MamZ protein plays an essential role in magnetosome maturation process of Magnetospirillum gryphiswaldense MSR-1*
2021-12-09ShaWUQingWANGXuWANGRuixueGUOTongweiZHANGYongxinPANFengLIYingLI
Sha WU , Qing WANG , Xu WANG , Ruixue GUO , Tongwei ZHANG ,Yongxin PAN , Feng LI ,**, Ying LI ,**
1 Engineering Technology Research Center of Ecological Restoration and Comprehensive Utilization of Coal Mining Collapse Area, Anhui Province; Anhui Province Key Laboratory of Pollutant Sensitive Materials and Environmental Remediation,College of Life Sciences, Huaibei Normal University, Huaibei 235000, China
2 State Key Laboratories for Agro-biotechnology, China Agricultural University, Beijing 100193, China
3 Paleomagnetism and Geochronology Laboratory, Key Laboratory of Earth and Planetary Physics, Institute of Geology and Geophysics, Chinese Academy of Sciences, France-China Joint Laboratory for Evolution and Development of Magnetotactic Multicelluar Organisms, Beijing 100029, China
Abstract Based on analysis of gene structure of mamXY operon in Magnetospirillum gryphiswaldense strain MSR-1, we constructed a mamZ deletion mutant strain (Δ mamZ) and four complemented strains with diff erent mamZ fragment lengths. Various cell phenotypic and physiological parameters were evaluated and compared among the wild-type (WT), mutant, and complemented strains. Cell growth rates were not notably diff erent; however, magnetic response (Cmag) and iron uptake ability were signif icantly lower in Δ mamZ.High-resolution transmission electron microscopy (HR-TEM) showed that magnetosomes in Δ mamZ were small and irregular, and rock magnetic measurements suggested that they contained immature particles. In comparison to WT of MSR-1, intracellular iron content of Δ mamZ and the complemented strains cultured with 20 μ mol/L iron source was similar or slightly higher. The complemented strains were unable to synthesize mature or normal amounts of magnetosomes, apparently because of abnormal expression of the transmembrane domain of MamZ protein. Real-time reverse transcription polymerase chain reaction (RTqPCR) analysis showed that relative transcription levels of mamX and ftsZ- like genes in Δ mamZ were higher at 18 h than at 12 h, suggesting that MamXY proteins play cooperative functional roles in the magnetosome maturation process. Transcription level of mms6 was signif icantly upregulated in Δ mamZ (incubated at 12 h) and the complemented strains (incubated at 12 and 18 h), ref lecting possible interaction between MamXY and Mms6 proteins during magnetosome biosynthesis. These f indings, taken together, demonstrate the essential role of MamZ in the magnetosome maturation process in MSR-1.
Keyword: Magnetospirillum gryphiswaldense; mamZ; deletion; mamXY operon; magnetosome maturation
1 INTRODUCTION
Magnetotactic bacteria (MTB) are characterized by the ability to synthesize nano-scale magnetic particles (“magnetosomes”) that consist of a lipid bilayer and Fe3O4or Fe3S4(Frankel et al., 1979; Balk et al., 1980; Gorby et al., 1988; Heywood et al., 1990).Biosynthesis of magnetosomes is strictly controlled by a series of genes located mainly on a “magnetosome island” (MAI) (Schüler, 2004). InMagnetospirillumgryphiswaldensestrain MSR-1, MAI consists offeoAB1,mamAB,mamFDC,mamXY, andmms6operons (Wang et al., 2014). According to Murat et al.(2010), biomineralization of magnetosomes inM.magnetotacticumstrain AMB-1 involves f ive steps: (i) invagination of magnetosome vesicle from inner membrane, (ii) sorting of magnetosome proteins to magnetosome membrane, (iii) alignment of magnetosomes in a chain along a f ilamentous cytoskeletal structure, (iv) iron uptake and initiation of crystal formation, (v) crystal maturation. Each of these steps involves certain key proteins, and the steps are not entirely distinct from each other. The functions of MAI genes have been the focus of increasing study during the past decade. In 2014, Schüler’s group characterized the MAI genes inRhodospirillumrubrumand their roles in magnetosome biosynthesis(Kolinko et al., 2014). To better understand the molecular mechanism of magnetosome biomineralization, functions of specif ic MAI genes in other species and strains of MTB remain to be elucidated.
The present study was focused on four genes located in themamXYoperon of MSR-1:mamY,mamX,mamZ, andftsZ-like(Wang et al., 2014).MamY contains the Bin/amphiphysin/Rvs (BAR)domain, which displays direct binding activity to liposomes composed of phospholipids. In sedimentation assay, MamY was co-precipitated with the liposomes. Magnetosome vesicles inmamYdeletion mutant were larger than in wild-type (WT)strain (Tanaka et al., 2010). These f indings suggest that MamY functions to constrict the magnetosome membrane during vesicle formation. MamX contains two CXXCH heme-binding motifs typical ofc-type cytochromes (Raschdorf et al., 2013; Yang et al.,2013). FtsZ-like displayed both ATPase and GTPase activities, resulting in GTP-dependent polymerization into tubulin f ilament bundles in vitro (Ding et al.,2010).ftsZ-likeandmamXdeletion mutants formed small, superparamagnetic particles (Ding et al., 2010;Yang et al., 2013) that are similar to those of entiremamXYoperon deletion mutant (Lohße et al., 2011).
mamXandmamZhave a 32-bp region of sequence overlap in MSR-1 based on genome sequence and analysis in 2014 (Wang et al., 2014). The analysis ofmamXrevealed a close relationship withmamZ, and complementary functions among the proteins encoded bymamXYoperon (Yang et al., 2013). Schüler’s group proposed that MamZ, MamX, and MamH functionally interact in the magnetosome membrane to form an iron oxidoreductase / transport complex for magnetite biomineralization (Raschdorf et al.,2013). Nudelman and Zarivach (2014) presented bioinformatics analysis and structure predictions of important MAI genes that were consistent with our previous f indings (Ding et al., 2010; Yang et al.,2013). However, visual data of this sort must be conf irmed by various physiological and biochemical analyses.
In this study, we successfully constructed amamZdeletion mutant strain (ΔmamZ) and four complemented strains with diff eringmamZfragment lengths. The eff ects ofmamZdeletion on cell phenotypic and physiological characteristics in MSR-1, and on transcription levels of other genes were evaluated. Comparative analysis of the characteristics of these strains helped elucidate the function ofmamZ, and the relationships among key MAI genes.
2 MATERIAL AND METHOD
2.1 Bioinformatics analysis
Two templates were used for 3D structure prediction of the major facilitator superfamily (MFS)and the ferric-reduction superfamily (FRS) domains of MamZ: glycerol-3-phosphate transporter (GlpT),belonging to the MFS transport family fromEscherichiacoli(PDB ID: 1PW4), and partial cytochrome b of the cytochrome bc1 complex(complex III) fromSaccharomycescerevisiae(PDB ID: 3CX5). Homologue-modeling structures of the two domains were generated using the SWISSMODEL web server following target sequencing and uploading of corresponding template PDB format f iles. Generated PDB f iles were loaded into PyMOL molecular visualization system to produce visual protein models.
