong WANGZhigao ZHOUShun’an ZHENG and Xingxiang WANG
1Key Laboratory of Soil Environment and Pollution Remediation,Institute of Soil Science,Chinese Academy of Sciences,Nanjing 210008(China)
2School of Geography and Environment,Key Laboratory of Poyang Lake Wetland and Watershed Research,Ministry of Education,Jiangxi Normal University,Nanchang 330022(China)
3Macau Environmental Research Institute,Macau University of Science and Technology,Taipa 999078,Macau(China)
4Rural Energy and Environment Agency,Ministry of Agriculture and Rural Affairs,Beijing 100125(China)
5Ecological Experimental Station of Red Soil,Chinese Academy of Sciences,Yingtan 335211(China)
6University of Chinese Academy of Sciences,Beijing 100049(China)
(Received January 6,2020;revised March 13,2020)
ABSTRACT Cadmium(Cd)contamination in paddy soils means that the rice produced there may be unsafe for human consumption. A hydroponic study was conducted to enrich rice seedlings with zinc(Zn)or manganese(Mn),and the uptake and transport characteristics of Cd in these Zn-and Mn-rich seedlings were subsequently investigated using a greenhouse pot trial.The results showed that hydroponic cultivation in 10—50 μmol L-1 Zn(ZnSO4·7H2O)or 50—250 μmol L-1 Mn(MnSO4·H2O)for 30 d had no significant impact on rice growth,while the accumulation of Zn and Mn was 7.31—18.5 and 25.4—47.7 times higher,respectively,than in the control(no Zn or Mn addition).The accumulation of Cd in the Zn-and Mn-rich rice plants was 26.3%—38.6%and 34.4%—44.5%lower than that in the control,respectively,and the translocation factors of Cd from roots to shoots also decreased by 23.3%—41.3%and 25.3%—37.0%,respectively,after transplanting to Cd-contaminated soils.Furthermore,the relative expression levels of OsIRT1(Oryza sativa iron-regulated transporter 1)were downregulated by 40.1%—59.3%and 16.0%—25.9%,respectively,in the Zn-and Mn-rich seedling roots.This downregulation may indicate a possible mechanism contributing to the reductions in Cd absorption.Field experiments confirmed that the Zn-and Mn-rich seedlings produced brown rice(unpolished rice grains)with significantly decreased concentrations of Cd(34.2%—44.4%).This study provides an innovative method for reducing the food safety risks from rice grown on slightly to moderately Cd-contaminated paddy soils.
Key Words: absorption enrichment,Cd contamination,contaminated paddy soil,gene expression,hydroponics,translocation factor
INTRODUCTION
Owing to the increasingly uncontrolled inputs of industrial hazardous wastes (e.g., wastes from the mining and smelting industries),sewage,phosphate fertilizers,and organophosphorus pesticides to soils(Liuet al.,2009;Zhaoet al.,2015),large amounts of cadmium(Cd)can now be found in farmland soils. According to the national survey report on soil pollution in China,published in 2014,approximately 7%of the soil samples were contaminated with Cd,and it was especially common in southern China(MEP MLR,2014).Consequently,the concentrations of Cd in brown rice(unpolished rice grains) grown in some Cd-contaminated regions exceeded the food safety limit(0.2 mg kg-1);this socalled“Cd-rice”is a matter of considerable public concern(Duet al.,2013).Brown rice is the predominant food crop for the public in southern China,with an average consumption of 219 g capita-1d-1(Huet al.,2016).The consumption of Cd-rice results in the overaccumulation of Cd in the human body and may subsequently cause certain incurable diseases,such as itai-itai disease and liver and kidney damage(Kimet al., 2003; Skrderet al., 2015). To avoid health risks to humans in Cd-contaminated regions,it is imperative to reduce the absorption of Cd by rice from soil through some effective and low-cost methods.
In recent decades, many methods have been explored to reduce the accumulation of Cd in rice, including soiland plant-based methods.Soil-based methods mainly aim to reduce the content or bioavailability of Cd in the soil and include phytoextraction(Li Zet al.,2014;Hanet al.,2019),electrokinetic remediation(Tanget al., 2018), andin situimmobilization(Bianet al.,2014;Hamidet al.,2020).Plantbased methods mainly aim to weaken the absorption ability of the rice,by using methods like regulating the expression of Cd-related genes in rice roots using molecular technologies,especially transgenesis(Yamajiet al.,2013;Liuet al.,2019).Gene regulation by transgenesis avoids the influences of soil variability;thus,Cd reductions performed by the rice itself are always more stable than soil-based methods that are generally applied to a variety of soils.However,transgenesis technology is still unacceptable to the public,and furthermore,the knockout of Cd-related genes may decrease the uptake of some essential micronutrients that use the same transporters as Cd(Sasakiet al.,2012).Nevertheless,the expression of important Cd-related genes can also be regulated by the application of essential microelements,such as iron(Chenet al.,2017a).Moreover,zinc(Zn)and manganese(Mn) are also essential to rice, and they are actively absorbed through transporters.Iron-regulated transporter-like proteins(such as OsIRT1)and natural resistance-associated macrophage proteins(such as OsNramp5)are involved in the transport of Zn and Mn(Ishimaruet al.,2011;Shaoet al.,2017b).Interestingly,OsNramp5 is a major transporter involved in the uptake of Cd and Mn by rice roots(Sasakiet al.,2012).OsIRT1 has also been shown to be associated with Cd uptake in rice(Yanget al.,2016).In addition,some transporters related to the upward transport of Zn or Mn in rice roots also function in the transport of Cd, such as heavy metal ATPases(OsHMA2 and OsHMA3)(Yamajiet al.,2013;Cai H Met al.,2019).Therefore,the up-or down regulated expression of these transporter genes in response to the requirements of Zn or Mn for rice growth, would also change the absorption of Cd (Shaoet al., 2018; Luet al.,2019).Accordingly,we hypothesized that regulating the expression of Cd-related genes is possible,by enriching the absorption of Zn or Mn in rice,and thus decreasing the ability of the rice to absorb Cd.
