Synthesis of Quasi-MIL-53(Fe)Photocatalysts for Enhanced Visible Light Photocatalytic Degradation of Organic Dyes
2021-12-09ZOUQiChaoMAYanCHIDianJunQIAOHongBinZHANGJunYingCHENQianSUNYuDieZHANGJianZHANGKuiLIUShengJun
ZOU Qi-ChaoMA YanCHI Dian-Jun QIAO Hong-Bin ZHANG Jun-Ying CHEN Qian SUN Yu-Die ZHANG Jian ZHANG KuiLIU Sheng-Jun
(School of Chemistry and Chemical Engineering,Anhui University of Technology,Ma′Anshan,Anhui 243032,China)
Abstract:Quasi-metal-organic-frameworks(MOFs)derivative-based MFe-T(T℃stands for calcination temperature)photocatalysts with a porous structure were synthesized by the pyrolysis of MIL-53(Fe)(termed MFe hereafter).Among the tested catalysts,MFe-250 exhibited the highest photodegradation performance,degradation of 99% methylene blue(MB)within 90 min.From the photocurrent and electrochemical impedance spectroscopy results,the electronic transmission capability of MFe-250 exceeded that of MFe.Furthermore,trapping experiments revealed that while hydroxyl radicals(·OH)were essential intermediates in the photocatalytic degradation of MB.Additionally,a mechanism for the photocatalytic process was proposed.
Keywords:metal-organic frameworks;visible light;photodegradation;organic dyes
0 Introduction
The rapid development of modern industry has led to water pollution,including contamination with organic dyes.One harmful pollutant is methylene blue(MB).Although the adsorbent can effectively remove MB from water,its high cost limits practical applications[1].In contrast,the solar photocatalytic degradation of organic pollutants has proven to be a clean technology that can address such environmental problems.Some successfully developed photocatalysts are metal oxides,metal sulfides and graphitic carbon nitride[1-2].However,their low quantum and solar energy conversion efficiencies limit their practical applications.Therefore,the development of new and more efficient photocatalysts is necessary.
Metal-organic frameworks(MOFs)are structures composed of organic ligands and metals(or metal clusters)and have a wide range of uses,such as in separation,storage,detection,and catalysis[3-21].In particular,MOFs featuring numerous active sites that generate numerous electron-hole pairs under adequate illumination can be effectively used as photocatalysts.The first of such reported MOF catalysts used for photocatalytic degradation ofphenolwas MOF-5[22].Thereafter,numerous other studies have focused on the development of MOF photocatalysts for CO2reduction[23-24],water splitting[25-28]and organic pollutant degradation[29-34].
The active sites of MOFs are important for the improvement of catalytic performance.Recent studies by Jiang′s group revealed that structurally defective UiO-66-NH2enhanced the efficiency of photocatalytic hydrogen production[35].Huang et al.reported that the synthesis of MIL-53(Fe)(termed MFe hereafter)using hydrochloric acid as a modulator exposed more active sites than as-prepared MFe without hydrochloric acid,leading to the efficient photocatalytic degradation of tetracycline[36].Further,Xu et al.reported that the use of quasi-MOFs obtained by thermal regulation enabled strong interactions between metal nanoparticles and the MOF,resulting in increased catalytic activity[37].Furthermore,we reported the preparation of quasi-MFe at lower temperatures to improve their photocatalytic activity.
Herein,a series of MFe-based photocatalysts,collectively termed as MFe-T(T℃stands for calcination temperature),were activated at different temperatures in air and employed in the visible-light-assisted degradation of MB.Studying the photocatalytic performance of different samples allowed for the identification of the most suitable catalyst for optimized photodegradation.The resulting MFe-250 exhibited high photocatalytic activity for MB degradation,reaching a degradation rate of 99% with in 90 min.Importantly,MFe-250 had excellent performance and chemical stability.
1 Experimental
1.1 Materials
N,N-dimethyl formamide(DMF,GC,99.5%),FeCl3·6H2O(AR,99.0%),ethanol(EtOH,AR,99.7%)and 2-propanol(AR,99.7%)were purchased from General-Reagent.Methylene blue(MB,AR),ethylenediamine tetraacetic acid disodium salt dihydrate(EDTA-2Na,AR)and hydrogen peroxide solution(30%,w/w)were purchased from Sinopharm Chemical Reagent Co.,Ltd.(Shanghai,China).Terephthalic acid(AR,99%)and ascorbic acid(VC,AR,99.0%)were purchased from Aladdin.All chemicals were purchased from commercial sources and used without further treatments.
