The occurrence of vanadium in nature: its biogeochemical cycling and relationship with organic matter—a case study of the Early Cambrian black rocks of the Niutitang Formation, western Hunan, China
2021-11-04RizwanSarwarAwanChenglinLiuShangfengYangYupingWuQibiaoZangAsharKhanGuoxiongLi
Rizwan Sarwar Awan • Chenglin Liu • Shangfeng Yang • Yuping Wu •Qibiao Zang • Ashar Khan • Guoxiong Li
Abstract Vanadium in the black rocks has economic and environmental impacts. In sediments, it is broadly disseminated as a multivalent metal element mainly sensitive to redox settings. Globally in petroleum, it is considered an abundant component. Vanadium is an essential tool to determine the relationship of the Earth with extra-terrestrial bodies. In the Yangtze region, the black rocks of the Early Cambrian Niutitang Formation are highly enriched in the concentration of V, Co, Ni and Mo. These sediments are comprised of a high total organic carbon content, and the average concentration of vanadium is over 240 ppm. Here we discuss the mechanisms and conditions that were responsible for the accumulation of vanadium in these black sediments in the Yangtze region. The oxygenated ocean water is favorable for the dissolved vanadate species V(V). Therefore, in oxic ocean-water, it can be reduced by organic matters or by H2S to vanadyl ions V(IV), which can facilely be adsorbed to the tiny particles and finally deposit into the sediments with the settling of the particles.The presence of V2O3 in the Niutitang Formation indicates the isomorphism state of vanadium existence in the clay minerals. Clays and pyrite are the most favorable mineral for vanadium enrichment. However, it is suggested the quartz of non-biogenic origin might be unfavorable for vanadium enrichment. Vanadium is mainly derived from the diagenetic transformation of its precursor (porphyrin pigments and chlorophyll) from the organism. During the Early Cambrian period, the massive transgression in the sea level created a favorable environment for organisms to survive. Additionally, the hydrothermal activities brought massive nutrient supply in the form of vanadium and other metal elements from the deep Earth. These creatures consumed the vanadium-rich nutrients, which became a part of their bodies in the form of hard and soft parts. Later on,when these organisms died and were submerged in the sediments. After the diagenetic actions, this vanadium became a part of these black sediments along with organic carbon. Therefore, these black rocks in the Yangtze region are enriched in vanadium and organic carbon. It is suggested the various processes such as adsorption, complexation, and reductions are the main factors responsible for the precipitation of dissolved vanadium into the organically rich sediments.
Keywords Vanadium ∙Organic matter ∙Chlorophyll ∙Porphyrin ∙Anthropogenic ∙Isomerization
1 Introduction
The black rock sediments are widely distributed around the globe (Fig. 1). The Early Cambrian is a unique geological span due to diversity in the abundance of ancient remains(fossils), also known as a period of Cambrian Explosion(Amthor et al. 2003; Pi et al. 2013). Variation in the ambient conditions signifies a feasible rationale for evolutionary heterogeneity; nevertheless, it is a subject of discussion. Early Cambrian marine sedimentary rocks are broadly deposited in entire South China. The Niutitang Formation in South China comprises various types of black sediments, e.g., shale, carbonaceous shale, siliceous shale,sandstone, silty shale, mudstone, and silty mudstone, also known as the black rock series (Awan et al. 2020). These rocks became a new type of mineral resource in China due to the enrichment of some precious elements, e.g.,molybdenum, vanadium, and uranium, but so far without substantial exploitation of these elements. In the environment, vanadium is a prevalent metal element. It is widely used nearly in all fields of geochemistry. A Spanish mineralogist, Andres Manuel Del Rio has discovered vanadium in 1801 during working on blue Pb ore (Pb5(VO4)3Cl)sample from Mexico. That has also been called vanadinite(Weeks 1932). There are two isotopes of indigenous vanadium (i)50V (radioactive), (ii)51V (stable). The concentration of vanadium (V) in the Earth’s crust is identical to zinc (Zn). It is nearly ten times greater than lead (Pb) and twice copper (Cu). In the Earth’s crust, it is the fifth most frequently occurring transitional element. The average amount of vanadium in the ocean, sediments, and in the atmosphere is 1.8 μg/L, 150 mg/kg, and 1000 ng/m3,respectively (Fig. 2) (Imtiaz et al. 2015; Langeslay et al.2018). Vanadium is broadly disseminated in rocks (igneous and sedimentary) and minerals as moderately incompatible lithophilic elements. Vanadium has not been studied extensively relative to other transitional metal elements.Vanadium, chiefly in the sediments, can occur in three forms (a) trivalent V (III), (b) tetravalent V (IV), and(c) pentavalent V (V) (Larsson 2014). The enrichment of vanadium has been noticed in oil shale, peat, crude oil,bitumen, and asphalt, and the sediments (rocks and soils) of the volcanic regions. Vanadium, as a VO-porphyrins in crude oils, can reach up to 0.12% (Aydin et al. 2012). In the sediments, the concentration of vanadium is used to examine the mineralogy of the parent rock. Almost 65 minerals in nature contained vanadium. The more significant minerals containing vanadium are vanadinite, carnotite, mottramite, patronite, and roscoelite (Lide 2008).Organic matter (OM) and vanadium have a significant relationship. It is due to its considerable attraction towards OM. The organic matter can form complex aggregates with vanadium or can reduce to its lower valence state, particularly at a lower pH level (Lu et al. 1998). Vanadium could be adsorbed or integrates into clays; therefore, the concentration of vanadium could be higher in organically rich sediments or peats. In metallic form, it can’t exist in soils,whereas commonly, it could occur with oxides of aluminium, iron, manganese, lead, copper, calcium, and zinc.In sediments, the most typical form of vanadium is vanadates (Venkataraman and Sudha 2005). In the oceans,vanadium is the second most abundant transitional element after molybdenum and is found as an ion pair (Na+H2-VO4-) with a typical concentration of 30–35 nM. Macroalgae utilize vanadate (V) from the ocean water in the form of vanadium. Marine organisms such as fan worms, sea squirts (ascidians) use vanadate without any distinct biological advantages (Rehder 2015). Currently, the distribution of vanadium under differentiation of magma in elevated temperature and pressure, and the new features to study the stable isotopic composition of vanadium is a hot topic (Nielsen et al. 2014; Prytulak et al. 2013). The possible distribution and the accumulation of the vanadium in the Earth’s interior are shown in Fig. 2. Nearly 80% of the produced world vanadium is chiefly utilized in the steel industry as an additive. As vanadium is an essential raw material, it plays a vital role in the steel-iron industry and other manufacturing units, e.g., shipyard, automobiles,fertilizers, etc. Its complexes are valuable and have a wide range of applications in catalysts-ceramics, industries,batteries, pigments. Vanadium is resistant to rust, utilized in various nuclear applications. It is also utilized in highspeed iron-steel tools and superconductive magnets. In the medical industry, vanadium supplements are being utilized to remedy various diseases (Emsley 2011). In the past few decades, the occurrence of vanadium (V) in the source rocks containing kerogen, bitumen, and crude oil has become a hot topic in the field of geochemistry. Vanadium is the most prevalent and abundant trace element in petroleum. The relationship of vanadium with the OM relies on various factors related to diagenetic changes, such as biological precursors, water incorporation, or sediments. It can be controlled by sedimentary redox settings (Eh and pH), type of OM, and organometallic compounds (Monaco et al. 2002). The following are the key objectives of this research:

Fig. 1 Global distribution of black rock/shales (Awan et al. 2020)

Fig. 2 The accumulation of vanadium on the earth. Modified after Huang et al. (2015)
1. This research broadly discusses the geochemical constraints of vanadium under various environments.
2. It is a holistic approach to elucidate the favorable minerals for vanadium accumulation.
3. To determine the biological origin of vanadium in organic-rich black rocks of the Niutitang Formation.
2 Geology and tectonics
A multistage action caused the fragmentations of Rodina during the Neoproterozoic time. The final phase of splitting and drifting took place during the period of 750–690 Ma(Liu et al. 2015). In North China, Cathasian and Yangtze blocks were isolated during the period of Ediacaran-Early Cambrian. The rifted deep marine basin had produced at the surrounding of the Yangtze block’s periphery (Liguo 1997). At that period, the Yangtze block was situated at the equatorial zone closer to the S–W of the Australian Plate(Fig. 3) (Liu and Xu 1994). Due to tectonics progression in Ediacaran to the Early Cambrian period, paleo-settings of the research area were transformed from shallow to a deeper marine shelf or slope environment (carbonates to argillaceous rocks) (Liu et al. 2015; Zhu et al. 2003).Recent studies revealed that Early Cambrian was characterized as a maximum flooding span in the Yangtze region.OAE (anoxic ocean event) extensively prevailed during the Early Tommotian and created anoxic bottom water settings on a massive scale (Jiang et al. 2011). Niutitang and its geological equivalent rocks are on a wide-scale comprised of black shale deposits on the Yangtze black, especially on the slope. Generally, it is suggested that phosphorites at the bottom of Cambrian are a clue of strong upwelling (Clark et al. 2004; Wille et al. 2008). Among them, some black shales are related to metallic ores (Fe–Ni–Mo). These ores were deposited due to hydrothermal events in the Cambrian period (Orberger et al. 2007; Steiner et al. 2001). Another indicia to prove Cambrian hydrothermal activity is higher values of Eu anomaly of the phosphate nodules within the shales (Zhu et al. 2014). Various metallic ratios (Co–Cu–Ni-PGE’s) suggest the ultramafic and mafic source for these rocks (Han et al. 2015). Few studies indicate the primary source for these metallic ores in these black sediments is due to seawater in a silled basin via durable stratigraphic condensation (Lehmann et al. 2007; Och et al.2013). In the eastern Yunnan region, the lower part of the Niutitang Formation’s geologic age is 540–536 Ma based on U–Pb dating. The Guizhou area shows relatively younger ages suggesting territorial diachroneity, for instance, 522.7 ± 4.9 Ma (Wang et al. 2012) and 532.3 ± 0.7 Ma (Jiang et al. 2011). The central part of the black Niutitang Formation is dated to 521.0 ± 5.0 Ma using Re-Os (Xu et al. 2011). The deposition age of the Niutitang Formation based on recent researches is 3–12 Ma year, which was started at 532–523 Ma and stopped at approximately 520 Ma years from the bottom to the top. The average thickness of Niutitang Formation in the research area is 76 m, which exhibits the rate of deposition was about 6–7 m/Ma or nearly 25 m/Ma (Xu et al. 2012).
