Characterization and diversity of magnetotactic bacteria from sediments of Caroline Seamount in the Western Pacif ic Ocean*
2021-12-09KaixuanCUIWenyanZHANGJiaLIUCongXUYicongZHAOSiCHENHongmiaoPANTianXIAOLongFeiWU
Kaixuan CUI , Wenyan ZHANG , Jia LIU , Cong XU , Yicong ZHAO ,Si CHEN , Hongmiao PAN ,**, Tian XIAO ,**, Long-Fei WU
1 CAS Key Laboratory of Marine Ecology and Environmental Sciences, Institute of Oceanology, Chinese Academy of Sciences,Qingdao 266071, China
2 Laboratory for Marine Ecology and Environmental Science, Pilot National Laboratory for Marine Science and Technology(Qingdao), Qingdao 266237, China
3 University of Chinese Academy of Sciences, Beijing 100049, China
4 Center for Ocean Mega-Science, Chinese Academy of Sciences, Qingdao 266071, China
5 International Associated Laboratory of Evolution and Development of Magnetotactic Multicellular Organisms (LIA-MagMC),CNRS-CAS, Qingdao 266071, China
6 Aix Marseille Univ, CNRS, LCB, IM2B, IMM, Marseille 13009, France
7 National Oceanographic Center, Qingdao 266071, China
Abstract Magnetotactic bacteria (MTB) are a group of microorganisms capable of orientating and swimming along magnetic f ields because they contain intracellular biomineralized magnetosomes composed of magnetite (Fe 3 O 4) or/and greigite (Fe 3 S 4). They are ubiquitous in freshwater, brackish, and marine habitats, and are cosmopolitan in distribution. However, knowledge of their occurrence and distribution in seamount ecosystems is limited. We investigated the diversity and distribution of MTB in the Caroline Seamount (CM4). The abundance of living MTB in 12 stations in depth varying from 90 to 1 545 m was 1.1×10 3- 43.7×10 3 inds./dm 3. Despite diverse shapes of MTB observed, magnetotactic cocci were the dominant morphotype and could be categorized into two types: 1) typical cocci that appeared to have peritrichous f lagella; and 2) those characterized by having a drop-shaped form and one bundle of f lagella located at the thin/narrow end of the cell. Transmission electron microscopy (TEM) analysis revealed that the magnetosomes formed by those magnetotactic cocci are magnetite (Fe 3 O 4) with octahedral crystal habit.A total of 41 operational taxonomic units (OTUs) of putative MTB (2 702 reads) were acquired from nine stations, based on high-throughput sequencing. Of these, 40 OTUs belonged to the Proteobacteria phylum and one belonged to the Nitrospirae phylum. We found apparent connectivity between the MTB populations on the Caroline and Kexue ( Science in Chinese) seamounts, although the diversity of MTB on Caroline was much richer than on the Kexue Seamount. Our results imply that the unique topography of seamounts and other as-yet unclear environmental factors could lead to evolution of diff erent f lagella arrangements in magnetotactic cocci, and the occurrence of octahedral magnetite magnetosomes.
Keyword: magnetotactic bacteria; Caroline Seamount; abundance; diversity; magnetosome
1 INTRODUCTION
Magnetotactic bacteria (MTB) are a group of Gram-negative bacteria that biomineralize unique membrane-enveloped, single magnetic domain, iron oxide (Fe3O4, magnetite) or/and iron sulf ide (Fe3S4,greigite) nanoparticles termed magnetosomes. Within MTB, magnetosomes act as a compass needle, and the f lagellar apparatus enables it to swim along magnetic f ield lines in a behavior termed magnetotaxis(Bazylinski and Frankel, 2004). MTB are a morphologically, phylogenetically, and metabolically diverse group of prokaryotes (Lefèvre and Wu, 2013).Diverse morphologies of MTB have been described,including coccus, ovoid, vibrio, rod, spirillum, and multicellular aggregates (Farina et al., 1983; Abreu et al., 2007; Isambert et al., 2007; Lefèvre et al., 2007,2011a; Lin et al., 2009). Based on NCBI taxonomy,the MTB are phylogenetically affi liated with diverse phyla of the domain Bacteria, including the Proteobacteria, Nitrospirae, and Planctomycetes phyla, and the candidate Omnitrophica (previously named as candidate division OP3) and Latescibacteria(previously named as candidate division WS3) phyla(Lefèvre and Bazylinski, 2013; Lin and Pan, 2015;Kolinko et al., 2016; Lin et al., 2017). Recent studies of reconstructed metagenome-assembled MTB genomes have expanded knowledge of the taxonomy of MTB. For example, 168 genomes were retrieved and classif ied as belonging to 13 bacterial phyla,based on GTDB (Genome Taxonomy Database)taxonomy, and 7 phyla have been classif ied based on NCBI taxonomy (Lin et al., 2020). In another study,38 novel MTB draft genomes were reconstructed,several of which were related to the phyla Elusimicrobia,CandidatusHydrogenedentes, and Nitrospinae (Uzun et al., 2020).
MTB are model organisms for investigating biomineralization processes because of their capacity for intracellular production of magnetite or greigite crystals in a process controlled by the magnetosome gene cluster (MGC) (Lin et al., 2017). MTB biomineralize diverse shapes of magnetosomes,including octahedral, cubo-octahedral, elongatedprismatic or parallelepipedal, bullet-shaped, and pleomorphic forms (Bazylinski et al., 2013a; Amor et al., 2020; Li et al., 2020d). Intriguingly, it has been reported that the morphological diversity of magnetosomes is strongly correlated with specif ic phylogenetic taxa of MTB (Pósfai et al., 2013; Li et al., 2020d; Liu et al., 2021). For example,cuboctahedral or elongated-prismatic magnetite magnetosomes are synthesized in the groups of the Alpha-, Eta-, and Gamma-proteobacteria of MTB,while bullet-shaped magnetite magnetosomes are produced by the Deltaproteobacteria class, and the phyla of Nitrospirae and Omnitrophica (Pósfai et al.,2006; Li et al., 2010b, 2015, 2017, 2019, 2020c, d;Lefèvre et al., 2011c, 2012; Lin et al., 2011; Kolinko et al., 2012; Zhou et al., 2012; Lefèvre and Bazylinski,2013; Zhang et al., 2013, 2017;Amor et al., 2020).However, diverse morphologies of greigite crystals,including bullet-shaped, cubo-octahedral, elongatedprismatic, and pleomorphic, only can be synthesized in Deltaproteobacteria MTB (Lefèvre and Bazylinski,2013).