1.2 Synthesis of MFe and MFe-T
MFe was synthesized based on minor modifications to the existing process[38],using the solvothermal method.In the process,ferric chloride hexahydrate(90 mg),terephthalic acid(55 mg)and DMF(8 mL)were mixed into a homogeneous solution.The solution was sealed in a 25 mL autoclave and heated at 180℃for 12 h.After the autoclave cooled to room temperature,the product was washed with DMF and ethanol several times,and finally placed in a vacuum oven at 60℃for 6 h for drying.Five samples were then taken from the dried product,following which each was successively placed in a muffle furnace and calcined in an air atmosphere for several hours at temperatures of 100,200,250,300,and 400℃.The resultant samples were denoted as MFe-100,MFe-200,MFe-250,MFe-300,and MFe-400,respectively.
1.3 Characterizations
The powder X-ray diffraction(PXRD)patterns of samples were tested on a Bruker D8 Advance X-ray diffractometer with Cu Kα radiation(λ=0.154 07 nm,40 kV,40 mA,5(°)·min-1from 5°to 80°).The FTIR spectra of the sample was tested on a Nicolet 6700 spectrometer.The Brunauer-Emmett-Teller(BET)surface area was measured at 77 K with a Micromeritics ASAP2460 instrument(The air-dried sample was activated in a vacuum at 120℃for 4 h).UV-Vis diffuse reflectance spectra(UV-Vis DRS)were recorded on a UV-Vis-NIR spectrophotometer (Shimadzu 3600).Scanning electro nmicroscopy(SEM,FEINANO SEM430)was used to analyze the morphology of the samples.X-ray electron spectroscopy(XPS)measurements were performed with a Thermo Scientific KAlpha+XPS system using Mg Kα as an excitation source.Photodegradation solutions were analyzed by UV-Vis spectroscopic measurements(Persee TU-1810)in a range from 200 to 800 nm in ambient conditions.Thermogravimetric(TG)analyses were performed on a Shimadzu DTG-60H integration thermal analyzer from 25 to 600℃ at a heating rate of 10℃·min-1under an air atmosphere.
1.4 Evaluation of photocatalytic activity
The photocatalytic activity of each MFe-T was evaluated by its degradation rate of MB under visible light.The photocatalytic system consisted of photocatalyst(20 mg),hydrogen peroxide(H2O2)(50 μL),and rhodamine B(RhB)(100 mL 20 mg·L-1)solution.After stirring for 60 min in the dark,the suspension was illuminated with a 300 W Xe visible-light lamp(λ>420 nm,MC-XF300,Beijing Merry Change Co.,Ltd.).During the process,the solution was sampled every 10 min,filtered,and analyzed using a UV-Vis detector.
1.5 Photoelectrochemical measurements
The photoelectrochemical properties of the materials were evaluated using an electrochemical workstation(CHI-760E,Chenhua Instrument,Shanghai,China).The sample(10 mg)and mass fraction of 5% Nafion solution(10 μL)were added to ethanol(1 mL),and the mixture was sonicated for 1 h to form a uniform slurry.The slurry(50 μL)was added dropwise onto the conductive side of an indium tin oxide glass(1 cm×1 cm)and then dried at 100℃for 5 h to obtain a working electrode.The reference electrode was Ag/AgCl(saturated KCl solution)while the counter electrode was a Pt sheet.A three-electrode system with a Na2SO4(0.5 mol·L-1)electrolyte was used to determine the photocurrent and perform electrochemical impedance spectroscopy(EIS).The light source was a 300 W Xe lamp(λ>420 nm).
2 Results and discussion
2.1 Characterization
PXRD patterns of pristine MFe and MFe-T are shown in Fig.1a.The XRD patterns of MFe,MFe-100,MFe-200,and MFe-250 are in good agreement with simulated patterns(MFe-Sim),indicating that the MFe structure was stable only at low temperatures(Fig.1a).From the patterns for MFe-300 and MFe-400,the absence of MFe peaks in the range of 5°-20°implied that the MFe framework was destroyed.Moreover,the diffraction patterns for MFe-300 and MFe-400 were consistent with that of Fe2O3(PDF No.33-0664),indicating the complete decomposition of MFe at high temperatures.