3 Methods and material
In this research, a geological field trip of the Xiangxi area in western Hunan, South China, has been organized to collect samples from two well-exposed outcrop sections Longbizui (LA) and Sancha (CR) section (Fig. 4). Various geochemical experiments were performed on these samples to accomplish this research.
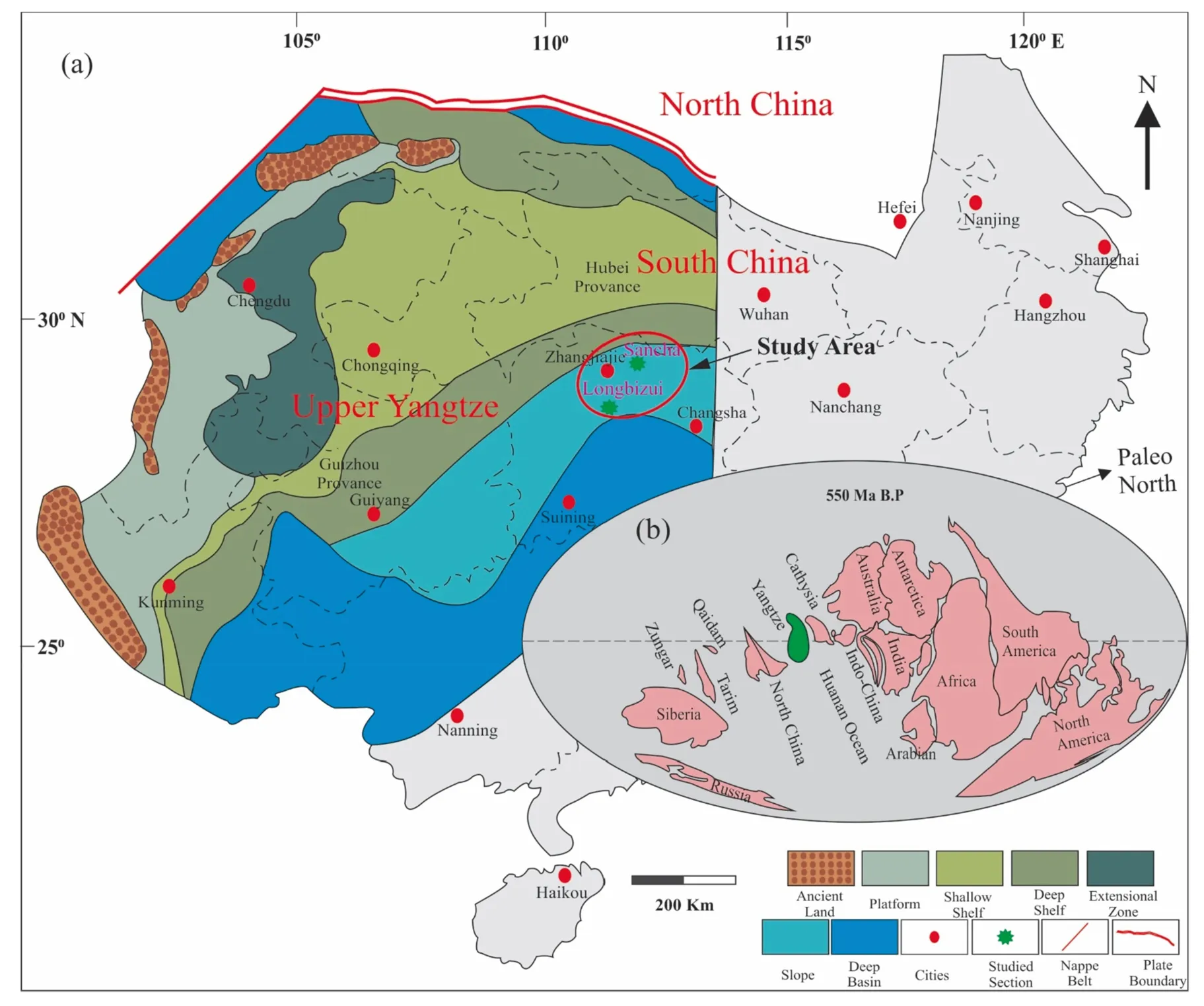
Fig. 3 a The Paleogeographic map of the upper Yangtze region, deciphering the different lithofacies (modified after Luo 2014; Steiner et al.2001; Zhang et al. 2018), b displaying global paleogeography during the early Cambrian period (modified after Liu et al. 2015)
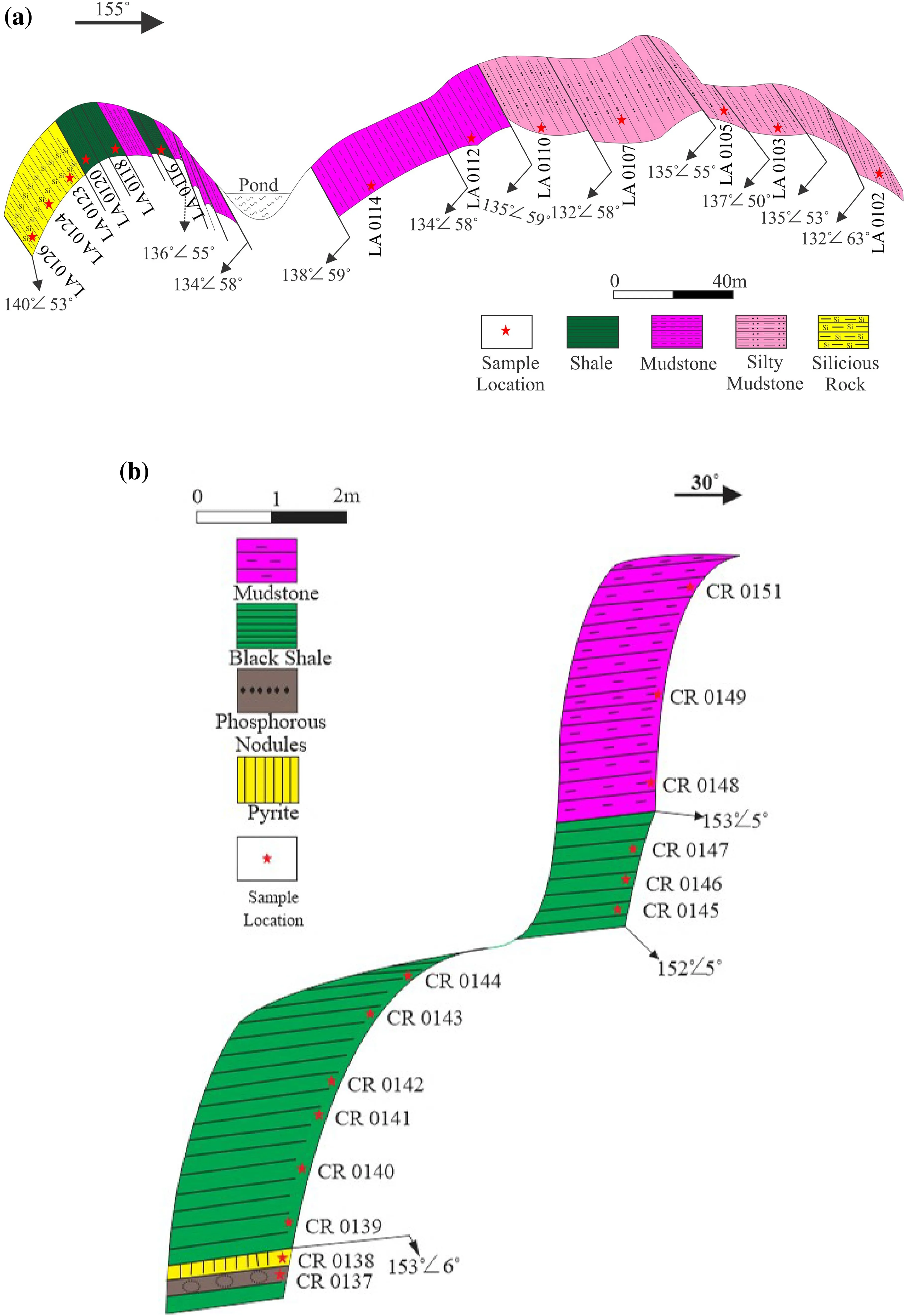
Fig. 4 The studied outcrop sections revealing the lithology and the sample collection points (a). Longbizui section (b). Sancha section
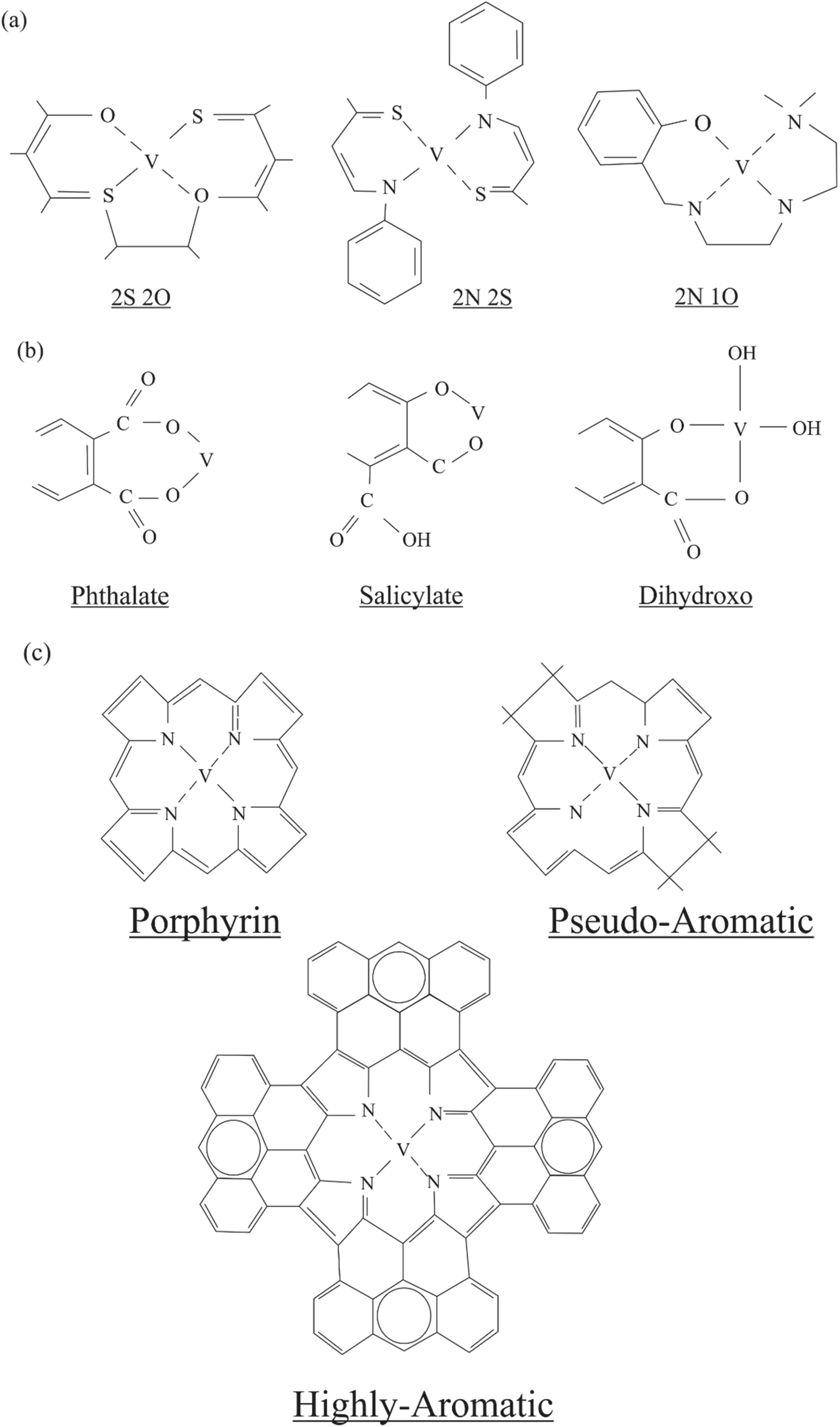
Fig. 5 The Metallo-organic compounds containing vanadium in naturally occurring substances
Total organic carbon (TOC)quantifies the total amount of organic carbon in the samples. In this research, the amount of TOC is measured by the LECO CS230. At first,rock specimens were crushed and sieved to a smaller size(<200) and cleaned with a dilute HCL (5%) for 24 h at 60 °C to eradicate carbonated and inorganic carbon. Subsequently, the treated HCl was eliminated with the purified water. Then the test specimens were desiccated to 50 °C in the oven for 24 h. The samples were combusted at high temperatures (900 °C) using an oxygen-enriched carrier gas. The quantity of organic C was entirely oxidized to CO2 from a specimen, and the bulk volume of produced CO2 was utilized to compute TOC.
X-ray diffractometer (XRD)using Bruker D2 PHASER X was applied to determine the mineralogy of the studied samples. A small amount (5 g) of grounded (300 mesh size) samples were utilized. Continuous scanning (183–6)in the device was selected at the step of 0.002 with an accuracy of ± 0.1%. The quantitative mineralogical results of the specimens were obtained via MacDiff software.
The concentration of trace elementsin these sediments was analyzed by incorporating Nex ION300D. Samples were formerly dissolved into an acidic solution. The trace elements were evaluated using the standard internal technique with a relative temperature and humidity below 21 °C and 31%, respectively. The error in this technique was below 10%.
The scanning electron microscope (SEM), with the aid of energy dispersive spectroscopy (EDS), was operated for the examination. The compositional analysis of catalyst,surface morphology, and rock minerals was investigated via Field Emission Microscope (FEI Quanta200F) along with a focused ion beam system. Initially, the surface of the samples was gold-coated. The gold coating process is adopted for a better resolution before the scan with an electrostatic beam positioned in the cylinder. Both the secondary ion and secondary electron produced by the ion beam’s bombardment on the sample were gathered and capture a focused ion beam microscopic image.
The electron probe micro-analyzer (EPMA)JEOL EPMA8230 equipment was used to determine various minerals and the concentration of the V2O3in the Hebei Institute of regional geology and mineral resource survey.The ambient temperature and the relative humidities were 24 °C and 52%, respectively.
4 Discussion
4.1 Mode of vanadium occurrence