Previous studies have considered the application of Zn(Huanget al., 2019)or Mn(Sunet al., 2018)for the safe exploitation of Cd-contaminated soils. Basal, topdressing or foliar applications of Zn at appropriate levels can reduce the accumulation of Cd in plants (Saifullahet al., 2014;Qaswaret al., 2017; Huanget al., 2019). However, the long-term effects are restricted by the gradually decreased availability of exogenous Zn,due to the aging effects in the soil(Donneret al.,2012).The continuous applications of Zn or Mn in each season can maintain their efficiency.However,long-term Zn or Mn inputs will induce new ecological risks to the soil and aquatic environments (Markset al., 2017;Baranet al.,2018).Similarly,soil or foliar applications of Mn fertilizer at high levels,are also unsuitable for enriching rice with Mn.Therefore,an innovative Zn or Mn utilization method should be developed to enrich rice with Zn or Mn and avoid environmental risks.Rice planting often involves transplanting young seedlings cultivated for approximately one month in a seedbed. Accordingly, it is possible to produce Zn-or Mn-rich seedlings by applying appropriate amounts of Zn or Mn during seedling growth.Subsequently,transplanting Zn-or Mn-rich seedlings into Cd-contaminated soil is a potential way to decrease the absorption of Cd by rice,however,this technique requires further investigation.In addition,Zn-Cd or Mn-Cd antagonism during the external uptake and internal transport processes in plant tissues was considered to be the main driving factor for Cd reduction(Huanget al., 2017; Cai Y Met al., 2019). However, the effects of Zn or Mn on the expression of Cd-related genes are still unclear.
Therefore, the aim of this study was to obtain rice that was safe for consumption even when grown on Cdcontaminated soils,by enriching the seedlings with Zn or Mn. The study included three aspects as follows: 1) the production of Zn-or Mn-rich rice seedlings by culturing in different amounts of exogenous Zn or Mn,2)investigation of the uptake and transport characteristics of Cd, Zn, and Mn in rice plants after transplanting the Zn-and Mn-rich seedlings into Cd-contaminated soils,and 3)exploration of the possible mechanisms involved in the reduction of Cd in rice plants,by studying the effects of Zn and Mn enrichment on the expression of Cd-related transporters in the roots.
MATERIALS AND METHODS
Rice seedling cultivation and hydroponic experiment
Wuyunjing21,a conventional japonica rice(growth period of 151 d), obtained from the Wujin District Institute of Agricultural Sciences in Changzhou City, was used as the experimental cultivar.The rice seeds were sterilized in 30%(volume/volume)H2O2for 15 min,soaked in deionized water at 25°C in the dark for 24 h,and then germinated in moist gauze for another 24 h.Seeds with similar size were then selected to become the experimental seedlings.Kimura B solution(pH 5.6)is a common nutrient solution for rice that is frequently used to culture seedlings in hydroponic experiments(Leeet al.,2013;Shaoet al.,2017a)and was used in this work.The seedlings were thus grown hydroponically in the Kimura B nutrient solution, which contained 0.36 mmol L-1(NH4)2SO4,0.55 mmol L-1MgSO4·7H2O,0.18 mmol L-1KNO3,0.37 mmol L-1Ca(NO3)2·4H2O,0.18 mmol L-1KH2PO4,20 μmol L-1FeSO4·7H2O,20 μmol L-1EDTA-2Na,0.5 μmol L-1MnCl2·4H2O,3 μmol L-1H3BO3,1 μmol L-1(NH4)6Mo7O24·4H2O,0.4 μmol L-1ZnSO4·7H2O,and 0.2 μmol L-1CuSO4·4H2O.The seeds were cultured in half-strength Kimura B solution(half of the concentration)for the first 3 d and then treated with Zn or Mn. According to the studies of Chenet al. (2013)and Chenet al.(2018),the addition of Zn2+at levels below 211 μmol L-1or Mn at levels below 500 μmol L-1had no negative effects on the growth of rice seedlings.Therefore,two safe levels of Zn2+(10 and 50 μmol L-1)and Mn2+(50 and 250 μmol L-1)were used for this study.Five different treatments were applied in the hydroponic experiment as follows:1)the control(CK),nutrient solution with no Zn or Mn addition; 2)Zn1, nutrient solution with 10 μmol L-1Zn(ZnSO4·7H2O);3)Zn2,nutrient solution with 50 μmol L-1Zn(ZnSO4·7H2O);4)Mn1,nutrient solution with 50 μmol L-1Mn(MnSO4·H2O);and 5)Mn2,nutrient solution with 250 μmol L-1Mn(MnSO4·H2O).The nutrient solution and the Zn and Mn solutions were renewed every 4 d.After cultivation for 30 d,some of the seedlings(referred to as “rice seedlings”) were sampled to measure their Zn and Mn concentrations and the relative expression of Cdrelated transporter genes.Other seedlings were transplanted to Cd-contaminated soil to participate in the pot experiment.