Magnetotactic cocci were the dominant morphotype of MTB in natural freshwater and marine habitats(Spring et al., 1998; Flies et al., 2005a; Lin et al.,2009; Du et al., 2017) including sediments in lakes(Lin et al., 2009), rivers (Kozyaeva et al., 2017),estuaries (Zhang et al., 2017), intertidal zones (Pan et al., 2008; Chen et al., 2015), lagoons (Du et al., 2017),continental shelves (Xu et al., 2018), and seamounts(Liu et al., 2017). Magnetotactic cocci belonged to the order Magnetococcales have recently been transferred from the Alphaproteobacteria to“CandidatusEtaproteobacteria”, based on ribosomal proteins, MTB core genes, whole genome analysis of the MO-1 and MC-1 strains, and a large-scaled identif ication and phylogenetic analysis on twenty novel magnetotactic cocci (Ji et al., 2017; Zhang et al., 2017; Lin et al., 2018; Liu et al., 2021). Many novel magnetotactic cocci species have been described as having diff erences in magnetosome chain conf igurations, and the number, crystal size, and morphology of magnetosomes (Meldrum et al., 1993;Lefèvre et al., 2009; Zhang et al., 2017; Liu et al.,2021). For instance, various models of MTB chain conf iguration have been reported among the cocci,including single chain, double separated chains,double parallel chains, double bundles containing two parallel chains in each bundle, particle aggregates,and other irregular forms (Liu et al., 2021). Elongated prismatic and (elongated) cuboctahedral magnetite crystals have commonly been reported to occur in the order Magnetococcales and had a relatively consistent morphology (Pósfai et al., 2013). However, a unique form of (elongated) octahedral magnetosomes have been described in the uncultured freshwater magnetotactic coccus strains SHHC-1 and MYC-5,the former having the crystals arranged in two double chains and the latter in two chains (Zhang et al., 2017;Liu et al., 2021). Similar crystals arranged in a chain have also been observed in the cultured MTB strainMagnetofabaaustralisIT-1, which was isolated from sediments of a brackish to marine coastal lagoon(Araujo et al., 2016).
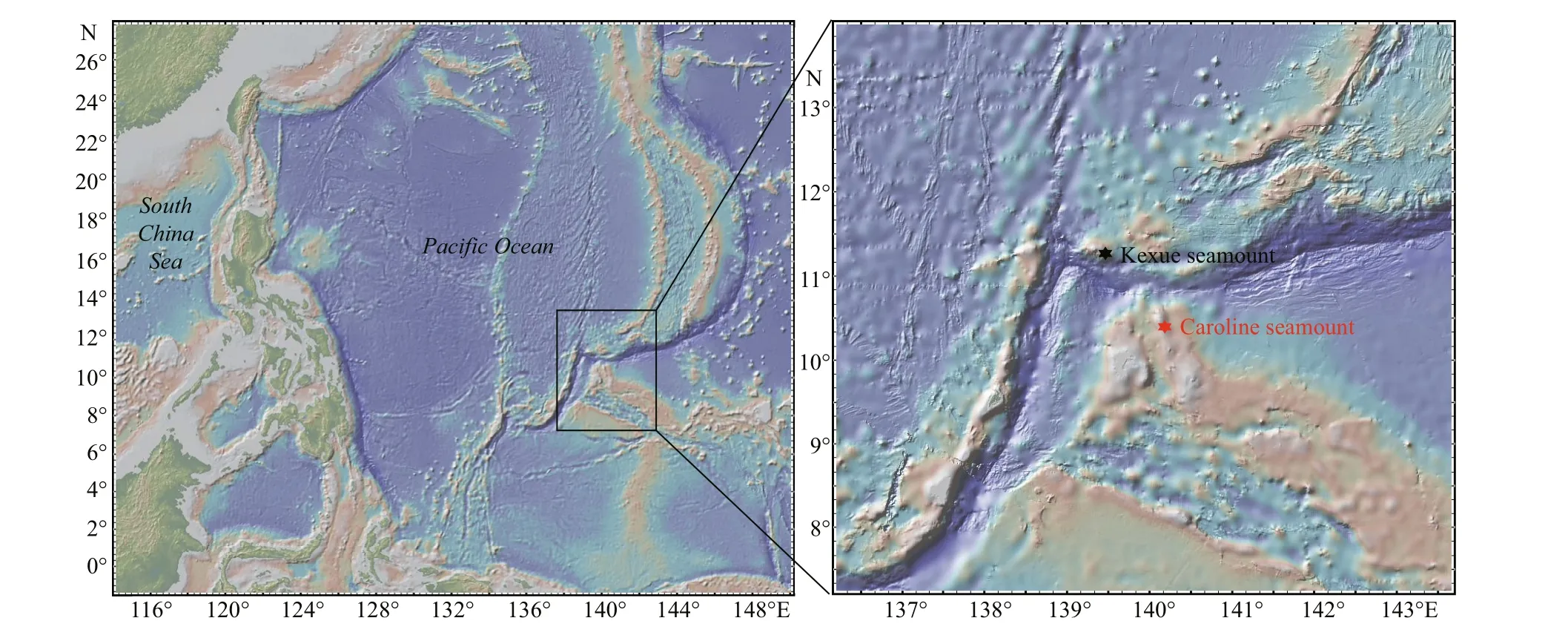
Fig.1 Location of the sampling area and the Caroline Seamount
Magnetotactic bacteria are ubiquitous in natural environments, and peak in abundance at the oxicanoxic transition zone (OATZ) in sediments or chemically stratif ied water columns in freshwater,brackish, marine, and hypersaline habitats (Bazylinski and Frankel, 2004; Moskowitz et al., 2008; Bazylinski et al., 2013a). Recent investigations of MTB diversity at the Kexue (Sciencein Chinese) Seamount (also designated M2) have extended our knowledge of the adaptability of MTB to this tropical oligotrophic marine habitat (Liu et al., 2017). Seamounts have numerous unique topographic characteristics including acceleration and recirculation of currents(Dower et al., 1992), Taylor columns (Hogg, 1973;Genin and Boehlert, 1985), and internal waves (Kunze and Sanford, 1996) that potentially support diversity hotpots in oligotrophic oceans (Mendonça et al.,2012). The Kexue Seamount is situated at the junction of the Yap and Mariana trenches, where complex dynamic hydrological variations can occur dramatically. Intriguingly, 16 novel MTB species specif ic to this seamount were identif ied, and phylogenetic analysis showed endemic adaptation of the MTB to the seamount. Some of the magnetotactic cocci from this seamount have the most sophisticated f lagellar apparatus known, involving 19 f lagella organized in a 3꞉4꞉5꞉4꞉3 array enveloped in a f lagellar bundle (Liu et al., 2017). However, studies of the distribution, diversity, and characterization of MTB from seamount ecosystems remain limited. In this study, sediments were collected from the Caroline Seamount (CM4) in the Western Pacif ic Ocean.Various shapes of MTB were identif ied using transmission electron microscope (TEM)investigations. The dominate MTB population was magnetotactic cocci that formed magnetite-type magnetosomes with octahedral morphology. Analysis using high-throughput 16S rRNA sequencing indicated the occurrence at this seamount of 41 putative MTB operational taxonomic units (OTUs)affi liated to the phyla Proteobacteria and Nitrospirae.We further compared the diversity and characteristics of MTB between the Caroline and Kexue seamounts,and discussed possible explanations for the specif icity of MTB and their magnetosomal biomineralization in these seamount habitats.