Vanadium may have been divided into three primary groups in naturally occurring Metallo-organic compounds.(i). Mixed ligands tetradendate, (ii). tetrapyrrole complexes, (iii). Humate complexes. This basic categorization is following the kinds of ligands that involve the interaction of metal bounding with the Metallo-organic compounds.Due to the structural complexity and the higher molecular weight of the precise structural and chemical nature of Metallo-organic compounds are not always displayed. The stability between the complexes and the metal bounding is an indication of ligands type and their coordination.
In mixed-ligands tetradentate, four ligands can be bonded with the metals. It might contain various combinations of oxygen, nitrogen, and sulfur (Fig. 5a) (Yen 1975).Usually, bivalent metallic cations are required in this kind of complex, and their coordination is not an octahedral form of stable square planer (Boucher and Yen 1968).Additionally, these ligands in less stable coordination of square pyramidal or tetrahedral are distorted around the metal. In dilute acids, vanadium demctallation may occur,and it may have low stability in mixed ligand tridentate(Yen 1975).
Humate complexes are defined as Metallo-organic compounds containing oxygen as bonding ligands(Fig. 5b). This kind of compound naturally occurs in humic complexes. Typically they contain oxygen atoms in phenolic hydroxide or carboxyl functional groups (Gamble and Schnitzer 1973; Schnitzer and Khan 1972). It is suggested that the functional group of phenolic hydroxide is bonded with metals up to some degree. In humate compounds, the primary ligands as a carboxyl functional group contain an oxygen atom (Vinkler et al. 1976). At low pH, the humate compounds may form by the cations of vanadyl (VO2+). Its stability is significantly lower than the desired trivalent cations of ferric and aluminum (Krˇı´bek et al. 1977), supported by the higher amount of Fe and Al than vanadium in naturally found humic compounds (Cheshire et al. 1977). It is also observed in humic compounds of marine origin,which contains a greater amount of divalent cations relative to trivalent (Rashid 1971). In some marine humic compounds, it is due to the increasing sulfur and nitrogen functional groups that act as a ligand, which contains oxygen atoms as a functional group (Nissenbaum and Swaine 1976). This kind of ligands might preferably form the divalent mixed ligands tetradenates. Moreover, in humate compounds, vanadiums are not preferred due to their instabilities, which suggests they may not survive in diagenetic settings. During early diagenetic conditions,OM’s thermal maturation via decarboxylation may result in humate compounds’ demetallation.
Tetrapyrrole complexes can be determined as Metalloorganic compounds containing four atoms of pyrrole-nitrogen serving as a ligand (Fig. 5c). Additionally, based on the extensive research of metallated porphyrins, the present definition includes the higher aromatic and the pseudoaromatic tetrapyrroles (Fig. 5c). This kind of structure may present in the subunits of macromolecules or as a free molecule of OM (Oehler et al. 1974). Tetrapyrole complexes and the vanadyl cation, in contrast to other Metalloorganic compounds, make tenacious covalent bonds having higher thermal stability, resistance to strong acid, and having inertness behavior to cation exchange reaction(Lewan and Maynard 1982). Even though, it is not possible to identify all the Metallo-organic compounds related to vanadium. The tenacious bonding of vanadium in asphalt,crude oil, and bitumens is thought to be associated with the tetrapyrrole compounds (Lewan 1981).
4.2 Vanadium occurrence and biogeochemical cycling
The biogeochemical cycling of the vanadium is the natural pathway in which the concentration of vanadium circulates in various environments via chemical, geological, and biological aspects (Fig. 6).