2.2 Bacterial strains and growth conditions
Bacterial strains and plasmids used in this study are listed in Table 1. MSR-1 WT and mutant strains were cultured in sodium lactate medium (SLM) at 30 °C with 100 r/min shaking (Rong et al., 2008).SLM was autoclaved before sterile ferric citrate added.E.colicells were cultured in Luria broth (LB)at 37 °C with 200 r/min shaking. Antibiotics used in culture media were as follows: for MSR-1 strains,gentamicin (Gm), tetracycline (Tc), and nalidixic acid(Nx) all at 5 μg/mL; forE.coli, ampicillin (Amp) at 100 μg/mL, Gm at 20 μg/mL, kanamycin (Km) at 50 μg/mL, and Tc at 12.5 μg/mL.
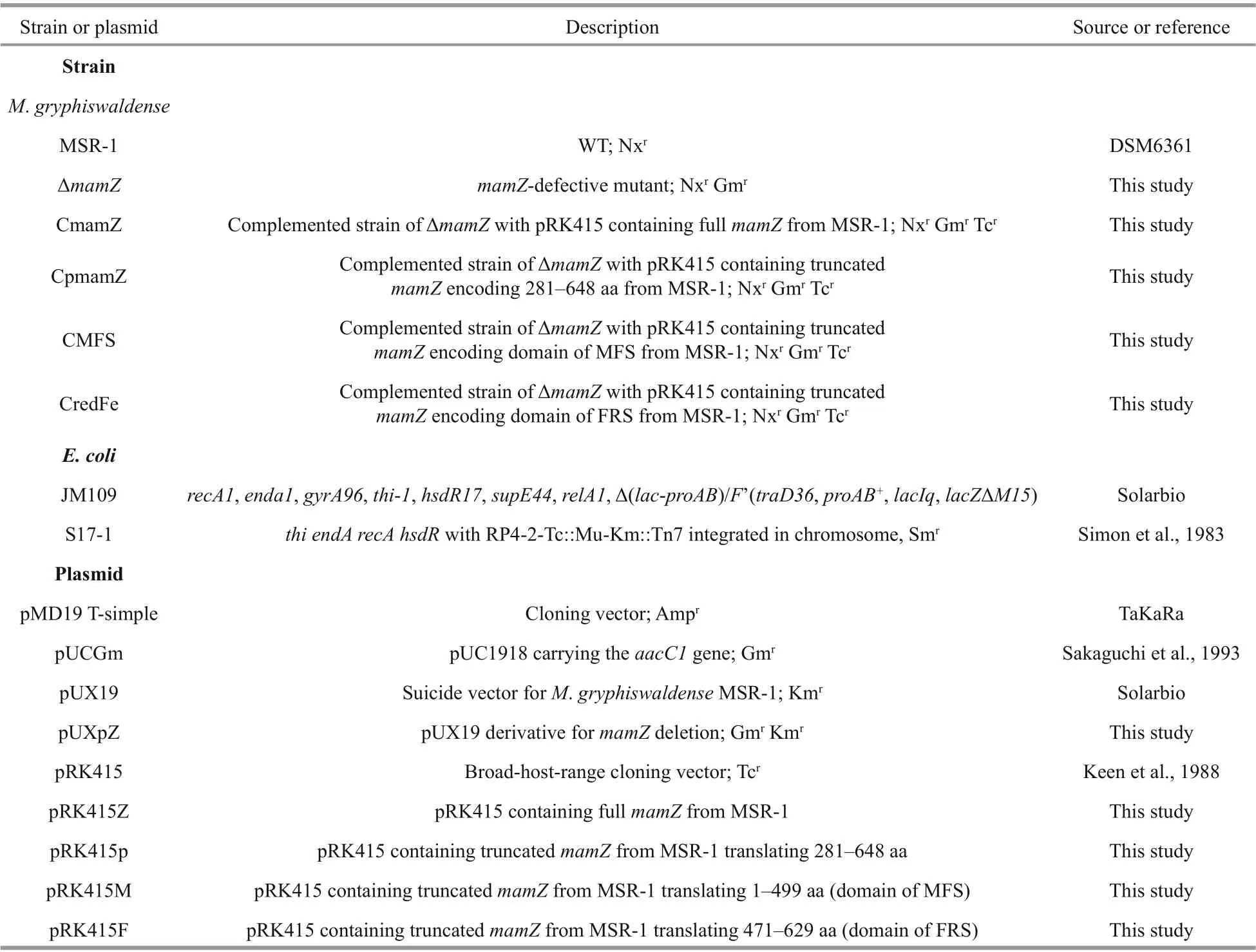
Table 1 Strains and plasmids used in this study
2.3 Construction of mamZ deletion mutant and its complemented strains
AmamZdeletion mutant of MSR-1 (termed ΔmamZ) was constructed by conjugation and subsequent homologous recombination. The primer sequences used are listed in Supplementary Table S1.Construction of ΔmamZis illustrated in Supplementary Fig.S1, and method described as previous (Yang et al., 2013). PCR primers for each fragment length to construction of complemented strains are listed in Supplementary Table S1. The respective truncatedmamZgenes were cloned into pRK415 and then introduced into ΔmamZby biparental conjugation.The four correct complemented strains (conf irmed by rigorous examination) were termed CmamZ (full lengthmamZ; 1 947 bp; 648 aa), CpmamZ (partial fragment; 281-648 aa), CMFS (N-terminal; MFS superfamily; 1-449 aa), and CredFe (C-terminal;ferric-reduction superfamily; 471-629 aa),respectively.
2.4 Cell growth curve and magnetic response(Cmag)
Cells were cultured in SLM at 30 °C with 100 r/min shaking (Wang et al., 2015a). Cell growth (OD565) and Cmag for each strain were measured at various sampling times as described previously (Wang et al.,2015a).
2.5 Transmission electron microscopy (TEM)
Cells were collected at 24 h, placed on copper or carbon grids, washed thrice with double-distilled H2O, and observed by TEM (model JEM-1230, JEOL;Tokyo, Japan) or high-resolution TEM (HR-TEM)(model JEM-2100, JEOL). Numbers and diameters of magnetosomes were analyzed statistically using ImageJ (National Institutes of Health; Bethesda, MD,USA), a Java-based image-processing program. Any two groups were compared with the analysis of independent sample T test by IBM SPSS Statistics.
2.6 Rock magnetic measurements
For rock magnetic measurements (Yang et al.,2013), room-temperature hysteresis loops and f irstorder reversal curves (FORC) of cells were measured by an alternating gradient force magnetometer (model MicroMag 3900, Princeton Measurements Corp.;Princeton, NJ, USA; sensitivity 5.0×10-10Am2) (Wang et al., 2015b).