Soil preparation and pot experiment
An acidic paddy soil was collected from Guixi City,Jiangxi Province,China(28°01′N,117°13′E).The tested soil was air-dried and partly sieved through 2-mm mesh for the measurements of soil pH,cation exchange capacity,clay content,and available contents of Cd,Mn,and Zn.Another part of the soil was sieved through a 0.15-mm mesh for the measurements of the organic matter and the total contents of Cd,Mn,and Zn.The soil pH was 4.81,and the total Cd content was 0.68 mg kg-1.Other principal properties of the topsoil(0—20 cm)are shown in Table SI(see Supplementary Material for Table SI).
The pot experiment was conducted during the seedling growth period in a greenhouse at the Institute of Soil Science, Chinese Academy of Sciences, Nanjing, Jiangsu Province,China.Some PVC pots(18 cm diameter×20 cm height)were filled with 4 kg of dry soil,and basal fertilizers,N at 0.20 g kg-1(CO(NH2)2),P2O5at 0.15 g kg-1(CaH2PO4·H2O), and K2O at 0.20 g kg-1(KCl), were evenly mixed with the soil.The soil was kept moist for one week, and two uniform seedlings were then transplanted into each pot(each treatment was replicated three times).After cultivation in flooded conditions with a water layer of 2—3 cm for 60 d,all rice plants(referred to as“late-tillering rice”(90 d))were collected,and the concentrations of Cd,Zn,and Mn were measured.
Field experiments
The effect of the Zn or Mn enrichment of the rice seedlings,on reducing the accumulation of Cd in brown rice,was verified under field conditions.Two field experiments were conducted in Guixi City,Jiangxi Province(Guixi site,28°01′N,117°13′E)and in Tongling City,Anhui Province(Tongling site, 31°01′N, 117°53′E), China. The field in Guixi had been contaminated by the irrigation of wastewater from a copper smelting factory since the 1990’s.The area of Cd contamination was over 150 ha,with Cd levels ranging from 0.3 to 0.9 mg kg-1. The field in Tongling had been contaminated by sewerage from a copper mine since the 1990’s, and the contaminated area was over 200 ha. The Cd levels in Tongling were similar to those in Guixi. The soil pH of the experimental plot at the Guixi site was 4.76,and the total Cd content was 0.61 mg kg-1. The soil pH of the experimental plot at the Tongling site was 5.41,and the total Cd content was 0.55 mg kg-1.Other principal soil properties of the two sites are shown in Table SI.
This experiment at each site included four treatments.Three of the treatments corresponded to the CK(seedlings in nutrient solution with no Zn or Mn addition), Zn2(seedlings in nutrient solution with 50 μmol L-1Zn),and Mn2(seedlings in nutrient solution with 250 μmol L-1Mn)treatments employed in the above pot trial,and a conventional seedling cultivation practice(seedlings grown on paddy soil)was used as a contrast treatment(CT).Two local cultivars were used in the corresponding field experiments:Meixiangxingzhan(conventional indica rice,123 d,obtained from the Jiangxi Xing’an Seed Industry Co.,Ltd.,China)in Guixi,and Jingliangyouhuazhan(hybrid indica rice,138 d,obtained from the Longping High-Tech,China)in Tongling.The rice seedlings used in each treatment were transplanted into a plot(4 m×5 m),and each treatment was replicated three times.The water and fertilizer management practices used were consistent with the local practices,briefly described as follows:basal application of compound fertilizers at 450 kg ha-1(N:P2O5:K2O=15%:15%:15%)and then topdressing with 75.0 kg ha-1of urea during the greening-up period and 50.0 kg ha-1of compound fertilizers at the ear emergence stage,with water management of submergence until the late stage of filling(water layer with a depth of 3 cm),followed by wet irrigation and then field-drying for a week before the harvest.In the harvesting process,the rice grains were separated from the spike using a reaping machine equipped with a drying device.The yield of all rice in each plot was weighed and recorded using an electronic platform scale.Approximately 500 g grains from each plot were randomly collected for laboratory analysis.After rinsing,oven-drying,and decladding using a sheller(JLG-II,Institute of Grain Storage in Chengdu,China),brown rice was obtained.The concentrations of Cd, Zn, and Mn in the brown rice were determined.
Chemical analysis of samples
Some of the seedling samples from the hydroponic experiment and some of the rice plants from the pot experiment were collected and divided into roots and shoots(both stems and leaves). The roots were first soaked in 5 mmol L-1CaCl2solution for 20 min and then rinsed in both tap and deionized water.Thereafter,the roots and shoots were oven-dried to constant weights at 75°C and then milled to powders using a blender(A11 basic,IKA,Germany).The brown rice collected from the field experiments was also pretreated in a similar manner.The powder samples were digested using a DigiBlock ED54-Itouch digester system(LabTech,Beijing,China).The concentrations of Cd,Zn,and Mn in the digestion solution were measured using flame and graphite furnace atomic absorption spectrometry(SpectrAA 220FS and 220Z,Varian,USA).Detailed information on these methods is described in Huanget al.(2018).
The remaining seedling samples collected from the hydroponic experiment were quickly rinsed in tap water and then stored at-80°C. The relative expression levels ofOsIRT1andOsNramp5(associated with root Cd uptake)andOsHMA3andOsHMA2(associated with Cd transport in the root)were then determined.The entire root was separated from each seedling and stored in liquid nitrogen.Total RNA was isolated using a UNlQ-10 column TRIzol total RNA isolation kit(Sangon Biotech,Shanghai,China),and 1 μg of total RNA was used to synthesize first-strand cDNA using a RevertAid™first strand cDNA synthesis kit (Thermo,USA), following the manufacturer’s instructions. The relative expression levels ofOsIRT1,OsNramp5,OsHMA3,andOsHMA2were quantified using a quantitative RT-PCR system(BIO-RAD CFX96,USA),andOsActinwas used to normalize the expression ratio of each gene.The primers for all genes are referenced in Shaoet al.(2017a)and Chenet al.(2017b).
