A new species Lobophora tsengii sp. nov. (Dictyotales;Phaeophyceae) from Bach Long Vy (Bailongwei) Island,Vietnam*
2021-12-09ZhongminSUNMinhDongDAOQuocToanTRANDucTienDAM
Zhongmin SUN , Minh Dong DAO, Quoc Toan TRAN , , Duc Tien DAM,5,6 ,
1 Department of Marine Organism Taxonomy and Phylogeny, Institute of Oceanology, Chinese Academy of Sciences, Qingdao 266071, China
2 University of Chinese Academy of Sciences, Beijing 100049, China
3 Graduate University of Science and Technology, Vietnam Academy of Science and Technology (VAST), Hanoi 100000, Vietnam
4 Institute of Natural Products Chemistry, Vietnam Academy of Science and Technology (VAST), Cau Giay, Hanoi 100000, Vietnam
5 Institute of Marine Environment and Resources (IMER), Vietnam Academy of Science and Technology (VAST), Haiphong 180000, Vietnam
6 Haiphong University of Medicine and Pharmacy, Haiphong 180000, Vietnam
Abstract A new species Lobophora tsengii is described from Bach Long Vy (Bailongwei) Island,Haiphong Province, Vietnam. The plants inhabit the subtidal zone and have predominantly erect fan-shaped thalli that attach to the substrate by a basal holdfast. The thallus is commonly composed of a single layer of large medullary cells with four layers of cortical cells on either side of the medulla. The newly collected specimens from the island are morphologically similar to those from Hainan Island, China, but diff er from the New Caledonian ones in having thicker thallus. In molecular phylogenetic analyses based on concatenated rbcL and cox3 s equences, the specimens from the island were 100% identical to those from Hainan Island,China, and they formed a clade separating from other Lobophora species. Additionally, based on analyses of numerous cox3 s equences, our specimens were also distinguished from the closely related taxa occurring in New Caledonia, Kenya, and West Australia. Combining the morphological and molecular analyses, we conclude that our specimens represent a new species of Lobophora, which is apparently endemic to the Beibu Gulf (Gulf of Tonkin) and adjacent waters.
Keyword: Bach Long Vy (Bailongwei) Island; cox3; Lobophora; molecular phylogeny; morphology; rbcL
1 INTRODUCTION
The brown algaLobophora(Dictyotales,Phaeophyceae) occurs in tropical and subtropical seas around the world. The genus is characterized by a marginal row of meristematic cells and a singlelayered large central medulla in the thallus(Womersley, 1967, 1987). It is diffi cult to identify species based on morphological criteria alone, and the recent molecular phylogenetic analyses suggest that the species level divergence is considerably underestimated; as consequence, a dozen new species have been described (Sun et al., 2012; Vieira et al.,2014, 2016, 2019; Schultz et al., 2015; Camacho et al., 2019). So far, 45 specif ic epithets ofLobophoraare listed in AlgaeBase (Guiry and Guiry, 2020), and it is estimated that more than 100 species are present in this genus (Vieira et al., 2017).
Previously, a single species ofLobophora, i.e.L.variegata(J. V. Lamouroux) Womersley ex Oliveira, was reported from Bach Long Vy(Bailongwei) Island, a small island located in the center of the Beibu Gulf (Gulf of Tonkin) (Dam,1997). This species with erect fan-shaped from Vietnam was identif ied asL.variegatausing gross morphological characters (Pham, 1969; Nguyen et al., 1993; Dam, 1997, 2004; Van Nguyen et al., 2013);however,L.variegatais not geographically distributed in the Pacif ic (Schultz et al., 2015; Vieira et al., 2016).In recent years, some new species have been reported from Vietnam and the neighbor waters (Sun et al.,2012; Phang et al., 2016; Vieira et al., 2016), and therefore in the present study, we conducted a taxonomic study ofLobophorafound in Gulf of Tonkin, Vietnam,using morphological and molecular data.