2 MATERIAL AND METHOD
2.1 Sediment sampling
Sediment samples were collected in the Tropical Western Pacif ic Ocean from the Caroline Seamount(CM4, 10.3°N- 10.9°N, 139.9°E- 140.4°E) during cruise WPOS-M4 conducted by the Chinese research vessel (R/V)Kexue(Sciencein Chinese) from 7 August to 5 September, 2017. The peak of the seamount is 57 m below the sea surface and it has a height of 5 950 m. Samples were collected from 13 stations at various depths (90- 2 805 m) in the seamount area (Figs.1 & 2a) using the remotely operated vehicle (ROV)Faxian(Discoveryin Chinese) (Fugro Geosolutions, Shenzhen, China). On return to the main vessel the sediments and seawater(ratio 1꞉1) were immediately transferred into 500-mL plastic bottles and incubated in dim light at ambient temperature (~20 ° C) for subsequence research. In parallel, approximately 50-g sediment samples from each station were transferred to sterile sealed bags and stored at -80 ° C for later DNA extraction.
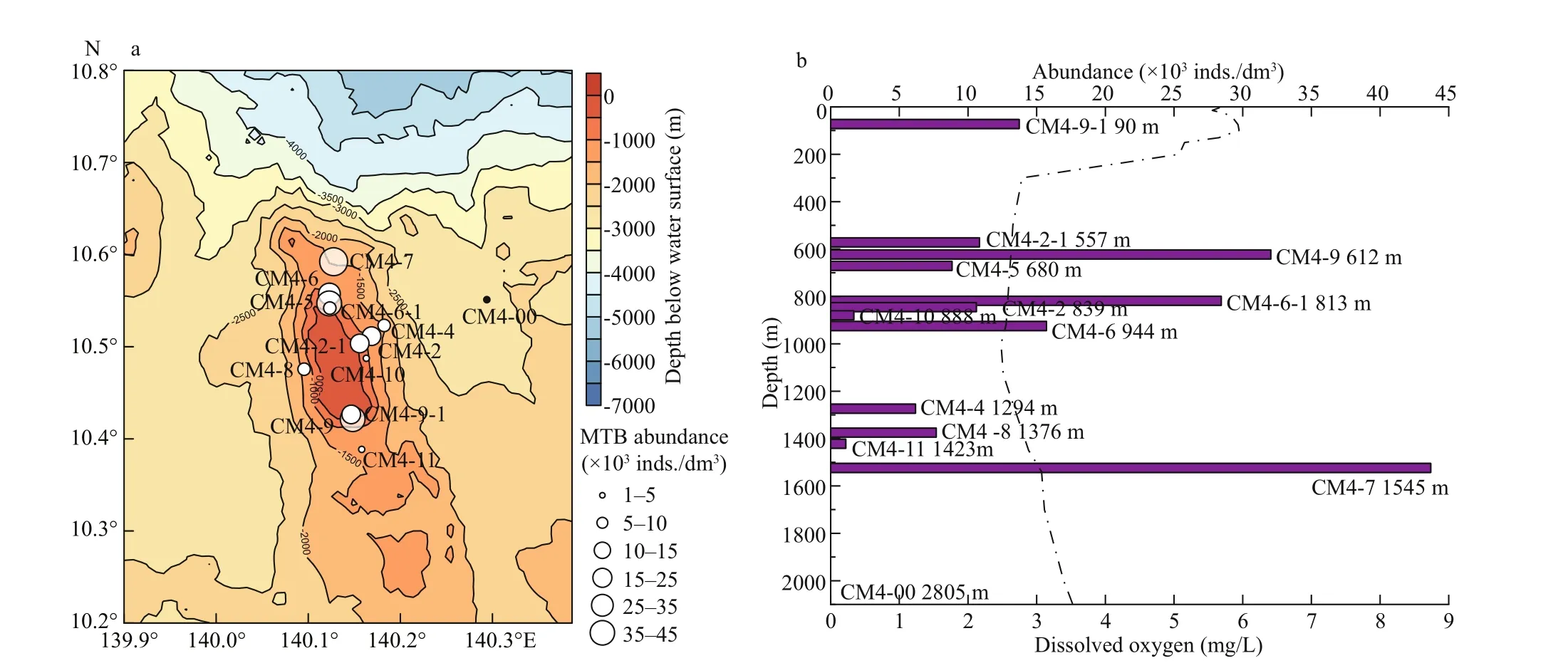
Fig.2 Distribution of MTB at the Caroline Seamount
2.2 Optical and electron microscopy
MTB in the sediments were enriched magnetically for observation on aboard immediately after sampling.Each plastic bottle was shaken to mix the sediment and seawater, then two magnets with opposite sides were attached to the outside of the bottle adjacent to the interface of the seawater and sediment. After 40 min the MTB that concentrated at the interface adjacent to the magnets were removed using a Pasteur pipette and transferred into a clean 1.5-mL tube. The MTB were inspected and counted using the hanging drop technique (Schüler, 2002) and an Olympus CX31 microscope. The abundance of MTB was calculated according to the formula A=N/V (A:abundance; N: number of MTB; V: volume of sediment), as previously described (Xu et al., 2018).
Subsequently, the enriched MTB were purif ied using the modif ied race-track method with a 1-mL pipette tip to collect MTB cells (Wolfe et al., 1987; Li et al., 2010a) and examined with TEM observations.Approximately 5-μL purif ied cells from the bottom of tube was deposited on a carbon-coated copper TEM grid and the grid was washed with Milli-Q water, then dried in air. The morphology of cells and the magnetosomes was investigated using a Hitachi HT7700 TEM operating at 100 kV. Cell size and cell diameter, and the length and width of magnetosome particles were measured from the TEM images of MTB. The detailed morphology, and the composition and crystal structure of magnetosomes were examined using high-resolution transmission electron microscopy (HRTEM; JEM-2100, JEOL) in a system equipped with energy dispersive X-ray spectroscopy(EDXS) operating at 200 kV.
