Synthesis,Structure and Properties of Three Copper(Ⅱ)Complexes Based on a Bifunctional Ligand 2,2′∶6′2″-Terpyridine-4′-carboxylic Acid
2021-12-09HEChunYuYANGXiaoQingZHANGYanHong2JIANGShuang
HE Chun-YuYANG Xiao-QingZHANG Yan-Hong*,,2JIANG Shuang
(1College of Chemistry and Environment Science,Key Laboratory of Excitonic Materials Chemistry&Devices,Inner Mongolia Normal University,Hohhot 010022,China)
(2Key Laboratory of Advanced Energy Materials Chemistry(Ministry of Education),Nankai University,Tianjin 300071,China)
Abstract:Two copper binuclear complexes[Cu(tpyc)(H2btc)]2(1),[Cu2(tpyc)2(suc)(H2O)2](2)and one coordination polymer{[Cu3(tpyc)3(OH)2(H2O)2]ClO4}n(3)(H3btc=1,3,5-benzenetricarboxylicacid,H2suc=succinicacid,Htpyc=2,2′∶6′2″-terpyridine-4′-carboxylic acid)have been synthesized under solvothermal conditions and characterized by elemental analysis,FT-IR spectroscopy,single-crystal X-ray diffraction,powder X-ray diffraction,thermogravimetric analysis and magnetic analysis.Structural analysis suggests that complexes 1-3 display 3D supramolecular networks by multiple hydrogen-bonding interactions.Magnetic studies show that ferromagnetic coupling exists between Cu(Ⅱ)ions in complex 1,while antiferromagnetic interaction exists between Cu(Ⅱ) ions in complex 2.CCDC:1950190,1;1950191,2;1950202,3.
Keywords:binuclear complex;coordination polymer;crystal structure;magnetic property
The design and construction of coordination polymers(CPs)have attracted tremendous interest due to their fascinating structures and intriguing topologies,as well as their potential applications in gas storage and separation,molecular recognition,luminescence,drug delivery,catalysis and magnetism[1-7].Although great progress and developments have been made in the construction of diverse architectures,it remains a considerable challenge to rationally design and synthesize the desired coordination polymers with exact structures,because the structural diversity of such solid materials is deeply influenced by many factors,such as temperature,solvent,pH values,coordination geometry of metal ions,connectivity of organic ligand and molar ratio of reactants,as well as the nature of auxiliary ligand involved[8-15].It is well known that the judicious choice of organic bridging ligands,such as flexibility,symmetry,length,substituent group plays a vital role to tune the structures and functions of the target polymer.Among all the extensively studied ligands,the polycarboxylate and the N-heterocyclic ligands are two types of excellent candidates for constructing CPs with interesting topologies and properties because they usually have good ligating ability to metal ions and readily adopt adjustable geometry[16-19].
Although a substantial number of coordination polymers incorporating various kinds of the abovementioned ligands have been reported,the examples constructed from the bifunctional organic ligands,which contain both carboxyl group and pyridine ring,are comparatively rare.This is mainly due to the distinct selectivity and competition of the N/O donors coordinated to the specific metal centers.Up to now,only limited bifunctional organic linkers have been extensively studied,such as isonicotinic acid[20-21],pyridine-polycarboxylic acid[22-26],bipyridine-dicarboxylic acid[27-28],pyrazine carboxylic acid[29-30]and azole-containing carboxylic acid[31-33].In this work,an asymmetricalrigid ligand 2,2′∶6′2″-terpyridine-4′-carboxylic acid(Htpyc)has been tentatively employed.The carboxylate groups may rotate around the C—C single bond,and the potential hydrogen-bonding,as well as the π-interactions generators can result in materials with good thermal stabilities and functionalization.The strong coordinating ability of the pyridine ring can not only facilitate the binding to metal centers,but also afford considerable structural complexity and diversity of CPs.
On the other hand,copper(Ⅱ)coordination polymers have been received considerable attention during the past decade.This is mainly due to their distinguished magnetic properties because Cu(Ⅱ)ion is paramagnetic and can readily take on a great variety of coordination spheres[34-35].The flexibility of the coordination mode,in combination with steric and crystal packing forces,leads to structural diversity and in turn,allows for a detailed investigation of their magnetostructural relationships and the further potential applications.
Taking inspiration from the above contexts,we have prepared two copper binuclear complexes[Cu(tpyc)(H2btc)]2(1),[Cu2(tpyc)2(suc)(H2O)2](2)and one coordination polymer{[Cu3(tpyc)3(OH)2(H2O)2]ClO4}n(3)(H3btc=1,3,5-benzenetricarboxylic acid,H2suc=succinic acid).Their crystal structures and thermal stabilities have been studied.Meanwhile,the magnetic properties of complexes 1 and 2 have also been investigated.
1 Experimental
1.1 Materials and physical measurements
All reagents used in the syntheses were commercially available and used as purchased.Elemental analyses for C,H,N were performed on a Perkin Elmer 2400 Elemental Analyzer.FT-IR spectra were recorded using a KBr pellet on a Nicolet 6700 FTIR in the 4 000-600 cm-1region.The powder X-ray diffraction(PXRD)data were collected on a Bruker D2 Phaser at room temperature with Cu Kα radiation(λ =0.154 06 nm)in a range of 7°≤2θ≤50°,operated at 30 kV and 10 mA.Thermogravimetric analyses(TGA)were performed on an SDT Q600 V20.9 Build 20 analyzer under a nitrogen atmosphere at a heating rate of 10℃·min-1in a range of 30-800℃.The variable temperature magnetic susceptibility data were collected on a Quantum Design MPMSXL-7 SQUID magnetometer.