Fig.1 Map showing the collection sites around Bach Long Vy (Bailongwei) Island (red arrows) in the present study
2 MATERIAL AND METHOD
2.1 Sampling and morphological analysis
New specimens were collected by snorkeling or Self-Contained Underwater Breathing Apparatus(SCUBA) diving from three sites (site 1: 20°08′15″N,107°43′36″E; site 2: 20°08′01″N, 107°44′06″E; site 3: 20°07′39″N, 107°44′03″E) around Bach Long Vy(Bailongwei) Island (Fig.1). Specimens for molecular studies were desiccated immediately in silica gel.Other specimens were dried and mounted on herbarium sheets. All the herbaria were deposited in the Marine Biological Museum of the Institute of Marine Environment and Resources (MBMIMER),Haiphong, Vietnam. For morphological observations,thalli were sectioned manually using a razor blade and mounted on glass slides in Karo Syrup/seawater.Photographs were taken with a Motic BA300 microscope (Taiwan, China) equipped with a ZEISS A-Plan 40X/0, 65 N. A. Phase contrast 2 objective(Zeiss, Germany) and an Olympus TG 5 digital camera (Japan).
2.2 DNA sequencing and phylogenetic analysis
Genomic DNA was extracted from the silica geldried specimens and herbarium specimens(morphology analyzed later) using a DNeasy®Plant Mini Kit (Qiagen, Hiden, Germany), according to the manufacturer’s instructions. TherbcL andcox3genes were PCR amplif ied with TaKaRa Ex Taq enzyme in 25-μL reaction column (TaKaRa, Japan). Primers and PCR conditions were as those described by Sun et al.(2012). The DNA sequencing was performed by Shanghai Sangon Biotechnology Co. Ltd. (Shanghai,China).
Eight specimens from Bach Long Vy Island were newly sequenced. The sequences of specimens from Hainan Island (Sun et al., 2012) were also added to analyze. A concatenated data set consisting of 24rbcL and 24cox3sequences from GenBank, respectively corresponding to 21 specimens, included 15 species ofLobophoraand two species ofZonaria(outgroup).A secondcox3data set consisting of sequences from 47 specimens ofL.rosacea, 6 specimens ofLobophorasp. 43 (Vieira et al., 2017, 2019), and 3 specimens of the new species in this study.
The concatenated nucleotide matrices were generated with PhyloSuite (Zhang et al., 2020). The new sequences and the previously published ones for phylogenetic analyses were aligned with MAFFT v7.313 (Katoh and Standley, 2013) and then manually adjusted. The maximum likelihood (ML) and Bayesian inference (BI) analysis were carried out to construct phylogenetic trees by using the software of IQ-TREE v1.6 (Nguyen et al., 2015) and MrBayes v3.2.6 (Ronquist et al., 2012), respectively. The best substitution model for each analysis was evaluated by Partitionf inder 2.0 (Lanfear et al., 2017). For BI analysis, two independent analyses were run with four chains each for 100 million generations, with the f irst 25% of the resulting trees was discarded as burnin. FigTree v.1.4.2 (http://tree.bio.ed.ac.uk/software/f igtree/) was used to visualize the trees.
3 RESULT
3.1 Molecular phylogenetic analysis

Fig.2 Maximum-likelihood tree based on rbcL and cox3 gene sequences
Although numerous specimens were obtained from the three sites of Bach Long Vy Island and from two sides of Hainan Island (Changjiang in the west coast,Changpo in the east coast), a single identical sequence was obtained for therbcL gene (1 348 bp) and thecox3gene (690 bp) respectively. The specimens from Bach Long Vy Island, Vietnam were 100% identical to those from Hainan Island, China, but diff ered fromLobophorarosaceaVieira, Payri et De Clerck from New Caledonia. In the phylogenic tree based on concatenatedrbcL andcox3sequences data set(Fig.2), the two species ofZonariawere designated the outgroup, and the remaining 13 species plus several unnamedLobophoraspp. formed a largeLobophoraclade. An Atlantic speciesL.dispersa(LAF06738, LAF06786) Camacho, Freshwater et Fredericq showed the closest relation with the two taxa ofLobophorafrom Vietnam and China mentioned above.