2.7 Iron content and iron uptake of experimental strains
Strains were grown in SLM supplemented with 20-or 60- μ mol/L ferric citrate at 30 °C for 24 h, and harvested by centrifugation (8 000×g, 5 min). The precipitate was digested by nitric acid as described previously (Rong et al., 2008; Wang et al., 2013), and total intracellular iron content was assayed by inductively coupled plasma optical emission spectrometry (ICP-OES) (model Optima 5300DV,Perkin Elmer; Waltham, MA, USA).Strains were cultured in 100-mL SLM with 60-μmol/L ferric citrate and centrifuged at various sampling times. The supernatant was used for detection of iron concentration remaining in the medium by the ferrozine standard curve method(Wang et al., 2013). Ferric citrate was prepared in various concentrations, reduced to ferrous ions by hydroxylamine hydrochloride, reacted with ferrozine as a chromogenic reagent, and detected at OD562. A standard curve was generated based on values obtained. Residual iron concentration ( μ mol/L Fe2+)in supernatant was measured and calculated at each sampling time using the standard curve.
2.8 RNA extraction and RT-qPCR
Cells were cultured in 100-mL SLM and harvested by centrifugation (12 000×g, 1 min, 4 °C). Total RNA was purif ied using TRIzol reagent (Tiangen Biotech;Beijing, China) according to the manufacturer's instructions. Remaining genomic DNA in RNA was digested with RNase-free DNase I (TaKaRa, Japan).Quality and quantity of the extraction were assessed by A260nm/A280nmratio.
cDNA was obtained as described in our previous study (Wang et al., 2013). RT-qPCR was performed using a LightCycler 480 RT-PCR system and LightCycler 480 SYBR Green I Master Kit (Roche;Mannheim, Germany). The primers used for amplif ication of MAI genes are listed in Supplementary Table S2. TherpoCgene encoding RNA polymerase subunit β’ was chosen as internal control and reference. Transcription levels were analyzed by the 2-ΔΔCtmethod (Wang et al., 2013).
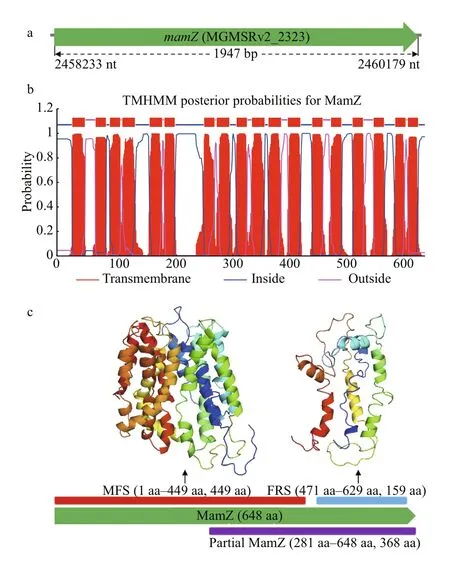
Fig.1 Protein structure analysis of MamZ
3 RESULT
3.1 Structural and functional prediction of mamZ gene
Orientation and composition ofmamZ, based on bioinformatics analysis, are summarized in Fig.1.mamZcontains 1 947 bp, encoding 648 aa residues.The genome number ofmamZin MSR-1 is MGMSRv2_2323 (Fig.1a). MamZ has 18 transmembrane helices (Fig.1b) containing two conserved domains. The 12 N-terminal helices belong to MFS (449 aa; Fig.1c), and have 64% identity with MamH in MTB. MFS, member of the large class of secondary active transporters presenting in cells of all kingdoms, is predicted to utilize the electrochemical potential of one substance to drive the transport of another substance (Madej et al., 2013; Madej and Kaback, 2013; Yan, 2013). The 6 C-terminal helices belong to FRS (159 aa; Fig.1c), and have high identity with YedZ inE.coli(Richter et al., 2007; Raschdorf et al., 2013; Nudelman and Zarivach, 2014). InE.coli,YedZ (a membrane-intrinsic cytochromebwith six putative transmembrane helices) and YedY (a soluble catalytic subunit with a twin arginine leader sequence)form a YedYZ complex that is a sulf ite oxidase homologue (von Rozycki et al., 2004; Brokx et al.,2005; Drew et al., 2005). MamZ appears to be responsible for iron transport using the N-terminal MFS region and ferric-reduction-related redox reaction of iron using the C-terminal FRS region.Sites for interaction with other DNAs, RNAs, and proteins are scattered throughout the gene sequence,but concentrated mainly in the C-terminus, particularly the 281-648 aa region (partial MamZ; Fig.1c).
Taking into account the multiple transmembrane domains of MamZ, we considered that complementation of the full-length gene would be diffi cult and problematic. Hence, we divided the complementary gene fragment into encoding fulllength MamZ (1-648 aa), N-terminal MFS (1-449 aa), C-terminal FRS (471-629 aa), and part of the extension of the C-terminal regions (partial MamZ,281-648 aa). The associated experimental design is summarized in Fig.1c.
3.2 mamZ deletion has no eff ect on cell growth, but results in loss of magnetic response
Careful examination and sequencing analysis conf irmed that the deletion mutant (ΔmamZ) strain showed no change relative tomamZat either the left or right side, thus ruling out a polarity eff ect. WT,ΔmamZ, and four complemented strains (integrity of MAI genes was ensured in each strain) were cultured in SLM supplemented with 20- or 60-μmol/L ferric citrate. Compared to WT, except that ΔmamZand CredFe lagging ~6 h, the growth rates of the other strains (CmamZ, CpmamZ, and CMFS) did not diff er notably (Fig.2a & c). Moreover, the maximal OD565of various strains distributed in 1.5-1.7 range. Cmag values for the strains are shown in Fig.2b & d. For WT under two iron concentrations, maximal Cmag values were observed at 20 h (0.788 under 20-μmol/L Fe3+; Fig.2b) and 14 h (1.10 under 60-μmol/L Fe3+;Fig.2d). Cmag value was zero for ΔmamZthroughout the incubation period, regardless of iron concentration.For full-length complemented strain CmamZ,maximal Camg values were 0.06 under 20-μmol/L and 0.10 under 60-μmol/L ferric citrate conditions that generally 10% of WT (Fig.2b & d). Under both conditions, Cmag values of CmamZ lagged ~2 h behind that of WT (Fig.2b & d). However, Cmag values of the other complemented strains (CpmamZ,CMFS, and CredFe) were the same as that of ΔmamZ.Thus, segmentalmamZwas insuffi cient to ΔmamZto generate enough Cmag value. RT-qPCR analysis showed thatmamZtranscription level was ~10-fold lower in CmamZ than in WT (Supplementary Fig S2).This result is consistent with Cmag values as compared to that of WT. The expression of full-length and segmentalmamZin the four complemented strains was reduced obviously, which might be another reason of the low Cmag values.