1.2 Synthesis of[Cu(tpyc)(H2btc)]2(1)
A mixture of Htpyc(27 mg,0.10 mmol)and H3btc(21 mg,0.10 mmol)was dissolved in DMF(4 mL)in a 15 mL glass vial,then a solution of Cu(ClO4)2·6H2O(44.5 mg,0.12 mmol)in 2 mL H2O was added dropwise into the above solution.After ultrasonication for about 30 min,the resulting solution was placed in an autoclave and heated at 90°C for 4 d.Blue block crystals were isolated after the reaction system was slowly cooled to room temperature.Yield:36 mg(54% based on Cu).Anal.Calcd.for C50H30Cu2N6O16(%):C,54.70;H,2.75;N,7.65.Found(%):C,54.77;H,2.69;N,7.73.FT-IR(KBr pellet,cm-1):3 064(w),1 855(w),1 694(m),1 608(m),1 583(m),1 563(m),1 479(w),1 382(m),1 246(m),1 174(m),1 090(w),1 057(w),1 018(m),973(w),927(w),902(w),805(w),772(m),753(m),694(m).
1.3 Synthesis of[Cu2(tpyc)2(suc)(H2O)2](2)
Htpyc(27 mg,0.10 mmol),H2suc(12 mg,0.10 mmol)and Cu(ClO4)2·6H2O(44.5 mg,0.12 mmol)were dispersed in 6 mL DMF/H2O(2∶1,V/V)and sealed into a 15 mL glass vial and stirred for 30 min,then heated to 100℃for 3 d.After slow cooling to the room temperature,green block crystals of the complex 2 were obtained,washed with DMF and dried in air.Yield:22 mg(42% based on Cu).Anal.Calcd.for C36H28Cu2N6O10(%):C,51.99;H,3.39;N,10.10.Found(%):C,52.34;H,3.01;N,10.37.FT-IR(KBr pellet,cm-1):3 389(w),3 032(w),1 610(s),1 551(s),1 471(m),1 398(s),1 318(s),1 226(m),1 167(w),1 048(w),1 021(w),909(w),790(m),730(m),684(m).
