Evidence for the role of KIF17 in f ish spermatid reshaping:expression pattern of KIF17 in Larimichthys polyactis spermiogenesis*
2021-12-09JingqianWANGXinmingGAOXuebinZHENGChenDUCongcongHOUQingpingXIEBaoLOUFengLIUShanJINJunquanZHU
Jingqian WANG , Xinming GAO , Xuebin ZHENG , Chen DU , Congcong HOU ,Qingping XIE , Bao LOU , Feng LIU , Shan JIN , Junquan ZHU ,**
1 Key Laboratory of Applied Marine Biotechnology by the Ministry of Education, School of Marine Sciences, Ningbo University,Ningbo 315211, China
2 Zhejiang Academy of Agricultural Sciences, Hangzhou 310021, China
Ab stract The homodimeric kinesin-2 protein KIF17 functions in intracellular transport and spermiogenesis in mammals. However, its role in f ish spermiogenesis has not been reported. Here, we aimed to clone full-length kif17 cDNA and determine the molecular characteristics and expression patterns of KIF17 in Larimichthys polyactis spermiogenesis. The full-length cDNA of L. polyactis kif17 ( Lp- kif17) was sequenced and found to contain a 332-bp 5′ untranslated region, 480-bp 3′ untranslated region, and 2 433-bp open reading frame encoding 810 amino acids. Bioinformatics analyses showed that L. polyactis KIF17( Lp-KIF17) shared high sequence similarity with homologs in other animals and possessed an N-terminal motor domain with microtubule-binding sites and adenosine triphosphate (ATP) hydrolysis sites, a stalk domain containing two coiled-coil regions, and a C-terminal tail domain. The Lp- kif17 mRNA was widely expressed in various tissues, with the highest level in the brain, followed by that in the testis. Fluorescence in situ hybridization (FISH) analysis revealed that Lp- kif17 was continuously expressed in spermiogenesis,showing that it had potential functions in this process. Using immunof luorescence (IF) analysis, we found that Lp-KIF17 colocalized with tubulin and was transferred from the perinuclear cytoplasm to the side of spermatid where the tail forms during spermiogenesis. These f indings suggested that KIF17 is involved in L. polyactis spermiogenesis. In particular, it may participate in nuclear shaping and tail formation by interacting with perinuclear microtubules during spermatid reshaping. In addition to providing evidence for the role of KIF17 in f ish spermatid reshaping, this study provides important data for studies of reproductive biology in L. polyactis.
Keyword: KIF17; spermiogenesis; Larimichthys polyactis; spermatid reshaping
1 INTRODUCTION
Spermiogenesis, or the post-meiotic development of spermatogenic cells, is a highly complex but orderly process. During spermiogenesis, spermatid reshaping occurs, in which round spermatids form streamlined sperm by a series of changes, including acrosome and tail formation, nuclear reshaping, and the disposal of extra cytoplasm (Wong-Riley and Besharse, 2012; Ma et al., 2017). Disruptions in spermatid reshaping result in poor sperm quality and even male infertility (Yan, 2009). Therefore, the molecular mechanisms underlining spermatid reshaping are crucial for understanding spermiogenesis and a focus of reproductive biology research.
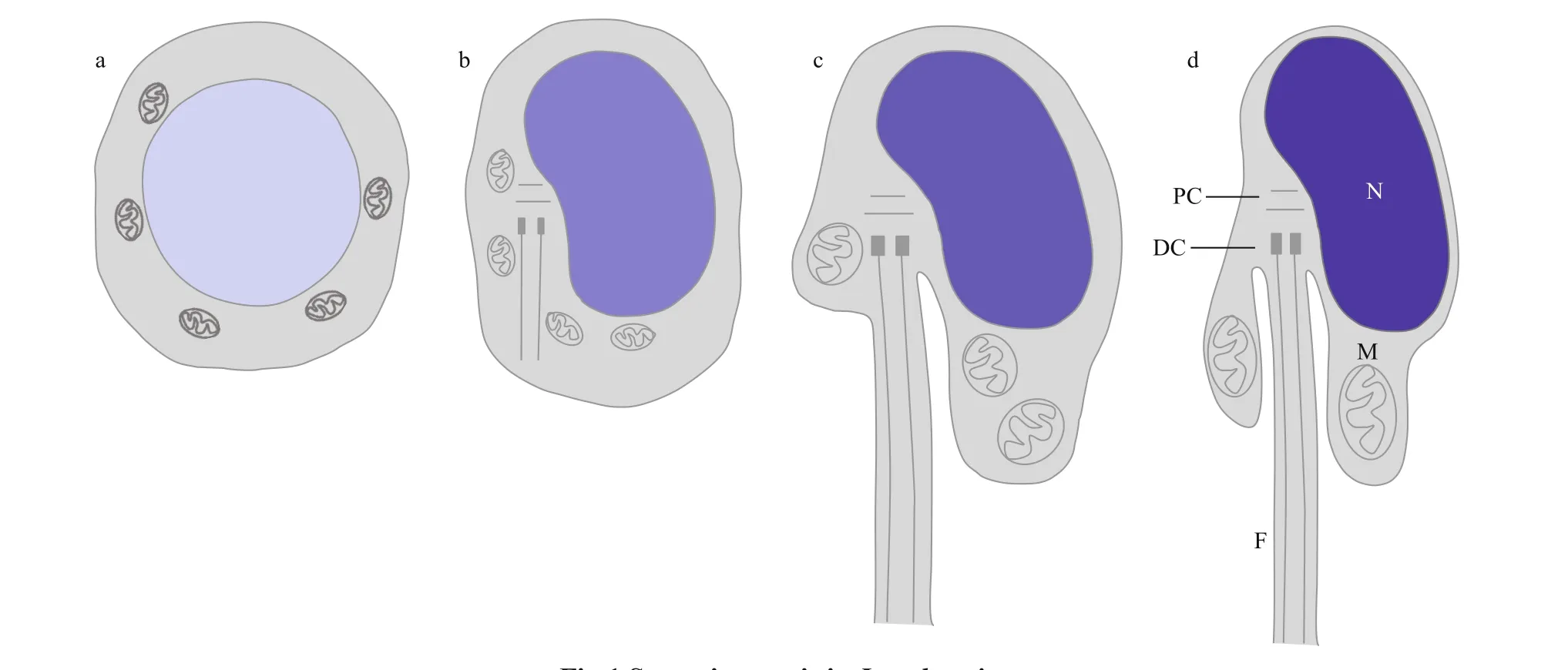
Fig.1 Spermiogenesis in L. polyactis
Kinesins are a class of microtubule-dependent motor proteins (Hirokawa et al., 2009) with important roles in spermatid reshaping. Kinesins are highly conserved during evolution and transport many diff erent cargos along the microtubule to the acrosome, the sperm tail, and the midpiece during spermiogenesis (Olson and Winfrey, 1990; Lehti et al., 2013; Ma et al., 2017). They may interact with the manchette, a transient perinuclear microtubule structure involved in spermatid nuclear shaping and sperm tail formation (Kierszenbaum, 2001; Lehti and Sironen, 2016; Ma et al., 2017).
