Visible Light-Assisted Synthesis of Platinum Nanoparticles for Catalytic Reduction Reaction of p-Nitrophenol
2021-12-09XIANLiangSUBiQuanFENGYinXiaCAONingJingSHENGLiMAJingXIBei
XIAN LiangSU Bi-QuanFENG Yin-XiaCAO Ning-JingSHENG Li MA JingXI Bei
(1School of Chemical Engineering,Northwest Minzu University,Lanzhou 730124,China)
(2School of Chemical and Biological Engineering,Lanzhou Jiaotong University,Lanzhou 730070,China)
Abstract:Here,platinum nanoparticles were synthesized on multi-walled carbon nanotubes(MWCNTs)in one step under the irradiation of visible light without any extra additives except ethylene glycol(EG)as the reducing agent and stabilizer,and Pt/MWCNTs composites were successfully prepared.The catalytic performance of Pt/MWCNTs was studied in the reduction of p-nitrophenol(p-NP).The morphology and crystal structure of as-synthesized materials were characterized by Fourier transform infrared spectroscopy(FT-IR),X-ray diffraction(XRD),transmission electron microscopy(TEM)and X-ray photoelectron spectroscopy(XPS).Visible light irradiation promotes the hydrolysis of a[PtCl4]2-precursor in EG aqueous solution.By the electronic effect of the metal interface,the reduction of platinum precursors makes the formation of the uniformly dispersed Pt metal ultra-small particles with an average of 2.1 nm size.The as-prepared Pt/MWCNTs effectively catalyzed the reduction of p-NP to p-aminophenol(p-AP)with NaBH4,exhibiting a high catalytic performance with an apparent rate constant of 0.25 min-1.Furthermore,high reusability without significant activity loss presents that Pt/MWCNTs prepared can be an excellent and stable catalyst.The experimental results prove that besides traditional light irradiation methods,e.g.ultraviolet,the proper utilization of visible light is also a very effective method for preparing platinum metal catalysts and the morphology control can be achieved in some simple ways rather than complex reaction conditions.
Keywords:platinum nanoparticles;multi-wall carbon nanotubes;visible light;catalysis;p-nitrophenol
0 Introduction
Nano-scale precious metal materials have become the most dynamic sub-discipline in the field of nanotechnology due to their outstanding catalytic,electrical,magnetic,and optical properties[1].However,reducing costs and improving activity are still the directions to solve the industrial application of precious metal[2-3].Although the research of platinum group metal(PGM)catalysts has made great progress in recent years,it still faces many challenges[4].An almost inevitable problem is that the increase of specific surface area can improve the atom utilization[5],but consequently,the high surface energy of the metal atoms or clusters usually leads to unstoppable and extensive aggregation,and then reduces the catalytic activity.Up to now,some typical methods,i.e.,carrier restriction,“substrate-carrier”interaction and the addition of capping agents,have been adopted to solve the problem[6-7].Photochemistry is another kind of way different from the above methods.It highlights to control the reaction rate and reaction process so as to control the morphology and size distribution of PGM nanoparticles(NPs).Compared with others,this method is relatively green,convenient,reliable and relatively easy to control the reaction.In this field,researchers mostly adopt intensive irradiation sources,viz.,ultraviolet,near-ultraviolet,and laser,to achieve dynamic control[8].On contrast,visible light is seldom to be considered in the preparation of PGM NPs in the precursor solution due to its weak irradiation.However,our previous research presents that visible light does have a strong influence on the hydrolysis equilibrium of K2[PtCl4]precursor and subsequently the formation of Pt NPs in the aqueous solution[9].With the existence of methanol as a reducing agent,the intermediate[Pt(CH3OH)Cl3]-was first found in the reaction process by195Pt NMR analysis.That means the process to form Pt NPs has some neglected and complicated steps which can influence the final Pt NPs products.These previous works indicate it is worthy to probe deeply the role of visible light on the formation of Pt NPs[10-11].
p-nitrophenol(p-NP)is one of the common environmental pollutants and is hard to be degraded to a harmless compound p-aminophenol(p-AP),a lowtoxicity reduction product and an analgesic and antipyretic intermediate[12].The reduction of p-NP is often adopted to characterize the catalytic performance of Pt NPs catalyst.