Data analysis
The accumulations(A)of Zn,Mn,and Cd in the whole plants in the rice and the translocation factor(TF)values of Zn, Mn, and Cd from the roots to shoots were calculated using the following equations:
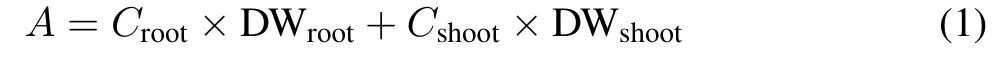

whereCrootandCshootrepresent the concentrations of Zn,Mn,or Cd in the roots and shoots,respectively,and DWrootand DWshootrepresent the dry weights of the roots and shoots,respectively.Real-time fluorescence quantification data were analyzed using Bio-Rad CFX Manager software(BIO-RAD,USA).
Statistical analysis
Data for each parameter are presented as the means±standard deviations(SDs).The differences in each parameter between different experimental treatments in the same tissues were analyzed by one-way analysis of variance(ANOVA)after checking the homogeneity(Levene’s test)and normality(Shapiro-Wilk test)of variances,with a significantPvalue of 0.05, followed by a least significant difference (LSD)post hoctest.Two-way ANOVA was applied to analyze the main effects of the treatment, the growth stage, and their interactions on the biomass and elemental concentrations of rice tissues. All statistical analyses were conducted using SPSS 19.0 software(IBM SPSS Statistics,USA).All graphs were produced by OriginPro 2016 software (OriginLab,USA).
RESULTS
Rice growth
Compared with CK,the Zn1,Zn2,Mn1,and Mn2 treatments had no significant impact on the dry weights of the roots or shoots of rice seedlings or late-tillering rice(Fig.1).A two-way ANOVA showed that the dry weights of the rice roots and shoots were mainly influenced by their growth stage(Table SII,see Supplementary Material for Table SII).
Absorption of Zn and Mn in rice tissues
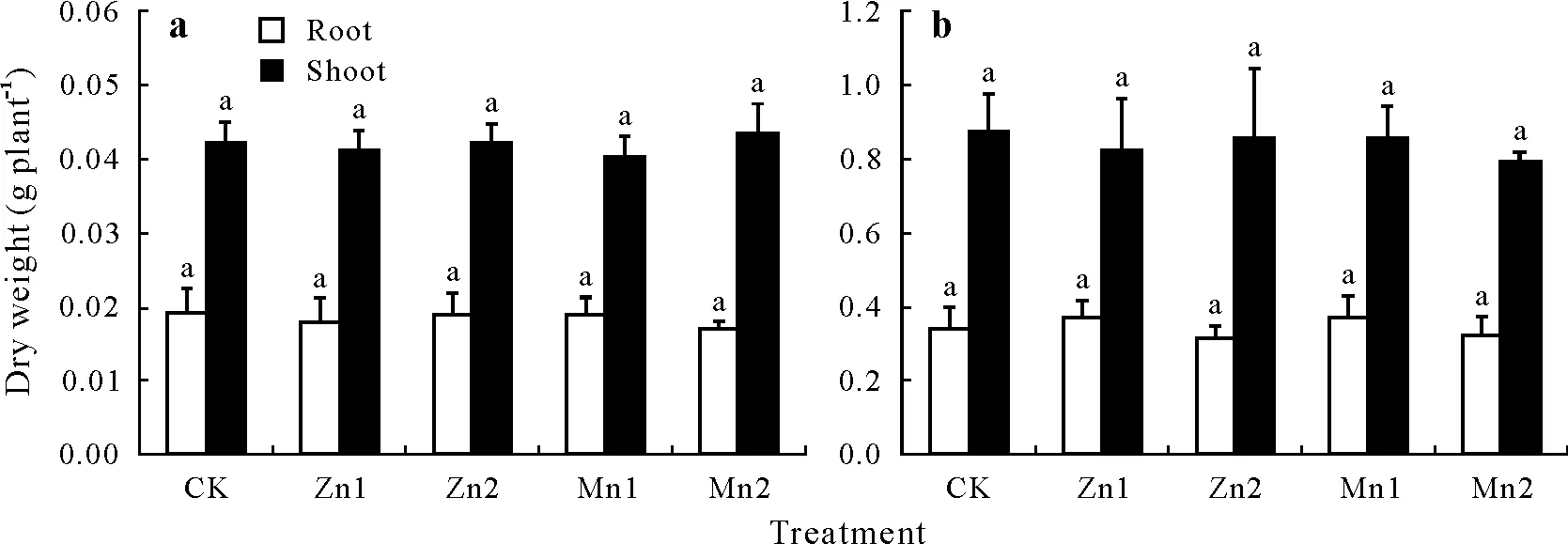
Fig.1 Changes in the dry weights of roots and shoots(both stem and leaf)of rice seedlings(30 d)(a)and late-tillering rice(90 d)(b)under different treatments.Bars are standard deviations of means(n =3);within the same tissues,bars with the same letter indicate no significant differences between different treatments at P <0.05.CK=the control,seedlings in nutrient solution with no Zn or Mn addition;Zn1 and Zn2=seedlings in nutrient solution with 10 and 50 μmol L-1 Zn,respectively,Mn1 and Mn2=seedlings in nutrient solution with 50 and 250 μmol L-1 Mn,respectively.