The homodimeric KIF17, and the heterotrimeric kinesin-II complexes, namely KIF3A/3B/KAP3 and KIF3A/3C/KAP3, constitute the kinesin-2 family in vertebrates (Silverman and Leroux, 2009).Homodimeric KIF17 is formed by the dimerization of identical polypeptide chains, both containing a pair of N-terminal head motor domains, a stalk domain containing two coiled-coil regions (CC1-CC2), and a C-terminal tail domain (Grüter et al., 1998; Miki et al., 2005; MacAskill et al., 2009). KIF17 mainly functions in the transport of diff erent cargos in an adenosine triphosphate (ATP)-dependent manner toward the plus ends of microtubules to the tips of dendrites, cilia, or outer segments of photoreceptor cells (Setou et al., 2000; Chu et al., 2006; Kayadjanian et al., 2007; Takano et al., 2007; Insinna et al., 2009).In mice and rats, KIF17 is highly expressed in male germ cells and is localized in the manchette and the portion of the sperm tail implicated in spermiogenesis(Setou et al., 2000; Kierszenbaum, 2002; Macho et al., 2002; Chennathukuzhi et al., 2003; Kierszenbaum and Tres, 2004; Kimmins et al., 2004). Therefore,KIF17 probably contributes to nuclear shaping and tail formation by interacting with the manchette in mammals. However, the function of KIF17 in f ish spermiogenesis has not been reported.
Larimichthyspolyactis, a benthopelagic f ish species in the family Sciaenidae, is an important economic f ishery resource (Zhang et al., 2016).Therefore, studies of spermiogenesis inL.polyactisare crucial to obtain a theoretical basis for its breeding and culture. Kang et al. (2013) and Wang et al. (2019)examined the ultrastructural characteristics of spermiogenesis inL.polyactis. In early spermatid, the nucleus is oval or round, and the mitochondria randomly distributed in the perinuclear cytoplasm. In middle spermatid, the nucleus is round, and the nuclear chromatin is condensed into clumps. The mitochondria fuse and migrate to one side of the nucleus, where the sperm midpiece forms. At this time, the f lagellum extends from the implantation fossa. In late spermatid, the nucleus is kidney-shaped,the nuclear chromatin is more condensed, the residual cytoplasm migrates gradually to the bottom of the implantation fossa, and the f lagellum extends gradually. In mature sperm, the nuclear chromatin is highly condensed, and mitochondria are typically found in the sperm midpiece. Overall,L.polyactisspermiogenesis involves nuclear reshaping, tail formation, and the removal of excess cytoplasm(Fig.1). These cytological changes of the spermatids duringL.polyactisspermiogenesis are similar to those in many f ish species (Zhang et al., 2017; Zhao et al., 2017). However, it is not clear that the molecular mechanism underlying spermatid reshaping during spermiogenesis inL.polyactisand in other taxa.
To investigate the functions of KIF17 in spermatid reshaping duringL.polyactisspermiogenesis, we performed cDNA cloning and analyzed the tissue distribution and expression ofLp-kif17, as well as the colocalization ofLp-KIF17 and microtubules. Our results provide evidence thatLp-KIF17 is involved in spermatid reshaping and, in particular, may promote nuclear reshaping and tail formation during spermiogenesis.
2 MATERIAL AND METHOD
2.1 Tissue sampling
The maleL. polyactisindividuals were sampled from Zhoushan (Zhejiang, China). Seven tissues (the testis (II-V stage), intestine, heart, brain, muscle,liver, and kidney) were extracted and stored at -80 ℃.Experiments were approved by the Animal Ethics Committee of Ningbo University and strictly enforced according to the Experimental Animal Management Law of China.
2.2 RNA extraction and reverse transcription
Total tissue RNA was extracted from each tissue type ofL.polyactisusing the TRIzol Reagent(Invitrogen, San Diego, CA, USA). Reverse transcription for real-time quantitative PCR (qPCR)was performed using the PrimeScript®RT Reagent Kit (TaKaRa, Dalian, China). the synthesis of f irststrand cDNA of 5′ Rapid Amplif ication of cDNA Ends (RACE) and 3′ RACE were performed using the Smart RACE cDNA Amplif ication Kit (Clontech,Mountain View, CA, USA) and 3′ Full RACE Amplif ication Kit (TaKaRa, Kusatsu, Japan),respectively. All cDNAs were stored at -20 °C.
2.3 Cloning of full-length kif17 cDNA
The open reading frame (ORF) ofLp-kif17was obtained from the full-length genome(ASM1011929v1). The specif ic primers (Table 1) for 5′ RACE and 3′ RACE were designed base on the ORF sequence ofkif17cDNA. The cDNA products were separated, and the target bands were extracted,purif ied, ligated, transformed and ultimately sequenced using GENEWIZ (Suzhou, China).
2.4 Bioinformatics analysis of KIF17
TheLp-KIF17 sequence was predicted using the Sequence Manipulation Suite (http://www.bio-soft.net/sms/index.html). Multiple alignments ofLp-KIF17 amino acid sequences were generated using Vector NTI10. A phylogenetic tree of KIF17 was constructed by the neighbor-joining method using MEGA 5.0. The functional domains and tertiary structure of KIF17 proteins were predicted using the ProtParam tool (http://web.expasy.org/protparam/)and I-TASSER (http://zhanglab.ccmb.med.umich.edu/I-TASSER/), respectively.