2.3 DNA extraction, 16S rRNA gene sequencing,and dataset analysis
Genomic DNA was extracted from 5 g of sediments following the modif ied methods described by Kvist et al. (2007). The genomic DNA was purif ied using the Wizard@DNA Clean-up System (Promega), following the manufacturers protocol. The hypervariable region V3- V4 of the bacterial 16S rRNA gene was amplif ied using the bar-coded universal primers 338F(5′-ACTCCTACGGGAGGCAGCA-3′) and 806R(5′-GGACTACHVGGGTWTCTAAT-3′). Paired-end reads were generated using the Illumina MiSeq platform, and were overlapped using FLASH (Fast Length Adjustment of Short reads, version 1.2.11)(Magoč and Salzberg, 2011). High quality reads were clustered into operational taxonomic units (OTUs)using UPARSE (Edgar, 2013) with a 97% threshold,and UCHIME (version 4.2.40) was used to f ilter and remove chimeras (Edgar et al., 2011). MTB were identif ied using BLAST and the NCBI nt database.The alignment of MTB sequences was performed using CLUSTAL X (version 2.0) with manual correction (Larkin et al., 2007). A phylogenetic tree was constructed using MEGA (version 7.0.26)software via the neighbor-joining method (Saitou and Nei, 1987). Bootstrap values were calculated based on 1 000 replicates.
3 RESULT
3.1 Sampling stations and the distribution of MTB in sediments of the Caroline Seamount
The Caroline Seamount (CM4) is located approximately 129 km to the southeast of the Kexue Seamount, and they are separated by the Mariana Trench (Fig.1). Optical microscopy observations were performed immediately following magnetic enrichment of MTB. Living MTB cells were observed in samples from 12 of the 13 stations, and were present at an average abundance of 1.5×104inds./dm3(1.1×103- 43.7×103inds./dm3), with magnetotactic cocci being the dominant morphotype. However, no live MTB were observed in samples from the deepest station CM4-00, which was far from the summit area of the Caroline Seamount at a depth of 2 805 m(Fig.2a).
MTB were mainly detected on the north, south,and east slopes of the Caroline Seamount (Fig.2a).The average abundance of MTB in the northern region (including stations CM4-7, CM4-6, CM4-6-1,and CM4-5) was 2.4×104inds./dm3, and was greater than in the southern (1.6×104inds./dm3; stations CM4-9-1, CM4-9, and CM4-11), western(7.7×103inds./dm3; station CM4-8 only), and eastern(7.3×103inds./dm3; stations CM4-2-1, CM4-10,CM4-2, and CM4-4) regions. The vertical distribution of MTB abundance was illustrated by three groups def ined according to the water depth and the dissolved oxygen in the seawater (Fig.2b). Above the 200-m layer (near the euphotic layer), sediments were sampled at station CM4-9-1 only (90-m depth), which had a MTB abundance of 1.4×104inds./dm3. In the 200- 1 000-m layer (twilight zone) there were seven stations that had an average MTB abundance of 1.5×104inds./dm3. In this layer the number of MTB at the various stations decreased slightly with increasing water depth. Below 1 000 m (the bathypelagic layer)there were four stations that had an average MTB abundance of 1.5×104inds./dm3. In this layer, the abundance tended to increase with increasing water depth, and the abundance was particularly high at 1 545-m depth (station CM4-7). Nevertheless, the highest and lowest abundances of MTB both occurred at depths beyond 1 000 m: 4.4×104inds./dm3at station CM4-7 (1 545-m depth) and 1.1×103inds./dm3at station CM4-11 (1 423-m depth). The most abundant station CM4-7 is located in an oxic-anoxic transition region with a dissolved oxygen content of 3 mg/L(Fig.2b).
3.2 Diverse morphologies of MTB and characterization of magnetosomes
Using TEM, diverse morphologies of MTB(coccus, vibrio, and spirillum) were observed (Fig.3)in the sediments of merely six stations of the Caroline Seamount in situ (Supplementary Table S1), but magnetotactic cocci were the dominant morphology.Four types of MTB were categorized based on characteristics including the morphology of cells, the f lagellar apparatus, and the magnetosome shapes. The f irst and most abundant type was typical cocci (Fig.3a)having an average diameter of 1.5±0.4 μm (n=38);one of these appeared to have peritrichous f lagella(approximately 35) that were distributed over the cell(Fig.3a). The second type was drop-shaped cocci that had numerous f lagella in a tuft at one end of the cell(Fig.3b). They had an average cell size of(2.1±0.3) μm×(1.7±0.3) μm (n=11), and the f lagella length varied from 5.3 to 26.2 μm, which exceeded the cell length by a factor of 5.7 in average (the longest f lagellum is 9.8 times larger than the corresponding length of the cell). Magnetotactic spirillum (Fig.3c) and vibrio (Fig.3d) cells comprised the other two MTB categories, having cell sizes of 4.2×0.4 μm (n=1) and 2.0×0.4 μm (n=1), respectively.
The magnetosomes within the MTB cells were all organized in chains having diverse arrangements.Most magnetotactic cocci biomineralized a single magnetosome chain in each cell (Fig.4a), while others biosynthesized two parallel (Fig.4b) or intertwining(Fig.4c) magnetosome chains. Three chain forms were observed in some cocci (Fig.4d). In the spirillum and vibrio, the magnetosome particles were arranged as a single chain along the long axis (Fig.3c & d).
Two main morphologies of magnetosome were observed including the octahedral and prismatic shapes (Fig.3e- h). The average sizes of the octahedral magnetosomes in these three types of MTB were(57.3±11.4) nm×(53.9±10.4) nm (n=554), (60.3±12.9) nm×(56.6±12.4) nm (n=204), and (48.1±4.8) nm×(45.3±4.3) nm (n=22), respectively, and the average width/length ratio of these MTB types was 0.94±0.04 (n=780). The magnetosome crystals in the vibrio cells were predominantly elongated prismatic,and were (66.9±3.9) nm×(53.0±3.2) nm in size and had a width/length ratio of 0.79±0.01 (n=5). HRTEM and EDXS analysis indicated that all magnetosomes types analyzed were composed of magnetite (Fe3O4).