4.2.1 Vanadium in the soilVanadium is a unique transitional metal element due to its redox-sensitive nature. It can be found in various rocks,especially in the black rocks under different environments.Vanadium in the soils is primarily derived from the parental rocks (Kabata-Pendias and Pendias 1993). However,recent studies showed that the vanadium in soils could be enriched by anthropogenic activities such as burning fossil fuels, mining, pesticides, industries, fertilizers, and recycling domestic wastes (Nriagu and Pirrone 1998; Taner 2002). Titanomagnetite deposits are considered a fundamental vanadium source in which vanadium minorly substitutes the iron (Evans and White 1987). Similarly, organic fractions, Fe-oxide compounds, and argillaceous minerals are also comprised of vanadium (Kabata-Pendias and Pendias 1979). In soils, the concentration of vanadium ranges from 2–310 mg/kg with a mean concentration of 90 mg/kg (Fig. 2). The global vanadium distribution in soils is presented in Table 1. It has been noticed the soil globally in Japan (Andosols) contains a higher concentration of vanadium 250 mg/kg (Takeda et al. 2004). Vanadium is highly enriched in organically rich-soils, and peats,which shows a strong tendency towards organic matter(OM). Different lithologies contain various concentrations of vanadium, such as shales are abundant in the concentration of vanadium (130 mg V/kg) than carbonates and sandstones (20 mg V/kg) (Adriano 1986; Alloway 1990).In the Earth’s continental crust, vanadium is broadly distributed as a vital element that ranges from 96 to 230 mg/kg (Fig. 2). It stands at 22nd in all discovered and at 5th in all transitional elements in the Earth’s crust. Furthermore,it is usually found in argillaceous and alkaline rocks as well as in the lithosphere (Adriano 1986; Anke 2004; Baroch 2005). The vanadium’s pH and oxidation state are the two significant factors that control vanadium’s geochemical attributes. Previous studies describe the higher cont of vanadium in limestone soil relative to other soils (Ovari et al. 2001; Połedniok and Buhl 2003).
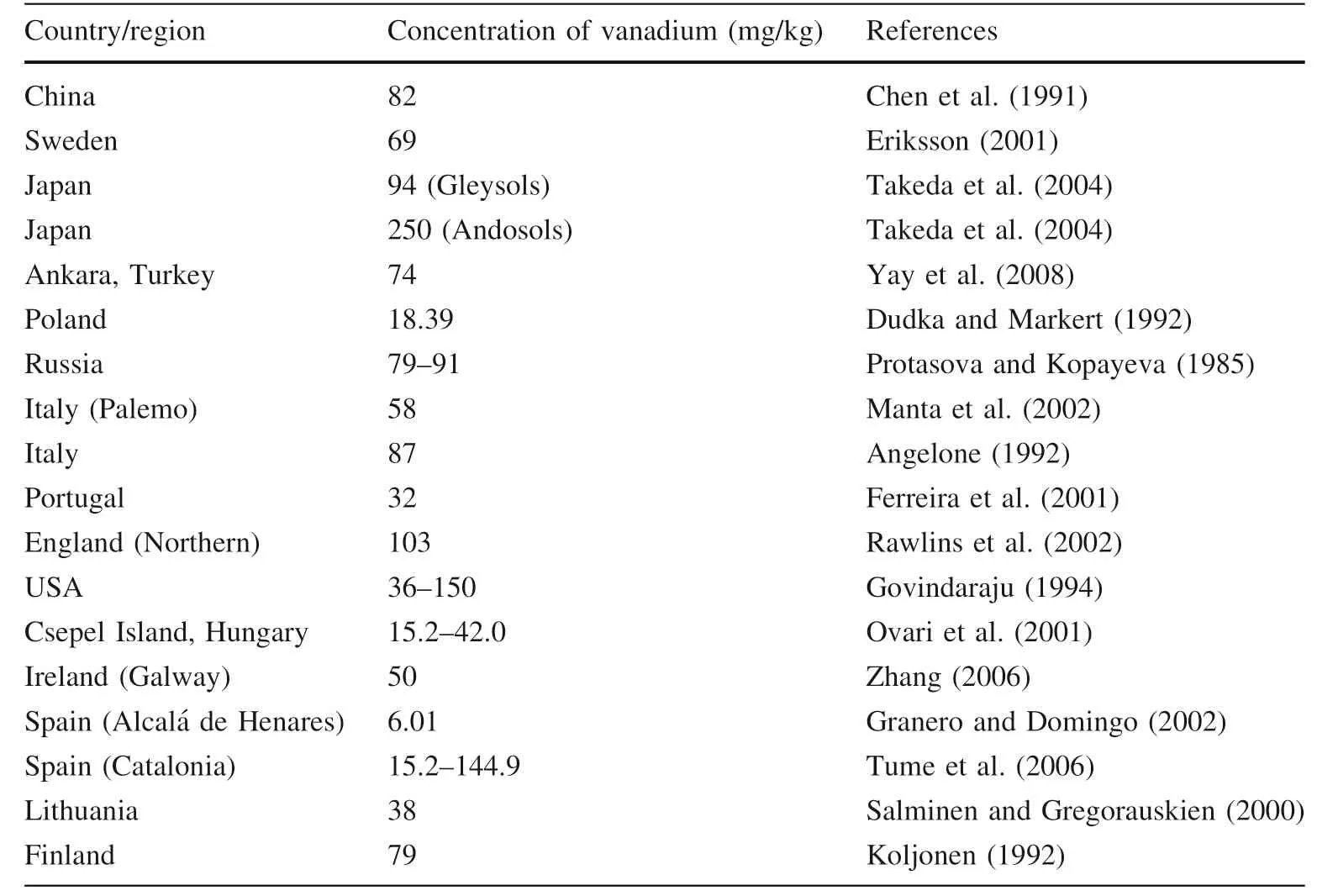
Table 1 Global distribution of vanadium in soils

Fig. 6 Schematic diagram revealing the vanadium biogeochemical cycle
4.2.2 Vanadium in the water sources
Earth is the distinct planet amongst all planets, known as the blue planet. It is due to water’s presence in a massive area. Additionally, 97% of the water on the Earth is salty,only 1% useable, and 2% is frozen. The concentration of dissolved vanadium in rivers is 0.78 ug/L (Fig. 2). In different environments, the relationship of various environmental factors and vanadium in solution may fluctuate,e.g., there could be a low content of dissolved vanadium in streams that might be due to a strong relationship with particulate and colloidal Fe (Wa¨llstedt et al. 2010). In contrast, the concentration of dissolved vanadium in major rivers is closely associated with the origin of rock and the weathering. However, it has a weak linkage with colloids or OM (Shiller and Boyle 1987). The concentration of dissolved vanadium in the water close to the mining horizons could be higher up to several hundred; for example,the dissolved vanadium in the Wyoming River of the United States is 30–220 mg/L (Schroeder 1970). In water,the concentration of vanadium is chiefly dependent on seasonal variations. During summer or in warm conditions,the concentration of dissolved vanadium in marine and freshwater systems has been reported at a higher level in Florida Bay (Caccia and Millero 2003), Biwa Lake and Toyko Bay (Sato and Okabe 1978), and Long Island Sound(Wang and Wilhelmy 2009). This seasonal mobility of vanadium could be affected by various factors. The relationship between the water particulates and the vanadium could be due to the substantial dependency of Eh and ph settings, which can vary seasonally. Light as a photoreduction could also impact the fluctuating concentration of vanadium in acidic streams (Kay et al. 2011). In the open ocean, the average dissolved vanadium concentration is thought to be 35–45 nmol/L (Emerson and Huested 1991).Vanadium keeps a conservative form in oceans with its high concentration. However, it exhibits lower concentration and a non-conservative behavior in coastal waters(Wang and Wilhelmy 2009). The suspended particulate form of vanadium reservoir is relatively smaller(1.8 × 1010g) in the global oceans than the dissolved vanadium reservoirs (2.7 × 1015g) (Hope 2008). Oceanic sediments are the biggest vanadium pool (1.7 × 1020g) on the Earth’s surface(Hope 2008). Moreover, the ultimate vanadium sink of the global circulated V is the ocean floors(Bengtsson and Tyler 1976; Miramand and Fowler 1998).In marine sediments, volcanic activities play a significant role in vanadium accumulation with a higher concentration(greater than 400 mg/kg) at the active volcanic ridge sediments (Bostro¨m and Fisher 1971).