Table 2 Mean diameter and number of magnetosomes in WT, mamZ deletion mutant, and four complemented strains
3.3 Magnetosomes in Δ mamZ are smaller and more irregular
Magnetosomes in the six strains (WT, ΔmamZ,CmamZ, CpmamZ, CMFS, CredFe) were observed by HR-TEM. WT cells contained regular cubooctahedral magnetosomes with mean diameter 28.22±0.45 nm and typical magnetite crystal lattice(Table 2; Fig.3a-c). In ΔmamZ, compared to WT,magnetosomes had lower mean number (11.89±1.068),smaller mean diameter (17.58±0.304 nm) (Table 2;Fig.3d & e), and were more irregular with poor crystal lattice (Fig.3f). Magnetosomes in CmamZ (Fig.3g-i),CpmamZ (Fig.3j-l), CMFS (Fig.3m-o), and CredFe(Fig.3p-r) were irregular and smaller than in WT,whereas mean magnetosome numbers in the four complemented strains were higher than in WT. The complemented strains evidently lacked the ability to synthesize mature magnetosomes, and instead formed many abnormal, smaller crystals (Table 2). It implied that cells lost the ability to synthesize normal magnetosomes due tomamZexpression deleted or disrupted.
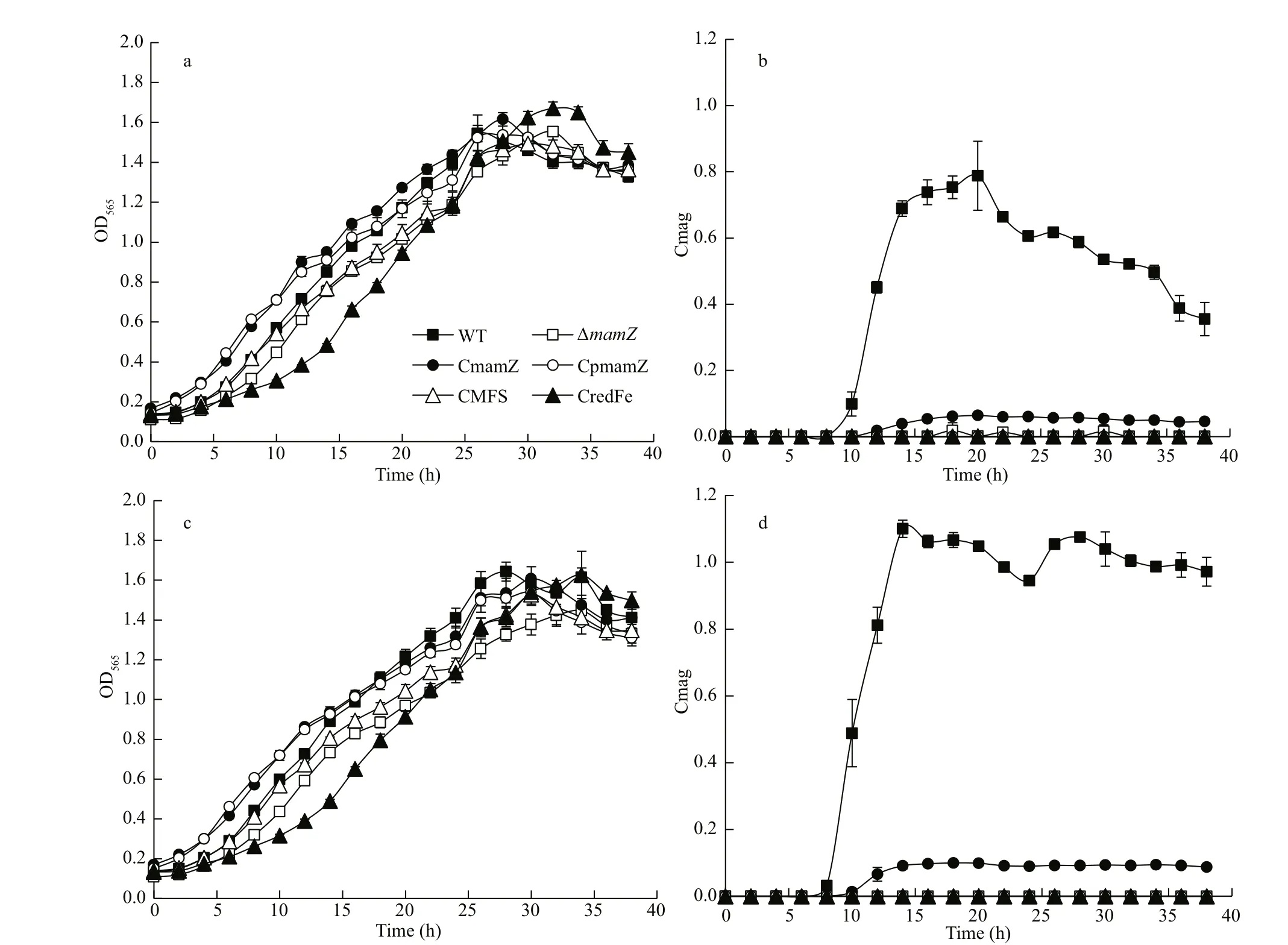
Fig.2 Comparison of cell growth and magnetic response (Cmag) in six strains cultured with 20-μmol/L (a, b) and 60-μmol/L(c, d) ferric citrate
3.4 Crystals in Δ mamZ are almost non-magnetic particles
The six strains were cultured with shaking for 24 h,and cells were harvested and collected by centrifugation. Magnetic properties of these minerals were analyzed using room-temperature hysteresis loops and FORC as described in Section 2. WT displays a typical Stoner-Wohlfarth type loop withHc,Hcr,Hcr/Hc, andMrs/Msvalues of 14.18 mT, 19.39 mT,1.37, and 0.40, respectively (Fig.4a1). The FORC diagram for WT (Fig.4a2) have closed inner contours centered around ~20 mT along the horizontal axis and with narrow distribution along the vertical axis, which further indicates the dominance of stable-singledomain-like particles. However, the hysteresis loop and FORC diagrams became noisy whenmamZwas deleted (Fig.4b1 & b2), indicating the extremely weak magnetism of cells. Apparent immature particles were still detected in some ΔmamZcells by HR-TEM,but were in the form of poor-lattice. Compared with WT, the coercivity values of CmamZ, CpmamZ,CMFS, and CredFe decreased to 4.39, 7.59, 4.08, and 10.67 mT, respectively (Fig.4c1, d1, f1), and FORC diagrams have closed contours for single domain particles centered below 20 mT and some outer contours for superparamagnetic particles perpendicular to the vertical axis close to the origin of FORC diagrams (Fig.4c2, d2, e2, f2). These results indicated that the four kinds of complemented strains synthesized magnetic particles, but the magnetism of complemented strains was signif icantly weaker than the magnetism of WT. That ismamZexpression,whether full-length or segmental, could repair ΔmamZpart capability of magnetosome biosynthesis.However, the magnetism of magnetic particles biosynthesized by complemented strains fails to reach that of WT.
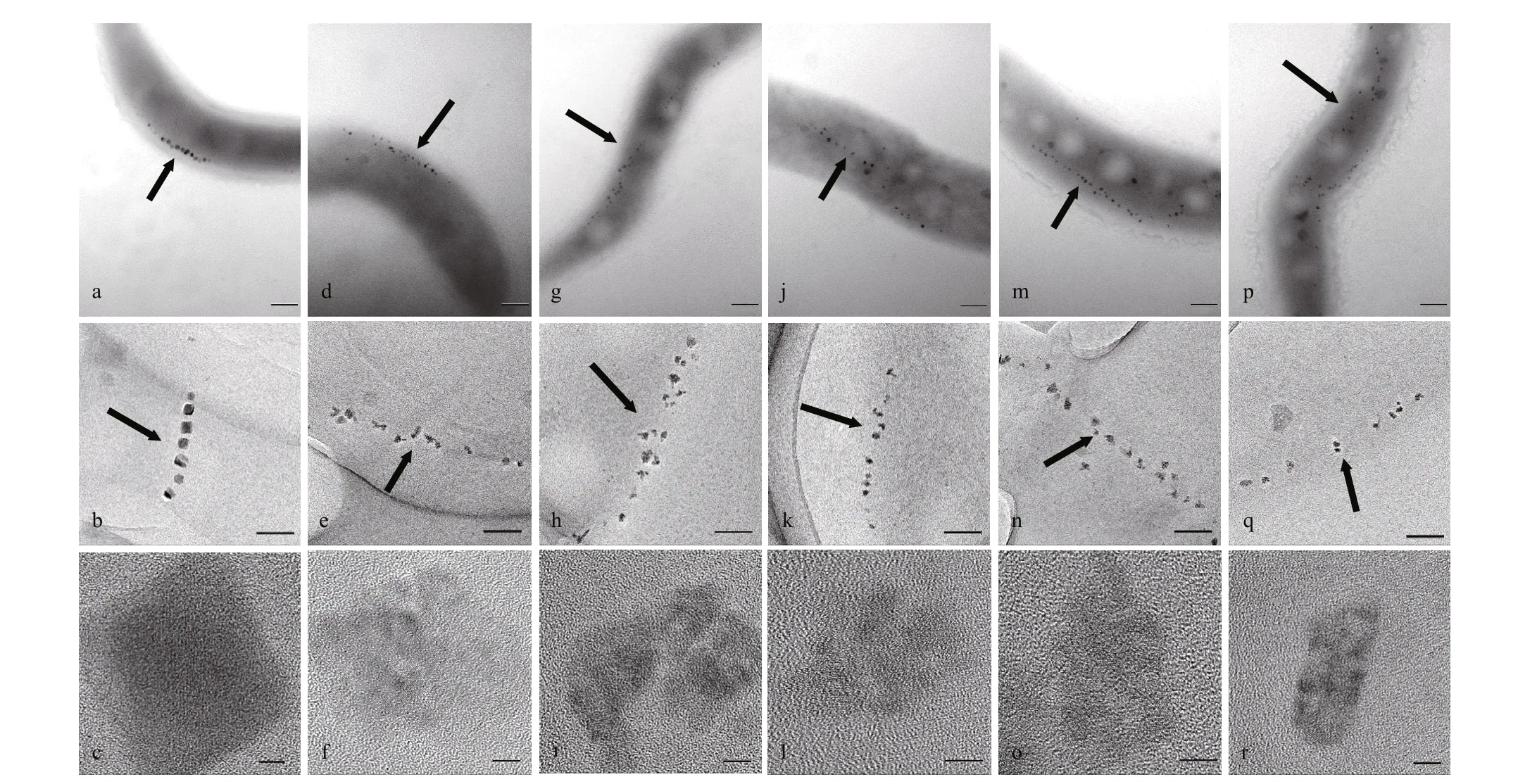
Fig.3 TEM and HR-TEM observations of six strains
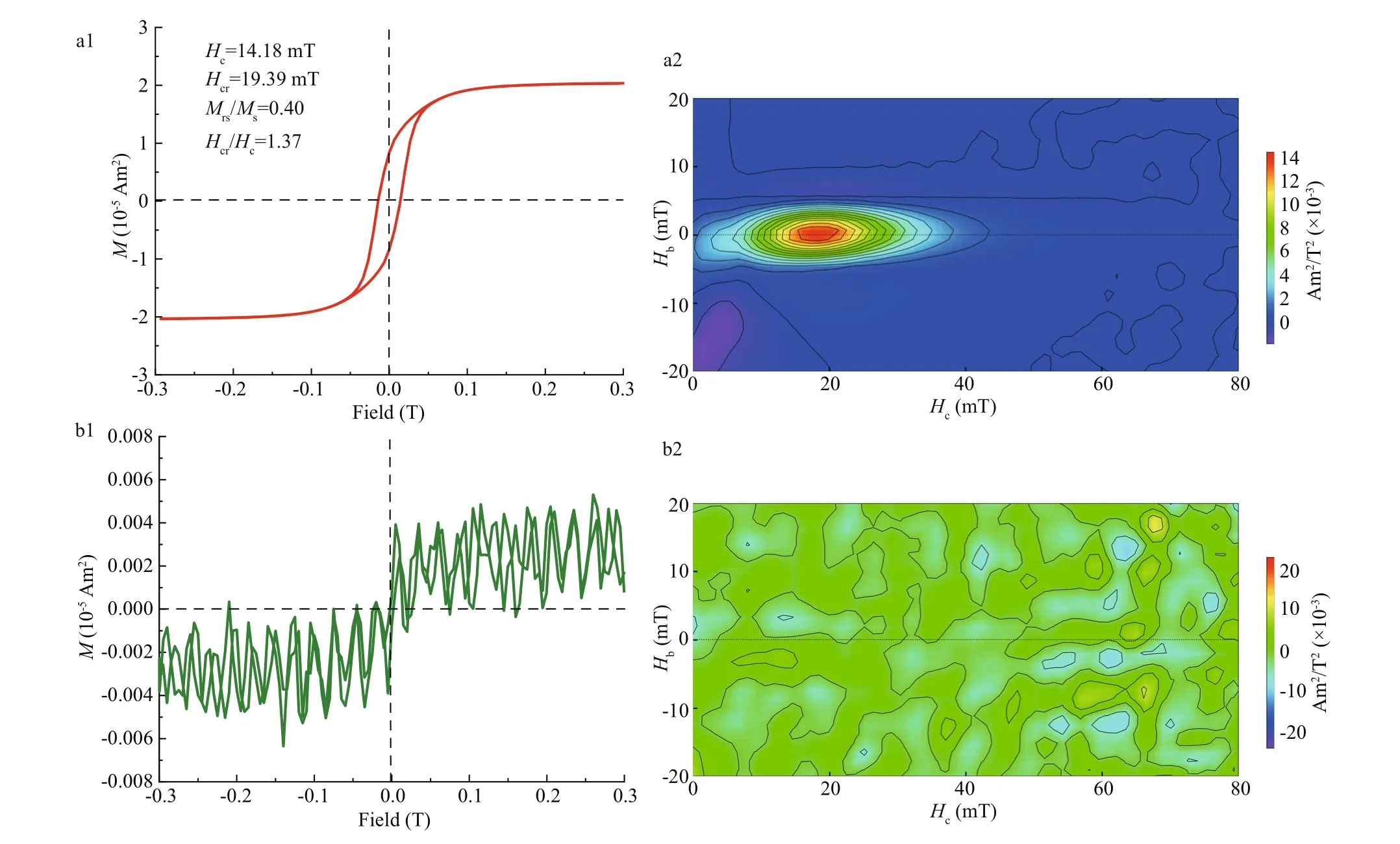
Fig.4 Room-temperature hysteresis loop and FORC for six strains
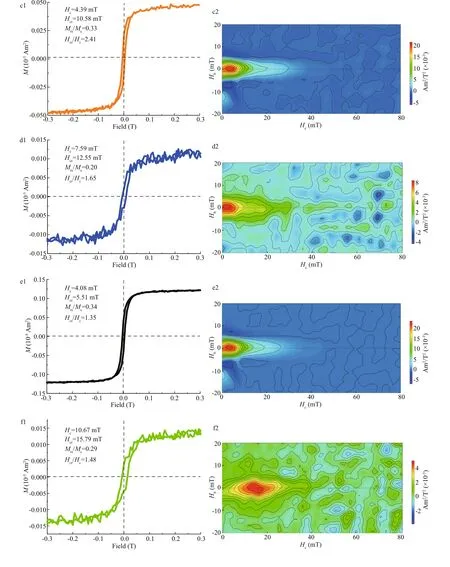
Fig.4 Continued
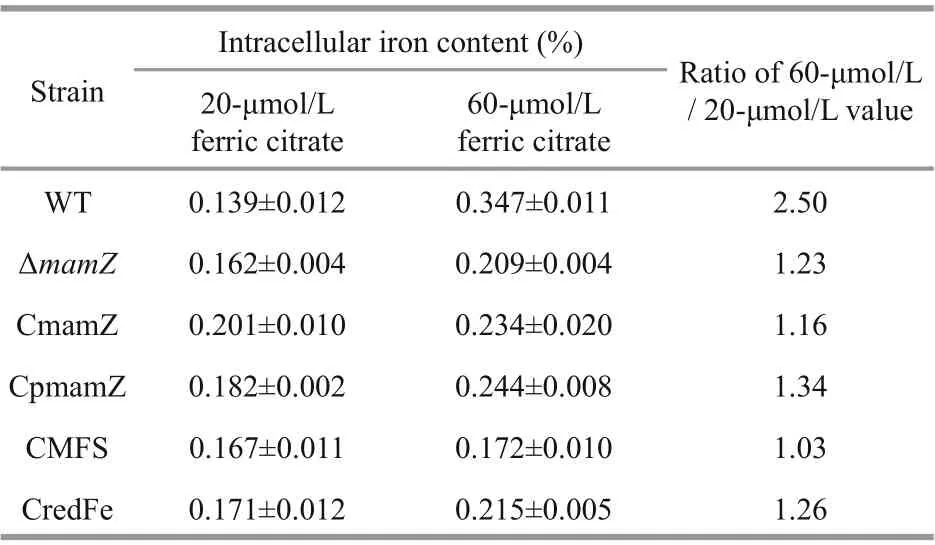
Table 3 Intracellular iron content of six strains cultured with 20- and 60-μmol/L ferric citrate
3.5 Increasing iron concentration reduces iron uptake ability of Δ mamZ
Previous studies demonstrated that 20- μ mol/L iron concentration is suffi cient for normal magnetosome biosynthesis in MSR-1 (Rong et al., 2008), but that cells could tolerate higher concentrations (Zhang et al., 2013). To further characterize ΔmamZand the four complemented strains, we measured iron uptake ability of cells cultured in media containing 20- and 60-μmol/L ferric citrate. Under 20-μmol/L ferric citrate condition, intracellular iron content was slightly higher in ΔmamZand the complemented strains than in WT, but otherwise did not diff er notably among the various strains (Table 3). For WT, iron content was 2.5-fold higher under 60-μmol/L compared to 20-μmol/L ferric citrate condition,indicating that iron uptake was stimulated by increasing iron concentration in medium. The other strains showed iron content enhancement in the 1.0-to 1.3-fold range (Table 3). In regard to iron uptake,the strains were next compared under 60- μ mol/L ferric citrate condition, including sampling at regular intervals of cell growth (OD565) and testing residual iron ion content in medium. Iron content in medium decreased gradually over time for all strains; however,residual iron content in medium during the culture process was highest for ΔmamZ(Supplementary Fig.S3). Thus,mamZdeletion resulted in reduced iron uptake when cells were cultured under high iron concentration. Among the complemented strains,residual iron content in medium was lower for CmamZ and CMFS than for CpmamZ and CredFe at each sampling time (Supplementary Fig.S3). This f inding is presumably due to the fact CmamZ and CMFS contain the integrated MFS domain of MamZ.Transformation of MFS domain into ΔmamZenhanced iron uptake under high-iron condition,consistent with predictions of MamZ structure.