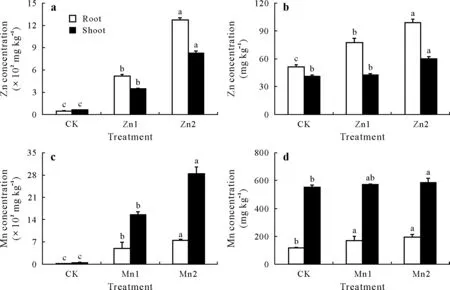
Fig.2 Concentrations of Zn and Mn in roots and shoots(both stem and leaf)of rice seedlings(30 d)(a and c)and late-tillering rice(90 d)(b and d)under different treatments.Bars are standard deviations of means(n=3);within the same tissue,bars with the same letter(s)indicate no significant differences between different treatments at P <0.05.CK=the control,seedlings in nutrient solution with no Zn or Mn addition;Zn1 and Zn2=seedlings in nutrient solution with 10 and 50 μmol L-1 Zn,respectively;Mn1 and Mn2=seedlings in nutrient solution with 50 and 250 μmol L-1 Mn,respectively.
As shown in Fig.2,the Zn concentrations in the roots and shoots of the rice seedlings were greatly increased with increased levels of Zn. Compared with the CK, the root and shoot Zn levels were 15.6 and 6.09 times higher in the Zn1 treatment and 27.1 and 14.8 times higher in the Zn2 treatment (Fig. 2a), respectively. Sixty days after the rice seedlings had been transplanted into the soil, the Zn concentrations in the roots and shoots of the late-tillering rice under the Zn treatments,were also higher than those of the CK.However,the differences were significantly reduced.Compared with the CK,the root Zn was 51.4%higher in the Zn1 treatment, and the root and shoot Zn was 93.3%and 45.9%higher in the Zn2 treatment(Fig.2b),respectively.The two-way ANOVA showed that the concentrations of Zn in the roots and shoots were also significantly influenced by the growth stage, and the interaction of the treatment and growth stage(Table SII).
The Mn changes in the roots and shoots under different treatments were similar to those of the Zn(Fig.2c and d).The concentrations of Mn in the roots and shoots of rice seedlings were 21.0 and 28.0 times those of the CK,under the Mn1 treatment,and 32.0 and 51.1 times those of the CK,under the Mn2 treatment(Fig.2c).However,the root and shoot Mn levels of the late-tillering rice were only 1.48 and 1.03 times those of the CK,under the Mn1 treatment,and 1.67 and 1.06 times those of the CK,under the Mn2 treatment(Fig.2d).The two-way ANOVA showed that the concentrations of Mn in the roots and shoots were also significantly influenced by the growth stage and the interactions of the treatment and growth stage(Table SII).
As shown in Fig.3a,during the early seedling growth period,the accumulation of Zn in rice seedlings was 7.31 and 18.5 times higher than that of the CK,under the Zn1 and Zn2 treatments, respectively.However, during the growth period following seedling transplantation,the accumulation of Zn under the Zn1 and Zn2 treatments was only 80.4%and 45.5%that of CK,respectively.Throughout the entire growth period, the accumulation of Zn in rice under the Zn2 treatment was 53.1%higher than that of the CK.Similar results were observed in the Mn treatments(Fig.3b).Mn accumulation during the early seedling growth period was 25.4 and 47.7 times that of the CK, under the Mn1 and Mn2 treatments,respectively.However,the accumulation of Mn during the growth period following seedling transplantation was 89.2%and 85.8%that of the CK under the Mn1 and Mn2 treatments, respectively. Throughout the entire growth period, the accumulation of Mn in rice under the Mn2 treatment was only 11.8%higher than that of the CK.
Absorption of Cd in rice

Fig.3 Total accumulation of Zn(a)and Mn(b)in rice during the early seedling growth period(30 d)and the growth period following transplantation(60 d)under different treatments.Bars are standard deviations of means(n =3);bars with the same letter indicate no significant differences between different treatments during the same growth period at P <0.05.CK=the control,seedlings in nutrient solution with no Zn or Mn addition;Zn1 and Zn2=seedlings in nutrient solution with 10 and 50 μmol L-1 Zn,respectively;Mn1 and Mn2=seedlings in nutrient solution with 50 and 250 μmol L-1 Mn,respectively.
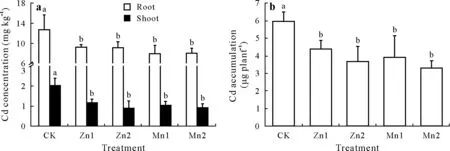
Fig.4 Concentrations of Cd in the roots and shoots(both stem and leaf)(a)and accumulation of Cd in the whole plants(b)in late-tillering rice(90 d)under different treatments.Bars are standard deviations of means(n=3);bars with the same letter indicate no significant differences between different treatments within the same tissue at P <0.05.CK=the control,seedlings in nutrient solution with no Zn or Mn addition;Zn1 and Zn2=seedlings in nutrient solution with 10 and 50 μmol L-1 Zn,respectively;Mn1 and Mn2=seedlings in nutrient solution with 50 and 250 μmol L-1 Mn,respectively.
In the late-tillering rice,the Zn and Mn treatments significantly decreased the concentrations of Cd in the roots and shoots,compared with those in the CK(Fig.4a).Compared with those of the CK,the concentrations of Cd in the roots decreased by 27.3%,28.3%,37.6%,and 37.0%under the Zn1,Zn2,Mn1,and Mn2 treatments,respectively.The Cd concentrations in the shoots decreased by 42.9%, 56.0%,48.4%,and 54.7%,respectively.Similarly,the accumulation of Cd in whole plants in the late-tillering rice also decreased by 26.3%,38.6%,34.4%,and 44.5%under the Zn1,Zn2,Mn1,and Mn2 treatments,respectively,compared to that in the CK(Fig.4b).