Fig.1 (a)PXRD patterns of MFe and MFe-T;(b)FTIR spectra of MFe and MFe-T
At 100℃,the loss of adsorbed water molecules occurred,whereas at 200℃,the loss of coordinated water molecules commenced.At 250℃,Fe—O sites are exposed by the loss of organic ligands as CO2,while at temperatures above 300℃,MFe structure began to collapse.Hence,MFe structure is only retained below a certain temperature.To confirm this,TG analysis of MFe was carried out by heating the sample from 25 to 700℃in the air(thermal calcination;Fig.S1,Supporting information).When the temperature reached 427℃,the weight decreased sharply by 60%,indicating that the original MOF structure was critically damaged.The removal of the organic ligand terephthalic acid occurs at such temperatures,resulting in the exposure of widely distributed Fe—O active sites.As the temperature approached 700℃,the weight dropped further by approximately 10%,suggesting the complete degradation of the MOF skeleton and the substantial oxidation of the iron to Fe2O3.These results show that TG analysis was a suitable method for sample analysis in this study.
To further confirm the presence of organic ligands in MFe-100,MFe-200,and MFe-250,FTIR spectra of MFe-T samples was compared to that of MFe sample(Fig.1b).The FTIR spectra of MFe-100,MFe-200,and MFe-250 were very similar to that of MFe,suggesting that MFe structure was largely retained.Conversely,pyrolysis at 300℃led to the disappearance of the peaks at 1 650-1 300 cm-1(carboxylate groups),indicating the partial decomposition of MFe structure.The temperature at which most significant degradation of MFe structure occurred was 400℃.
Next,UV-Vis DRS was conducted.The spectra for MFe and MFe-T are shown in Fig.S2a.As the literature described[39],MFe absorbs visible light in the rededge region(approximately 650 nm).For MFe-T samples,the initial increase in Fe2O3(at temperatures below 300℃)led to a redshift(shift of the absorption edge towards higher wavelengths/lower energy),thereby enhancing light absorption.Consequently,the band gaps of MFe decreased from 2.50 to 2.15 eV(MFe-200).Hence,thermally treated MFe-T potentially exhibits photocatalytic properties.
Fig.S3 shows the nitrogen(N2)adsorption-desorption isotherms of MFe-T samples.The N2adsorptiondesorption isotherms of the samples pyrolyzed at 100 and 200℃were the same,indicating that MFe porosity was preserved.MFe-300 and MFe-400 exhibited slightly increase in the surface area,whereas their porosity was more or less maintained.Furthermore,the Horvath-Kawazoe(H-K)micropore size distributions of MFe and MFe-T were very similar(Fig.S4),although MFe crystals shattered upon heat treatment(Fig.S5).These results indicate that the integrity of MFe structure was maintained in MFe-T,because it retained numerous Fe—O active sites.
XPS was employed to determine the surface composition and element valence of the samples(Fig.2).The XPS survey spectra for both MFe and MFe-250 confirmed the presence of Fe,O,and C atoms,and did not contain peaks corresponding to any distinct impurities(Fig.2a).The results indicate a similarity in the composition and structure of MFe and MFe-250.The Fe2p3/2and Fe2p1/2XPS peaks for MFe were located at 711.39 and 725.09 eV,respectively[35],whereas those of MFe-250 were located at 711.34 and 724.84 eV,respectively(Fig.2b).The close similarity between these values indicates that MFe-250 structure did not collapse.In the XPS O1s spectrum of MFe(Fig.2c),the main peak corresponding to the carboxyl group of the terephthalic acid(H2BDC)linkers and the Fe—O bonds was located at 531.40 eV[40].In contrast,the corresponding O1s peak for MFe-250 shifted to 531.48 eV because some Fe—O bonds remain after decarboxylation[40].The high-resolution C1s XPS spectrum of MFe(Fig.2d)exhibited two peaks with binding energies of 288.43 and 284.50 eV,corresponding to the Fecarboxylate moiety and benzoic acid linker,respectively[39].The decrease in the intensity of the C1s peak in the spectrum for MFe-250 further indicates the heatinduced loss of organic ligands.Hence,the thermal treatment led to MFe decarboxylation and the corresponding generation of metal oxides,as revealed by the changes in the XPS peak intensity and position.