3.6 Interaction and complementary characteristics of MamZ in the mamXY operon
To evaluate eff ects ofmamZdeletion on other genes in themamXYoperon, we measured relative transcription levels ofmamY,mamX, andftsZ-likein the six strains at diff erent growth stages, e.g., the six strains were cultured for 12 h (logarithmic phase of cell growth; OD565~0.45) and for 18 h (early stage of stationary phase; OD565~0.80). Relative transcription level ofmamYwas slightly higher in ΔmamZ, CmamZ,CMFS, and CredFe than in WT at 12 h and 18 h, and at 18 h was 1- to 2-fold higher in CpmamZ than in the other strains (Fig.5a).mamXlevel at 12 h was slightly higher in ΔmamZ, CmamZ, and CredFe than in WT(Fig.5b). Compared to 12 h,mamXlevel at 18 h was enhanced signif icantly in WT, but to a lesser degree in the other strains (Fig.5b).ftsZ-likelevel was upregulated moderately at 12 h in ΔmamZand the complemented strains, and signif icantly (1- to 5-fold)at 18 h, particularly in CmamZ, CpmamZ, and CMFS(Fig.5c). Hence, the relative transcription levels ofmamY,mamX, andftsZ-likein ΔmamZor the complemented strains were upregulated with varying degrees. In the model of MamXY proteins during magnetosome formation, MamZ was expressed at last stage and formed complexes with MamX and FtsZlike (Wang et al., 2019). However, there had no interaction between MamX and MamZ, and they were separately recruited by FtsZ-like at diff erent stages, respectively. It is a pity that only one interaction site was detected in MamZ with FtsZ-like. This might be the reason thatftsZ-likeexpressed most signif icant,especially at 18 h in CmamZ, CMFS, and CpmamZ,the next wasmamX, and the last wasmamY. Two of themamXYoperon genes (mamXandftsZ-like) were clearly upregulated during the stationary phase of cell growth, which proved the corresponds to the gradual maturation stage of magnetosomes once again.