Fig.3 Morphology of the magnetotactic bacteria and characteristics of magnetosomes
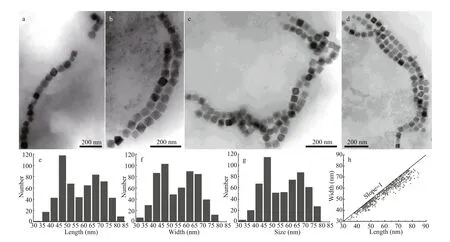
Fig.4 Magnetosome chain conf igurations and the features of magnetosomes in magnetotactic cocci
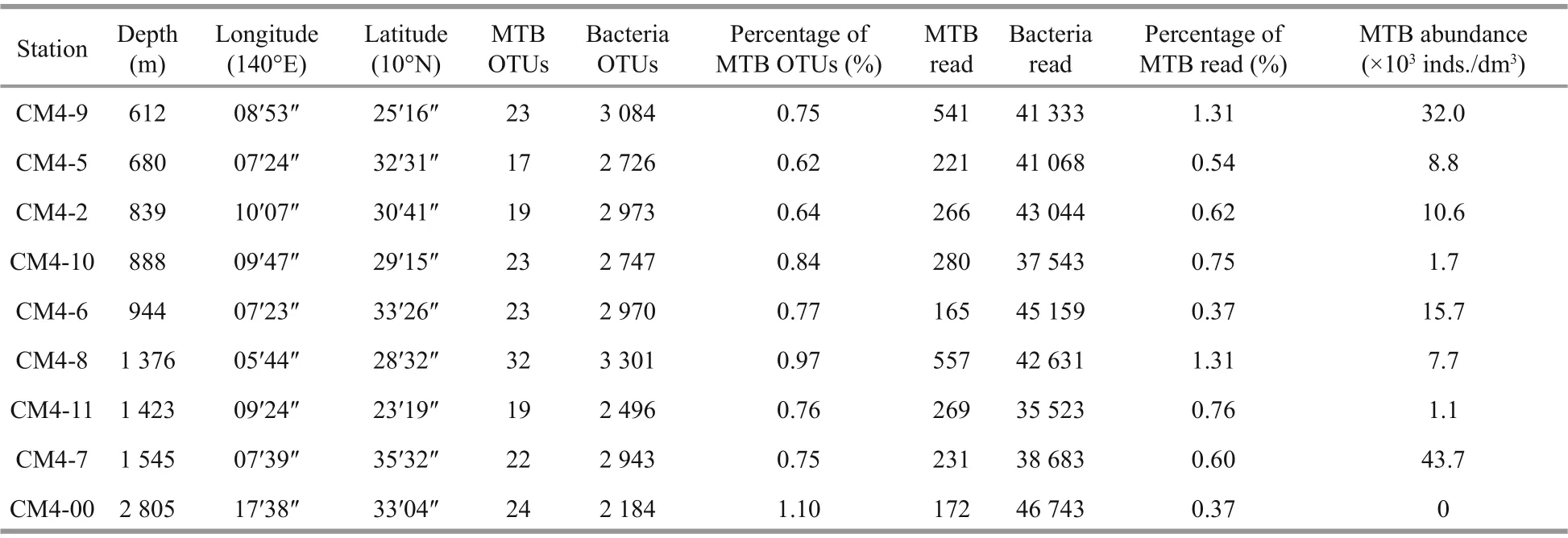
Table 1 MTB OTUs, reads, and abundances at nine stations on the Caroline Seamount
3.3 Phylogenetic diversity of MTB in sediments of the Caroline Seamount
Suffi cient DNA for analysis was extracted from sediments from nine stations (CM4-2, CM4-5, CM4-6, CM4-7, CM4-8, CM4-9, CM4-10, CM4-11, and CM4-00; depth range: 612- 2 805 m). High-quality 16S rRNA gene sequences for each station ranging from 35 523 to 46 743 were retrieved from the raw data. The rarefaction curves indicated that the sequencing depths were suffi cient, and the sequencing results were reliable. These reads were classif ied into 2 184- 3 301 OTUs, based on a threshold of 97%sequence similarity (Tindall et al., 2010). In total, 41 OTUs (2 702 reads) were affi liated with MTB, based on a BLAST search of the NCBI nt database (Table 1& Fig.5). Among these putative MTB sequences,17- 32 OTUs of putative MTB were detected at each station (Fig.6a). The largest number of putative MTB OTUs (32) occurred at 1 376-m depth at station CM4-8, while the lowest value (17) occurred at 680-m depth at station CM4-5. The maximum number of MTB reads (557) was found at station CM4-8 (1 376-m depth), and the next greatest number of reads (541)was found at station CM4-9 (612-m depth). The lowest number of reads (165) was found at station CM4-6 (944-m depth), and the second lowest number(172) was found at station CM4-00 (2 805-m depth)(Fig.6b).
All the putative MTB OTUs were related to the Alphaproteobacteria, except for OTU4156 and OTU1871. Based on the most up-to-date NCBI taxonomic database, OTU4156 (4 reads) and OTU1871 (7 reads) were the closest to unculturedMagnetococcusclone OTU9 (MF099880) belonging to “CandidatusEtaproteobacteria”, and “CandidatusMagnetoovummohavensis” strain LO-1 (GU979422)belonging to the phylum Nitrospirae, respectively.Among the 39 OTUs affi liated to the Alphaproteobacteria, 24 were closest toMagnetospirasp. QH-2 (EU675666), with seven of these closest toMagnetospirathiophilaMMS-1 (EU861390), four closest to unculturedMagnetospiraclone (KP183009),two closest to unculturedMagnetospirillumclones(HQ222269 and HQ154657), and two closest toMagnetovibrioblakemoreiMV-1 (L06455). The phylogenetic tree for 41 MTB OTUs and related sequences was constructed by analysing sequence alignments for 16S rRNA gene using the neighborjoining method, and is shown in Fig.5.Remarkably, the most common f ive OTUs,including OTU102 (718 reads), OTU154 (432 reads),OTU356 (190 reads), OTU94 (155 reads), and OTU594 (139 reads), accounted for 60.5% (1 623 reads) of the total MTB reads. They all belonged to the genusMagnetospira, and were distributed among almost all the nine stations on the Caroline Seamount;the exception was OTU594, which was not detected at station CM4-7 (Fig.6c).