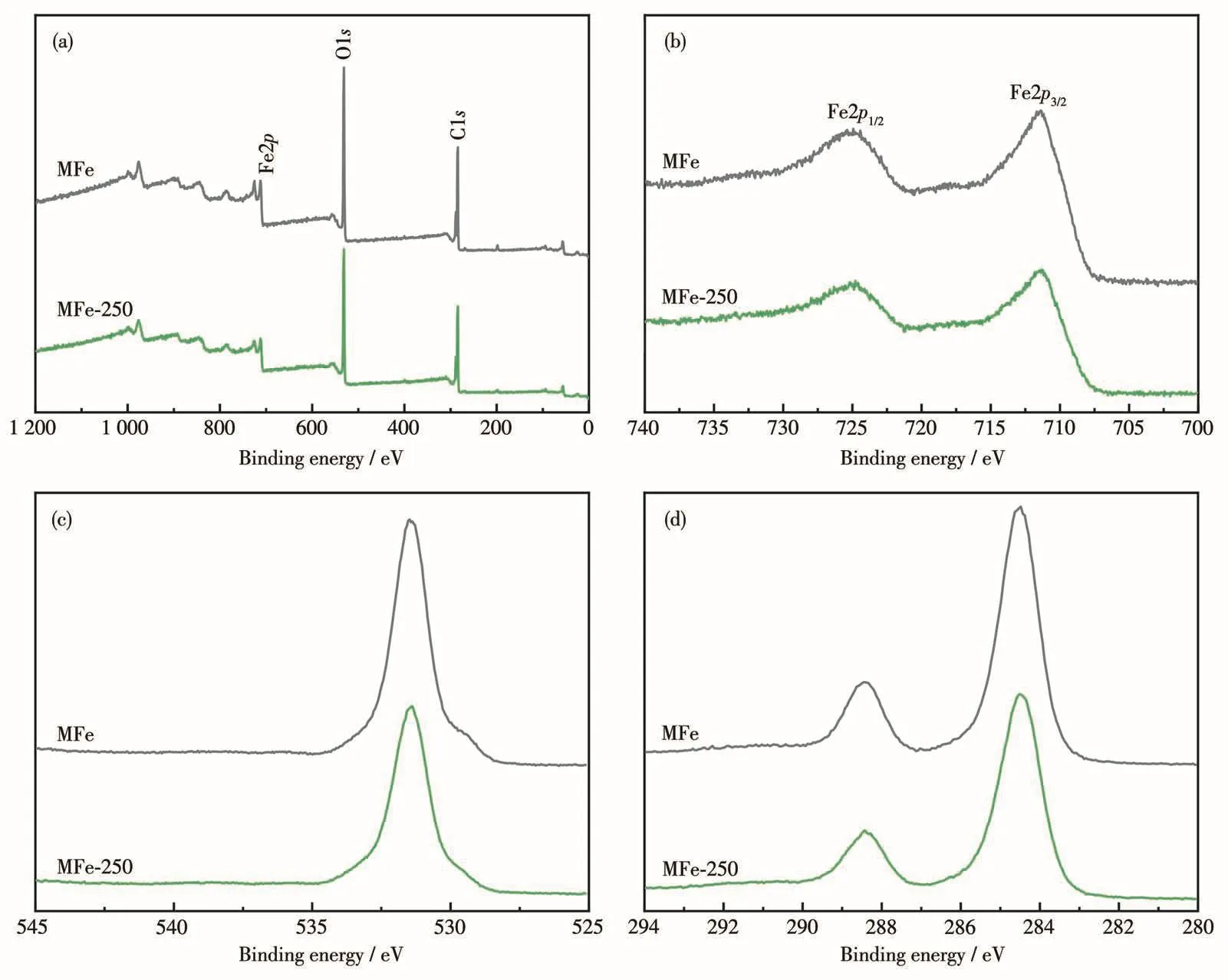
Fig.2 XPS spectra of MFe and MFe-250:(a)survey,(b)Fe2p,(c)O1s,and(d)C1s
2.2 Photocatalytic activities
The MB degradation activities of MFe-T catalysts were evaluated at 25℃in a jacketed glass reactor containing photocatalyst,H2O2,and MB in aqueous under visible light irradiation.Fig.3a shows the photodegradation rate of MFe,MFe-100,MFe-200,MFe-250,MFe-300,and MFe-400;all samples exhibited catalytic activities towards MB degradation.Under the same conditions,non-calcined MFe showed a more general cata-lytic performance,degrading 88% of the MB within 90 min.The photocatalytic activity of the sample increased when the calcination temperature was raised from 100 to 250℃owing to the generation of more structural defects.Hence,MFe-200 and MFe-250 photodegraded 97% and 99% of MB,respectively,within 90 min,suggesting that the Fe—O sites play a critical role in the process.Among the tested catalysts,MFe-250 exhibited the highest photodegradation rate(99% within 90 min),suggesting that it is a novel and effective photocatalyst for the degradation of MB under visible light.In contrast,MFe-300 and MFe-400 photodegraded 23% and 16% of MB,respectively,within the same 90 min(Fig.3a).Therefore,it can be said that the photocatalytic activity is proportional to the number of Fe—O sites,and reaches a maximum for MFe-250.As the pyrolysis temperature increased further,conversion of MFe structure to Fe2O3was accompanied by a decrease in the catalytic activity.A further increase in the calcination temperature causes the collapse of MFe framework[41].In the cases studied,Fe2O3photocatalysts exhibited significantly lower MB degradation activity compared to pristine MFe.Hence,the MFe framework is necessary for a high photocatalytic MB degradation performance.