2.5 Quantitative analysis of Lp- kif17 mRNA expression
The mRNA expression levels ofkif17in the heart,intestine, muscle, liver, brain, kidney, and testis (II-V)were measured by qPCR, as described previously(Zhao et al., 2018).Lp-kif17-specific primers (Table 1)were designed using Primer Premier 5 and synthesized by GENEWIZ (Suzhou, China). The β-actin (Actb)gene was chosen as a positive control. The relative mRNA levels ofLp-kif17were analyzed using the 2-ΔΔCtmethod. Statistical analyses performed with SPSS 16.0 were using one-way analysis of variance(ANOVA) followed by the Duncan’s multiple comparison, whereP<0.05 indicated a signif icant diff erence.
2.6 Fluorescence in situ hybridization (FISH)
The IV stage testes of maleL.polyactiswereextracted, and frozen sections were produced and preserved as described previously (Zhao et al., 2017).Thekif17probe (Table 1) was synthesized by GENEWIZ (Suzhou, China) and was conjugated to f luorescein with f luorescein isothiocyanate (FITC);Probe specif icity was evaluated using the BLAST.The experiment was conducted based on the methods of Zhao et al. (2018). The f luorescence signal were observed and photographed using a confocal laserscanning microscope (LSM880; Carl Zeiss,Oberkochen, Germany).
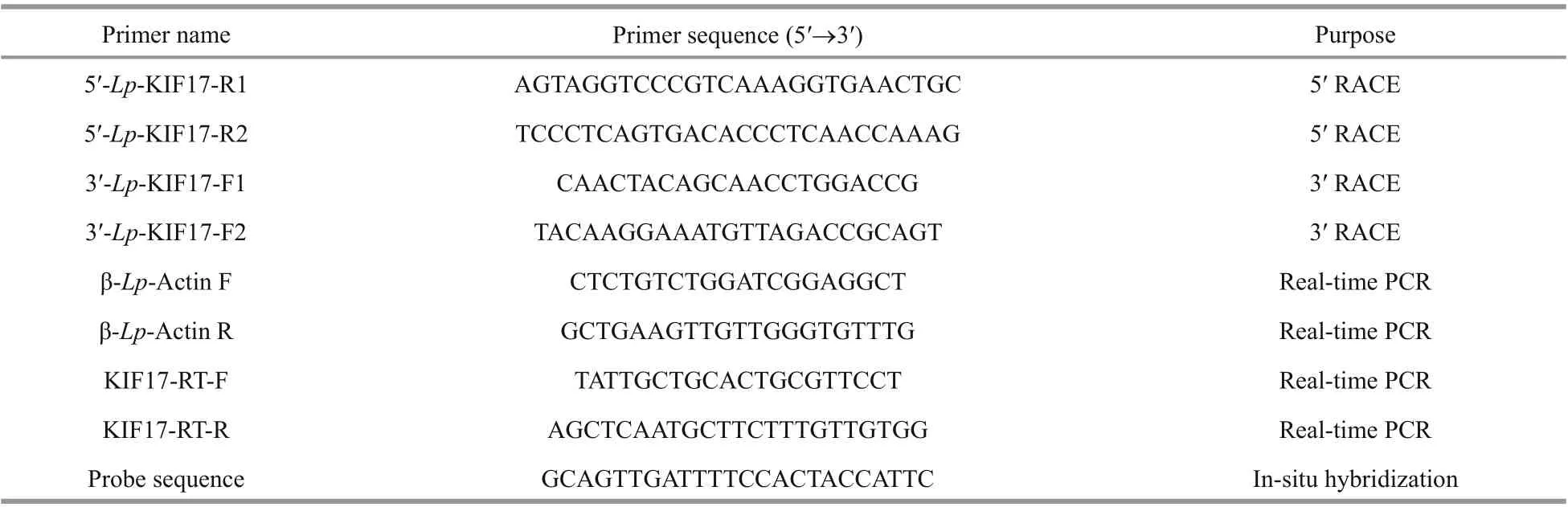
Table 1 Primers and probe sequences used in this study
2.7 Antibodies
A mouse anti-Lp-KIF17 antibody was prepared as reported by Gao et al. (2019). The sequence encoding the tail domain of KIF17 (aa 567-725) was amplif ied,and the polypeptide was approximately 17.6 kDa. The cDNA sequence was inserted into the pEASY-Blunt E2 vector (TransGen, Beijing, China) to construct the prokaryotic expression vector. The primers Anti-KIF17-F is GACACCAGGCAGAGGAAGAAC and the primers Anti-KIF17-R is TCTGTTGATGTTGGGGATGCA. The constructed pEASY-Blunt E2-KIF17 plasmid was transformed into Transetta (DE3)competent cells. The transformed cells were incubated until the OD600reached 0.6, and the expression of KIF17 was induced by isopropyl-β-dthiogalactopyranoside (IPTG) at a f inal concentration of 1 mmol/L. The recombinant protein KIF17 was purif ied using the His-tag Protein Purif ication Kit(Beyotime, Shanghai, China) following the manufacturer’s instructions. Finally, the KIF17 recombination protein was used for ICR mouse immunization to produce the antiserum according to Gao et al. (2019).
2.8 Western blotting
The quality of the mouse anti-Lp-KIF17 antibody was tested by western blot analysis, following a previously described method (Zhao et al., 2018).HRP-conjugated goat anti-mouse IgG (H+L)(Beyotime) was used as the secondary antibody.Protein bands were visualized by chemiluminescence imaging (Tanon 5200; Shanghai, China).
2.9 Immunofluorescence staining
The IF experiment was conducted according to Wang et al. (2019). The frozen tissue sections were incubated with a mouse anti-KIF17 antibody (diluted 1꞉70) and rabbit anti-tubulin antibody (diluted 1꞉100;Beyotime) at 4 °C overnight. Then the sections were incubated with Alexa Fluor 555-labeled Goat Anti-Mouse IgG (H+L) (Beyotime) and Alexa Fluor 488-labeled Donkey Anti-Rabbit IgG (H+L)(Beyotime), for 1 h at 37 °C. The immunostained tissues sections were mounted and observed using a confocal laser-scanning microscope. The control group was processed without the addition of the primary antibody.