1 Experimental
This article presents a facile one-step reaction associated with visible light in an aqueous solution with multi-walled carbon nanotubes(MWCNTs)as carriers and ethylene glycol(EG)as a reducing agent,as well as a stabilizer to control Pt NPs from aggregation.The research attempts to seek more possibilities to broaden the photochemical sources from commonly used light irradiations of ultraviolet,near-ultraviolet and laser light to visible light in the preparation of Pt NPs catalyst.Undoubtedly,this novel and relatively simple approach of visible light irradiation without rigorous reaction conditions conforms with the trend of green chemistry.
1.1 Materials
Potassium chloroplatinate(K2[PtCl4],AR)was purchased from Sigma-Aldrich Co.;EG(AR)was purchased from China National Pharmaceutical Group Chemical Reagent Co.,Ltd.;MWCNTs were purchased from Sigma-Aldrich Co.;Sodium borohydride(NaBH4,AR)was purchased from Shanghai Zhongqin Chemical Reagent Co.,Ltd.;p-NP(AR)was purchased from Tianjin Kaixin Chemical Industry Co.,Ltd.All chemicals were not further purified before use.Aqueous solutions were prepared with Mili-Q ultrapure water(18.2 MΩ·cm).
1.2 Preparation of Pt/MWCNTs
LED regular lights(6 000 K,15 W,wavelength region from 400 to 800 nm,Yuanbo Lighting,Guangdong)was adopted as the visible light irradiation source.24 mg MWCNTs pre-treated[13]by concentrated HNO3mixed with concentrated H2SO4at the ratio of 1∶1(V/V)was mixed with 100 mL of EG and then the flask was kept sonicate for 30 min at 40 kHz.After forming a stable black suspension,100 mL of 2 mmol·L-1K2[PtCl4]aqueous solution was added in and the pH of the solution was adjusted to 4.0,7.0 and 11.0 sepa-rately by adding a tiny amount of NaOH solution with a capillary glass tube.The reaction time was 8 h under the irradiation of LED lamp at room temperature.After the reaction was over,the solid particles were collected by centrifugation and cleaned with distilled water several times.The samples were dried and recorded as Pt/MWCNTs.
1.3 Characterizations of Pt/MWCNTs
Fourier transform infrared spectroscopy(FT-IR)was performed on Bruker TENSOR 27 infrared spectrometer(Germany)with a wavenumber range of 4 000-500 cm-1.X-ray photoelectron spectroscopy(XPS)was characterized on Kratos AXIS Ultra DLD instrument(Japan)using Al Kα as the excitation source.X-ray diffraction(XRD)was analyzed on D/MAX-RC(Japan),operating at 40 kV and 100 mA,scanning rate of 2(°)·min-1from 2θ=10°to 90°,using Cu Kα as a radiation source and λ was 0.150 6 nm.Transmission electron microscopy(TEM)and high-resolution transmission electron microscopy(HR-TEM)images were obtained from the JSM-7000 equipment(Japan)and the acceleration voltage was 300 kV.Energy-dispersive X-ray spectroscopy(EDS)was obtained on the Apollo XLT SDD energy spectrometer(USA).Ultraviolet-visible spectroscopy(UV-Vis)absorption spectra were performed on U-3900H ultraviolet-visible spectrophotometer(Japan).
1.4 Catalytic performance experiments
3 mL of p-NP solution(0.1 mmol·L-1)and 3 mL of NaBH4solution(2 mmol·L-1)were mixed in a small beaker,subsequently,2 mg of catalyst was added to start the catalysis.During the reaction process,the reaction solution was centrifuged at a certain interval(2 min)and the supernatant was taken to detect its UVVis absorption spectrum at 275-500 nm.After the reaction,the solid particles at the bottom were collected by centrifugation,washed with distilled water for several times,and then dried for the next cycle experiment.