1.4 Synthesis of{[Cu3(tpyc)3(OH)2(H2O)2]ClO4}n(3)
Htpyc(27 mg,0.10 mmol),4,4′-bipyridine(16 mg,0.10 mmol)and Cu(ClO4)2·6H2O(44.5 mg,0.12 mmol)were dissolved in 5 mL DMF/H2O(4∶1,V/V)in a 15 mL glass vial.After ultrasonication for about 30 min,the resulting solution was placed in an autoclave and heated at 90℃for 3 d and then slowly cooled to room temperature.Green block crystals of 3 were collected by filtration,washed with DMF and then dried in air.Yield:23 mg(48% based on Cu).Anal.Calcd.for C48H36ClCu3N9O14(%):C,48.49;H,3.05;N,10.60.Found(%):C,48.61;H,3.01;N,10.47.FT-IR(KBr pellet,cm-1):3 090(w),1 615(s),1 563(m),1 472(m),1 395(m),1 330(m),1 246(m),1 174(w),1 070(vs),895(m),850(w),778(m),740(m),681(m),649(m).
1.5 Crystal structure determination and refinement
Single crystals of complexes 1 and 3 were mounted on a Bruker Smart Apex CCD diffractometer with graphite-monochromatized MoKαradiation (λ=0.071 073 nm)by using theωscan technique at 193 K.Diffraction data of 2 were collected on an XtaLAB AFC12(RINC):Kappa single diffractometer with CuKαradiation(λ=0.154 178 nm)at 120 K.Empirical absorption corrections were applied by using the SADABS program[36].The structures were solved by direct methods and refined by the full-matrix leastsquares onF2using the SHELXTL-2014 software package for 1 and 3 and Olex 2 for 2[37-39].All nonhydrogen atoms were refined with anisotropic displacement parameters.The