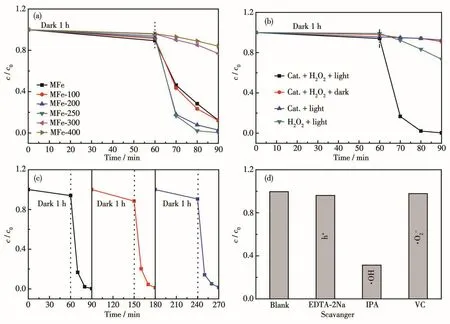
Fig.3 (a)Photocatalytic degradation of MB under visible-light irradiation over different samples;(b)Degradation rate of MFe-250 on MB under different conditions;(c)MB removal in the repeated tests over as-prepared MFe-250;(d)Photocatalytic degradation of MB over MFe-250 under visible light irradiation in the presence of different scavengers
Control experiments were conducted to verify the photocatalytic degradation properties of MFe-250.Fig.3b shows the concentration(c/c0)of MB in different photocatalytic degradation systems.In the absence of catalyst and H2O2,no degradation of MB under irradiation was observed[42].The addition of H2O2to the MB solutions led to 27% degradation within 90 min of visible-light irradiation.This resulted from the lightinduced formation of·OH upon the irradiation of H2O2.When only MFe-250 and visible light alone was employed,8% MB was degraded.This suggested an H2O2-dependence of the photoactivity of the catalyst.In the dark,a mixture of MFe-250 and H2O2only led to a degradation rate of 9% MB within 90 min.Furthermore,99% MB was degraded by using MFe-250 and H2O2under visible light irradiation.Additionally,the changes in the UV-visible spectra of MB solution containing MFe-250 and H2O2under visible-light irradiation(Fig.S6)corroborate the high catalytic performance of MFe-250 in the presence of H2O2.
Catalyst stability is pivotal to the choice of practical applications in photocatalysis.The stability of MFe-250 was assessed by recycling it during several experiments.After each reaction,the recovered catalyst was washed with H2O and EtOH to remove adsorbed impurities,after which it was dried at 60℃.The same amount of sample was then added to the next experiment,which was conducted under the same conditions as the previous one.As shown in Fig.3c,nearly no loss of activity was observed in MFe-250,even over three cycles.The stability of MFe-250 was confirmed by PXRD analysis,where the same results were obtained for both,the as-prepared and the recovered catalysts(Fig.S7).Hence,MFe-250 catalyst is both recyclable and stable under photocatalytic reaction conditions.
The origin of the excellent photocatalytic activity of MFe-250 was obtained from the photocurrent and EIS results.The photocurrent intensity of MFe-250 was almost twice that of MFe(Fig.4a).Meanwhile,EIS,which reflects the charge separation capability of a photocatalyst,revealed a higher light-induced charge separation rate in MFe-250 than in MFe.The Nyquist arc radius of MFe-250 was smaller than that of MFe(Fig.4b),indicating a corresponding smaller charge/electron transfer resistance in MFe-250 compared to that in MFe[2].Therefore,the high charge separation efficiency of MFe-250 can be attributed to its low electron transfer resistance.Thus,the thermal treatment improved the charge separation efficiency of MFe and ultimately enhanced its photocatalytic performance.