Transportation of Zn,Mn,and Cd in rice
The Zn and Mn treatments also affected the transportation of Zn,Mn,and Cd within the rice(Fig.5).In the late-tillering rice, compared with those of CK, the TFvalues from the roots to shoots decreased by 31.9%—37.0% for Zn under the Zn treatments,and 30.1%—33.8%for Mn under the Mn treatments. The TFvalues of Cd from the roots to shoots significantly decreased by 23.3%,41.3%,18.3%,and 30.0%under the Zn1,Zn2,Mn1,and Mn2 treatments,respectively,compared to those under the CK.
Relative expression of Cd-related genes in seedling roots
After growth in a hydroponic solution containing high levels of Zn or Mn for 30 d,the relative expression levels of two genes(OsNramp5andOsIRT1)associated with Cd uptake by the roots were downregulated to different degrees(Fig. 6). Compared with that of the CK, the relative expression level ofOsNramp5was slightly downregulated by 18.3% under the Mn2 treatment, but no significant differences were observed in the other treatments.ForOsIRT1,the relative expression levels were downregulated by 40.1%,59.3%, 16.0%, and 25.9%under the Zn1, Zn2, Mn1, and Mn2 treatments,respectively,compared to those of the CK.No significant differences were observed in the relative expression levels of the two genes(OsHMA3andOsHMA2)associated with the transport of Cd in the roots.
Yield and Cd concentration of field-grown brown ric e
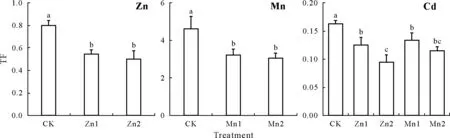
Fig.5 Translocation factor(TF)values of Zn,Mn,and Cd from the roots to shoots(both stem and leaf)of the late-tillering rice(90 d)under different treatments.Bars are standard deviations of means(n=3);bars with the same letter(s)indicate no significant differences between different treatments at P <0.05.CK=the control,seedlings in nutrient solution with no Zn or Mn addition;Zn1 and Zn2=seedlings in nutrient solution with 10 and 50 μmol L-1 Zn,respectively;Mn1 and Mn2=seedlings in nutrient solution with 50 and 250 μmol L-1 Mn,respectively.
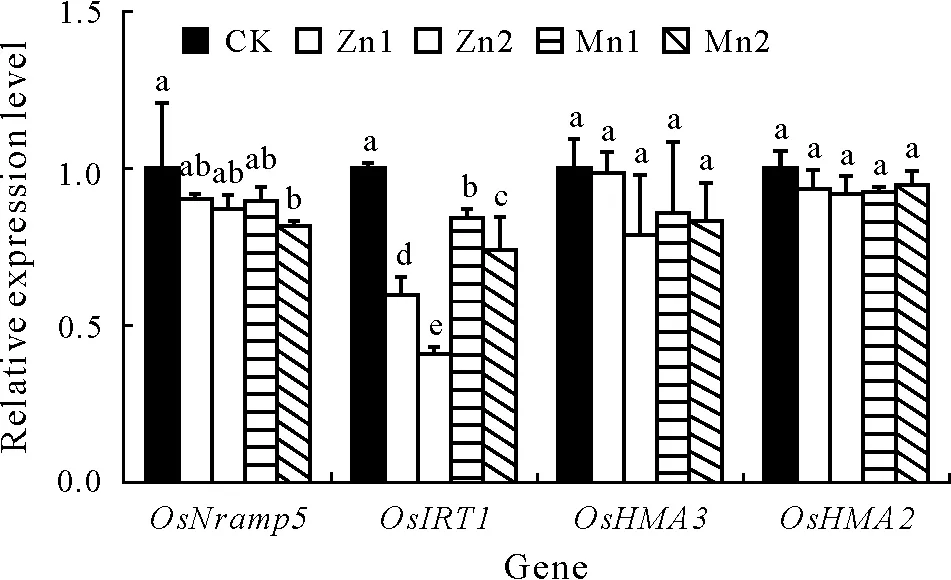
Fig. 6 Relative expression levels of the Cd-related genes, OsNramp5,OsIRT1, OsHMA3, and OsHMA2, in rice seedling roots (30 d) under different treatments.Bars are standard deviations of means(n=3);for the same gene,bars with the same letter(s)indicate no significant differences between different treatments at P <0.05.CK=the control,seedlings in nutrient solution with no Zn or Mn addition; Zn1 and Zn2 = seedlings in nutrient solution with 10 and 50 μmol L-1 Zn,respectively;Mn1 and Mn2 = seedlings in nutrient solution with 50 and 250 μmol L-1 Mn,respectively.
In the two field experiments at the Guixi and Tongling sites,the addition of 50 μmol L-1Zn or 250 μmol L-1Mn to the rice seedlings had no significant impact on rice yield or Zn and Mn concentrations in the brown rice;however,the Cd concentrations in the brown rice significantly decreased compared with the CK and CT (Table I). Compared with the CK,the Cd concentrations in the brown rice decreased by 39.0% and 31.7% under the Zn and Mn treatments,respectively, at the Guixi site, and by 32.4% and 26.5%,respectively,at the Tongling site.Compared with the CT,the concentrations of Cd in the brown rice decreased by 44.4%and 37.8%under the Zn and Mn treatments,respectively,at the Guixi site,and by 39.5%and 34.2%,respectively,at the Tongling site.