3.7 Relationship between mamXY and mms6
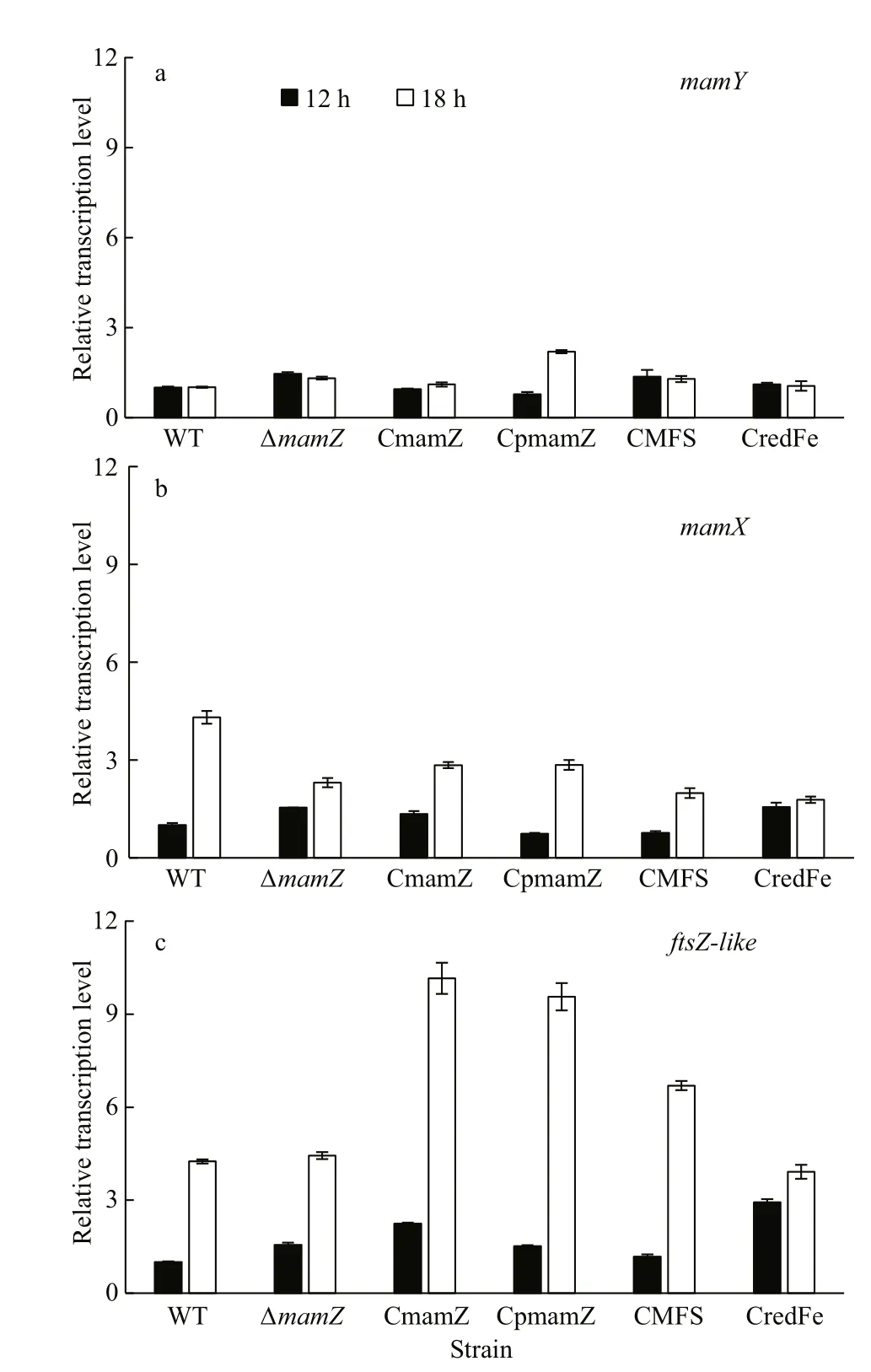
Fig.5 Relative transcription levels of mamY, mamX, and ftsZ- like in WT, Δ mamZ, and the four complemented strains incubated for 12 h and 18 h
There is sequence overlap (32 bp) betweenmamXandmamZ(Wang et al., 2014), and deletion of either Transcription levels ofmamXand (particularly)ftsZ-likewere upregulated at 18 h for all strains, indicating that the encoded proteins play essential roles in the magnetosome maturation process.gene led to formation of similar poor-lattice magnetosomes (Yang et al., 2013). In addition to ΔmamZand its complemented, we therefore performed additional experiments on ΔmamXand its complemented strain (CmamX), simultaneously.
The MAI consists offeoAB1,mamAB,mamFDC,mamXY, andmms6operons. We randomly selected important genes located in operons other thanmamXY,and determined relative transcription levels of these genes in ΔmamZand its complemented strains. The seven selected genes, and primers used, are listed in Supplementary Table S2. Transcription level ofmms6was much higher than that of the other genes in ΔmamZand the four complemented strains at both 12 h (Fig.6a) and 18 h (Fig.6b). In particular,mms6transcription level was 10- to 50-fold higher than those of the other genes in ΔmamZat 12 h, and >50-fold higher in the complemented strains at 18 h.Levels ofmamBandmamFwere slightly enhanced in CredFe at 12 h, and those ofmamFandmamEwere two-fold higher in CmamZ, CpmamZ, and CMFS at 18 h. Similar, the results were obtained for CmamX(Fig.6c & d). Levels of certain genes were higher in complemented strains; e. g.,mamF,mamN,mamBincreased ~2-fold.mms6level was increased 15-fold in CmamX at 12 h (Fig.6c), and 12-fold at 18 h(Fig.6d). The other genes did not show notable changes relative to WT at 18 h (Fig.6d).
mamZdeletion caused a dramatic increase inmms6transcription during the logarithmic phase of cell growth (12 h). In the complemented strains, maximal levels ofmms6transcription were observed during the stationary phase (18 h), coinciding with the gradual maturation stage of magnetosomes. To rule out a possible eff ect of the plasmid itself, a control strain containing an empty plasmid was subjected to RT-qPCR analysis.mms6was not upregulated at either logarithmic phase or stationary phase(Supplementary Fig.S4). Our f indings suggested that exogenous fragments caused signif icant upregulation ofmms6, and that some type of interaction occurred among the fragments. We propose that MamXY interacts with Mms6 during the magnetosome maturation process.
4 DISCUSSION
4.1 MamZ proteins function primarily during the magnetosome maturation process in MSR-1 cells
Based on the present and previous f indings, we hypothesize that the four proteins encoded by genes of themamXYoperon play important roles in the magnetosome maturation process (corresponding to the stationary phase of cell growth). To elucidate the function of the highly conserved MamZ protein in MSR-1, we constructed a mutantmamZdeletion strain (ΔmamZ), f irstly.mamZdeletion had no eff ect on growth curve, but notably reduced magnetic response.
ΔmamZcells showed very weak magnetism of immature magnetosomes. Magnetosomes in ΔmamZcells were small, irregular, and consisted of poorlattice crystals, as demonstrated by HR-TEM images,similar to those ofmamX,ftsZ-like, and integratedmamXYoperon deletion mutant strains (Ding et al.,2010; Murat et al., 2010; Yang et al., 2013), consistent with f indings by Schüler’s group (Raschdorf et al.,2013). Besides, Yang et al. (2013) show that transcription level ofmamZis much higher than those of othermamXYoperon genes during the period of magnetosome biosynthesis. MamZ evidently plays as important cooperative role in the magnetosome maturation process.

Fig.6 Relative transcription levels of genes in MAI operons other than the mamXY operon
Bioinformatics analysis revealed two conserved domains in MamZ consisting of 18 transmembrane helices. The N-terminal domain, MFS, is homologous to MamH encoded within themamABoperon(Raschdorf et al., 2013). The ability of MFS transporters was to move a variety of small compounds across biological membranes (Marger and Saier,1993; Pao et al., 1998; Reddy et al., 2012). The C-terminal domain (helices belonging to FRS) shows highest similarity to YedZ inE.coli(Richter et al.,2007). YedZ is a heme B-binding membrane protein consisting of six putative transmembrane helices,while YedY is a soluble catalytic subunit inE.coli(Loschi et al., 2004; Brokx et al., 2005). YedY anchored to YedZ is a highly conserved bacterial heme-molybdoenzyme of the sulf ite oxidase class found in a variety of bacteria. The importance of YedZ-like C-terminal domain in MamZ was demonstrated by Schüler’s group, who deleted the YedZ-like domain and observed magnetosomes in resulting strainmamZΔ438-639 similar to those in ΔmamZ(deletion of full-lengthmamZ) (Raschdorf et al., 2013). Therefore, the N-terminal MFS domain was focused on in this study. Besides, sites in MamZ for interaction with other DNAs, RNAs, and proteins mainly scatter in 281-648 aa region. Four complemented strains with diff eringmamZfragment lengths (CmamZ, CpmamZ, CMFS, and CredFe)were constructed. Results from room-temperature hysteresis loops and FORC showed that the magnetism of cells was strengthen whenmamZ, whether fulllength or segmental, expressed in ΔmamZ. However,Cmag values of cells were still zero as long as partialmamZwas expressed in ΔmamZ. These suggested that physical properties of magnetosomes might be changed (e.g., composition, structure) (Ding et al.,2010; Murat et al., 2010; Yang et al., 2013) and various domains in MamZ might play indispensable roles in magnetosome maturation process. In iron uptake study, strains showed various iron uptake capability from medium during 0-24 h in a declining order of CmamZ, CMFS, CpmamZ, and CredFe(Supplementary Fig.S3). The diff erence implied the importance role of MFS in MamZ to transport iron,consistent with the analysis of bioinformation.Compared to CmamZ, CMFS, and CredFe, CpmamZ exhibited a mid-state in all of phenotypic and physiological experiments. These might be related to interaction sites scattering in partial MFS and integrated FRS. Multiple transmembrane helices and low transcription levels of MamZ could be other reasons leading to complemented strains, even CmamZ, lack the capacity for normal magnetosome maturation, and instead produce large numbers of immature particles.
4.2 Relationships (interactions) among mamZ and other key MAI genes
We determined relative transcription levels of important MAI genes by RT-qPCR. Deletion ofmamZresulted in upregulation of three other genes in themamXYoperon, indicating a close relationship among the four encoded proteins (Yang et al., 2013). In view of the overlapping region betweenmamZandmamXgene, we evaluated transcription levels of MAI genes in the corresponding deletion mutants (ΔmamZ,ΔmamX) and their complemented strains, in comparison to WT. Of these genes, onlymms6showed greatly increased transcription; particularly, a 10- to 50-fold increase in the complemented strains.Previous studies have shown that Mms6 protein has a hydrophobic N-terminal region involving an interlaced structure of intra- and inter-molecular interactions, and a hydrophilic C-terminal region including an iron-binding site that plays a major role in regulation of magnetite crystal size and morphology(Arakaki et al., 2003, 2010, 2014; Amemiya et al.,2007; Feng et al., 2013). Mms6 was proposed as a specif ic magnetite nucleation protein and the key features for self-assembly to display a charged surface for specif ic iron binding, with the curvature of the surfaces determining the particle size (Staniland and Rawlings, 2016). The C-terminal region of MamZ is predicted to be a ferric-reduction domain with high similarity to YedZ inE.coli, including homologous conserved histidyl residues in the transmembrane domains that function in heme binding (von Rozycki et al., 2004). MamX contains two CXXCH hemebinding motifs typical ofc-type cytochromes, which may function in electron transport or as redox buff ers during magnetite formation (Siponen et al., 2012;Yang et al., 2013). Deletion ofmamZ,mamX, ormms6resulted in reduced magnetosome size (Amemiya et al., 2007; Tanaka et al., 2011; Raschdorf et al., 2013;Yang et al., 2013; Kashyap et al., 2014).
We hypothesize that MamX and MamZ proteins, in addition to having a close complementary or cooperative relationship with each other, are associated with Mms6, a protein that plays an important role in promotion of magnetosome biosynthesis both in vitro and in vivo. The several proteins encoded by MAI genes cooperate closely with each other and are all involved in magnetosome biosynthesis and maturation; none of them can be omitted. The proteins encoded bymamXYoperon genes are closely related to Mms6, which was also supported by Wang et al. (2019). It indicates that Mms6 is also essential to the magnetosome biosynthesis process. Due to the presence of multiple transmembrane domains, heterologous expression of the MamZ protein is quite diffi cult and we are trying to perform analysis of protein interaction in vitro. In addition, most of the current studies on the function of key proteins (such as Mms6) have been conducted in vitro (Nguyen et al., 2016; Peigneux et al., 2019;Rawlings et al., 2020), and our next experiments aim at intracellular analysis of the relationship between MamZ, Mms6, and several key proteins. Moreover,the MamZ structure includes numerous binding sites for proteins and nucleic acids (both DNA and RNA),indicating that this protein interacts with such macromolecules. Studies are in progress to test this idea using a new experimental strategy for in vivo analysis.
5 CONCLUSION
In this study, amamZdeleted mutantMagnetospirillumgryphiswaldenseMSR-1 (ΔmamZ)was obtained successfully. Phenotypic and physiological experiments proved that MamZ evidently plays an important role in the magnetosome maturation process. The results of transcriptional levels by RT-qPCR showed the close relationship betweenmamZand other genes inmamXYoperon during the maturation process of magnetosome,especially the interaction between Mms6 and MamXY worthy for further attention.
6 DATA AVAILABILITY STATEMENT
All the data supporting the results of this study are available within the article and the supplementary material.
7 ACKNOWLEDGMENT
The authors are grateful to Dr. Yinzhao WANG(Institute of Geology and Geophysics, Chinese Academy of Sciences) for technical help, and to Dr. S.ANDERSON for English editing of the manuscript.
杂志排行
Journal of Oceanology and Limnology的其它文章
- Maximum sustainable yield estimation of enhancement species with the characteristics of movement inside and outside marine ranching*
- Magnetotactic bacteria from the human gut microbiome associated with orientation and navigation regions of the brain*
- How light aff ect the magnetotactic behavior and reproduction of ellipsoidal multicellular magnetoglobules?*
- Biocompatibility of marine magnetotactic ovoid strain MO-1 for in vivo application*
- Determination of the heating effi ciency of magnetotactic bacteria in alternating magnetic f ield*
- An approach to determine coeffi cients of logarithmic velocity vertical prof ile in the bottom boundary layer*