3 RESULT
3.1 Characterization of the full-length cDNA of Lp- kif17 and the Lp-KIF17 protein
The clonedkif17cDNA had a 332-bp 5′ untranslated region (UTR), 480-bp 3′ UTR, and 2 433-bp ORF encoding 810 amino acids (aa) (Fig.2). The predicted molecular weight ofLp-KIF17 protein was 90.3 kDa.The deducedLp-KIF17 protein possessed three domains: a motor domain (3-343 aa), a stalk domain(344-652 aa) containing two coiled-coil regions(398-439 aa/ 615-653 aa), and a tail domain (654-810 aa) (Fig.3b). Furthermore, the tertiary structure ofLp-KIF17 was predicted (Fig.3c), including a motor domain (Fig.3f), a stalk domain containing two coiled-coil regions (Fig.3g), and a tail domain(Fig.3h). As in other kinesins,Lp-KIF17 had putative ATP hydrolysis sites (Fig.3d) and microtubulebinding motifs (Fig.3e) in the motor domain. A model of the putativeLp-KIF17 homodimeric complex is depicted in Fig.3i.
We aligned theLp-KIF17 amino acid sequence with sequences of homologs in other species (Fig.3).The sequence identities between theLp-KIF17 sequence and sequences of homologs inMusmusculus,Notechisscutatus,Xenopuslaevis,Daniorerio,Carassiusauratus,Larimichthyscrocea, andPomaceacanaliculatawere 52.8%, 56.7%, 55.6%,66.2%, 67.1%, 94.4%, and 74.7%, respectively. As shown in Fig.4, a phylogenetic analysis showed thatLp-KIF17 formed a group with orthologues in other teleosts, distinct from other kinesin-2 family clusters.Moreover,Lp-KIF17 was most closely related to the KIF17 ofL.crocea.
3.2 Quantitative analysis of Lp- kif17 mRNA
As determined by qPCR,Lp-kif17expression showed a wide distribution in all tested tissues, with high expression level in the brain and testis and low expression level in other tissue types (Fig.5a). We further evaluatedLp-kif17expression during II-V stages of testis development (Fig.5b). The mRNA expression ofLp-kif17increased gradually, peaking at stage IV, and then decreased dramatically at stage V.
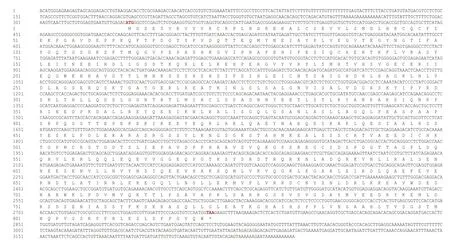
Fig.2 Full-length cDNA of Lp- kif17 and the deduced amino acid sequence

Fig.3 Multiple alignments and structure prediction of Lp-KIF17
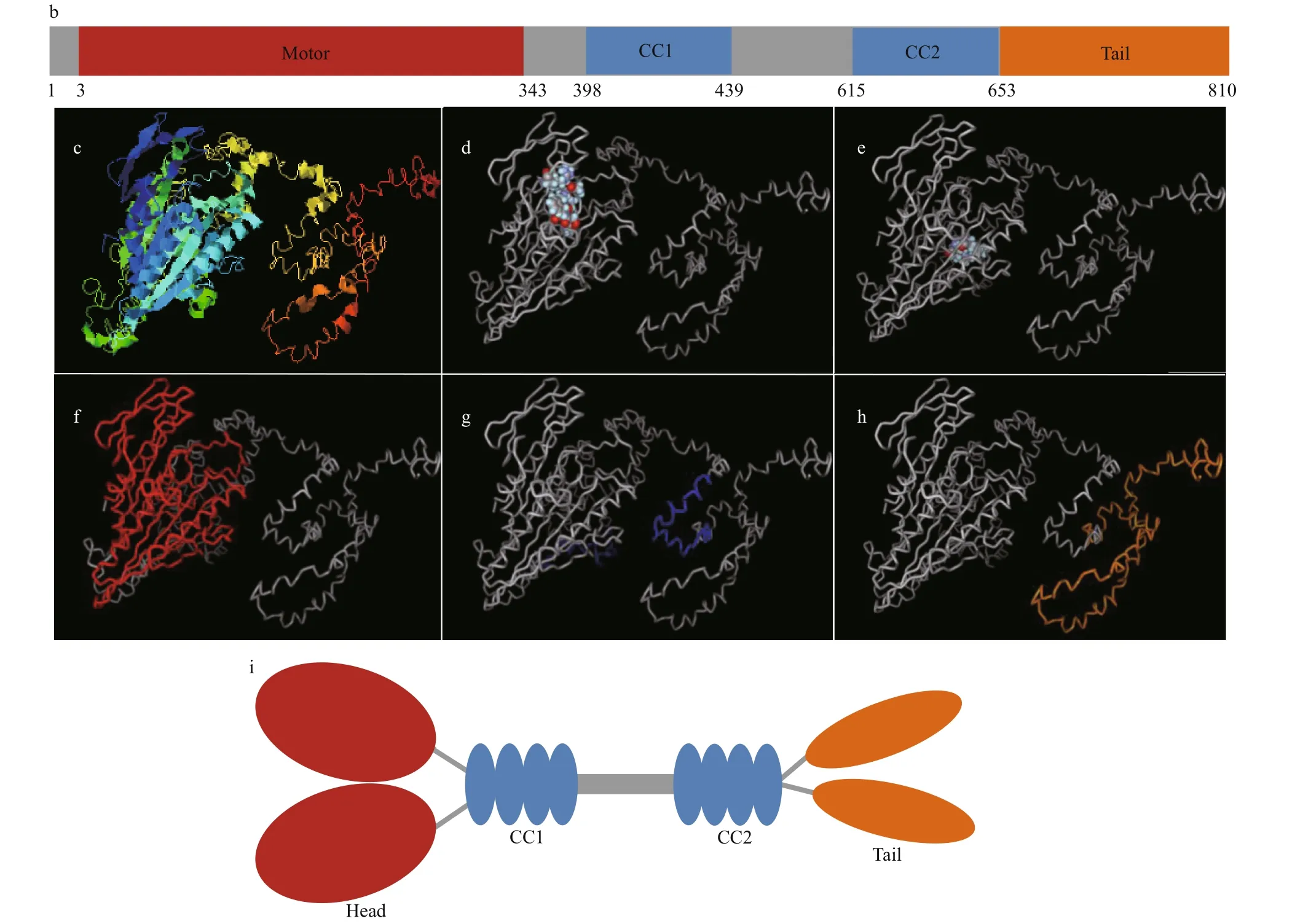
Fig.3 Continued
3.3 Spatiotemporal expression of Lp- kif17 mRNA during spermiogenesis
The expression and distribution ofLp-kif17were evaluated by FISH (Fig.6). In early spermatid, theLpkif17mRNA signals were evenly distributed in the perinuclear cytoplasm (Fig.6a1-a4). During middle spermatid, the signals were enhanced and were concentrated around the nucleus (Fig.6b1-b4). In late spermatid, the signals mainly accumulated on one side of the nucleus where the tail forms (Fig.6c1-c4).In mature sperm, mRNA was detected at the midpiece of sperm (Fig.6d1-d4).