DISCUSSION
Seedlings enriched with Zn inhibit the uptake and transport of Cd in rice
As an essential micronutrient,Zn is vital for plant growth,but a deficiency or an excess of Zn is harmful to plant physiological processes and can inhibit photosynthesis or induce oxidative stress(Wang and Jin,2005;Sagardoyet al.,2010;Songet al.,2011).In the present study,the Zn addition levels(10—50 μmol L-1)were significantly lower than those associated with rice toxicity(Songet al.,2011;Chenet al.,2018);thus,no adverse influences occurred on the growth of the rice seedlings.The Kimura B nutrient solution provided a normal level of Zn(0.4 μmol L-1)to the rice seedlings,so the control seedlings were not deficient in Zn.
During the Zn uptake processes within the rice root,Znregulated transporters and Fe-regulated transporter-like proteins,such as OsZIP1,OsZIP3,OsZIP4,OsZIP5,OsIRT1,and OsIRT2,play vital roles(Ramesh,2003;Ishimaruet al.,2006;Yanget al.,2009;Leeet al.,2010).By regulating the expression of these transporters, the uptake and transport of Zn in rice can be regulated. Unfortunately, several Zn transporters,such as OsIRT1 and OsIRT2,also function in the uptake of Cd(Nakanishiet al.,2006;Lee and An,2009).The upregulated expression of OsIRT1, which is induced by Fe deficiency,also enhances the uptake of Cd(Nakanishiet al.,2006).In the current study,the expression level of theOsIRT1gene was downregulated when plants were cultivated with a high level of Zn.This result indicates that a sufficient supply of Zn may inhibit the expression of Znrelated transporters.Subsequently,the uptake of Zn greatly decreased after Zn-rich seedlings were transplanted into the soil.Cd is not essential for rice growth,and the inhibition of the expression ofOsIRT1may have weakened the uptake of the Cd by the rice roots after the Zn-rich seedlings were transplanted(Fig.S1,see Supplementary Material for Fig.S1).In addition,the Zn absorption ability was also weakened in Zn-rich rice,indicating that the high accumulation levels of Zn in seedlings may weaken the rice tissue’s requirements for Zn following transplantation.As a result,the absorption of Cd was subsequently reduced.
Once Cd is taken up into rice roots,its upward transport is vital for its allocation to the aerial parts of the plant,andcertain transporters,such as OsHMA3 and OsHMA2,play an important role in this process(Uenoet al.,2010;Yamajiet al.,2013).OsHMA3 is a tonoplast-localized transporter that functions in the sequestration of Cd to the tonoplast;therefore,the expression of theOsHMA3gene inhibits the transport of Cd in root cells (Uenoet al., 2010). Perhaps because OsHMA3 is a Cd-specialized transporter that is insensitive to other metals(Uenoet al.,2010),enrichment with Zn did not influence the expression of theOsHMA3gene in the seedling roots.Xylem loading is important for the transport of Cd to aerial plant parts;OsHMA2 is a key transporter in this process and also has a function in the xylem loading of Zn(Takahashiet al.,2012).Although Zn enrichment had no effect on the expression ofOsHMA2in seedling roots,the translocation factors of Cd from the roots to shoots significantly decreased.This could be because Zn is an essential nutrient for rice tissue growth,and OsHMA2 preferentially transports Zn to meet rice growth needs(Yamajiet al.,2013).Therefore,Zn enrichment could enhance the competition between Zn and Cd for adsorption sites on OsHMA2 and thus result in the inhibition of the upward transport of Cd(Fig.S1).
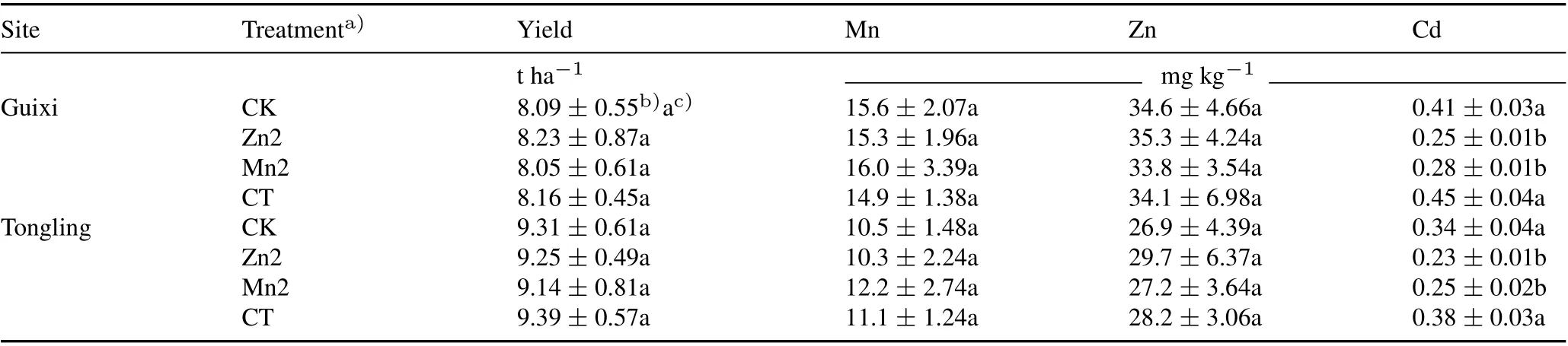
TABLE I Yields and concentrations of Zn,Mn,and Cd in brown rice(unpolished rice grains)of two field experiments in Guixi and Tongling,China
Seedlings enriched with Mn inhibit the uptake and transport of Cd in rice
Similar to Zn, Mn also participates in many plant metabolic processes, such as photosynthesis and the redox process(Shenkeret al.,2004).Both a deficiency in Mn and its overaccumulation are detrimental to plant growth.Previous studies have demonstrated that a Mn level ranging from 0.5 to 500 μmol L-1is safe for rice growth (Sasakiet al.,2011;Chenet al.,2013).Therefore,the Mn addition levels in this study(50 and 250 μmol L-1)had no negative impacts on rice growth.