Vanadium in groundwater is considered a significant heavy metal, and the surface water is mainly influenced by vanadium in the mining regions (Ortiz-Bernad et al. 2004).In the aqua sphere, vanadium is a more abundant transitional element similar to the average concentration of zinc(Rehder 1991). The pollution of vanadium is further noticed in different water resources, such as lakes, rivers,and seas. In oceans, the average amount of vanadium is 1.8 ug/L. However, the vanadium quantity at mid-ocean ridge sediments is pretty much higher at 400 mg/kg (Fig. 2).Vanadium concentration at the bottom of the Persian Gulf is higher than 100 μg/g, similar to the fresh dry samples(Pourang et al. 2005). The groundwater specimens (about 10%) from the USA (California and some other states)contain more than 25 μg/dm3of vanadium. It is because of vanadium washout from water-bearing sediments (Wright and Belitz 2010). In oxic water settings, the most dominant form of vanadium is V(V). In contrast, the vanadyl ion VO2+(IV) is more stable in reducing conditions and tends to hydrolyze. In Colorado River Basin, the amount of vanadium is 0.2–49.2 μg/L, with its maximum concentration close to the mining region of U-V (Linstedt and Kruger 1969). The most significant freshwater source in China is the Yangtze River, which contains vanadium from 0.24 to 64.5 μg/L (Licheng and Kezhun 1992). In water,the geochemical attributes of vanadium control the biogeochemical cycle of vanadium in the surface environs(Fig. 6). The speciation of V is a composite function of solution chemistry, biology, Eh, pH, and several surface environments. Vanadium in the natural water resources exists in three different states + 5, + 4, + 3. Various vanadate species V(III), V(IV), and V(V) are stable in anoxic, oxic, and suboxic environments, respectively(Sadiq 1988). Different spectroscopic and chemical techniques are shown chiefly V(IV) and V(V) species in a natural aqueous system (Colina et al. 2005; Wann and Jiang 1997; Zhao et al. 2006). Vanadium complexation with both inorganic and organic ligands can enhance its mobility at the surface of the interface (solid-water)(Wanty and Goldhaber 1992). Vanadium (IV) readily forms complexes with a broader range of inorganic and organic ligands relative to vanadium V(V). The typical compounds formed with the inorganic ligands are VSO4-for V(III); VO(OH)CO3-, VOCL+, VOF42-, VOF3-,VO(CH3COO), VO(CH3COO)2VOF+VOF2, VOCO3,VOSO4, VO(OH)C2O4-, VO(C2O4)22-, OS4, for V(IV);H4VO4C2O4-and H4VO4(C2O4)23-for V(V) (Wanty and Goldhaber 1992). A wide range of organic substances has an affinity with vanadium comprising humic substances and fulvic acid via oxygen groups. Moreover, the organic substances form comparatively weaker complexes with vanadate oxyanion than oxycation V(IV) (Wehrli 1987).Hence, the V(V) organic compounds are vital to reduce V(V), and vanadium can be stabilized against reductions via binding of humic compounds at pH values (Kustin 1974). The reduction of abiotic and biotic V(V) can produce both V(III) and V(IV) (Carpentier et al. 2003).Vanadium’s organic-metalliferous chemistry is well established, and the typical form is cyclopentadienyl. The most common type of reagent in synthetic organic chemistry is vanadocene dichloride (C5H5)2VCl2. Which can reduce to C2H5)2V, and C5H5)2V.
4.2.3 Vanadium in the atmosphere
One of the substantial elements of the vanadium cycle is the atmospheric vanadium. Vanadium is substantially increasing in the biosphere since the last few decades that is threatened for the future. The main factors responsible for this uprise are mining activities, wet and dry atmospheric deposition, weathering, etc. (Morrell et al. 1986;Ringelband and Hehl 2000). Vanadium exists in hot-water springs and with volcanic ashes, tephra due to the volcanic actions (Mizuno et al. 2008; Smichowski et al. 2003). The vanadium concentration added to the atmosphere by the natural and anthropogenic sources is comparable(37 × 109g/year). However, only 10% of the vanadium deposited in the global oceans is by anthropogenic sources(Duce and Hoffman 1976). Vanadium tends to stand for a prolonged time in the water, soil, and air and can react with the other metal elements in its surrounding (Miramand and Fowler 1998). In the contaminant list of Europe, vanadium(144t) ranks at 3rd owing to the combustion of power generation plants after Pb (191.5t) and Zn (341t). Hence due to vanadium, environmental contamination is more significant near oil fires and industries (Sabbioni et al.1984). The key natural sources which liberate vanadium in the atmosphere are volcanic eruption, marine aerosols,continental dust, fossil fuels, forest fires, soil erosion, and other biogenic activities (Nadal et al. 2004).
It is estimated that almost greater than 64,000 tons of the V are liberated to the atmosphere via anthropogenic and natural sources (Lin et al. 2004). Nearly 58,500 tons (91%)of the global vanadium emission is from oil combustion,heavy oils, coals, and mining activities (Nadal et al. 2004).Russia and Eastern Europe contribute about 14,500 tons of global vanadium emission, whereas the Asian developing countries share greater than 33,500 tons. 85% of the total produced global vanadium is utilized in the steel sector as a ferrovanadium. However, up to 10% of the remaining global vanadium as a V-Al-Ti alloy is utilized for aerospace industries (Moskalyk and Alfantazi 2003). The vanadium uses in various other sectors are glass coating,catalysts, rechargeable batteries, etc. However, its utilization in the generation and storage of energy might be increased in the future. In the ferrous metallurgy sector, the produced vanadium wastes are chiefly in the form of particulate and liquid (WHO 1988). 60%–70% of the vanadium is recovered from these wastes. However, the rest of them (30%–40%) is liberated into the environment through slag, wastewater, dust, and gas emission (Hope 1997; Jiao and Teng 2008). A significant amount of vanadium (up to several hundred mg/L) may be contained in the plants’wash water and liquid waste. In the mining industry,vanadium-containing leachates and effluents are mainly produced during the processing of Ti and U ores. Additionally, vanadium is a vital element of oxidation reactions and the chemical synthesis of the polymer and chemical industries, which also generates vanadium-containing waste (Nriagu 1998). The combustion of petroliferous fossil fuels rich in vanadium is an important source of V in the atmosphere (Duce and Hoffman 1976; Hope 1997).Only 60%–70% of the vanadium is recovered from refineries, and the remaining 30%–40% is discharged into the environment (Hope 1997). Vanadium (+ 4) is thought to be less toxic than vanadium (+ 5). It is because vanadium (+ 5) could affect certain enzymes’ actions (Assem and Levy 2009). Moreover, V (+ 5) harms various animals(Assem and Levy 2009). The amount of vanadium mined every year is about 130,000 tons (Monakhov et al. 2004).Coal exploration also causes a higher annual concentration of vanadium in the atmosphere as coals also contain vanadium traces. In oceans, approximately 100,000 years is the time for vanadium to reside (Whitfield and Turner 1979) and are mainly transported by riverine as particulate vanadium and mechanical weathering. The concentration of vanadium might be lower in various vanadium pools during geohistorical periods. However, the concentration of vanadium in recent sediments and various other vanadium pools could be higher mainly by anthropogenic sources,which also changes the global climatic conditions. Hope(1997) indicated the concentration of vanadium introduced to the environment via anthropogenic sources is significantly higher (230 Gg) and is increasing every year.Moreover, Liu et al. (2017) has also specified the increase of vanadium via anthropogenic activities in recent years is due to vanadium utilization in various industries. Due to these reasons, it is suggested the concentration of vanadium in modern sediments will be higher. However, it will be a hot, and debatable topic for future researchers.
4.2.4 Vanadium in the biological creatures
Some aquatic organisms pile up vanadium in their cells called vanadoytes. The concentration of vanadium in various marine organisms is presented in Table 2. Vanadium can enhance the metabolic action, yield, and size of some plants. Vanadium has similar phosphate characteristics due to its capability to motivate or/and inhibit various metabolizing phosphate enzymes (Rehder 1991). Furthermore,the smaller quantity of vanadium can inhibit the phosphorous transformation of enzymes in the organisms(Cantley et al. 1977). Biological networks, such as the membrane’s transport mechanism, can be damaged by the intermingling of several proteins with the vanadium oligomers (Arans 1995). It is noticed the reactive oxygen species (ROS) are regulated by vanadium (Zhang et al.2001). Certain micro-creatures can respire vanadium (V) or(IV) as an electron exceptor (Ortiz-Bernad et al. 2004;Zhang et al. 2014). The higher redox potentiality of vanadium for V(V/IV), and moderately lower toxicity insinuates that other electron acceptors such as Fe+3would prefer to utilize V. The concentration of vanadium might be higher in the sediments due to vanadium consumption and sorption by microbes that could be the main source of V in fossil fuels (Breit 1989; Lewan 1984).

Table 2 The concentration of vanadium in the marine organisms

Table 3 The average amount of total organic carbon and mineral content in the black rocks of the Niutitang Formation
4.3 Vanadium and the total organic carbon
Total organic carbon (TOC) is the quantity of organic carbon in sediments (Awan et al. 2021; Jian et al. 2021;Tahir et al. 2020). The average quantities of TOC from the Longbizui and the Sancha sections are 3.34 wt.% and 8.09 wt.%, respectively (Table 3). To develop and the relationship between vanadium and organic matter (OM), we have presented the cross plot between vanadium and TOC from various shales around the globe (Fig. 7). In marine shales, it is observed the vanadium has a strong positive relationship with total organic richness, as shown in Fig. 7.However, vanadium’s relationship with TOC in the Green River Formation of the lacustrine environment reveals a weak positive association. The strong positive association of V with the OM might be due to a strong association of vanadium with the microbial shells. This relationship reveals the linkage of vanadium with organic matter (OM)or is a sign of favorable conditions in which OM is preserved on dissolved vanadium and in the sediments. The variation in the slope lines of vanadium and TOC (Fig. 7)may be due to the following factors: (1) compositional discrepancies of the organic matter (OM), (2) variation in the sedimentation rate or the supply of vanadium, (3)during diagenesis compositional variation of the sediments,(4) different depositional environments (Lewan 1984;Lewan and Maynard 1982).