3.4 Expression and purif ication of Lp-KIF17
Lp-KIF17 was successfully expressed inEscherichia coliTransetta (DE3) (Fig.7a). A clear target band was found in induced cells (line 3) but not in un-induced cells (line 2) by sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE).The purif ied recombinant protein showed a single band (line 4) with an approximate molecular mass of 17.6 kDa. Finally, the recombination proteinLp-KIF17 was used for mouse immunization, and the antiserum was extracted as a mouse anti-KIF17 polyclonal antibody againstL.polyactis. Western blotting was used to evaluate the specif icity of this antibody. A ~90-kDa protein band was only detected(Fig.7b), consistent with the predicted molecular weight ofLp-KIF17. These results suggest that the mouse anti-KIF17 polyclonal antibody could be used for subsequent IF assays.
3.5 Expression and colocalization of Lp-KIF17 with tubulin during spermiogenesis
As determined by immunof luorescence,Lp-KIF17 was expressed and colocalized with microtubules during spermiogenesis inL.polyactis. In early spermatid,Lp-KIF17 and tubulin were evenly distributed in the cytoplasm (Fig.8a1-a4). In middle spermatid, the localization patterns ofLp-KIF17 and tubulin signals were similar to those of early spermatid and accumulated around the nucleus (Fig.8b1-b4). In late spermatid, the signals migrated to one side of the nucleus where sperm tail forms (Fig.8c1-c4). In mature sperm,Lp-KIF17 and tubulin were mainly detected in the midpiece of the sperm (Fig.8d1-d4).No signals were detected in negative control group(data not shown).
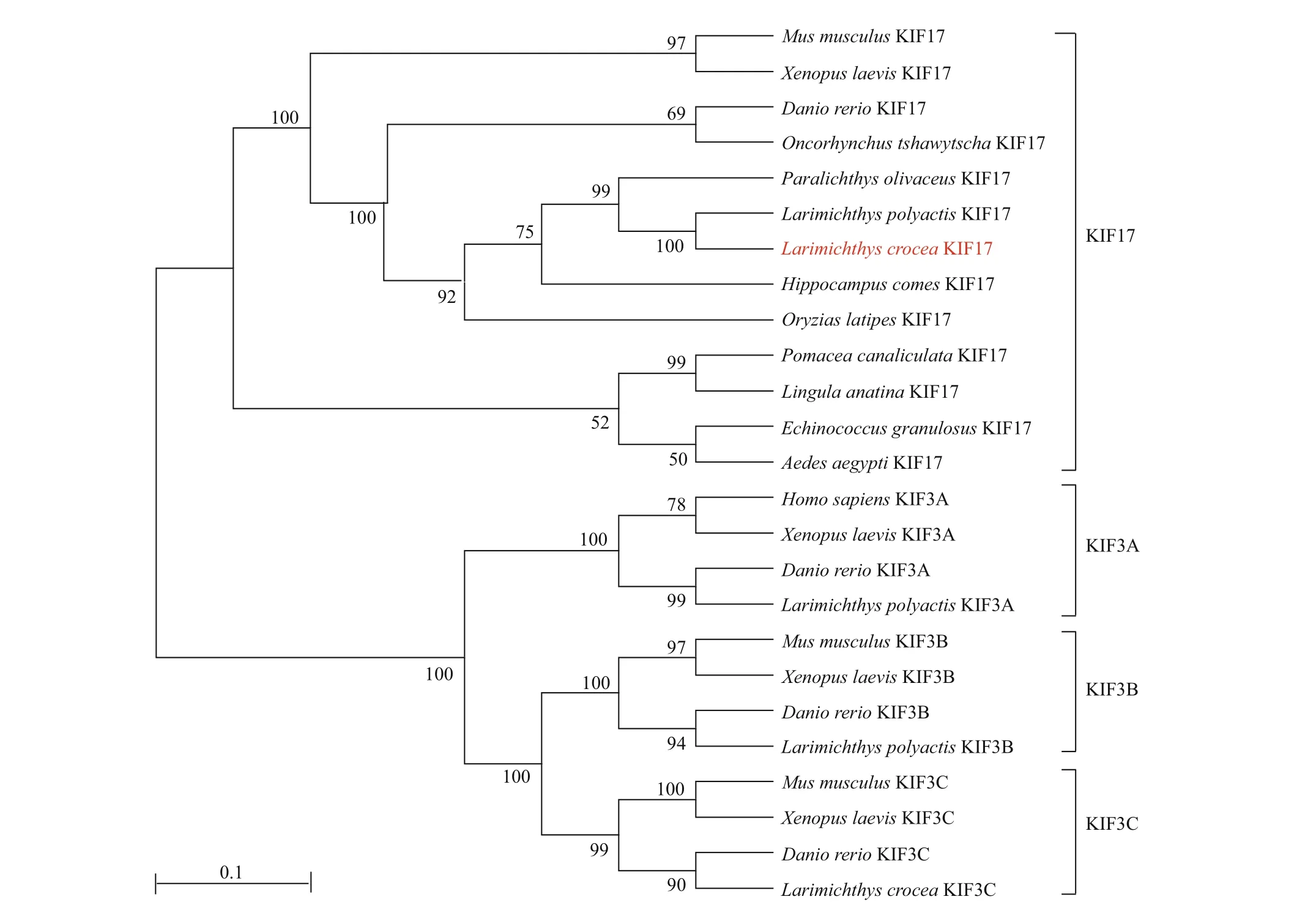
Fig.4 Phylogenetic tree of KIF17 and 25 available kinesin-2 proteins
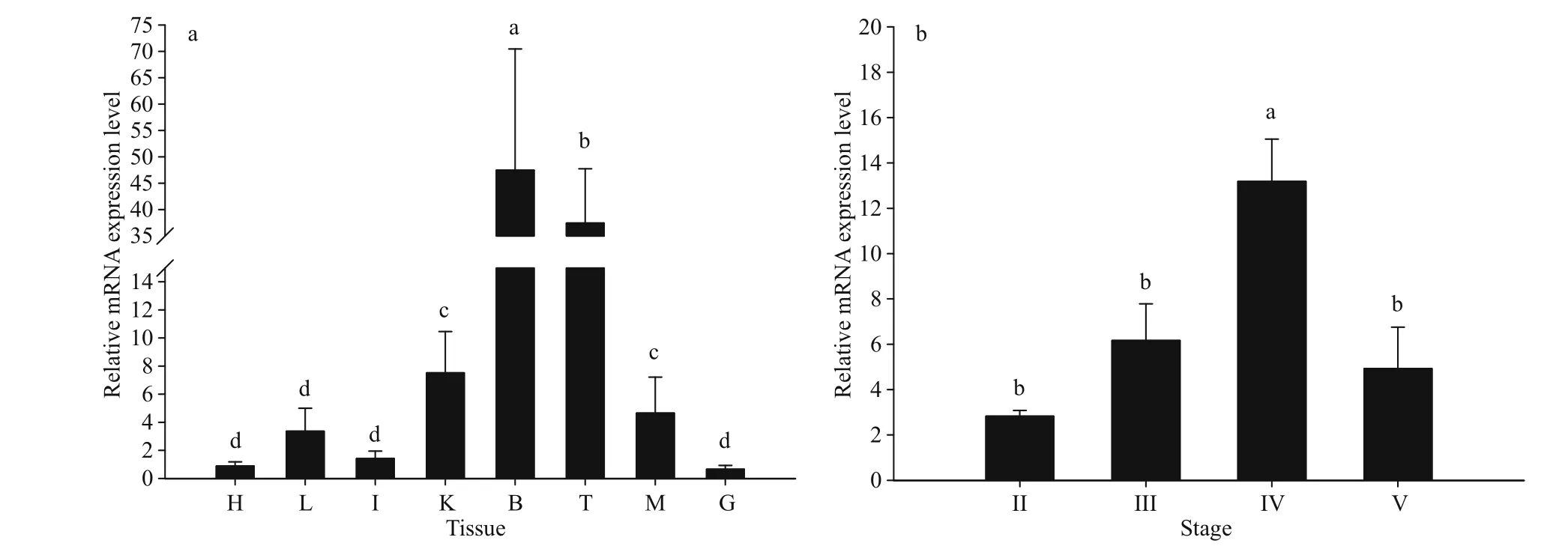
Fig.5 Quantitative analysis of Lp- kif17 mRNA

Fig.6 Fluorescence in- situ hybridization results of Lp- kif17 mRNA during spermiogenesis in L. polyactis
4 DISCUSSION
KIF17 is a homodimeric motor protein belonging to the kinesin-2 family (Silverman and Leroux, 2009).The KIF17 domain structure is well-characterized in mammals (Yamazaki et al., 1995; Miki et al., 2005;MacAskill et al., 2009). The motor domain functions in movement along microtubules by binding to microtubules and hydrolyzing ATP. The tail domain is a highly variable domain that binds to various cargos.The stalk region, containing two coiled-coil domains(CC1-CC2), is a regulatory region controller (Milic et al., 2017). The structural characteristics of KIF17 were similar to those of other kinesin-2 family members (KIF3A, KIF3B, and KIF3C) and N-terminal kinesins. However, the stalk domains of KIF3A,KIF3B, KIF3C, and other kinesins usually have a single coiled-coil domain, whereas KIF17 has two coiled-coil domains. The special structural features of the stalk domain of KIF17 may be related to its autoinhibition by folding around its coiled-coil domain to suppress motility in the absence