positions of hydrogen atoms attached to carbon atoms were generated geometrically and refined with isotropic thermal parameters.Detailed crystallographic data and structure refinement parameters for complexes 1-3 are listed in Table 1.Selected bond lengths and bond angles are listed in Table 2.The hydrogen bond parameters are listed in Table 3.
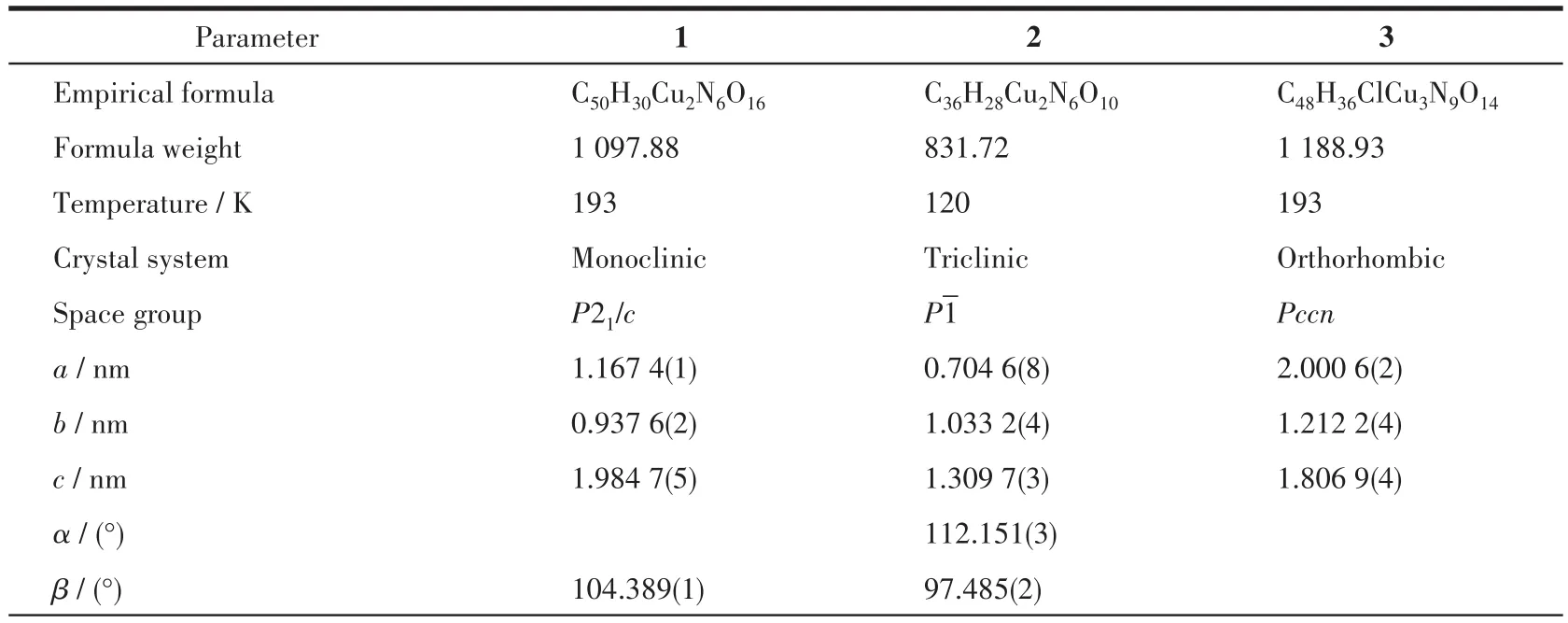
Table 1 Crystal data and structure refinement for complexes 1-3
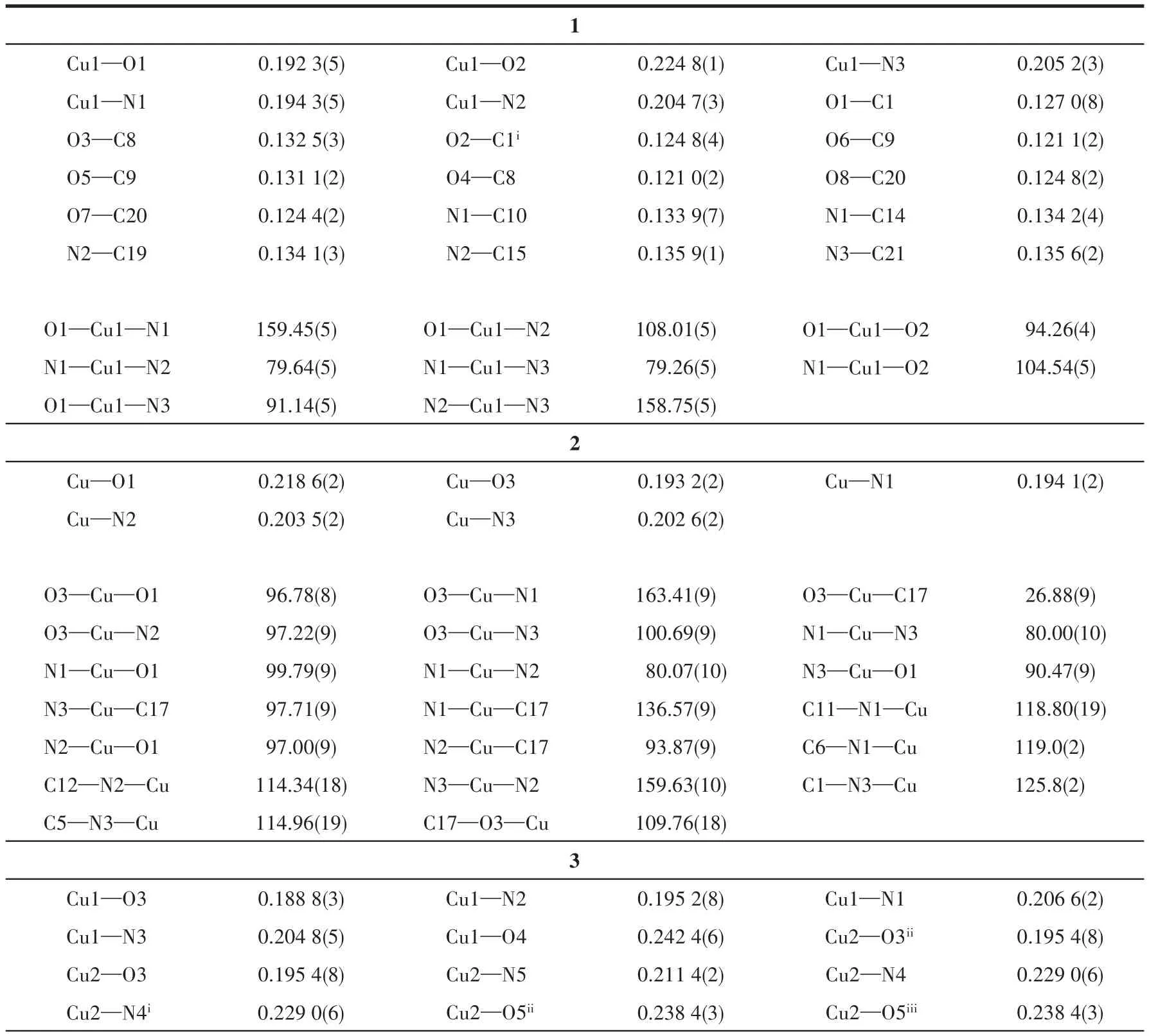
Table 2 Selected bond lengths(nm)and angles(°)for complexes 1-3
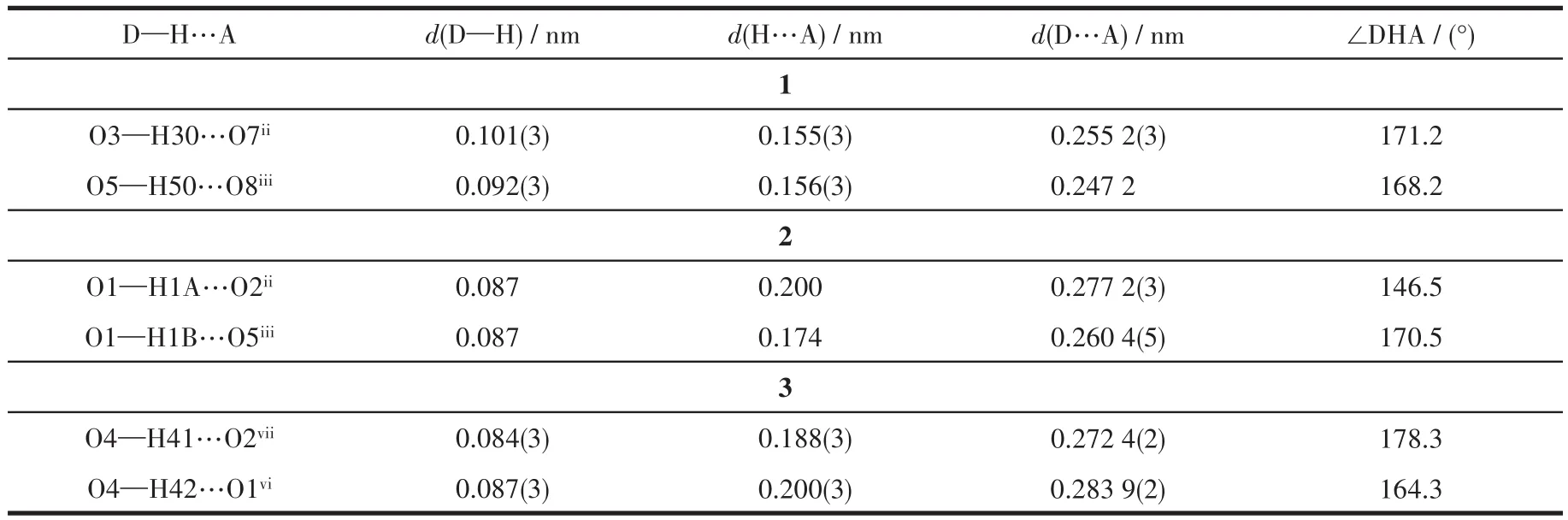
Table 3 Hydrogen bond parameters for complexes 1-3
CCDC:1950190,1;1950191,2;1950202,3.
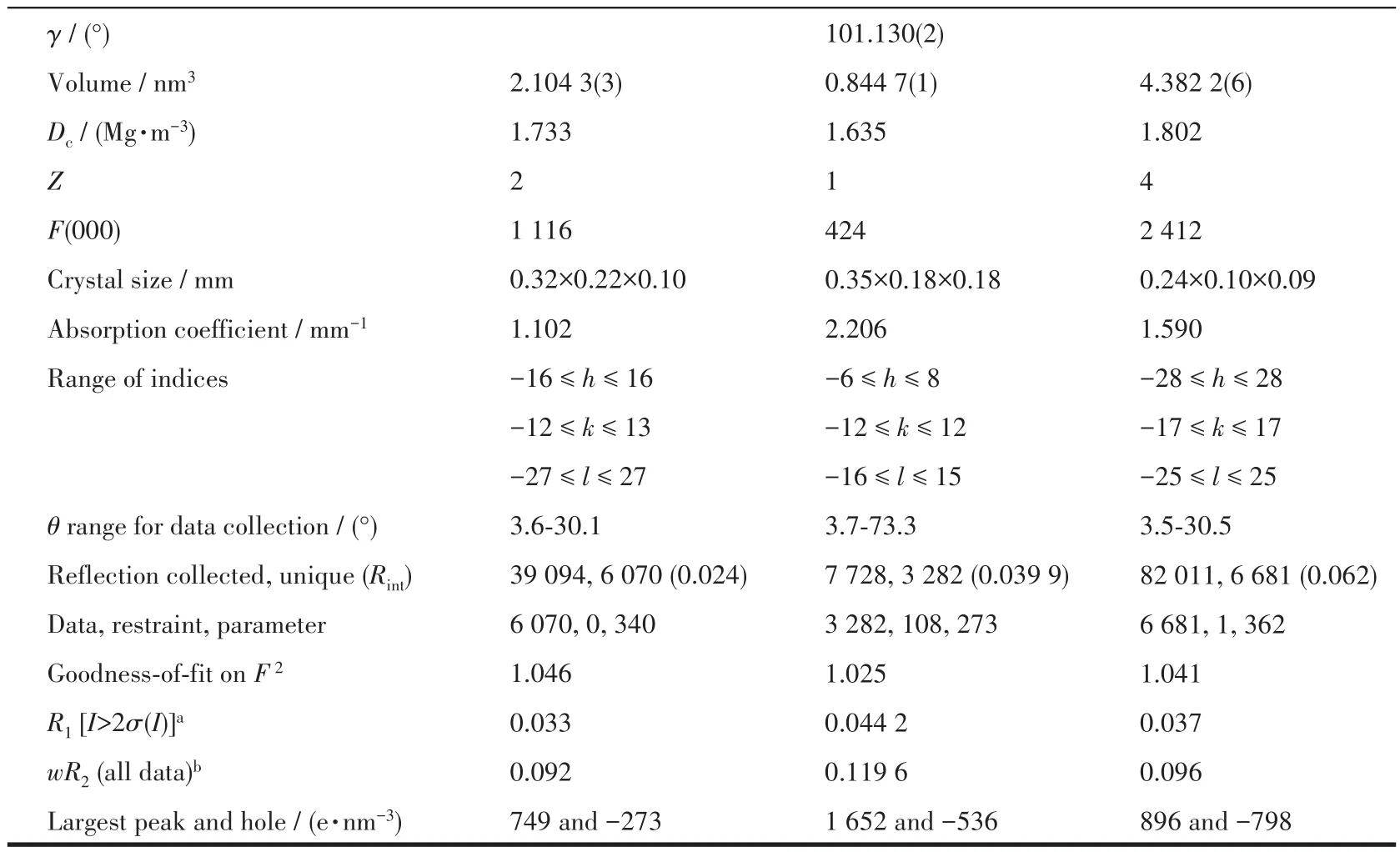
Continued Table 1
Continued Table 2
2 Results and discussion
2.1 Structure description of 1
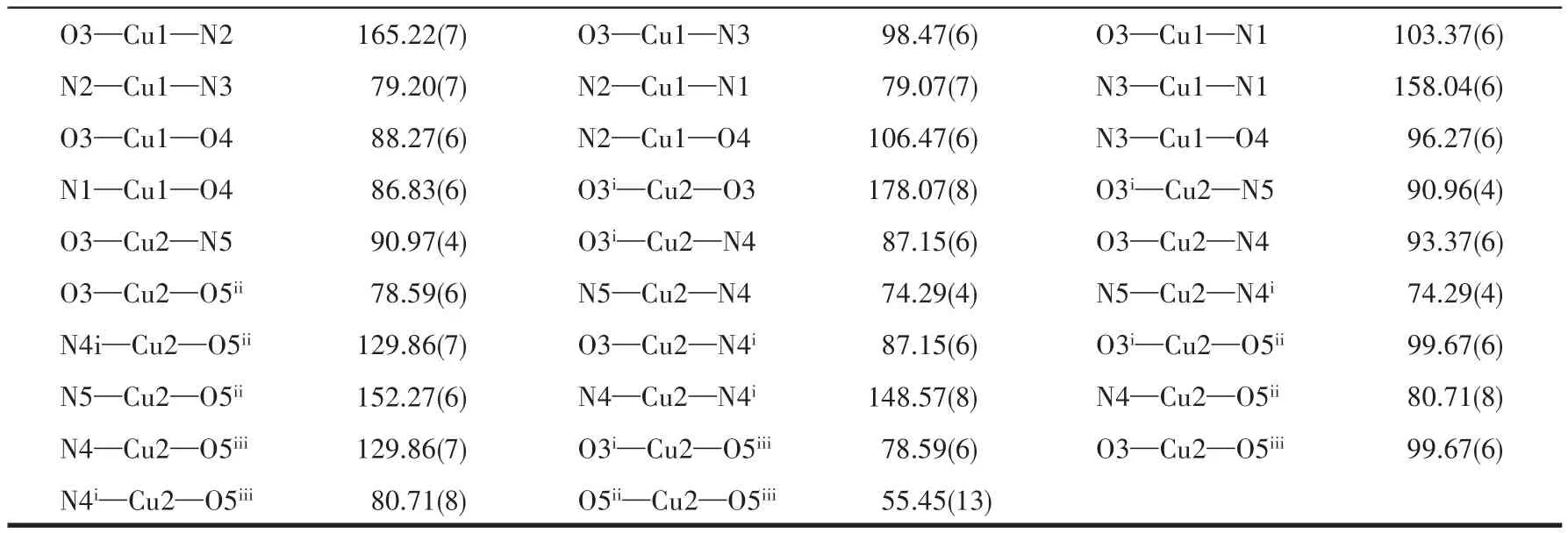
Single-crystal X-ray analysis reveals that complex 1 crystallizes in the monoclinic space groupP21/c.There are one crystallographically independent Cu(Ⅱ)ion,one tpyc-ligand,as well as one partially deprotonated H2btc-moiety in the asymmetric unit.As illustrated in Fig.1a,the Cu(Ⅱ)center is in a five-coordinated environment.In a five-coordinated complex,the structural index parameterτcan be applicable to describe the degree of trigonality between trigonal bipyramid(τ=1)and square pyramid(τ=0)[40].In complex 1,theτvalue is 0.012 and thus the coordination