The biological origin of vanadium in organic matter,crude oil, and asphalt in black rocks are unclear (Vine and Tourtelot 1970). Vanadium, as tetrapyrroles in these organic substances, is firmly bonded with the organic–metallic compounds. These tetrapyrroles form covalent bonds with the cations of vanadyl (VO2+). As a result,these macromolecules develop higher thermal stability(Lewan and Maynard 1982). V-porphyrins are structurally comprised of four polypyrrole macrocycles. These polypyrroles are linked with each other via methine bridges.These methane bridges are contained four heterocyclic pyrroles of nitrogen atoms that act as ligands. These macromolecules are mainly derived from the living organism’s diagenetic transformation, especially from the precursors of vanadium, such as heme porphyrin pigments and chlorophyll (Blumer and Snyder 1967). The diagenetic pathway of organic–metallic compounds is shown in Fig. 8. Here, the central cation Mg in the porphyrin ring is substituted by other cations, e.g., VO2+. These vanadyl cations are highly enriched in planktonic biomass and organic-rich sediments (Lewan and Maynard 1982). Furthermore, other oxygenated groups and the phytol chains are eliminated after an increase in thermal stresses via different bond cleavages (Fig. 8). Due to the experiences of environmental stresses in algae, the abundant distribution of chlorophyll-carotenoids in the cells actively responds(Minhas et al. 2016; Mulders et al. 2014). As the stress increases, the cysts developed from algae. These cysts comprise a prolific amount of chlorophyll carotenoids bodies. After the deposition of cysts in the sediments,diagenetically chlorophyll-carotenoids altered, leading to vanadium preservation throughout the fossil cysts (Minhas et al. 2016; Mulders et al. 2014). Therefore, it can be suggested that vanadium may be attributed to the organic walls of acritarchs as a remnant form of chlorophyll, which contains chloroplasts.

Fig. 7 The relationship of vanadium with the organic carbon in the various black rocks around the globe. The Green River Shale was deposited in a lacustrine environment, whereas the other was deposited in marine settings. Some part of the data is retrieved from Andersson et al. (1985), Derkey (1985), Gao et al. (2018),Gulbrandsen (1966), Hall (2012), McKelvey et al. (1987), Riley and Saxby (1986), Tang et al. (2017), Tuo et al. (2016), Tuttle et al.(1983), Wenger and Baker (1986) and Wu et al. (2016)
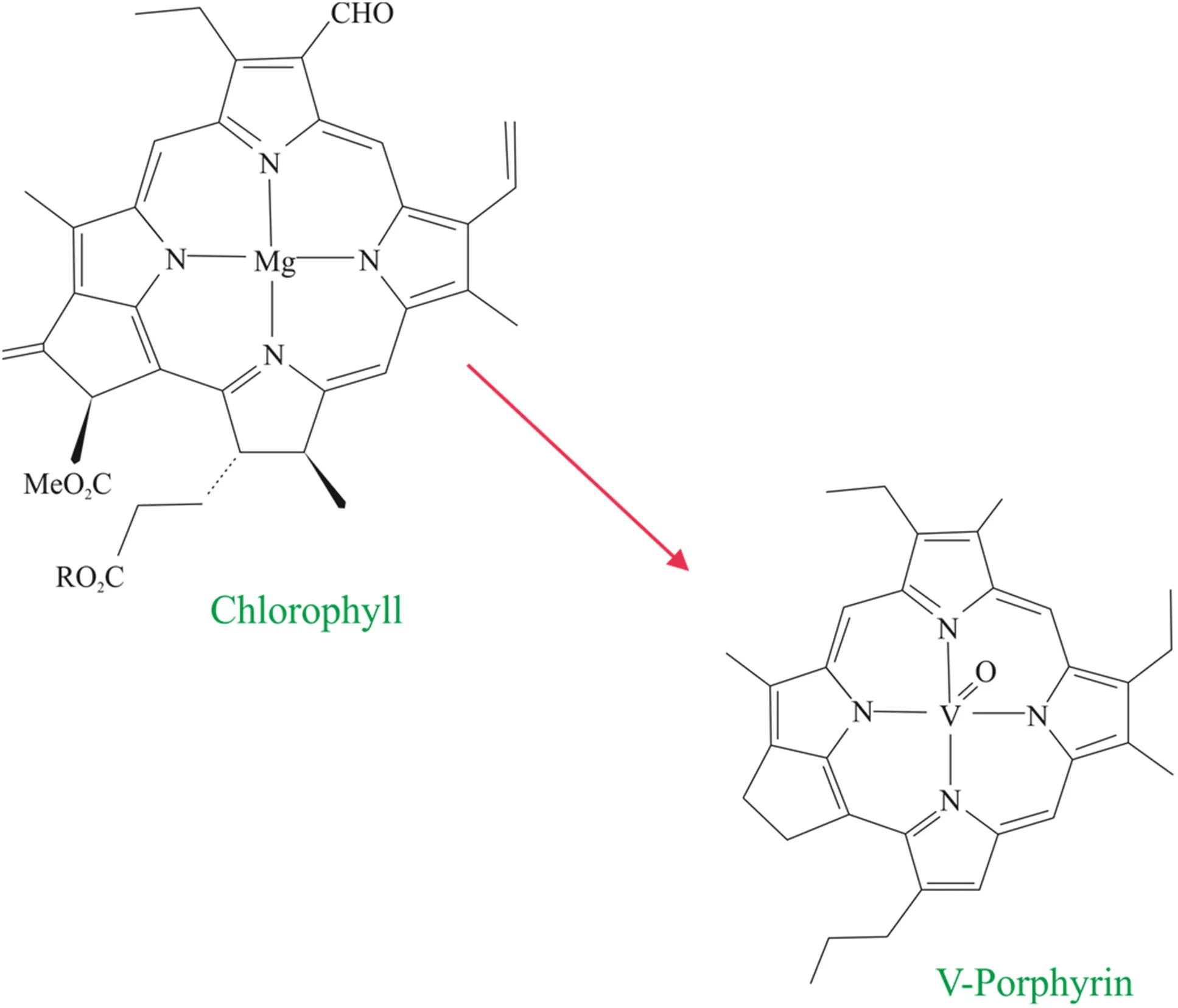
Fig. 8 Diagenetic alteration and the molecular structure of chlorophyll transformation into Vanadium porphyrin
4.4 Mineralogy and the vanadium association
Mineralogy plays a key role in vanadium accumulation.Therefore, It is important to discuss various minerals that are favorable for vanadium accumulation in sedimentary rocks. In the studied samples, the heterogenic mineral composition is observed. However, quartz, clays, and pyrite are the main mineral constituents (Table 3). The cross-plot between quartz and V displays a weak positive association (Fig. 9). Previous studies suggested the presence of quartz in the Niutitang Formation is chiefly derived from a biogenic origin (Awan et al. 2020). Some part of vanadium that is consumed by organisms becomes a part of their bodies. In this context, this weak positive association of quartz with vanadium might be due to quartz’s biogenic origin. Therefore, this research also suggests the quartz other than biogenic origin might be unfavorable for vanadium enrichment. In contrast, clays and pyrite depict a relatively positive relationship with vanadium. Pyrite is an essential mineral for predicting a reducing-anoxic environment. The anoxic environment is beneficial for the deposition of some trace elements, i.e., V, Ni, and Co.Hence, it can also suggest the higher amount of pyrite mineral in a rock formation is also favorable for the enrichment of vanadium. In various environmental conditions such as hydrothermal, sedimentary, and metamorphic,the clay mineral contains a significant vanadium concentration. The broader occurrence of vanadium in clay minerals suggests that the clay minerals can rapidly incorporate vanadium in nature over a broader range of temperature,pressure, and geochemical conditions. Redox settings mainly control the geochemistry of vanadium in natural water. In dissolved form, vanadium can be transported as V4+and/or V5+, and it can be reduced and precipitated as a V3+.