In the phylogenic tree based oncox3sequences data set (Fig.3), most specimens ofLobophorarosaceashared the same sequence, including the type specimens IRD10213 and one specimen from Kenya(ODC1571). In contrast, the specimens from Hainan and Bach Long Vy Islands had a long genetic distance from other ones and showed a closer relation to those identif ied asLobophorasp. 43 from West Australia.
3.2 Morphological analysis
Fan-shaped thalli grew on the substrate in the subtidal zone, attached with a basal holdfast. Plants frequently overlapped each other and formed a dense rosette (Fig.4a). Larger thalli were predominantly erect, spirally arranged, and up to 9-cm wide and 8-cm high. Fresh specimens were yellow to light brown in color but became dark brown when dried(Fig.4b). Anatomically, the mid-region of the thallus was commonly composed of nine layers, including a single layer of large medullary cells and four layers of small cortical cells on dorsal and ventral sides (Fig.4c& d). Sporangial sori were scattered on ventral surface of the mature thallus (Fig.4e), and sporangia were sessile and ovate without paraphyses in section view(Fig.4f). Male and female reproductive structures were undetected. Some mature specimens with sporangia had seven layers, the medullary layer surrounded by dorsal and ventral layers of cortical cells with three layers (Fig.4f).
3.3 Taxonomy
LobophoratsengiiD. Tien et Z. Sun sp. nov.
=Lobophorasp. 67 Vieira et al. (2016)
=LobophorarosaceaSun et al. (2017)

Fig.3 Maximum-likelihood tree based on cox3 gene sequences of closely related taxa
Description:
Large fan-shaped thallus, up to 9-cm wide and 8-cm high, strongly attaching to the substrate by a basal holdfast. Surface smooth, yellow to brown in color. Thallus 90-190-μm thick, composed of a single layer of large medullary cells and three to four layers of dorsal and ventral cortical cells. The gene sequences(MT779811 and MT779812) for therbcL andcox3genes from the type specimen (IMER19036) were unique.
Type locality: Bach Long Vy (Bailongwei) Island,Haiphong, Vietnam.
Holotype designated here: IMER19036, a dried herbarium specimen, deposited in the Marine Biological Museum of the Institute of Marine Environment and Resources (MBMIMER),Haiphong, Vietnam.
Habitat: Growing on hard substrates in subtidal zone 2-7-m deep, commonly growing with other macroalgae.
Etymology: Named after the Chinese phycologist and taxonomist Cheng Kwei Tseng.
Distribution: Haiphong, Vietnam; Guangdong,Hainan, China.
Specimens examined: Bach Long Vy Island,Haiphong, Vietnam, Dam Duc Tien, 28 July 1993(HIO93023, HIO93041); Bach Long Vy Island,Haiphong, Vietnam, Dam Duc Tien, 16 May 2003(HIO03216, HIO03244, HIO03310); Bach Long Vy Island, Haiphong, Vietnam, Dam Duc Tien and Dao Minh Dong, 14 July 2019 (IMER19036-10942).
Remark:Lobophoratsengiimostly closely resemblesL.rosaceabut diff ers from the later in having a thicker thallus caused by the incrassation of one or two cortical layers, and the distinctive DNA sequences MT779811 and MT779812.
4 DISCUSSION
The specimens ofLobophorafrom Beibu (Tonkin)Gulf and Hainan Island are morphologically similar to those from New Caledonia, in having a basal mound of rhizoids, a predominantly erect and fanshaped thallus that is arranged into a dense rosette(Table 1). Vieira et al. (2014) documented two morphotypes ofL.rosaceafrom New Caledonia,including the thinner type with f ive to seven cell layers, and the thicker type with f ive to eight cell layers. Although some thalli from Beibu Gulf and Hainan Island, such as those with sporangial sori,tend to be thinner, our specimens always have more than seven layers (commonly nine layers, with one medullary layer, four dorsal cortical layers, and four ventral cortical layers), and f ive layers are never present. Consequently, our new species are morphologically diff erent fromL.rosaceadue to the increase of one or two cortical layers.
In the concatenatedrbcL andcox3phylogenetic tree, the Vietnamese and Chinese specimens formed a clade that separated from otherLobophoraspecies(Fig.2). Unfortunately, only a few availablerbcL sequences ofL.rosaceaand its closely related taxa were present in GenBank, which were able to correspond to theircox3sequences. TherbcL sequence of the type specimen (IRD10213) ofL.rosaceawas probably mistaken, because it diff eredfrom those of other conspecif ic taxa in more than 8%base substitution. The specimens ofL.obscuraVieira,De Clerck et Payri (NVT093) andL.asiaticaSun,Tanaka et Kawai (NVT052), which were also present in the phylogenetic tree.
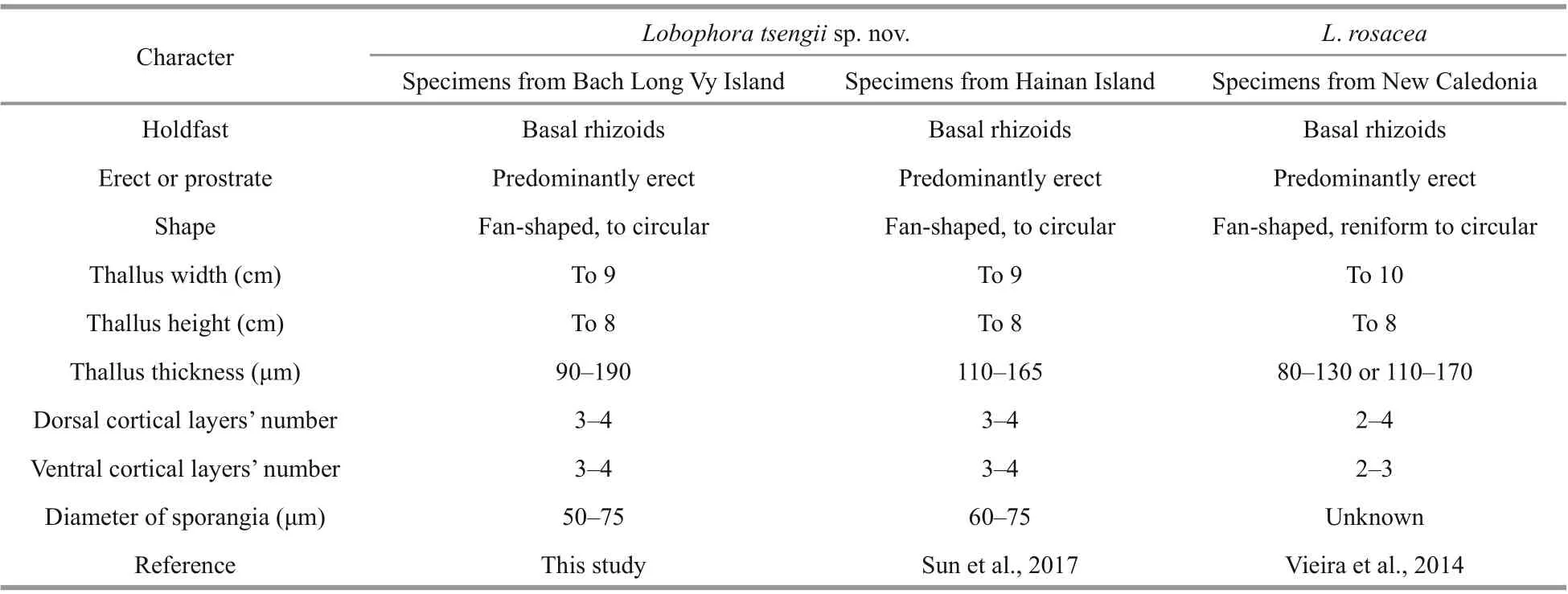
Table 1 Comparison of morphological characters among the specimens from Bach Long Vy Island, Hainan Island, and New Caledonia
Additionally, the phylogenetic tree based on all publishedcox3sequences closely related toL.rosacea,L.tsengii, andLobophorasp. 43 (Fig.3),demonstrated thatL.tsengiiwas separated from the related taxa. Vieira et al. (2016, 2017) had conducted species delimitation analyses withcox3sequences ofLobophoraand treated the specimen (MBM616)from Hainan Island as a separate species (Lobophorasp. 67), instead of merging it intoL.rosacea. Almost simultaneously, Sun et al. (2017) reported the same specimens from Hainan Island as a population ofL.rosaceawithout the references of Vieira et al.(2016, 2017). Subsequently, another closely relatedLobophorasp. 43 was detected from Coral Bay,Western Australia based oncox3sequences, without morphological description (Vieira et al., 2017, 2019).
Vieira et al. (2017) suggested that mostLobophoraspecies have small ranges limited to marine realms. It seemsL.rosaceais endemic in New Caledonia,expect one specimen (ODC1571) collected from Kenya, probably due to the equator warm current in the Indian Ocean.Lobophorasp. 43, which showed a closer genetic distance toL.tsengiithanL.rosacea,may be endemic in West Australia.L.tsengiiis distributed around Hainan Island, rare in the south coast and abundant in the west coast and Beibu Gulf.Since the morphological and molecular characters and geographic distribution of our specimens are diff erent from the previously described species, it is reasonable to identify them as a separate species.Considering Bach Long Vy Island where this species grows abundantly, we took this off shore Island as the type locality.
Dam (1997) had misidentif iedLobophoratsengiiasL.variegatabased on the specimens collected from Bach Long Vy Island. Luan et al. (2013) also misidentif ied this species from Hainan Island asL.variegata. However,L.variegatais not distributed in this region and geographically restricted to the Caribbean Sea (Schultz et al., 2015; Vieira et al.,2016). The misidentif ication may be due to the morphological resemblance in the erect fan-shaped thallus. As to the species with prostrate thallus,Lobophorasp. reported from Nha Trang, Vietnam(Tsutsui et al., 2005) should be identif ied asL.obscura(=L.crassaSun, Lim et Kawai in Sun et al., 2012),based on the distinguished morphological feature.Vieira et al. (2016) detected two prostate species,L.obscuraandL.asiaticafrom Vietnam based on molecular phylogenic analyses. To date, three species ofLobophorawere reported from Vietnam, includingL.asiatica,L.obscura, andL.tsengii(=Lobophorasp. 67 in Vieira et al., 2016; =L.rosaceain Sun et al.,2017).In our recent survey on the algal f lora in Vietnam,severalLobophora-like taxa were detected, and a further investigation should be carried out based on morphological and molecular phylogenic analyses in the future.
5 CONCLUSION
A new species ofLobophoratsengiiwas described from Bach Long Vy Island, which had been mistakenly identif ied asL.variegataandL.rosaceain China and Vietnam. Our study shows thatL.tsengiiis diff erent from the relatedL.rosaceabased on both morphological and molecular data, and the later should be endemic to the Southern Hemisphere.

Fig.4 Morphology of Lobophora tsengii sp. nov. from the Bach Long Vy Island, Haiphong, Vietnam
6 DATA AVAILABILITY STATEMENT
All datasets generated and analyzed during the current study are available from the corresponding author on reasonable request.
杂志排行
Journal of Oceanology and Limnology的其它文章
- MamZ protein plays an essential role in magnetosome maturation process of Magnetospirillum gryphiswaldense MSR-1*
- Magnetotactic bacteria from the human gut microbiome associated with orientation and navigation regions of the brain*
- How light aff ect the magnetotactic behavior and reproduction of ellipsoidal multicellular magnetoglobules?*
- Biocompatibility of marine magnetotactic ovoid strain MO-1 for in vivo application*
- Determination of the heating effi ciency of magnetotactic bacteria in alternating magnetic f ield*
- An approach to determine coeffi cients of logarithmic velocity vertical prof ile in the bottom boundary layer*