2 Results and discussion
2.1 FT-IR characterization
The FT-IR spectra of blank MWCNTs and Pt/MWCNTs in the wavelength range of 4 000-500 cm-1are shown in Fig.1a and 1b.The broad and strong absorption peaks at 3 401 and 3 430 cm-1belong to stretching vibration absorption peaks of O—H existing in carboxyl and water molecules.The peaks at 2 926 and 2 921 cm-1belong to the absorption peaks generated by C—H stretching vibration.The peak at 1 643 cm-1belongs to the stretching vibration of C=O in the carboxyl group[14].The absorption peaks of 1 159 and 1 032 cm-1are attributed to the C—O stretching vibration.The existence of these oxygen-containing groups is related to the acidification of MWCNTs.After loading Pt on MWCNTs,the absorption peaks of C—O all show a little bit shift(1 155,1 035 cm-1)[15],and the absorption peak at 2 355 cm-1is attributed to the adsorption of CO2[16].Compared with the blank MWCNTs,the intensity of some absorption peaks in Pt/MWCNTs is reduced.This is due to the formation of“Pt—O”coordination adsorption between Pt NPs generated and oxygen-containing groups on the surface of MWCNTs,thus reducing the polarity of oxygen-containing groups.This“anchoring effect”of oxygen-containing groups is conducive to the loading of Pt NPs on the surface of MWCNTs[17].
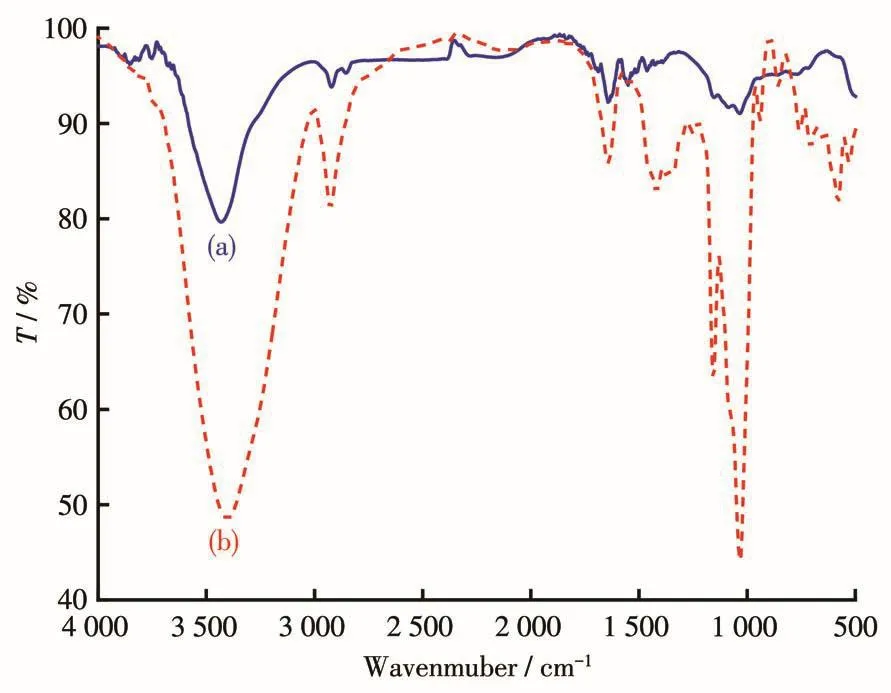
Fig.1 FT-IR spectra of(a)blank MWCNTs and(b)Pt/MWCNTs
2.2 XPS characterization
Fig.2 is the XPS spectra of Pt/MWCNTs.Fig.2a shows that the characteristic peaks of Pt4f,C1s and O1s appeared at 73.6,284.0 and 532.4 eV,respectively.After deconvolution,the XPS spectrum of Pt4f split into two pairs shown in Fig.2b.A pair of peaks at 71.0 and 74.2 eV corresponds to 4f7/2and 4f5/2peaks of Pt0,and a pair of peaks at 72.7 and 75.9 eV corresponds to 4f7/2and 4f5/2peaks of Pt2+[18].The peak of Pt2+is much weaker than that of Pt0,indicating that the visible light irradiation effectively promotes the reduction of[PtCl4]2-by EG as a reducing agent.Most of the precursors are reduced to Pt0.A small amount of Pt2+is proposed to be in the form of PtO or Pt(OH)2[19].Under the reaction conditions presented herein,the reaction was very effective and the solution after the reaction did not contain[PtCl4]2-precursor anymore according to chemical analysis.By calculation,the mass loading of Pt NPs on carriers was 1.6%.