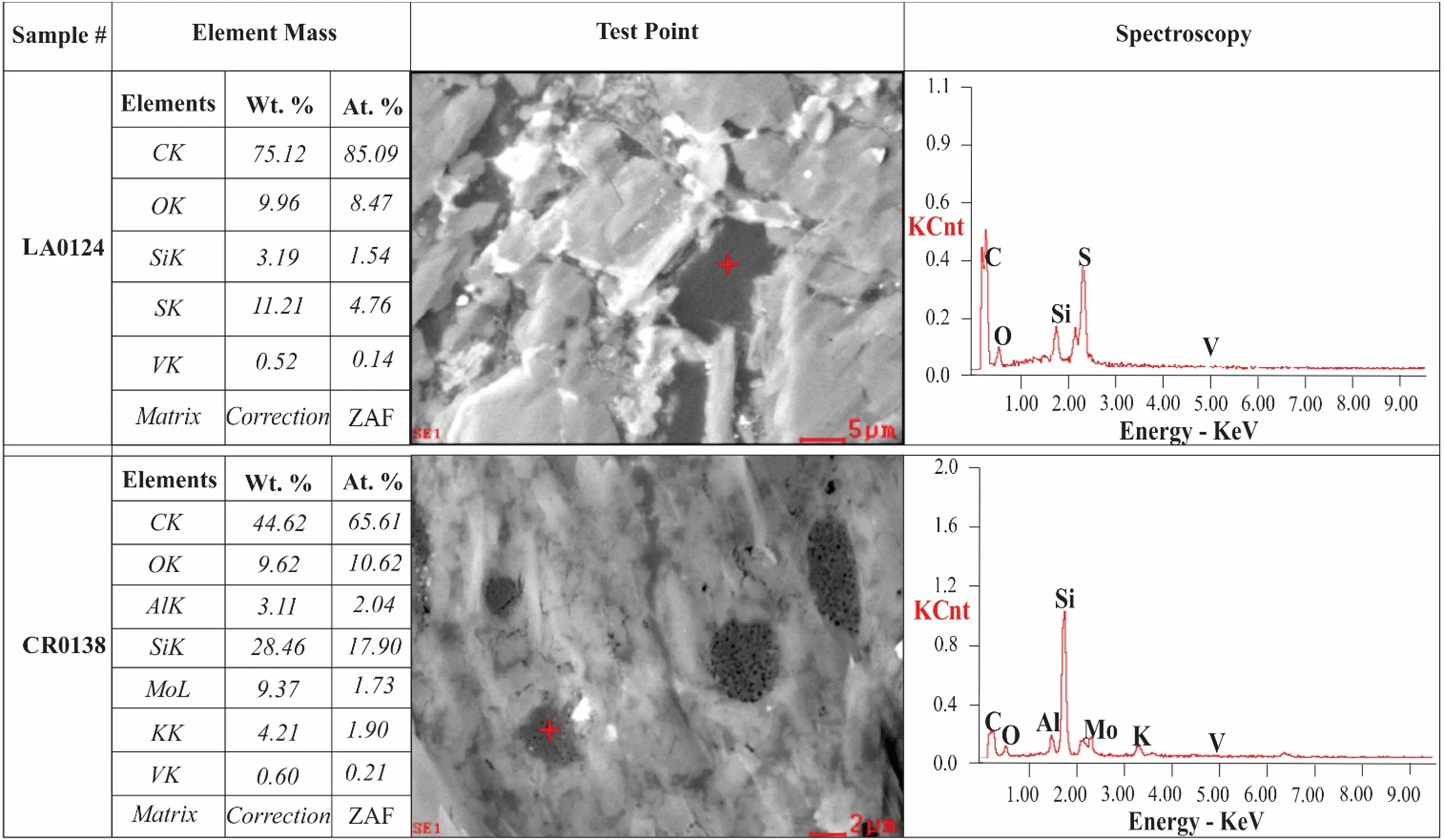
Fig. 10 Scanning electron microscopy revealing the distribution of the elemental energy spectrum from the black rocks of the Niutitang Formation

Table 4 Electron probe micro-analyzer (EPMA) results of the early Cambrian Niutitang Formation
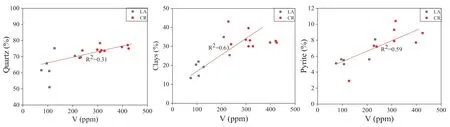
Fig. 9 The relationship of vanadium with various minerals (LA: Longbizui; CR: Sancha)
In sedimentary rocks, during the diagenetic action, the clay minerals usually incorporate vanadium (Breit and Wanty 1991). In clay minerals, vanadium can persist in its crystalline network as an isomorphism by substituting Al with V(IV) and V(III). However, a tiny quantity of vanadium amalgamates with OM and/or adsorbed on the surfaces of minerals, i.e., clays and pyrite, etc. (Zhang et al.2011). Hitherto, vanadium has been noticed in three groups of organisms, e.g., ascidians, certain amanita mushrooms(fly agaric), marine Polychaeta fan worms. The specialized blood cells in the ascidian (Vanddocytes) take vanadium,with a higher concentration in ascidia gemmate (350 nM).A wide range of bacteria utilizes vanadium for various biological functions. Vanadium nitrogenase is also termed iron-molybdenum enzymes. It has been commonly utilized to reduce the level of dinitrogen–ammonia in several plants. These vanadium nitrogenase are mainly derived from some specific bacterias nourished in molybdenum lean settings. However, their activity is inferior, then MONase (Sippel and Einsle 2017). In sedimentary rock,vanadium availability is chiefly dependent on the V-bearing minerals, e.g., V-micas, Cr-V garnet, and Ti-V garnet,or it can be adsorbed on clays or organic matters. Vanadium ions (V4+or V+3) in clay minerals substitute the aluminum (Al3+) and become a part of the crystal lattice via isomerization. The presence of vanadium in the black rocks of the Niutitang Formation is displayed in Table 4.These results reveal the presence of V2O3, which indicates the isomorphism state of vanadium existence in the clay minerals. The vanadium presence in the Niutitang Formation is also observed under a scanning electron microscope(Fig. 10) that shows a strong association of vanadium with organic carbon.
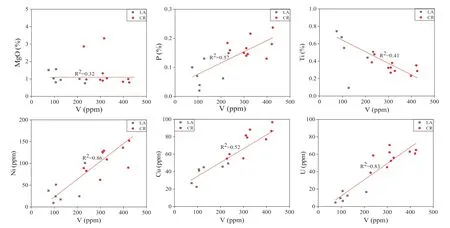
Fig. 11 The relationship of vanadium with various trace elements (LA: Longbizui; CR: Sancha)
4.5 The association of vanadium with other elements
The favorable geochemical settings for the vanadium accumulation can be discussed in the black rocks by determining its enrichment and geochemical constraints on other associated metal elements. This research discussed vanadium’s relationship with the presence of various metal elements (Mg, P, Ti, Ni, Cu, and U) in the Niutitang Formation (Fig. 11). Here we noticed the strong positive linkage of vanadium with Ni, Cu, P, and U, and the weak positive association with MgO. However, Vanadium and Ti have a negative relationship in the Niutitang Formation.The black rocks usually contain a higher concentration of metal elements (Awan et al. 2020; Brumsack 1983; Holland 1979). These elements, especially the abundance of Ni and Cr are the significant elements in determining the paleodepositional settings (Awan et al. 2020; Lewan 1984;Tang et al. 2017). Vanadium usually occurs in H2S rich strongly reducing settings relative to nickel and chromium(high V/Cr, and V/Ni) (Awan et al. 2020; Tang et al. 2017).The higher values of V/Ni and V/Cr are clues of anoxic settings. The discussion of Ni and V in the black rocks is due to their greater concentration in petroleum. The enrichment of these elements is due to remarkably stable porphyrin compounds in petroleum (Lewan and Maynard 1982). Due to the reduction of V via H2S and hindrance of Ni by NiS compounds, the ratio of V/Ni in sulfur-rich petroleums is at a higher level (Lewan 1984).However, systematically the ratio of V/Ni doesn’t upsurge with the rise of sulfur concentration. The maximum average ratio of V/Ni above 1.0 wt% of sulfur is between 4.0 and 5.0 (Lewan 1984), which is quite analogous to the modern ocean-water (Ni = 8.20 nmol/Kg (Bruland 1980)and V = 40.0 nmol/Kg (Jeandel et al. 1987). The measured ratios of V/Ni in petroleum and bitumen are consistent.However, it is different from the ratio of V/Ni in the whole rock. This fluctuation of V/Ni value in bitumen reveals Ni in sulfides and V in silicates.
The vanadium-rich black rocks are usually associated or interbedded with the phosphatic-rich sediments (Beier and Hayes 1989; Patterson et al. 1986). The phosphate-rich rocks stratigraphically occur above or below in vanadiumrich black rocks. Based on the spatial relationship, phosphorus and vanadium are genetically related. However,these elements accumulate in little diverse depositional settings. The phosphorous and vanadium relationship is persistent with the vanadium behavior in the water column.The vanadium depletion in estuaries water (Collier 1984)and the near-surface water (Shiller and Boyle 1987) are analogous to phosphate quantity. In various ways, i.e.,adsorption to biogenic particles, biogenic assimilation,vanadium may be linked with the phosphate cycle or via replacement of vanadate to phosphate (Jeandel et al. 1987).The sulfur-rich petroleum contains larger vanadium quantities, revealing an obvious relationship between sulfur and vanadium (Lewan 1984; Tissot and Welte 1984). Similarly,the black sediments with massive organically bound sulfur comprise a substantial amount of vanadium (Patterson et al.1986). The relationship between vanadium and sulfur in OM is considered due to the vanadium preferred bonding to the sulfur ligands (Baker and Louda 1986; Yen 1975). It might be due to the various chemical processes that occur during the deposition of these sediments (Lewan 1984).The organic-sulfur compounds are so far poorly featured,and the degree of bonding type is still unclear. Lewan(1984) suggests the enrichment of vanadium in carbonaceous black rocks linked with H2S. It is because the H2S creates a favorable condition in the formation of V(IV). In the overlying water, some of the H2S will be diffused due to the depletion of iron. Consequently, the strongly reduced bottom water will beneficial in the reduction of vanadium from V(V)–V(IV) or to V(III).
4.6 Vanadium accumulation and sources in Niutitang Formation
The enrichment of vanadium in these Early Cambrian sediments might be due to chemical, physical, and biological processes. Various processes like diffusion,adsorption, and particle settling may influence the efficiencies of these progressions. Biotas are thought to play a proactive role in the vanadium cycle. They can accumulate vanadium as their lifecycle or reduced them by excreting(Boyd and Kustin 1984). In the sediments, algae may inactively adsorb vanadium, which plays a vital role in vanadium flux (Lee 1983). A few ascidians are predicted to have an enriched quantity of vanadium, e.g., Urochordates(Zhang 1985). However, In anoxic environments, the assemblage of sessile benthonic creatures in carbonaceous rocks is hard to endure (Lewan and Maynard 1982).Brumsack (1983) suggested the enrichment of metal elements in marine sediments could be determined by the combination of metal elements in the average shales and planktons. However, the abundance of vanadium in metallic-rich rocks and sediments, e.g., sediments of the Black Sea, postulate other processes to enrich vanadium.Those processes may change the chemical environment,which can be beneficial for the enrichment of vanadium in sediments.