4 DISCUSSION
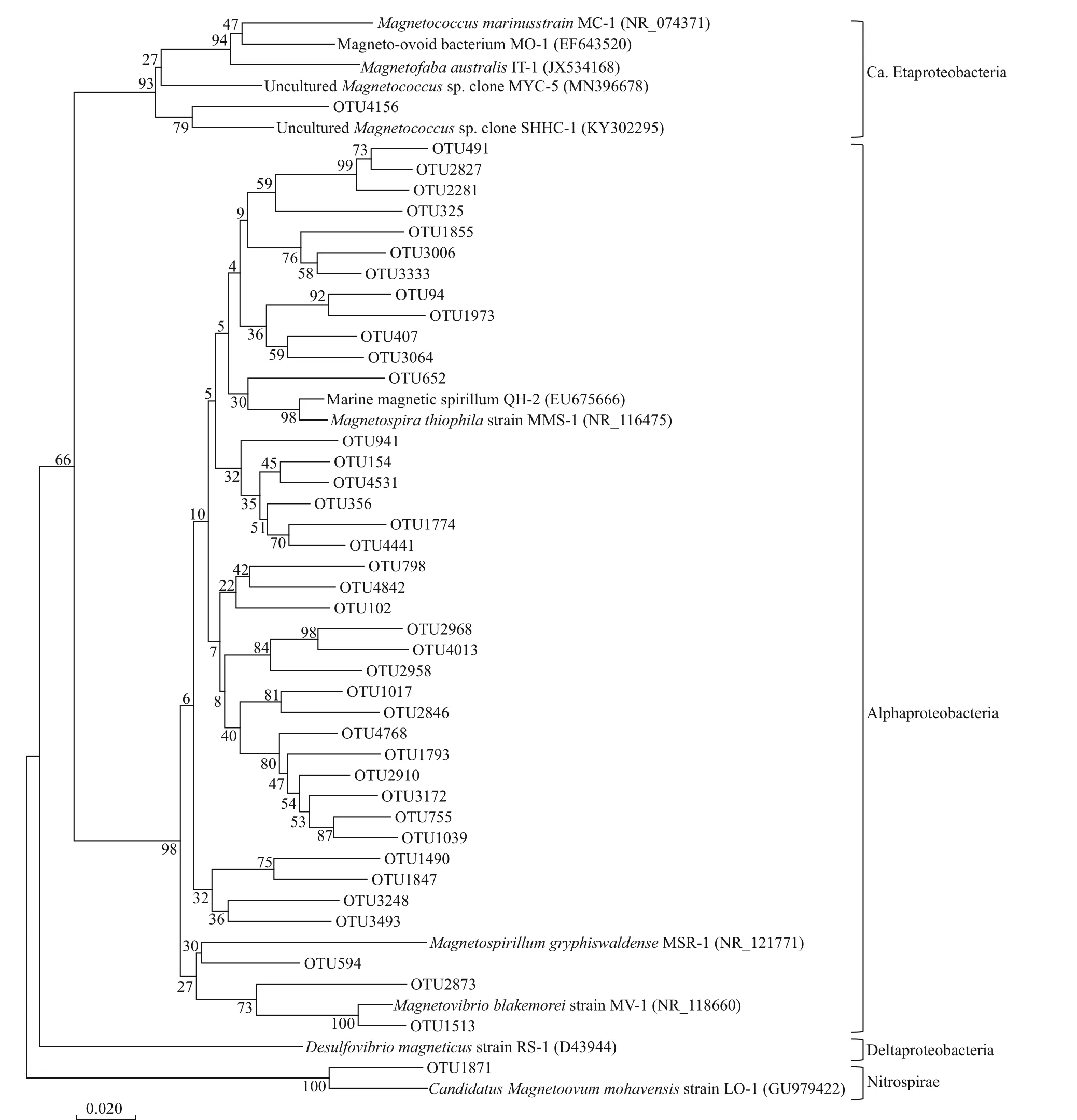
Fig.5 Neighbor-joining phylogenetic tree showing the relationship of the putative 41 MTB OTUs from the Caroline Seamount to the closest database matches
Based on direct microscope counts of MTB, their abundance in sediments of the Caroline Seamount averaged approximately 1.5×104inds./dm3, which is higher than the average abundance in sediments of the continental shelf of the Yellow Sea (290 inds./dm3in summer; 130 inds./dm3in winter) (Xu et al., 2018),but is orders of magnitude lower than the abundance of MTB at a seamount station (4×105inds./dm3at site 1726) in the South Atlantic Ocean (Petermann and Bleil, 1993), in the Mediterranean Sea (105-106inds./dm3in summer) (Lefèvre et al., 2007), and in intertidal sediments of the Yellow Sea including at Lake Yuehu (106- 107inds./dm3) (Du et al., 2017) and Xiaoshi Island (107- 108inds./dm3) (Chen et al.,2015). Notably, the MTB abundance at the Caroline Seamount was much higher than that at the Kexue Seamount (54.6 inds./dm3); these seamounts are adjacent to each other but separated by the Mariana Trench (Liu et al., 2017). Previous studies have suggested that many MTB populations have the ability to f ix nitrogen, as they contain the full suite ofnifgenes in their genomes (Bazylinski and Blakemore,1983; Bazylinski et al., 2000, 2013b; Schübbe et al.,2009). Recently, thenap,nar, andnirgenes were found in Mcas genome (strain MYR-1, Nitrospirae phylum), which also implied that the magnetotactic Mcas strain has the potential ability to transform nitrate to N2/NH4+(Lin et al., 2014; Li et al., 2020a).Investigation of the distribution of nutrients in the euphotic zone (0- 200-m depth) at the Kexue (also designated M2) and Caroline (also designated C4)seamounts has recently indicated that nitrogen limitation at the Caroline Seamount (N꞉P=13.5꞉1)was less than at the Kexue Seamount (N꞉P=3.7꞉1)(Ma et al., 2019). Thus, it is possible that the greater abundance of MTB at the Caroline Seamount contributes to this phenomenon, although further investigation of this is required.
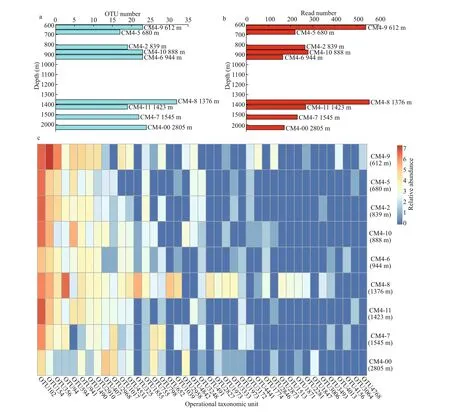
Fig.6 Vertical distribution of MTB OTUs (a) and MTB reads (b), and a heatmap of the various compositions of MTB OTUs and reads (c) at the nine stations
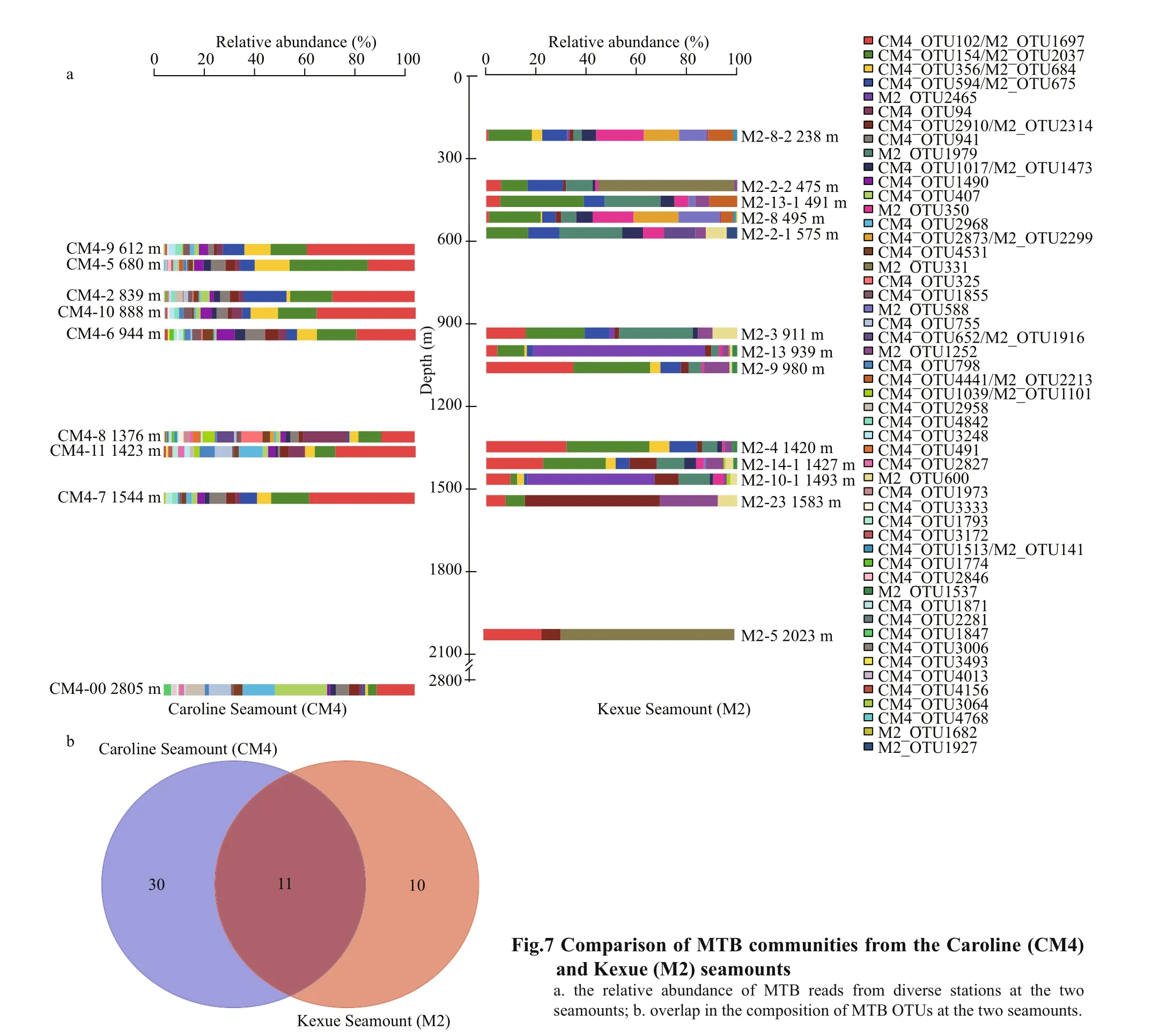
Based on high-throughput 16S rRNA sequencing analyses, the dominant group of MTB at the Caroline Seamount was related to theMagnetospira, while magnetotactic cocci were the most abundant group in the intertidal sediments of Yuehu Lake (Zhang et al.,2019), and Nitrospirae MTB dominated in sediments of the Yellow Sea continental shelf (Xu et al., 2018).Although MTB OTUs affi liated toMagnetospirawere the dominant groups at both the Kexue and Caroline seamounts, the communities of MTB in the sediments of the Caroline Seamount were more complex and diverse (Fig.7a). At the Kexue