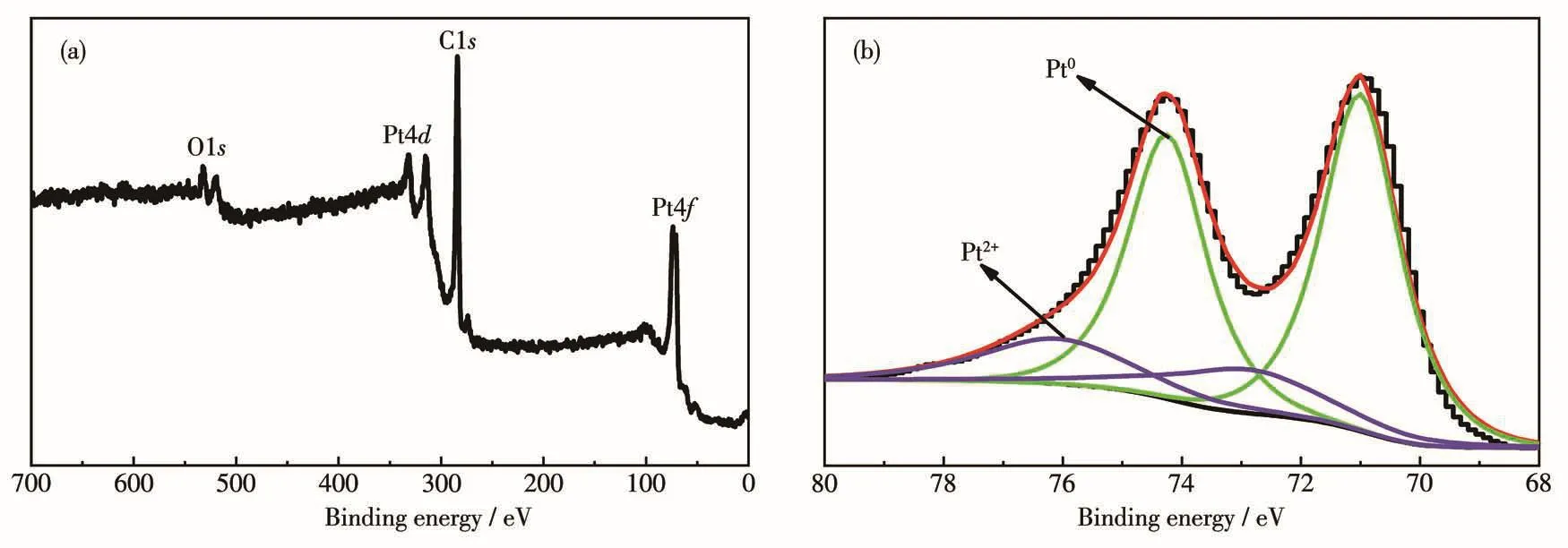
Fig.2 (a)XPS survey spectrum and(b)high-resolution Pt4f XPS spectrum of Pt/MWCNTs
2.3 XRD characterization
Pt/MWCNTs were characterized by XRD to evaluate the crystal structure of Pt NPs embedded on the surface of MWCNTs.The XRD pattern of blank MWCNTs is shown in Fig.3a.The diffraction peak near 2θ=25°is the characteristic diffraction peak of C(002).The XRD patterns of Pt/MWCNTs are shown in Fig.3b.The characteristic diffraction peaks of Pt near 2θ of 39.8°,46.2°,67.5°and81.4°correspondtothecrystalplanesof Pt(111),Pt(200),Pt(220)and Pt(311),respectively[20].The narrow and strong diffraction peaks indicate that Pt has good crystallinity.The results show that Pt NPs on the surface of MWCNTs exist in a typical facecentered cubic(fcc)structure.The average particle size of Pt/MWCNTs calculated by the Scherrer formula[21]was 2.1 nm.Obviously,the application of visible light irradiation in this synthesis of Pt nanocatalyst is very productive.Without rigorous reaction conditions,the particles size distribution of Pt NPs can also be effectively controlled.