of cargo (Imanishi et al., 2006; Hammond et al., 2010). In this study, a fulllength cDNA sequence was obtained forkif17from the testis ofL.polyactis. The predictedLp-KIF17 protein possessed all of the main structural features of previously-reported KIF17, including an N-terminal head motor domains containing microtubule-binding and ATP hydrolysis sites, a stalk domain containing two coiled-coil regions (CC1-CC2), and a C-terminal tail domain. Multiple sequence alignment of KIF17 proteins in various taxa illustrated thatLp-KIF17 is relatively structurally conserved and that its motor domain is more highly conserved than its non-motor domains. The high conservation of the motor domain may ref lect its involvement in ATP hydrolysis and microtubule-binding; the C-terminal domain, on the contrary, is involved in interaction with various cargos(Miki et al., 2005). The structural conservatism ofLp-KIF17 suggests that its function was conserved during evolution.
InL.polyactis,kif17mRNA levels were highest in the brain, followed by the testis, and were low in other tissues. This expression pattern is consistent with previous results (Nakagawa et al., 1997; Setou et al.,2000). KIF17 in the brain is likely to function in the transport of neuronal proteins to dendrites in a microtubule-dependent manner. In the mouse, high KIF17 expression in the central nervous system may be related to the transport ofN-methyl-d-aspartate receptor NR2B subunit, potassium Kv4.2 channels,and kainate receptor GluR5 from cell bodies to dendrites (Setou et al., 2000; Chu et al., 2006;Kayadjanian et al., 2007). InCaenorhabditiselegans,Osm-3 (a homolog of mammalian KIF17) shows highly specif ic expression in neurons and is thought to contribute to dendritic transport (Tabish et al.,1995). Hence,Lp-kif17in the brain may be related to dendritic transport. In addition, high KIF17 expression in the testis may be related to spermiogenesis (Wong-Riley and Besharse, 2012; Ma et al., 2017). In mice,KIF17 is highly expressed in the testis and may participate in spermiogenesis by interacting with the manchette to promote nuclear reshaping and tail formation (Saade et al., 2007). Similarly, the high expression ofkif17in the testis suggests that KIF17 is involved inL.polyactisspermiogenesis. In this study,we further determined thatkif17expression in the testis is highest at stage IV. In this stage, the testis ofL.polyactiscontains abundant spermatids, which undergo morphological transformation into spermatozoa by spermiogenesis (Kang et al., 2013).Therefore, we speculate thatLp-kif17in the testis may be related to spermiogenesis. To conf irm our conjecture, we tracked the spatiotemporal expression pattern ofLp-kif17mRNA by FISH during spermiogenesis. TheLp-kif17mRNA signals were continuously detected throughout spermiogenesis.Overall, these results suggested thatkif17is involved inL.polyactisspermiogenesis.
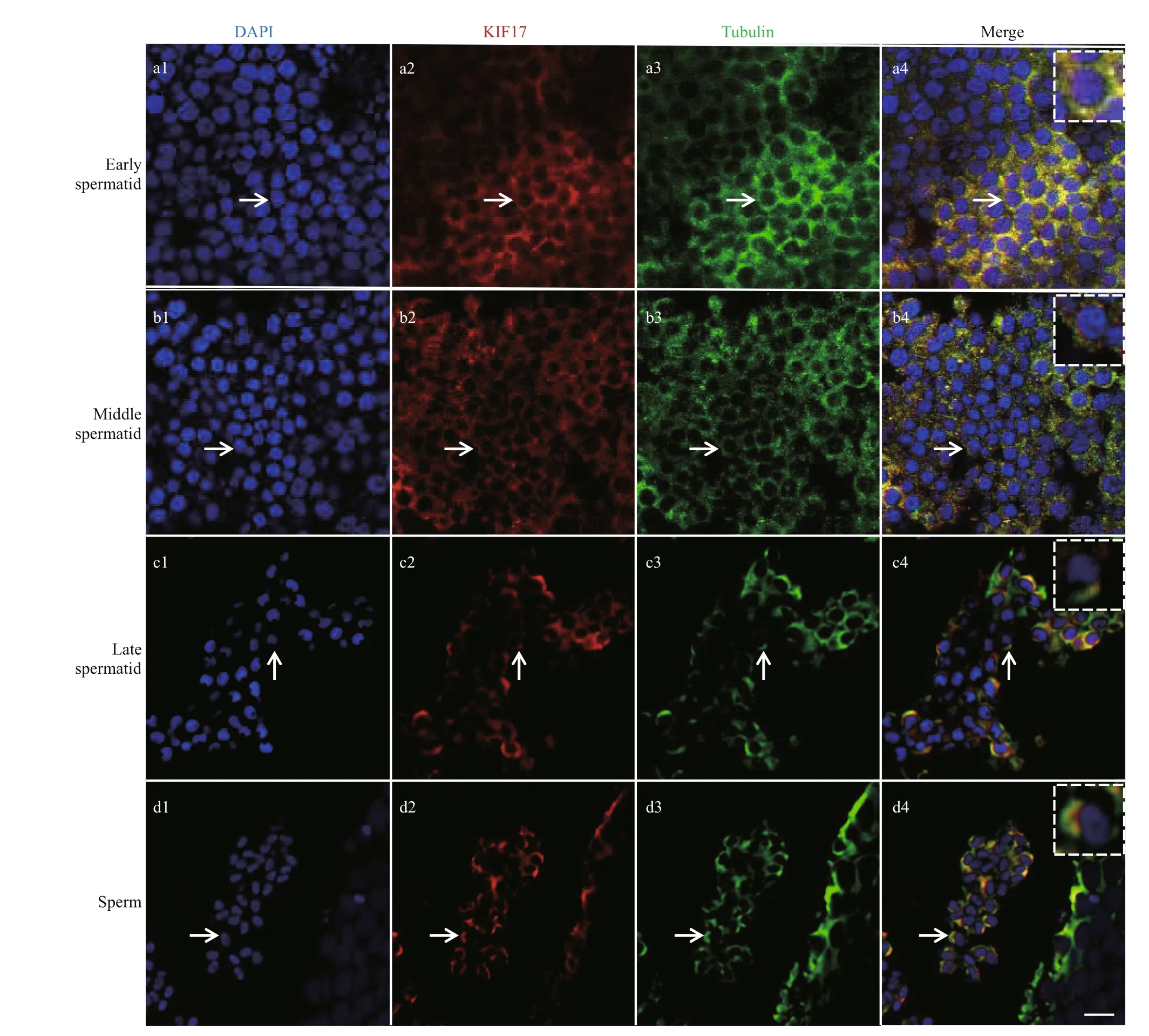
Fig.8 Immunof luorescence localization of Lp-KIF17 and tubulin during spermiogenesis in L. polyactis
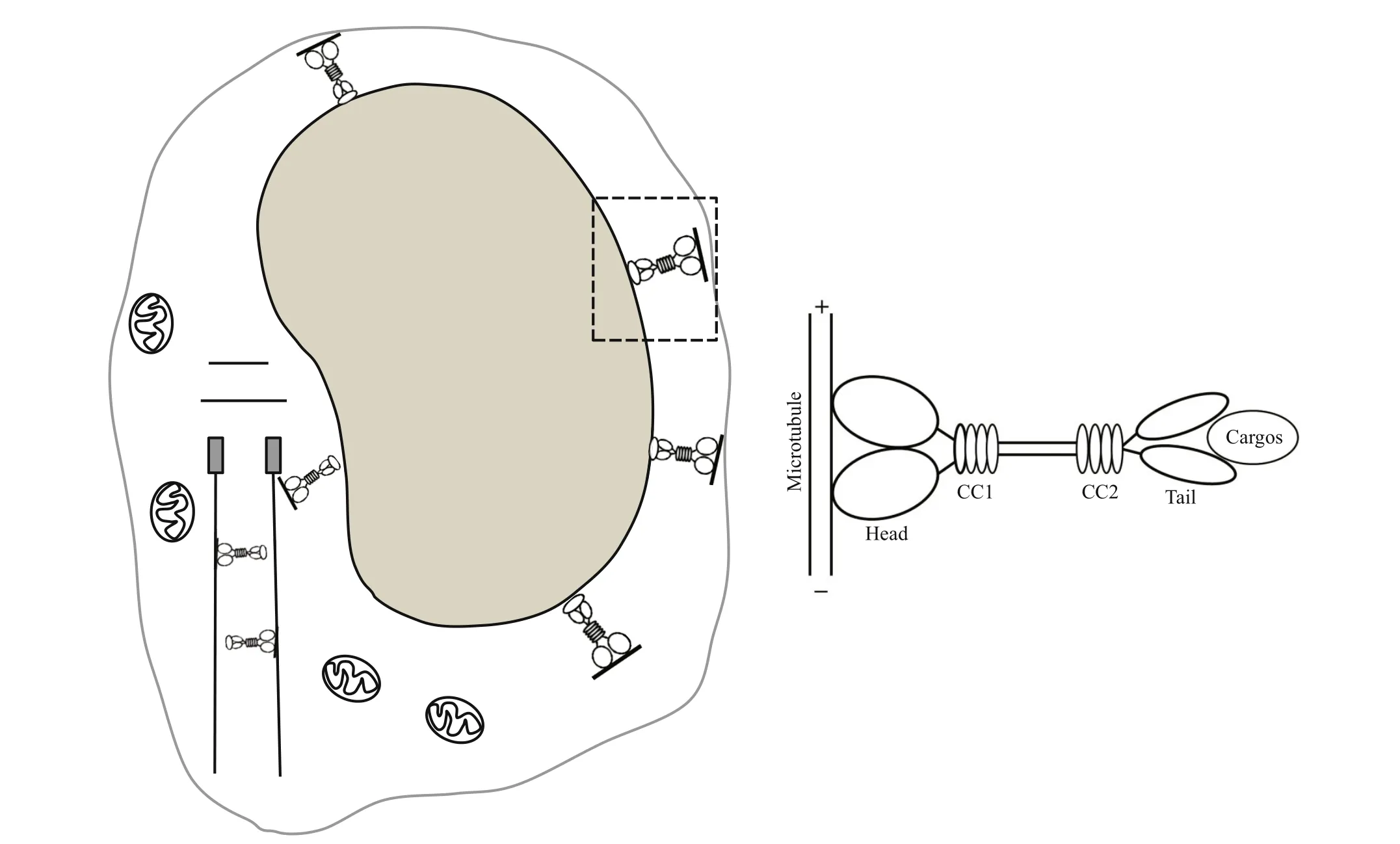
Fig.9 Overview potential function of Lp-KIF17 during spermiogenesis
During spermatid reshaping, the nucleus undergoes striking chromosome condensation and compression,eventually leading to nuclear elongation and tail formation (Ma et al., 2017). During spermiogenesis, a transient perinuclear microtubule structure, called the manchette, appears when the round nucleus begins to elongate and gradually moves toward the posterior half of the spermatid to elongate the nucleus by the formation of a connection between the microtubule and the nuclear skeleton. This structure disappears when the elongation and condensation of the spermatid nucleus are near completion (Kierszenbaum,2002). Previous studies have shown that the manchette plays a signif icant role in spermatid nuclear shaping and tail formation by sorting structural and functional molecules during nuclear shaping and sperm tail development (Kierszenbaum, 2002; Kierszenbaum and Tres, 2004). In azh (abnormal sperm head shape)mouse mutants, the abnormal position of the manchette is correlated with an abnormally-shaped spermatid nucleus and multiple coiling of the sperm tail (Mochida et al., 1999). The manchette displays complex motility via the activity of several kinesins.For instance, KIF3A knockout mice show abnormally manchettes and typical malformed heads, suggesting that kinesin KIF3A participates in spermatid reshaping by interacting with the manchette (Lehti et al., 2013).In mice, KIF17 protein signals are colocalized with the manchette, further supporting the relationship between KIF17 and the manchette (Saade et al.,2007). In our immunof luorescence analysis ofL.polyactis, tubulin signals were evenly distributed in the perinuclear cytoplasm in the early spermatid stage, primarily distributed around the nucleus in the middle spermatid stage, and distributed on the side where the midpiece forms in the late spermatid stage.In mature spermatozoa, the signals of tubulin were mainly detected in the midpiece of the sperm. The localization pattern of tubulin suggests thatL.polyactismay have a perinuclear microtubule structure, similar to the manchette, involved in spermatid nuclear shaping. We further found thatLp-KIF17 colocalized with tubulin during early spermatid development into mature sperm, and the signals shifted from the perinuclear cytoplasm to one side where the midpiece forms, suggesting thatLp-KIF17 participates in spermatid nuclear shaping and tail formation during spermatid reshaping by interacting with perinuclear microtubules. Based on these f indings, models ofLp-KIF17 involved during spermiogenesis is prosed (Fig.9).
5 CONCLUSION
In this study, we cloned theLp-kif17cDNA and characterized the expression patterns and potential functions of KIF17 duringL.polyactisspermiogenesis.Lp-kif17was widely expressed in most tissues, with the highest level in brain, followed by that in the testis. Furthermore,kif17was continuously detected during spermiogenesis, suggesting that it plays a key role in this process.Lp-KIF17 colocalized with tubulin at all stages, and the signals moved from the perinuclear cytoplasm to the side of tail formation.These f indings suggest that KIF17 may be involved inL.polyactisspermiogenesis and may specif ically participate in nuclear shaping and tail formation by interacting with perinuclear microtubules during spermatid reshaping. Based on these f indings, models ofLp-KIF17 involved during spermiogenesis is prosed. However, detailed function of KIF17 inL.polyactisspermiogenesis requires further study.
6 DATA AVAILABILITY STATEMENT
The data that support the f indings of this study are available from the corresponding author upon reasonable request.
杂志排行
Journal of Oceanology and Limnology的其它文章
- MamZ protein plays an essential role in magnetosome maturation process of Magnetospirillum gryphiswaldense MSR-1*
- Maximum sustainable yield estimation of enhancement species with the characteristics of movement inside and outside marine ranching*
- Neglectella glomerata sp. nov., a new species and implications for the systematics of the genus Neglectella (Oocystaceae,Trebouxiophyceae, Chlorophyta)*
- A new species Lobophora tsengii sp. nov. (Dictyotales;Phaeophyceae) from Bach Long Vy (Bailongwei) Island,Vietnam*
- Transcriptome prof ile of Dunaliella salina in Yuncheng Salt Lake reveals salt-stress-related genes under diff erent salinity stresses*
- Characterization and diversity of magnetotactic bacteria from sediments of Caroline Seamount in the Western Pacif ic Ocean*