Seamount,Liu et al. (2017) detected 21 putative MTB OTUs(1 130 reads), while 41 MTB OTUs (2 702 reads)were found at the Caroline Seamount. The numbers of both MTB OTUs and reads at the Caroline Seamount were approximately twice that at the Kexue Seamount.In addition, the MTB OTUs affi liated with Etaproteobacteria and Nitrospirae were only detected at the Caroline Seamount. At present, Nitrospirae MTB biomineralize bullet-shaped magnetosomes, but those characteristic particles were not examined within MTB individuals by TEM analyses unfortunately. It might result from the low number of Nitrospirae MTB cells dwelled in the seamount habitat, which made it diffi cult to detect them though they were present at the Caroline Seamount actually.Comparison of the mean Chao, Shannon, and Simpson diversity indices for MTB from diff erent habitats is shown in Supplementary Table S2. The data suggest that the diversity of MTB at the Caroline Seamount was richer than at the Kexue Seamount, but lower than in the Yellow Sea and Yuehu Lake. Discrepancies in the community compositions of MTB may be inf luenced by the environmental characteristics in the various habitats. Biogeographic studies have suggested that environments heterogeneous for factors including salinity, temperature, nitrate, or sulfur compounds may be responsible for diff erences in the abundance or community composition of MTB(Martins et al., 2009, 2012; Postec et al., 2012; Lin et al., 2012b, 2013; Silva et al., 2020).
Although the diversity of MTB at the Caroline Seamount was richer than at the Kexue Seamount, 11 MTB OTUs were shared between the two seamounts(Fig.7b), accounting for 26.8% and 52.4% of the total MTB OTUs at these two seamounts, respectively. The reads of shared MTB OTUs accounted for 63.6%(1 719 reads) and 54.8% (619 reads) at the Caroline and Kexue seamounts, respectively. More interestingly, among 11 shared MTB OTUs, four OTUs are the most common for Caroline Seamount(OTU102, 154, 356, and 594) which correspond to OTU1697 (third most common), 2 037 (most common), 684 (thirteenth most common) and 675(f ifth most common) for Kexue Seamount,respectively. This f inding suggests that the dominant MTB populations were consistent between the two seamounts. In addition, the phylogenetic analyses showed that most of the shared MTB OTUs were clustered into a single large branch in the Alphaproteobacteria. Notably, these two seamounts are both shallow (Genin, 2004), with the distance from summit to the sea surface being <200 m, and the sediment at both is mainly composed of coral and foraminiferan sands (Supplementary Fig.S1; Liu et al., 2017). The similar geological properties and hydrological conditions of the seamounts may be signif icant in determining the occurrence of MTB.Therefore, it is reasonable to infer that there is population connectivity between these two seamounts,at least for the MTB community.