Fig.3 XRD patterns of(a)blank MWCNTs and(b)Pt/MWCNTs
2.4 TEM characterization
Fig.4a is the TEM image of Pt/MWCNTs presenting Pt0metal atoms are loading on carriers as nanoparticles.Fig.4b is the HR-TEM image of Pt/MWCNTs.It can be seen the lattice fringes of Pt were clear,and the interplanar spacing was 0.23 nm,which corresponds to the crystal plane of Pt(111)[22].This result shows that visible light promotes Pt crystallites dispersed on MWCNTs with a good crystal orientation as proved by the XRD characterization above.A total of 111 particles was measured and calculated by Nano Measurer1.2.0 in Fig.S1(Supporting information).Fig.S2 presents the average diameter of Pt NPs was 2.1 nm and the size distribution was very narrow.This average size of Pt NPs is basically consistent with the XRD analysis result above.EDS spectrum of Pt/MWCNTs in Fig.S3 shows that Pt/MWCNTs contain a large number of Pt elements,indicating that Pt is successfully loaded on the surface of MWCNTs.Combining with the previous research[9-11],it is proposed that visible light irradiation induce the[PtCl4]2-precursor to undergo a hydrolysis reaction with a relatively low reaction rate,making the solution contain a large number of unstable hydrolysis intermediates such as [PtCln(H2O)4-n]2-n(n≤3),[PtCln(H2O)m(OH)4-n-m]m-2(n+m≤3),[PtCln(OH)4-n]2-etc.The bonding of these hydrolyzed products with the oxygen-containing functional groups on the surface of MWCNTs prevents effectively the occurrence of Pt NPs agglomeration.On the other hand,the strong interaction between Pt NPs and the carrier is also beneficial for the stability of Pt NPs in the following catalysis and keeping high catalytic performance.In addition,the ultra small particle size and narrow size distribution presented herein are very similar in our previous research[10-11].The main reason for such morphology obtained in the series research is proposed that the interaction between carbon carrier and Pt0atoms is strong and also the relative“mild”visible light irradiation is very suitable for the formation rate of Pt0ultra small particles avoiding or weakening“self-catalysis”of Pt0atom clusters.
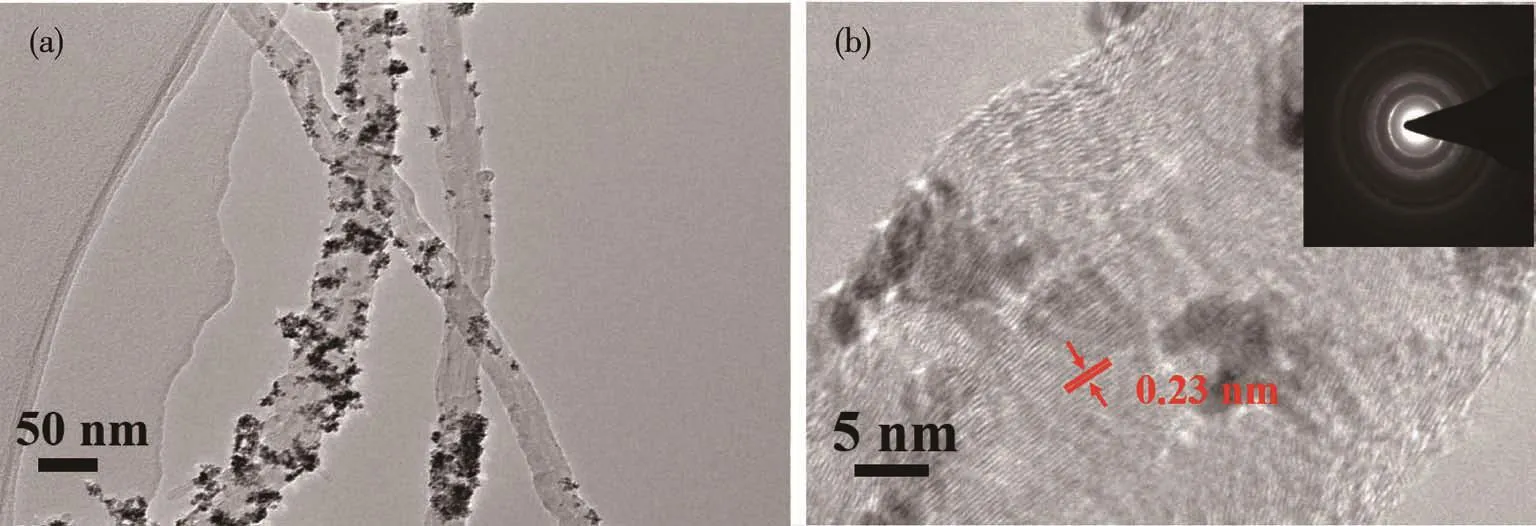
Fig.4 (a)TEM image and(b)HR-TEM image and selected area electron diffraction(Inset)of Pt/MWCNTs
2.5 Catalytic performance
The catalytic ability of Pt/MWCNTs was probed by the classic reduction of p-NP by NaBH4.The reaction with excess reducing agent follows first-order reaction kinetics[23].The absorption peaks intensity in UV-Vis has the following relationship[24]:ln(At/A0)=ln(ct/c0)=-kt,Herein,k is the apparent rate constant,c0and ctare the initial concentration and the concentration at t of p-NP,respectively.A0and Atare the initial absorbance and the absorbance at t of p-NP,respectively.k can be calculated according to the linear fitting slope of-ln(ct/c0)and t.
Fig.5a shows the UV-Vis results at 20℃.The characteristic absorption peaks of p-NP and p-AP were 400 and 300 nm,respectively[25].With the increase of reaction time,the characteristic absorption peak of p-NP gradually decreased,indicating that p-NP wasreduced to p-AP.Fig.5b presents the rate constants of Pt/MWCNTs catalysts for the reduction of p-NP at different temperatures.With the reaction temperature increasing from 20 to 80℃,the chemical reaction rate constant of the reaction increased from 0.25 to 0.55 min-1,which is higher compared with previous references[23].For calculating the activation energy according to the Arrhenius formula[26],1/T is plotted with ln k at different reaction temperatures.A straight line was obtained by linear fitting,as shown in the inset of Fig.5b.By calculation,the activation energy of the reaction,in this case,was 11.58 kJ·mol-1,which is less than that of the catalysts prepared by other methods[27].It is concluded from all the experimental data that the high catalytic activity of Pt/MWCNTs is from the stable interaction between MWCNTs and Pt NPs,small and uniform size distribution,and the relatively large specific surface area.