The most frequent process for the evacuation of vanadium from the seawater is its adsorption on the terrigenous or biogenic particles. Vanadate in various pH ranges of the natural waters can vigorously adsorb on the clay minerals and the oxides of Al and Fe, (Wehrli and Stumm 1989).Vanadyl ions adsorbed more powerfully than vanadates.However, its durability as the adsorbed species in the oxic water is modest (Wehrli and Stumm 1989). Besides the higher concentration of ligands, the vanadyl-oxides’adsorption is preferable relative to the complexity of the dissolve organic ligand (Micera and Dallocchio 1988).More than 40% of the transported vanadium from the Mississippi River is adsorbed to the detrital particles(Shiller and Boyle 1987). It is experimentally noticed that the adsorption of vanadate exacerbates when the pH raised over 4.5. Iron oxides can substantially adsorb the massive quantity of vanadium from the ocean water in the oxic basins (Shieh and Duedall 1988). These oxic basins may be a vital pool for dissolved vanadium (Trefry and Metz 1989). Another possible mechanism for the enrichment of vanadium in these black rocks of the Niutitang Formation could be due to diffusion phenomena. In these Early Cambrian sediments, vanadium diffusion from the bottom water is preferred by the declining quantity of V(V) species in the pores via complexation, reduction, and adsorption into the sediments. In sediments, due to the chemical transformation, the available concentration of vanadium may alter. The concentration of vanadium in the shallower pore water may increase due to the sediments reworking under the oxic environment or apparently due to vanadium’s mobilization from the degraded biogenic particles(Shaw et al. 1990). The reworked vanadium may be diffused upward or downward into the bottom water and reprecipitated into the reduced regions (Colley et al. 1984;Heggie et al. 1986; Jarvis and Higgs 1987; Shaw et al.1990). However, Shaw et al. (1990) compared the pore water vanadium in oxic and anoxic sediments and suggested the redistribution of vanadium does not occur in anoxic sediments.
Vanadium is extensively used to study ancient marine sediments due to its unique nature and abundance. It can be used to determine some invaluable facts regarding the source, diagenesis, and paleoenvironmental settings. In marine sediments, especially in shales, the origin of vanadium is still a source of contention. The abundance of vanadium in marine sediments is interesting due to the burial of vanadium-rich organisms (Vinogradov 1936). The rapid growth of massive algal-biomass in a specific time window (e.g., 100,000 years) could be a prerequisite in forming polymetallic beds. Li et al. (1999) and Awan et al.(2020) suggested the black shales in the Yangtze region during the Early Cambrian period were developed through massive organic production in an upwelling system. Various forms of algae have revealed a higher biosorption ability of V and Ni (Holan and Volesky 1994). In the photic zone, the annual concentration of green algae production could be elevated to 48.9 kg∙m-2(Xu and He 2006), which could enhance the storage of vanadium. Xu and Li (2015)observed algae’s presence in the black rocks of Niutitang Formation in the form of heavily sequized vesicles or trilete appearance. The red phosphatized algae were also noticed in the Doushantuo Formation in South China (Xiao et al. 2004). The reduction and adsorption of metal elements on organic substances (such as humic substances)are the probable mechanisms for the enrichment of vanadium in carbonaceous marine shales. Usually, the humic substance in the sediments forms soluble compounds with vanadium, which play a fundamental role in the fixation and transportation of vanadium (Cheshire et al. 1977;McBRIDE 1978). The chemical composition of igneous rocks and the volcanic-clastic material are considered to be identical. Basalts and some other igneous rocks comparatively contain a higher vanadium level (up to 0.11%).Similarly, these rocks contain a higher concentration of other transitional metal elements such as Cu, Mo, Ni, and Cr. Volcanic ash in the Niutitang Formation may be a source of vanadium in the form of VO2+porphyrin. In the marine Niutitang Formation, the VO2+porphyrins can be divided into three groups based on their physicochemical characteristics. (i) VO2+porphyrins are a product of kerogenization and polymerization phenomena with higher aromaticity or molecular weight, (ii) VO2+porphyrins,which reveals the nominal diagenetic evidence, (iii) and the VO2+porphyrins embedded with the kerogen configuration. Incorporating the nuclei of VO2+porphyrins into the matrix’s kerogen is certainly an abiotic geochemical alteration of biosynthesize pigmental chlorophyll. The inclusion of VO2+porphyrins into the sediments is not its ultimate fate. Several high aromatics and high /low extractable porphyrins are considered to be a product of kerogen thermal stress (Mackenzie et al. 1980). These processes are considered to be accountable to produce a broader spectrum of aliphatic hydrocarbons from kerogen.Various types of VO2+porphyrins may have been produced from biogenic pigment chlorophyll via diagenetic and catagenetic processes. Previous studies also indicated that volcanic ash in marine shales is an unusual source of metallic enrichment (Leventhal and Kepferle 1982;Wedepohl 1971). The chemical alteration of ash is led by various factors when the ash falls on the bottom of the sea:fine particle size, loose porous nature, broader surface region, and its unstable composition. Under the high reducing setting of vanadium, the chemical solutions of weathering become enriched. V(V) and V(IV) occur in various chemical forms, such as (i) sulfides, (ii) vanadates,or hydro-oxides, which are weakly adsorbed/precipitated on limestones or clays, (iii) vanadium porphyrin or humic compounds. At the bottom of the ocean, the vanadium could be abundant via the scavenged activity of sulfides and marine organic matter.
4.7 Vanadium accumulation model
The enrichment model of the vanadium in the black rock is presented in Fig. 12. The reason for this enrichment of vanadium in black rocks are (i) higher vanadium supply in the seawater, (ii) stratification, (iii) anoxic water settings,(iv) slow sedimentation rate. In the shallow water, biogenic particles adsorbed some of the vanadium. However, this concentration of adsorbed vanadium is inadequate for the sediments’ enrichment in the modern ocean. In the anoxic water column, the sediments adsorbed the vanadyl ion.Moreover, Due to adsorption and strong organic compounds, the vanadyl ion is stable in these settings. During the early diagenesis, organic ligands are destroyed by thermal maturation and consequently form complex vanadium. With an increased burial depth and thermal maturity,the liberated V from organic compounds become a part of petroleum via migration. In the black rocks, the diagenetic alteration to the mineral composition and the OM is due to the intricate mechanisms associated with temperature,pressure, and chemical settings. These variations in the black rocks transform both the existence and the abundance of vanadium that has been suggested by the fluctuations of V-rich sediments graphitic schist to immature oil shales. As the vanadium is deposited into the sediments, it forms complex compounds with the organic macromolecules.Previous research on estuarine humic compounds and soil reveals the rapid association of organic acid compounds with vanadium (Goncalves and Mota 1987; Mangrich and Vugman 1988; Templeton and Chasteen 1980). OM liberates a few functional groups gradually with condensation and maturity (Tissot and Welte 1984). Presumably, the redistributed V as a functional group develops more stable compounds, e.g., asphaltenes and porphyrins. During progressive diagenetic changes, both the increase and redistribution of porphyrins metallated percentages are consistent (Baker and Louda 1986). As the burial temperature upsurges, the vanadium compounds and the vanadyl porphyrins joins the migration hydrocarbons after separation from macromolecules. The concentration of vanadium may increase into the heavy hydrocarbons during the migration of petroleum. It is due to the depletion of light hydrocarbons or the occurrence of biodegradation (Filby and Van Berkel 1987). Most of the vanadium in the black rocks accumulates during sedimentation, and it remains in the sediments even during metamorphism and diagenetic actions (Sozinov 2018). The factors that are beneficial for the preservation and enrichment of vanadium in the black rocks are poorly described. However, the enrichment of vanadium may be affected by clay minerals and the nature of OM. Marine black rocks, sapropelic coals, and carbonaceous shale mainly encompass V-rich minerals (Zhang 1985; Coveney et al. 1987). In clay minerals, V chiefly occurs in the form of V(III). However, V(IV) species have also been noticed in the clay minerals (Premovic´ 1984;Wanty et al. 1990). It is thought V(III) might be formed at high temperature via reaction of V(IV) with residual OM or believed to form through the reaction of V(IV) with H2S(Wanty 1986). In clay octahedral sites AL readily substitutes by V(III). In clay’s structure, V(III) is stable, and it can exist also exist in extreme weather conditions. It is suggested the Niutitang Formation is extensively affected by volcanic activity during the Early Cambrian period due to extensional forces (Awan et al. 2020). These volcanic activities brought massive nutrients in the form of the various metal element (V, Ni, Co, Cr) from the deeper Earth through faults and fissures. Moreover, the sea level transgression during the Early Cambrian period enhanced biological activity. These organisms consumed the metal elements, which later become a part of their bodies. Later on, the vanadium and other metal-rich organisms were died and laid down at the bottom of the ocean surface and deposited in the sediments. Finally, when the initially deposited loose sediments were diagenetically altered, this vanadium became enriched along with organic matter in the Niutitang Formation. In the upper water column or in the photic region where macroalgae and phytoplanktons proliferate is a productive zone for photosynthesis. As a result of OM’s higher sedimentation rate, the black shales,phosphates, mudstone, chert, and adsorbed trace metal elements could accumulate onto the seafloor (Li et al.2013; Piper and Link 2002). Patrick (1978) suggests the growth of green algae and diatoms in the laboratory could be higher with the higher availability of vanadium concentration. Moreover, the favorable amount of the dissolved vanadate for the evolution of nitrogen-fixing bacterias is 10–1000 Nm, while the growth of these bacterias is limited under 10 nM (Bellenger et al. 2008a).However, vanadophores may also be produced and consumed by these bacterias under limited settings (Bellenger et al. 2008b). The previous studies have also explained that marine algae’s biomasses, e.g., red, brown, and green algae, have a substantial sorption ability for Ni, V, Cd, