龙葵碱对肝癌Treg细胞介导的肿瘤免疫逃逸的影响
2021-11-30高聚伟徐凯冉冉应吟吟
高聚伟 徐凯 冉冉 应吟吟

[關键词] 龙葵碱;肝癌;免疫逃逸;Treg细胞
[中图分类号] R285.5 [文献标识码] A [文章编号] 1673-9701(2021)18-0035-04
Impacts of solanine on tumor immune escape mediated by Treg cells in hepatocellular carcinoma
GAO Juwei XU Kai RAN Ran YING Yinyin
Department of Oncology, The Third Affiliated Hospital of Zhejiang Chinese Medical University, Hangzhou 310012, China
[Abstract] Objective To research the impacts of solanine on hepatoma cells immune escape mediated by regulatory T cells (Treg cells) in hepatocarcinoma bearing mice and its action mechanism. Methods The mice model of H22 cell transplantation tumor was established and they were divided into the control group, the solanine group, the transforming growth factor-β1 (TGF-β1) inhibitor group and the solanine combined with TGF-β1 inhibitor group. After 14 days of treatment, the weight of transplanted tumor in each group was measured, and the level changes of immune inhibitory cytokines such as IL-2, IL-10 and TGF-β1 in the peripheral blood of mice in each group were detected by the enzyme linked immunosorbent assay (ELISA) method. The expression levels of CD4+, CD4+CD25+Foxp3+Treg in spleen lymphocyte culture medium of mice in each group were detected by the flow cytometry method, and the immunofluorescence intensity of Foxp3+ in spleen lymphocyte culture medium of mice was detected by the immunofluorescence method. Results After 14 days, compared with the control group, the tumor weights of the hepatocarcinoma bearing mice in the solanine group, the TGF-β1 inhibitor group and the combined group were all significantly lower than that in the control group, with statistically significant differences (P<0.05). The levels of immunosuppressive cytokines such as IL-2, IL-10 and TGF-β1 in the peripheral blood of mice in the solanine group, the TGF-β1 inhibitor group and the combined group were all significantly decreased (P<0.05). The expression levels of CD4+ in the solanine group, the TGF-β1 inhibitor group and the combined group were higher than those in the control group, while the proportion of CD4+CD25+Treg decreased significantly (P<0.05). Immunofluorescence staining showed that the expressions of Foxp3+ in Treg cells of hepatocarcinoma bearing mice in the solanine group, the TGF-β1 inhibitor group and the combined group were significantly lower than those in the control group (P<0.05). Conclusion Solanine may play an anti-tumor role by reducing the content of Treg cells in hepatocarcinoma bearing mice to improve immune escape.
[Key words] Solanine; Hepatocellular carcinoma; Immune escape; Treg cells
原发性肝癌(Hepatocellular carcinoma,HCC)的发生发展机制复杂,免疫逃逸是其重要特征之一[1]。肿瘤免疫治疗,包括过继细胞疗法、肿瘤疫苗及细胞因子治疗等,尤其是近年来针对免疫检查点的免疫治疗在肝癌临床中取得了较好的疗效,成为肝癌治疗的重要手段之一[2-3],但目前仍面临疗效不确定、客观缓解率低等问题。研究表明,调节性T(regulatory T,Treg)细胞在肿瘤的免疫逃逸中发挥重要作用,肿瘤内高密度的Treg细胞常提示了较差的临床预后,如肾癌、肝癌和胃癌等的研究中证明了不良预后与瘤内Treg细胞密度增加相关[4-5]。龙葵碱是一种弱碱性糖苷,研究表明,龙葵碱在肝癌、胰腺癌、乳腺癌、结肠癌等多种肿瘤中具有广泛抗癌作用,其通过抑制细胞增殖,诱导细胞凋亡,阻断细胞周期等,发挥显著抗肿瘤效应[6-8]。但是,关于龙葵碱对肿瘤免疫功能影响的研究较少。本课题组前期研究发现[9-10],龙葵碱可恢复荷瘤小鼠抗肿瘤的免疫性反应,但对免疫逃逸的影响未明确。
1 材料与方法
1.1材料来源
实验动物SPF级雄性昆明种小鼠,6周龄,体重18-22 g,由浙江中医药大学实验动物中心提供,合格證号:SYXK(浙)2013-0184。H22肝癌细胞株购自中国科学院上海生科院细胞资源中心。龙葵碱(Solanine,HPLC≥95%)及TGF-β1抑制剂(SB-431542)均购自美国Sigma公司。
1.2 方法
1.2.1 H22荷瘤小鼠模型建立 选用1只实验小鼠,将正常传代的H22肝癌细胞株接种于小鼠腹腔,接种后第7天无菌抽取腹水,用生理盐水稀释成1.0×106个/mL瘤细胞悬液。取40只小鼠,每只小鼠右上肢腋部皮下接种0.2 mL瘤细胞悬液。
1.2.2 动物分组及给药 接种24 h后将小鼠随机分四组,分别为对照组(Control):生理盐水0.2 mL/d腹腔注射;龙葵碱组(Solanine):龙葵碱按37.5 mg/kg剂量每日0.2 mL腹腔注射;TGF-β1抑制剂组(SB-431542):生理盐水0.2 mL/d腹腔注射,用药第6、7天及第13、14天加用TGF-β1抑制剂SB-431542,按10 mg/Kg剂量0.2 mL腹腔注射;龙葵碱组联合TGF-β1抑制剂组(solanine +SB-431542):龙葵碱按37.5 mg/kg剂量每日0.2 mL腹腔注射,用药第6、7天及第13、14天加用TGF-β1抑制剂SB-431542,按10 mg/Kg剂量0.2 mL腹腔注射。
1.3 观察指标
1.3.1 抑瘤作用检测 末次用药24 h后,断颈牺牲小鼠,剥取瘤块称重,按如下公式计算肿瘤抑制率:肿瘤抑制率(%)=[(对照组平均瘤重g-给药组平均瘤重g)/对照组平均瘤重g]×100。
1.3.2 ELISA检测小鼠外周血中IL-2、IL-10、TGF-β1水平 在断颈牺牲小鼠前先摘眼球取血,4℃静置1 h,3000 rpm离心10 min,收集上层血清,置-20℃冰箱中冻存备用。细胞因子IL-2、IL-10、TGF-β1和ELISA检测试剂盒均购自武汉赛培生物科技有限公司。按ELISA检测试剂盒说明书推荐方法测定各组小鼠血清中IL-2、IL-10、TGF-β1的含量。
1.3.3 FCM测定小鼠脾细胞中CD4+、CD4+CD25+Foxp3+Treg的表达水平 实验结束后,将无菌分离的脾脏研磨匀浆制作细胞悬液,200目细胞筛过滤后移至新离心管,800×g离心5 min后去上清。细胞经PBS溶液洗涤2次后,离心弃上清。加适量细胞培养基,调整细胞密度为1×107个/mL。用淋巴细胞分离液处理使细胞分层为分层液和上清液之间的云雾层,用注射针吸取至新的离心管。同样PBS溶液洗涤2次,离心去上清,即得脾脏组织单个核细胞。分别取100 μL的细胞悬液于两个流式细胞管中,第1管加入PERCP标记的CD4抗体,第2管中加入PERCP标记的CD4抗体、CD25抗体。室温下避光孵育20 min后,加入500 μL细胞内膜阻断剂10 min后,离心去上清。加入PE标记的Foxp3抗体,室温下避光孵育20 min后,PBS溶液洗涤1次后悬浮。流式细胞仪测定各组小鼠脾淋巴细胞中CD4+CD25+Foxp3+/CD4+(Treg)的比例。
1.3.4 免疫荧光检测小鼠脾细胞中Foxp3+免疫荧光强度 脾细胞悬液玻片用4%的多聚甲醛固定爬片15 min,PBS浸洗玻片3次,每次3 min。0.5%Tritonx-100(PBS配置)室温通透20 min,PBS浸洗玻片3次,每次3 min,吸水纸吸干PBS,玻片上加入山羊血清,室温封闭30 min。每片玻片滴入一抗(FOXP3,Abcam,1∶100),放入湿盒,4℃孵育过夜。加入荧光二抗,湿盒中孵育1 h后,回收二抗,PBST洗涤3次,每次5 min。玻片上滴加DAPI,避光孵育5 min,对标本进行染核,吸水纸吸干玻片上液体,用含抗荧光淬灭剂的封片液封片,在荧光显微镜下观察和收集图像(日本尼康)。
1.4 统计学方法
应用SPSS 20.0统计软件进行相关数据分析,计量资料均用(x±s)表示,两组间的比较用t检验,多个样本率的比较用χ2检验,多组间比较采用单因素方差分析(one-way ANOVA)。以P<0.05或P<0.01表示差异有统计学意义。
2 结果
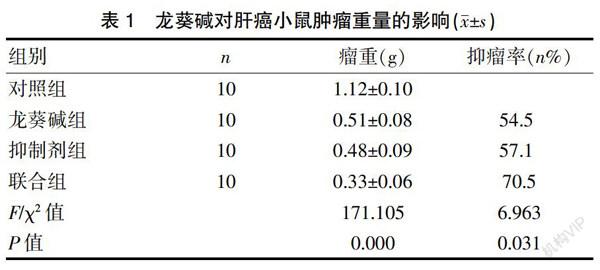
2.1龙葵碱可抑制小鼠肝癌移植瘤的生长
龙葵碱组(Solanine)、TGF-β1抑制剂组(SB-431542)、联合组(Solanine+SB-431542)的移植瘤重量均明显低于对照组(Control),差异有统计学意义(P<0.01),见表1。
2.2龙葵碱可降低小鼠外周血中IL-2、IL-10、TGF-β1水平
与对照组(Control)相比,龙葵碱组(Solanine)、TGF-β1抑制剂组(SB-431542)、联合组(Solanine+SB-431542)3组小鼠外周血中IL-2、IL-10、TGF-β1水平明显降低,差异有统计学意义(P<0.05),见图1。
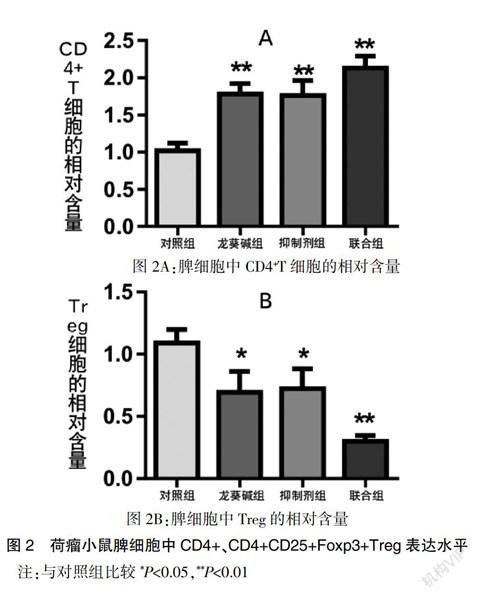
2.3龙葵碱可降低脾脏中CD4+CD25+Foxp3+Treg表达水平
为观察龙葵碱对肝癌荷瘤小鼠Treg的影响,本研究以CD4+CD25+Foxp3+作为Treg标记。结果显示,龙葵碱组(Solanine)、TGF-β1抑制剂组(SB-431542)、联合组(Solanine +SB-431542)三组CD4+表达水平均高于对照组(control),相应的,CD4+CD25+Treg在CD4+T细胞中的比例则明显下降,尤其是联合组(Solanine +SB-431542)下降更为明显,差异具有统计学意义(P<0.01)。见图2。
2.4小鼠脾细胞中Foxp3+免疫荧光强度
免疫荧光染色结果显示,龙葵碱组(Solanine)、TGF-β1抑制剂组(SB-431542)、联合组(Solanine+SB-431542)三组荷瘤小鼠Treg细胞中Foxp3+的表达明显低于对照组(Control),差异有统计学意义(P<0.05)。见图3,封三图3。
3 讨论
肿瘤免疫逃逸(Tumor immune escape,TIE)是肿瘤形成的重要特征之一,是肿瘤细胞通过多种机制逃避机体免疫系统识别和攻击,从而得以在体内生存和增殖的现象。Weinberg教授和Hanahan教授在2011年的“The Hallmarks of Cancer”将免疫逃逸作为肿瘤发生发展十大特征之一,其涉及基因、炎症、血管、代谢等多环节参与。免疫疗法通过逆转免疫逃逸、恢复机体免疫识别和清除肿瘤细胞,日益成为研究热门[11]。肝癌的免疫治疗主要有过继細胞疗法、肿瘤疫苗、细胞因子治疗以及新近热门免疫检查点疗法,但因肿瘤免疫逃逸机制处于一个复杂的免疫网络中,故目前免疫治疗效果也因此受到一定限制[2-3]。肝癌的微环境中包含大量巨噬细胞及固有免疫和适应性免疫细胞,形成了复杂的免疫耐受微环境[12]。研究发现[13-14],肝癌肿瘤微环境中CD4+CD25+Treg大量增加,这些CD4+CD25+Treg能够抑制肝癌中DC的免疫应答,高密度的肿瘤浸润性CD4+CD25+Foxp3+Treg与不良预后相关。抑制Treg可能是诱导肝癌免疫应答的重要途径,对肿瘤免疫治疗具有重要意义。
Treg细胞占正常人外周血中CD4+T淋巴细胞亚群的5%~10%,分为天然产生的自然调节性T细胞(nTreg)和诱导产生的适应性调节性T细胞(iTreg),肿瘤微环境中的Treg细胞主要是诱导产生iTreg[15]。Treg的表面标志物是CD4、CD25,胞核标志物Foxp3。在肿瘤微环境中,由肿瘤细胞分泌的TGF-β1与IL-2一起诱导初始CD4+CD25-细胞转化为CD4+CD25+细胞并表达FoxP3,使其具备CD4+CD25+细胞的特性(即CD4+CD25+FoxP3+Treg细胞)[16]。CD4+CD25+FoxP3+Treg细胞与肿瘤免疫逃逸密切相关,具有抑制CD4+细胞和CD8+T细胞活化、增殖的作用。叉头翼状螺旋转录因子(Foxp3)与免疫调节相关,在细胞的分化和功能维持方面具有重要作用,是Treg细胞的特异性标记物。研究表明[17],TGF-β1/Smad通路在肿瘤微环境中Treg的生成中发挥重要作用,其通过诱导Treg细胞表达其特异转录因子Foxp3,促进肿瘤免疫逃逸。而SB-431542为特异性的TGF-β1抑制剂,可以抑制下游信号分子Smad2/3的磷酸化,从而阻断依赖 Smad 途径[18]。研究表明[19-20],CD4+CD25+FoxP3+Treg主要通过两条途径调控肿瘤免疫:一是通过分泌白介素-2(Interleukin-2,IL-2)、白介素-10(Interleukin-10,IL-10)及转化生长因子-β1(Transforming growth factor-beta,TGF-β1)等抑制性细胞因子,抑制细胞毒性T细胞(CD8+)和自然杀伤细胞(NK)的活化、增殖而发挥其负性免疫调控作用;另一个是通过细胞与细胞间的直接接触发挥细胞抑制作用。龙葵(Solanum nigrum L.)是我国常用抗癌中药,为茄科茄属草本植物,其抗肿瘤药理活性成分为龙葵生物碱(Solanine),通过抑制细胞增殖、诱导细胞凋亡、阻断细胞周期等机制发挥抗癌作用,具有良好的药用前景[6-8]。本研究从龙葵碱影响肝癌荷瘤小鼠免疫逃逸的角度探讨了龙葵碱抗肿瘤的可能机制,结果表明,龙葵碱作用后肝癌荷瘤小鼠脾细胞中Foxp3+免疫荧光强度明显减低,Treg细胞表达水平明显下降,外周血中抑制性细胞因子IL-2、IL-10、TGF-β1的水平也明显下降,推测龙葵碱可能改善了细胞毒性T细胞(CD8+)和自然杀伤细胞(NK)的抗肿瘤活性,从而发挥抗肿瘤作用。
在本研究中,通过H22肝癌荷瘤小鼠动物实验验证了龙葵碱对肝癌的抑制作用。龙葵碱和TGF-β1抑制剂可显著降低荷瘤小鼠Treg细胞含量,降低免疫性抑制细胞因子IL-2、IL-10及TGF-β1的分泌。龙葵碱可能通过抑制Treg细胞中关键转录因子Foxp3的表达而发挥免疫调节作用。具体机制有待于进一步实验验证。
[参考文献]
[1] Ringelhan M,Pfister D,OConnor T,et al.The immunology of hepatocellular carcinoma[J].Nat Immunol,2018,19(3):222-232.
[2] JMichael P Johnston,Salim I Khakoo.Immunotherapy for hepatocellular carcinoma: Current and future[J].World J Gastroenterol,2019,25(24):2977-2989.
[3] Liu X,Qin S.Immune Checkpoint Inhibitorsin Hepatocellular Carcinoma:Opportunities and Challenges[J].Oncologist,2019,24(Suppl 1):S3-S10.
[4] Nicholas Arpaia,Jesse A Green,Bruno Moltedo,et al.A Distinct Function of Regulatory T Cells in Tissue Protection[J].Cell,2015,162(5):1078-1089.
[5] Tanaka A,Sakaguchi S.Regulatory T cells in cancer immunotherapy[J].Cell Res,2017,27(1):109-118.
[6] 黃苗苗,刘美英,李保环,等.龙葵碱通过调节miR-140和结肠癌转移相关基因1调控胃癌细胞增殖和凋亡[J].中国临床药理学杂志,2020,36(16),2440-2443.
[7] Mohsenikia M,Alizadeh AM,Khodayari S,et al.The protective and therapeutic effects of alpha-solanine on mice breast cancer[J].Eur J Pharmacol.2013,718(1-3):1-9.
[8] Wei-Feng Zhong,Si-Ping Liu,Bin Pan,et al.Solanine inhibits prostate cancer Du145 xenograft growth in nude mice by inducing cell cycle arrest in G1/S phase[J].Nan Fang Yi Ke Da Xue Xue Bao,2016,36(5):665-670.
[9] 陈培丰,潘磊,高聚伟,等.龙葵正丁醇萃取物对肝癌H22荷瘤小鼠的抑瘤作用及其免疫功能的影响[J].中国中医药科技,2013,20(2):141-142.
[10] 陈培丰,高聚伟,潘磊.龙葵氯仿及正丁醇提取物对lewis肺癌移植瘤增殖及其血清IFN-γ、IL-2和IL-4含量的影响[J].中华中医药学刊,2014,32(8):1799-1802.
[11] Vasquez L,Castro D,León J,et al.Cancer immunotherapy:from inception to Nobel Prize[J].Rev Peru Med Exp Salud Publica,2020,37(1):115-121.
[12] Kurebayashi Y,Ojima H,Tsujikawa H,et al. Landscape of immune microenvironment in hepatocellular carcinoma and its additional impact on histological and molecular classification[J].Hepatology,2018,68(3):1025-1041.
[13] Lee WC,Wu TJ,Chou HS,et al.The impact of CD4+CD25+T cells in the tumor microenvironment of hepatocellular carcinoma[J].Surgery,2012,151(2):213-222.
[14] Huang Y,Wang FM,Wang T,et al.Tumor-infiltrating FoxP3+Tregs and CD8+T cells affect the prognosis of hepatocellular carcinoma patients[J].Digestion,2012,86(4):329-337.
[15] Bluestone,J.A,A.K. Abbas.Natural versus adaptive regulatory T cells[J].Nat Rev Immunol,2003,3(3):253-257.
[16] Greten T F,Ormandy L A,Fikuart A,et al.Low-dose cyclophosphamide treatment impairs regulatory T cells and unmasks AFP-specific CD4+ T-cell responses in patients with advanced HCC[J].J Immunother,2010,33(2): 211-218.
[17] Chu C S,Boyer J,Schullery D S,et al.Phase I/II randomized trial of dendritic cell vaccination with or without cyclophosphamide for consolidation therapy of advanced ovarian cancer in first or second remission[J]Cancer Immunol Immunother,2012,61(5):629-641.
[18] Gu AD,Wang Y,Lin L,et al.Requirements of transcription factor Smad-dependent and independent TGF-β signaling to control discrete T-cell functions[J].Proc Natl Acad Sci USA,2012,109(3):905-910.
[19] Wolf D,Sopper S,Pircher A,et al.Treg(s) in Cancer: Friends or Foe?[J].Cell Physiol,2015,230(11):2598-2605.
[20] Zhang H,Podojil JR,Chang J,et al.TGF-beta-induced myelin peptide-specific regulatory T cells mediate antigen-specific suppression of induction of experimental autoimmune encephalomyelitis[J].J Immunol,2010,184(12):6629-6636.
(收稿日期:2021-01-11)