polyhedron around Cu(Ⅱ)ion can be regarded as a slightly distorted square pyramidal geometry.The basal plane consists of three nitrogen atoms(N1,N2,N3)from the same tpycligand and one oxygen atom(O1)from one H2btcligand with the bond length ranging from 0.192 3(5)nm(Cu1—O1)to 0.205 2(3)nm(Cu1—N3).The four donor atoms deviate from their mean plane by 0.012 nm(N1),-0.009 nm(N2),-0.011 nm(N3)and 0.008 nm(O1),and the Cu(Ⅱ)ion is-0.021 nm out of the plane.The apical position is occupied by the O2 atom from the other coordinated H2btc-ligand at a distance of 0.224 8(1)nm.The bond angles around the Cu(Ⅱ)center are lying in a range of 79.26(5)°-159.45(5)°.In H2btc-anion,only one carboxylic group takes part in coordination and adopts a bridging bidentate modeμ2-η1∶η1to connect two Cu (Ⅱ) ions,forming[Cu2(CO2)2]secondary building units(SBUs)with a Cu1…Cu1iseparation of 0.464 94 nm.
In the crystalline state,there are two kinds of O—H…O hydrogen bonds(Table 3)involving the free—COOH groups of H2btc-ligand and the oxygen atoms of tpyc-backbone.The neighboring[Cu2(CO2)2]SBUs are associated to form a 1D loop chain by hydrogen-bonding interaction of O3—H3O…O7ii(Fig.1b).The adjacent 1D chains are conjoined into a 2D layer network running parallel to theabplane through O5—H5O…O8iii(Fig.1c).These 2D layers are further interlinked via intermolecular interaction(O3—H3O…O7ii)to form a 3D supramolecular framework(Fig.1d).
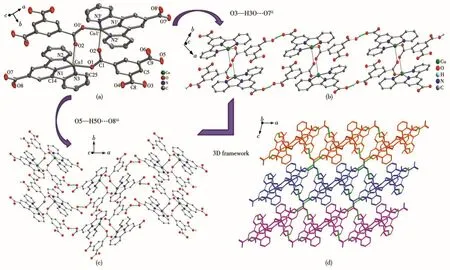
Fig.1 (a)Coordination environment of Cu(Ⅱ)in 1,where hydrogen atoms are omitted for clarity;(b)View of 1D loop chain of 1;(c)View of 2D layer of 1;(d)3D supermolecular framework of 1
2.2 Structure description of 2
Complex 2 crystallizes in the triclinic system,with the P1 space group.The asymmetric unit contains one crystallographically independent Cu(Ⅱ)ion,one tpyc-ligand,and half a fully deprotonated suc2-linker,as well as one coordinated water molecule.As shown in Fig.2a,the Cu(Ⅱ)center is five-coordinated to three nitrogen atoms(N1,N2,N3)from the same tpycligand,one oxygen atom(O3)from one suc2-ligand,and one oxygen atom(O1)from the coordinated water molecule,forming a distorted square pyramidal geometry with τ value of 0.063.The equatorial plane is made up of N1,N2,N3,O3 and the bond lengths of Cu—N/O fall in a range of 0.193 2(2)-0.203 5(2)nm.The axial position is occupied by O1 and the bond length of Cu—O1 is 0.218 6(2)nm.The suc2-anion adopts a bridging coordination mode μ2-η1∶η0∶η1∶η0to connect two neighboring Cu(Ⅱ)ions,forming[Cu2C2H2(CO2)2]SBUs with a Cu1…Cu1idistance of 0.910 46 nm.There are two different kinds of hydrogen bonds:O1—H1A…O2iioriginates from the coordinated water molecule and suc2-ligand;O1—H1B…O5iiicomes from the coordinated water molecule and the free oxygen atoms of tpyc-ligand.As depicted in Fig.2b,the neighboring[Cu2C2H2(CO2)2]SBUs are joined together by hydrogen bonds of O1—H1A…O2iito give an infinite 1D doublechain.Then the adjacent ladder chains are further extended into a 3D network through hydrogen bonds of O1—H1B…O5iii(Fig.2c).
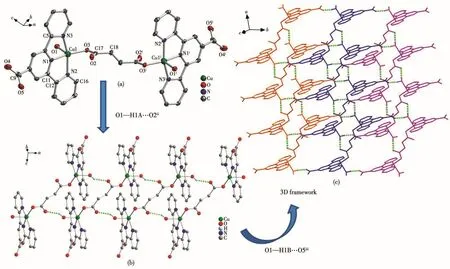
Fig.2 (a)Coordination environment of Cu(Ⅱ)in 2,where hydrogen atoms are omitted for clarity;(b)View of 1D double-chain of 2;(c)View of 3D network of 2
2.3 Structure description of 3
Complex 3 crystallizes in the orthorhombic Pccn space group.The asymmetric unit of 3 consists of one and a half Cu(Ⅱ)ions,one and a half tpyc-ligands and one coordinated water molecule,as well as one coordinated OH-and half a free ClO4-.As shown in Fig.3a,there are two kinds of Cu(Ⅱ)coordination environments.Cu1 is located in a distorted{CuN3O2}square pyramidal geometry(τ=0.119)and ligated by three nitrogen atoms(N1,N2,N3)from the same tpyc-ligand,one coordinated water molecule(O4)and one OH-anion(O3).The equatorial plane is defined by atoms N1,N2,N3 and O3(the mean deviation from the plane is 0.012 4 nm),and the axial position is occupied by O4.The bond lengths of Cu1—N/O are measured in a range of 0.188 8(4)-0.242 4(6)nm.Cu2 is seven-coordinated with a distorted{CuN3O4}pentagonal bipyramid coordination geometry.The equatorial plane is made up of three nitrogen atoms(N4,N4i,N5)from the same tpycligand,two carboxylate oxygen atoms from another tpyc-ligand(O5ii,O5iii).The apical positions are occupied by the coordinated OH-(O3 and O3i)with a trans angle of 178.06(9)°.The Cu2—N/O distances range from 0.195 4(8)to 0.229(1)nm.The angles around Cu2 vary from 55.45(13)°to 178.06(9)°.The coordinated hydroxyl ions reside in the opposite position of the tpyc-ligand plane connecting three copper ions(Cu1,Cu1iand Cu2)to form a trinuclear[Cu3(tpyc)3(OH)2(H2O)2]subunit.The adjacent discrete trinuclear subunits are interconnected by the carboxylate oxygen atoms from tpyc-ligand and the hydrogen bonds(O4—H42…O1vi)to form an infinite 1D chain along the c axis.The distance of Cu…Cu is 0.332 2(7)nm for Cu1…Cu2 and 0.5846(5)nm for Cu1…Cu1i(Fig.3b).These 1D chains are further linked together to afford a 3D supramolecular architecture through the intermolecular hydrogen interaction of O4—H41…O2vii(the distance of H41…O2viiis 0.188(3)nm and the angle of O4—H41…O2viiis 178.3°)between the coordinated water molecules and free carboxylate oxygen atoms of tpycligands(Fig.3c).
Fig.3 (a)Coordination environment of Cu(Ⅱ)in 3,where hydrogen atoms are omitted for clarity;(b)View of 1D chain of 3;(c)3D supramolecular architecture of 3
2.4 Structural diversities of complexes 1-3
In this work,the same main ligand Htpyc and metal salt Cu(ClO4)2·6H2O as well as similar reaction conditions have been adopted to synthesize the three complexes.However,changes in the auxiliary ligands lead to different dimensionality in the final structures.In complex 1,we have selected the tricarboxylate H3btc to expect the high dimensional framework.However,the carboxylic groups of H3btc are partially deprotonated and only one carboxylic group takes part in coordination.Meanwhile,the carboxyl oxygen atoms of Htpyc ligand did not participate in the coordination,which makes the structure not further extended,showing a dimer structure.And then the adjacent dimer units are associated by multiple hydrogen bonding interactions resulting in a 3D network structure.In complex 2,when the liner H2suc coligand was introduced into the reaction system,the two carboxylic groups are completely deprotonated and the suc2-anion bridges the two adjacent Cu(Ⅱ)ions in a bridging mode to yield a binuclear structure.The neighboring binuclear units are further interlinked by the hydrogen-bonding interactions into a 3D network.In complex 3,4,4′-bipyridine was employed as the auxiliary ligand to adjust the structure of the complex,however,an unexpected 3D supramolecular architecture was obtained,in which 4,4′-bipyridine is not present.Although 4,4′-bipyridine does not exist in the final product,it might play an important role to regulate the pH value of the reaction system.The only poorly defined microcrystalline product can be obtained without it.From the structural descriptions above,it is evident that the coligands have a profound influence on the overall structure of the complexes.
2.5 PXRD patterns and TGA
PXRD experiments were carried out at room temperature to examine the purities and homogeneities of complexes 1-3.As shown in Fig.S1(Supporting information),the peak positions of the measured patterns agreed well with the simulated ones,indicative of the good purity of the complexes.
To examine the thermal stabilities of complexes 1-3,TGA was performed on their single-crystal samples under an N2atmosphere.As shown in Fig.4,complex 1 was thermally stable up to 274℃and then displayed two steps of weight loss.The first weight loss of 50.2% between 274 and 308℃can be attributed to the release of tpyc-ligand(Calcd.49.2%).The second weight loss of 37.9% in a range of 308-380℃corresponds to the release of H2btc-ligand(Calcd.38.3%).The remaining residue is equivalent to the formation of CuO(Obsd.13.5%,Calcd.14.5%).The TGA curve of complex 2 showed three decomposition steps.The initial weight loss of 4.5% below 140℃can be assigned to the removal of two coordinated water molecules(Calcd.4.1%).The second abrupt weight loss of 58.1% between 220 and 290℃is due to the release of tpyc-ligand (Calcd.58.9%).Then suc2-ligand gradually broke down and the residual product of 23% corresponds to the component of CuO(Calcd.22.1%).For complex 3,the first weight loss was observed from 120 to 250℃,which can be attributed to the release of two coordinated H2O molecules and OH-anions,as well as the counter anions ClO4-.The weight loss of 15.6% is consistent with the calculated value(14.5%).Then the overall framework gradually collapsed until 580℃with a final residual mass of 17.2%,which is close to the calculated component of CuO(19.2%).
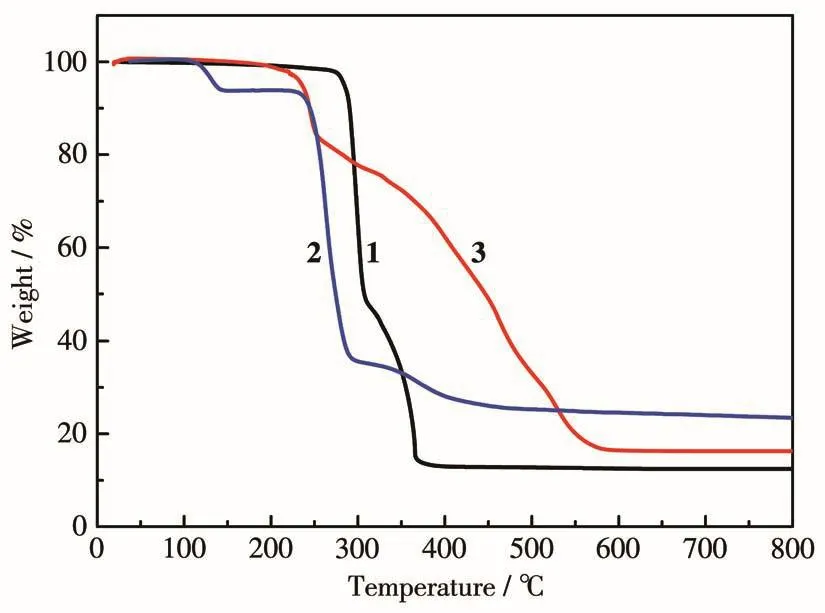
Fig.4 TG curves for complexes 1-3
2.6 Magnetic properties
Variable temperature(2-300 K)magnetic susceptibility measurements were performed for polycrystalline samples of complexes 1 and 2 in an applied magnetic field of 1 000 Oe.Diamagnetic corrections were estimated with Pascal′s constants for all the constituent atoms[41].Fig.5 shows the magnetic behavior of 1 and 2 in the forms of χMand χMT versus T plots.At room temperature,the χMT value was 0.82 cm3·mol-1·K for complex 1,which was slightly higher than the spinonly value(0.75 cm3·mol-1·K)for two magnetically isolated Cu(Ⅱ) ions(S=1/2).On cooling,the χMT increased gradually at first to reach a maximum value(0.85 cm3·mol-1·K)at 20 K and then decreased obviously.These features are indicative of the predominance of ferromagnetic interactions in the binuclear Cu(Ⅱ)species as observed in similar complexes[42-43].The diminishing in χMT at low temperatures may be due to either intermolecular antiferromagnetic interactions and/or the effect of the zero-field splitting(ZFS)of the ground state(S=1).Magnetic analysis of complex 1 has been made using the Bleaney-Bowers equation(1)derived from the Heisenberg spin Hamiltonian and modified with ZFS[42].
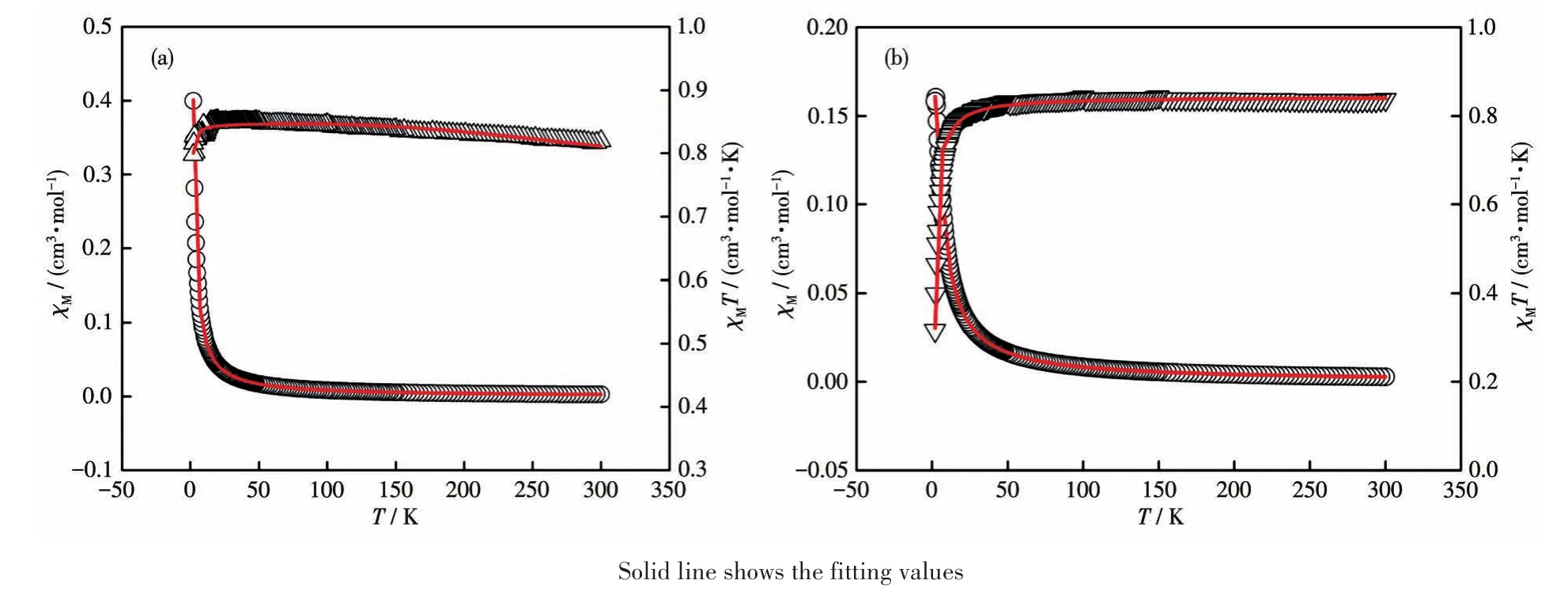
Fig.5 Plots of χMversus T(open circles)and χMT versus T(open triangles)for complexes 1(a)and 2(b)
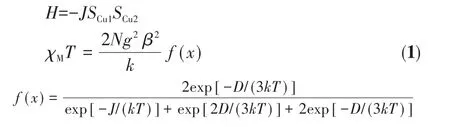
Where J is the exchange interaction parameter between the intrabinuclear Cu(Ⅱ)ions;D is the correction term for the ZFS and other symbols have their usual meanings.On this basis,the least-squares fitting of the experimental data led to J=26 cm-1,g=2.0 and D=-0.18 cm-1.These results indicate that ferromagnetic coupling interaction exists between the adjacent Cu(Ⅱ)ions within complex 1.
For complex 2,the χMT value at 300 K was 0.84 cm3·mol-1·K,which was also slightly higher than the spin-only value for two uncoupled Cu(Ⅱ)ions(S=1/2).With the decrease of temperature,the χMT decreased smoothly over the entire temperature region,and reached a minimum value(0.32 cm3·mol-1·K)at 2 K.These behaviors are indicative of an overall weak antiferromagnetic coupling.The magnetic analysis was carried out by using the magnetic susceptibility expression(2)based on the isotropic Heisenberg model[43].
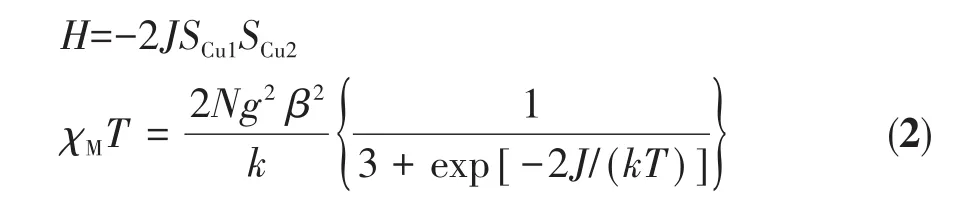
The symbols in equation 2 have the same meaning as in equation 1.The best least-squares fit was obtained with the parameters J=-1.54 cm-1and g=2.1.The small negative J value demonstrates a weak antiferromagnetic exchange propagated between the two paramagnetic Cu(Ⅱ)ions within complex 2.The significant difference between the magnetic properties of complexes 1 and 2 may be due to the coordination mode diversities of metal centers or the different distances between metal ions.
3 Conclusions
In summary,three new copper complexes have been solvothermally synthesized based on the same Htpyc ligand and three different coligands.In complex 1,the coligand H3btc is partially deprotonated and acts as a bridging ligand to connect adjacent Cu(Ⅱ)ions to form[Cu2(CO2)2]SBUs,which are finally extended into a 3D framework by hydrogen bonding interactions.In complex 2,the Cu(Ⅱ)ions are bridged by a fully deprotonated H2suc ligand to form[Cu2C2H2(CO2)2]SBUs,which are further linked into a 3D network by hydrogen bond interactions.In complex 3,although the auxiliary ligand 4,4′-bipyridine is not incorporated in the complex,its existence is crucial to the growth of the crystalline product.Complex 3 finally displays a fascinating 3D supramolecular architecture.These results confirm that the selection of suitable coligand is a feasible way to construct new coordination polymers with diverse structures.Magnetic investigation indicates that complex 1 exhibits ferromagnetic exchange interaction,while complex 2 displays antiferromagnetic exchange interaction between adjacent Cu(Ⅱ)centers.
Supporting information is available at http://www.wjhxxb.cn