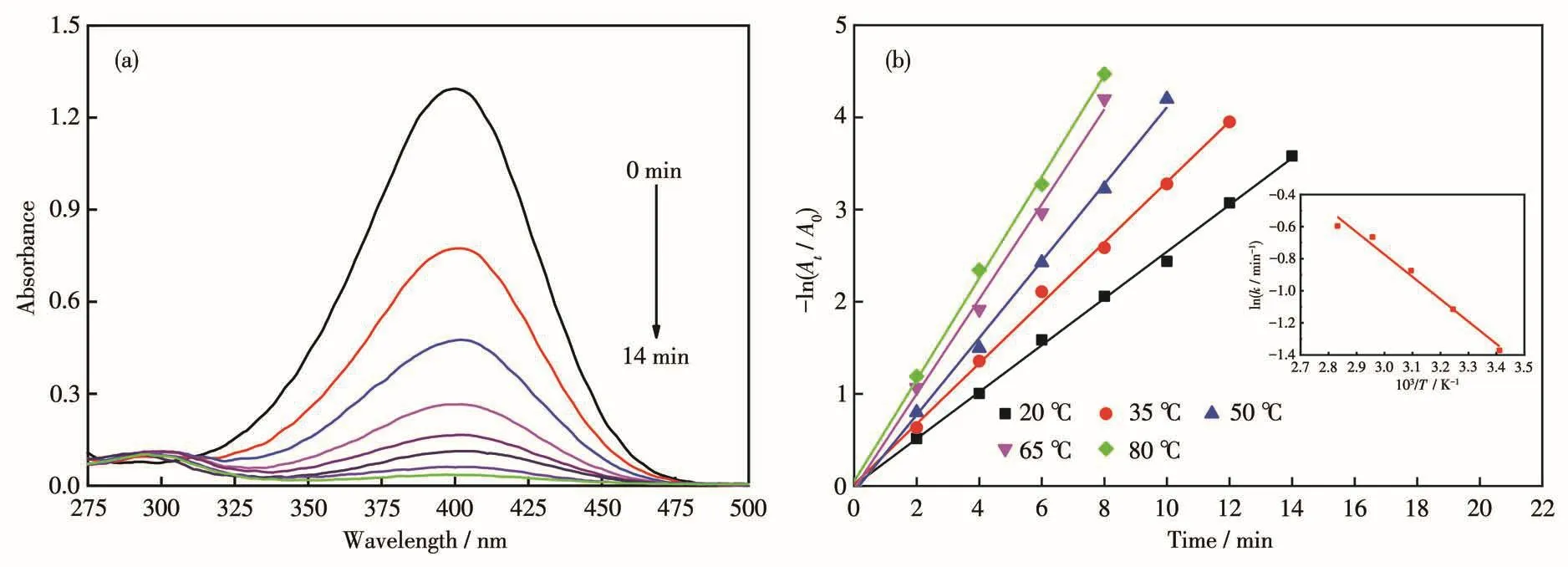
Fig.5 (a)UV-Vis absorption spectra of the p-NP solution catalyzed by Pt/MWCNTs at 20℃;(b)Graphs of the rate constants of Pt/MWCNTs catalysts for the reduction of p-NP at different temperatures(Inset:linear relationship between ln k and 1/T)
Fig.S4 shows the correlation between the conversion rate of p-NP and the number of the recycling of catalyst Pt/MWCNTs.After five cycles of use,the conversion rate of p-NP was still up to 84%.After about 10 catalytic cycles,the normalized conversion of p-NP was kept up to 80%.The decreasing of the conversion is proposed due to that there is a slight loss of unstably adsorbed Pt ultra small particles at the beginning of the catalytic cycles.
3 Conclusions
A green and facile method to prepare Pt NPs on the surface of MWCNTs was presented in this research.[PtCl4]2-precursors can be reduced by EG under the irradiation of visible light.Pt NPs with an average diameter of 2.1 nm were prepared.The characterizations of morphology,composition and valence state show that visible light can effectively control the reduction rate of[PtCl4]2-precursor and the formation of Pt NPs.The final products have a relatively high reaction rate constant and stability.This study provides a useful attempt to broaden the range of available light sources from ultraviolet,near-ultraviolet light to visible light for photochemical preparation of noble metal catalysts.By relatively simple approaches,the particles size distribution can also be controlled effectively and this kind of way conforms with the trend of green chemistry.
Supporting information is available at http://www.wjhxxb.cn