TEM observations showed that diverse morphotypes of MTB, including cocci, vibrio, and spirillum forms, were present in enrichments of the samples collected from the Caroline Seamount.Magnetotactic cocci were the dominant morphology,which was a f inding consistent with previous reports(Spring et al., 1998; Flies et al., 2005b; Lin et al.,2009; Lin and Pan, 2009; Du et al., 2017; Liu et al.,2017; Xu et al., 2018). Based on previous studies,most magnetotactic cocci affi liated to the Proteobacteria phylum have two tufts of f lagella. For example, bothMagnetococcusmarinusMC-1(Bazylinski et al., 2013b) and magnetotactic ovoid strain MO-1 (Zhang et al., 2012) have two sheathed bundles of f lagella, while magnetotactic cocci strains IT-1 (Araujo et al., 2016), SHHC-1 (Zhang et al.,2017), and QHL (Pan et al., 2008) have two bundles of lophotrichous f lagella lacking a sheath. At the Kexue Seamount, one type of magnetotactic cocci having two bundles of f lagella was found, with the 19 f lagella in each bundle arranged in a 3꞉4꞉5꞉4꞉3 array(Liu et al., 2017); this is unlike MO-1, which has only seven f lagella (Ruan et al., 2012). It has been speculated that the more complex f lagellar assembly is the result of adaptation to the seamount environment(Liu et al., 2017). In the present study we observed magnetotactic cocci having putative peritrichous f lagella (it remains possible that the spread of a single bundle of f lagella may have given the appearance of peritrichous f lagella) (Fig.3a), and drop-shaped cocci having a single bundle of f lagella (Fig.3b). MTB affi liated to the Nitrospirae phylum, including the large rod “CandidatusMagnetobacteriumcasensis”(Lin et al., 2014), the watermelon-shaped MWB-1(Lin et al., 2012a), and the large ovoid LO-1 (Lefèvre et al., 2011a) have been reported to have single bundles of f lagella. Magnetotactic cocci having two bundles of f lagella have been reported as one of the fastest natural micro-swimmers (Bente et al., 2020).These f indings imply that having a single bundle of f lagella is suffi cient to enable motility of MTB at the Caroline Seamount. During sampling of sediments from this seamount using the ROVFaxian(Discoveryin Chinese), the occurrence of strong ocean bottom currents was evident, and these may cause the surface sediment to drift. In addition, seamount slope topography is known to aff ect the spatial distribution of sediments. Thus, it is likely that the MTB inhabiting the Caroline Seamount surface sediment can move easily because of ocean currents and the seamount slope, which could result in the evolution of single bundle of f lagella.
Although the properties of magnetosomes are controlled by genes in the MTB, there are some evidences that they can be inf luenced by environmental conditions (see the recent review by Moisescu et al.,2014). For example, Fe uptake rates and pH seem to aff ect the size and morphology of magnetite crystals in the typical strain ofMagnetospirillumgryphiswaldenseMSR-1 (Faivre et al., 2008;Moisescu et al., 2011), and the size of magnetosomes inM.magneticumstrain AMB-1 varies with variations in the oxygen concentration or magnetic f ield intensity(Wang et al., 2008; Popa et al., 2009; Li and Pan,2012; Moisescu et al., 2014). In culture,Desulfamplusmagnetovallimortisstrain BW-1 synthesizes bulletshaped magnetite magnetosomes in the presence of<0.3-mmol/L sulf ide, and roughly rectangular greigite magnetosomes when the sulf ide concentration is>0.3 mmol/L (Lefèvre et al., 2011b). In the present study, octahedral magnetite magnetosomes were found to occur in magnetotactic cocci and the magnetotactic spirillum (Fig.3). And among living MTB reported elsewhere, only few appear to be able to synthesize octahedral magnetite magnetosomes,includingMagnetofabaaustralisIT-1, magnetotactic coccus strains SHHC-1 and MYC-5, and an unclassif ied magnetotactic spirillum from a pond(Vali et al., 1987; Araujo et al., 2016; Zhang et al.,2017; Liu et al., 2021). Intriguingly, the dominant magnetotactic cocci at the Kexue Seamount without growing in the bottles also synthesized octahedral magnetite crystals (Liu et al., 2017). It is notable that both the IT-1 and SHHC-1 strains appear to have a greater adaptability to changing environments, based on genomic data for IT-1 and the co-occurrence of various magnetosome forms in individual SHHC-1 cells (Araujo et al., 2016; Zhang et al., 2017). As noted above, the sediments at both seamounts are mainly composed of unconsolidated coral and foraminiferan sands (Supplementary Fig.S1), which can easily drift in ocean currents. Thus, it is possible that unstable habitats result in an increase in the biomineralization of octahedral magnetosomes by MTB. Further research should be undertaken to test this hypothesis. In contrast, octahedral magnetofossils seem to be common and the main magnetosome type in deep sea sediments (Vali et al., 1987; Yamazaki and Kawahata, 1998; Dong et al., 2016; Yamazaki et al.,2019; He and Pan, 2020; Li et al., 2020b) and especially red clay (Yamazaki and Shimono, 2013;Usui et al., 2017). In red clay, octahedral magnetofossils were found to be associated with high oxygen concentrations, inferring that some MTB species that synthesize octahedral magnetosomes may be oxygen tolerant (Yamazaki et al., 2019).Therefore, the MTB living in unstable seamount sediments may be subject to high oxygen concentrations, leading to the domination of octahedral magnetosomes in some MTB species.
5 CONCLUSION
In this study we described the diversity,characteristics, and distribution of MTB from the Caroline Seamount (CM4). Based on TEM observations, magnetotactic cocci were the dominant morphotype. Among the typical MTB cocci, one type appeared to have peritrichous f lagella (approximately 35); the other type of coccoid cells showed dropshaped, and had a single bundle of f lagella approximately a factor of 5.7 longer than the cell diameter in average. The dominant magnetosome morphology biomineralized in most MTB individuals with octahedral crystal habit; this study is the f irst to report the occurrence of octahedral magnetite particles among the diverse species of MTB living in the same seamount habitat. It was inferred that the biomineralization of magnetosome magnetite was related to both the species and environmental parameters. High-throughput 16S rRNA sequencing identif ied 41 putative MTB OTUs belonging to two phyla, Proteobacteria and Nitrospirae. Comparison of MTB diversity between the Caroline and Kexue seamounts suggested that there is both commonality(indicating connectivity) and specif icity in terms of the MTB populations on these two seamounts.
6 DATA AVAILABILITY STATEMENT
All data generated and/or analyzed during the study are available from the corresponding author on reasonable request.
7 ACKNOWLEDGMENT
We thank the crew and captain of R/VKexue, the ROV team for sediments sampling. We thank Wei LIU and Yuanyuan SUN at the IOCAS (Institute of Oceanology, Chinese Academy of Sciences) for their assistance in TEM observations. We thank the engineer Mr. Xu TANG at the IGGCAS (Institute of Geology and Geophysics, Chinese Academy of Sciences) for his assistance in TEM experiments.
杂志排行
Journal of Oceanology and Limnology的其它文章
- MamZ protein plays an essential role in magnetosome maturation process of Magnetospirillum gryphiswaldense MSR-1*
- Magnetotactic bacteria from the human gut microbiome associated with orientation and navigation regions of the brain*
- How light aff ect the magnetotactic behavior and reproduction of ellipsoidal multicellular magnetoglobules?*
- Biocompatibility of marine magnetotactic ovoid strain MO-1 for in vivo application*
- Determination of the heating effi ciency of magnetotactic bacteria in alternating magnetic f ield*
- An approach to determine coeffi cients of logarithmic velocity vertical prof ile in the bottom boundary layer*