Rice has a strong tolerance for Mn, and most of the Mn in the roots is transported to the aerial parts of the plant (Tsunemitsuet al., 2018). Therefore, the Mn-rich seedlings accumulated large amounts of Mn from the soil.Nevertheless, their Mn accumulation ability was slightly weakened.The uptake of Mn by rice roots is also controlled by several transporters,such as OsNramp5 and OsMTP9.OsNramp5 mainly functions in the transport of Mn from the rhizosphere to the parenchymal root cells(Sasakiet al.,2012;Uenoet al., 2015). A large amount of Mn accumulation in rice roots following Mn cultivation could thus induce the degradation of the transcribed or translated OsNramp5 and result in the inhibition of Cd uptake(Tsunemitsuet al.,2018).The slightly downregulated expression level of theOsNramp5gene under the Mn2 treatment in this study may also have contributed to the decrease in Cd accumulation in rice(Fig.S1).In addition,Mn cultivation inhibited the expression ofOsIRT1, which is involved in the uptake of Cd by roots(Nakanishiet al.,2006);this could be another reason for the decreased amounts of Cd in the Mn-rich rice.
Although high levels of Mn accumulation in seedling roots did not affect the expression of theOsHMA3orOsHMA2genes,the upward transport of Cd in rice plants was also inhibited by Mn enrichment.In addition toOsHMA3andOsHMA2,the transport of Cd and Mn in the roots is also associated with OsNramp5,which functions in transporting Cd and Mn from the endodermis to the stele and then moving them upwardsviaphloem transport and xylem loading(Shaoet al.,2017b).This same transporter(OsNramp5)in rice roots can induce strong competition between Cd and Mn(Fig.S1,see Supplementary Material for Fig.S1).The high accumulation of Mn in seedling roots could occupy adsorption sites on OsNramp5 in the endodermis,thereby inhibiting the transport of Cd.
Advantages and disadvantages of Zn or Mn-rich seedlings with respect to reducing Cd
Soil and foliar applications are the most commonly used methods for applying Zn and Mn to effectively reduce Cd accumulations in plants(Li Bet al.,2014;Saifullahet al.,2016).The present study innovatively proposes that Zn-or Mn-rich seedlings have the potential to reduce Cd accumulations in rice,with a reduction of approximately 40%.Furthermore, Zn- and Mn-rich seedlings had no negative impact on the subsequent accumulation of Zn or Mn in brown rice or on the rice yield. Enriching seedlings with Zn or Mn is easy to achieve at a low cost,as this method involves little risk to the environment and minimizes the potential for large amounts of Zn or Mn to accumulate in soils.For Cd-contaminated paddy fields,cultivating Zn-or Mn-rich seedlings at the early seedling stage,combined with other technologies(such as lime immobilization)during the period of transplantation,could be an effective method for ensuring the production of safe rice. However, the Zn- or Mn-rich seedlings in the present study were hydroponically cultured;it would be necessary to convert them to a growth matrix or seedbed when applying this method in the field.It is thus important to investigate a suitable method for cultivating Znor Mn-rich seedlings at the early seedling growth stage and to identify the appropriate amounts of Zn or Mn to be applied in this method.In addition,the effects of Zn-and Mn-rich seedlings on reducing the rice Cd in different rice cultivars or soil types are still unclear and need to be investigated,and addition level of Zn or Mn need to be optimized to enhance Cd reduction effect.
CONCLUSIONS
Enriching rice seedlings with Zn or Mn inhibited the expression ofOsIRT1and enhanced competition for Cd absorption and transport in rice roots.Therefore,enriching rice seedlings with Zn or Mn greatly reduced the accumulation of Cd in brown rice;moreover,no negative impacts on the rice yields were observed.The results indicate that enriching seedlings with Zn or Mn is an effective,low-risk method for safe rice cultivation in slightly to moderately Cd-contaminated soils.
ACKNOWLEDGEMENTS
This research was jointly sponsored by the National Key Technology Research and Development Program of China(No. 2015BAD05B04), the Natural Science Foundation of Jiangxi Province, China (No. 20202BAB215016), the Foundation of Jiangxi Educational Committee,China(No.GJJ191707),the Science and Technology Service Network Program of Chinese Academy of Sciences(STS Program),the Regional Soil Pollution Control Program of the Ministry of Agriculture and Rural Affairs of China, and the Agro-Environmental Protection Program of Jiangxi Province,China.
SUPPLEMENTARY MATERIAL
Supplementary material for this article can be found in the online version.
杂志排行
Pedosphere的其它文章
- Letter to the Editor Molecular characterization of an extensively drug-resistant Acinetobacter baumannii isolated from a corn culture soil
- Soil organic matter content and chemical composition under two rotation management systems in a Mediterranean climate
- Impacts of land use and salinization on soil inorganic and organic carbon in the middle-lower Yellow River Delta
- Responses of the methanogenic pathway and fraction of CH4 oxidization in a flooded paddy soil to rice planting
- Effect of biochar applied with plant growth-promoting rhizobacteria(PGPR)on soil microbial community composition and nitrogen utilization in tomato
- Soil chronosequence and biosequence on old lake sediments of the Burdur Lake in Turkey