Fig.4 (a)Photocurrent responses and(b)EIS spectra of MFe and MFe-250
2.3 Mechanism of MB photodegradation using MFe-250/visible-light/H2O2system
Hydroxyl radicals(·OH)play an important role in the photocatalytic degradation of MB[42].Isopropanol(IPA)was used as a scavenger to detect·OH generated at the surface of MFe-250[43].To gain a deeper understanding of the reaction mechanism,other scavengers such as EDTA-2Na[44-45]and VC[46]were used to detect the formation of photoexcited holes(h+)and superoxide radicals(·O2-)during the MB degradation experiments.Fig.3d reveals a decrease in the MB photodegradation rate of MFe-250 from 99% to 96% following the addition of EDTA-2Na.When VC was added,the photodegradation rate was 98%,whereas the addition of IPA significantly decreased the photodegradation rate to 31%,indicating that IPA greatly influences the degradation reaction.Hence,·OH plays a crucial role in the photocatalytic degradation of MB,whereas h+and·O2-are less important.Based on these results,a plausible mechanism for the photocatalytic degradation of MB was proposed(Fig.5).
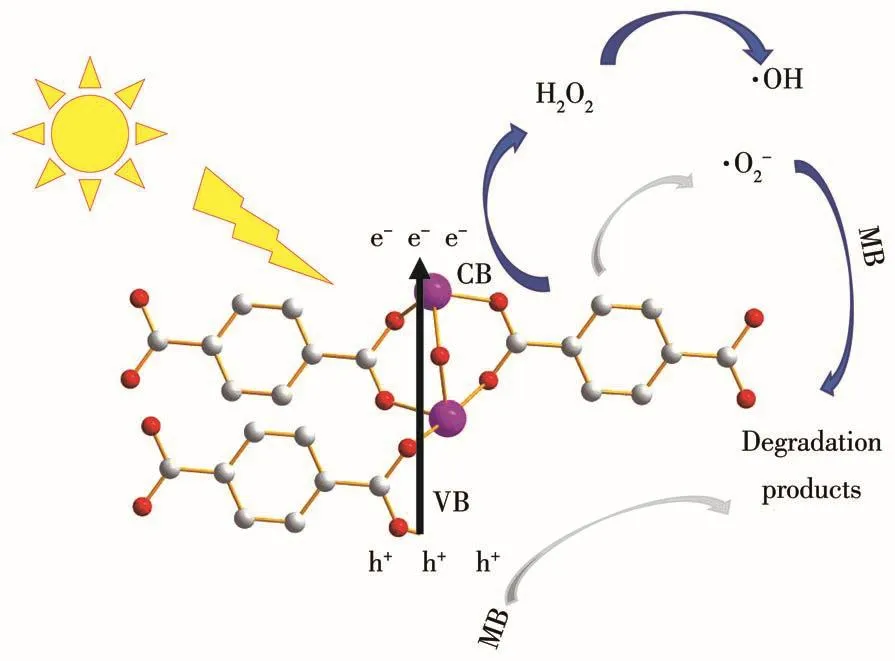
Fig.5 Possible mechanism for the photocatalytic degradation of MB and the charge transfer process
3 Conclusions
MFe-based quasi-MOF materials were synthesized by the solvothermal method to investigate the effect of the calcination temperature on their photocatalytic activity towards MB degradation.Among the tested samples,MFe-250 showed the best efficiency in the photocatalytic degradation of MB.Furthermore,it exhibited high chemical stability.To study the reaction mechanisms involved in the process,a series of control experiments were conducted.MFe was synthesized and calcined at different temperatures in air.The results showed that most of MFe porous structure remained intact in MFe-250,which exhibited the best photocatalytic degradation efficiency on MB among all tested materials.According to the photocurrent and EIS results,the electronic transmission capability of MFe-250 exceeded that of MFe.The improvement in the photocatalytic performance stems from the enhanced exposure of active sites and preservation of the framework.The porous structure favours the absorption of MB molecules,whereas the exposed active sites promote photocatalytic activity.In addition,scavenging experiments revealed that hydroxyl radicals are the main active species,whereas photoexcited holes and superoxide radicals are far less important.This study will likely inspire future designs and preparation methods of environmentally friendly photocatalysts.
Supporting information is available at http://www.wjhxxb.cn
Acknowledgments:This work was supported by the Natural Science Foundation of Anhui Province (Grant No.1908085QB75),National Natural Science Foundation of China(Grants No.62104003,21976002 and 22004003).
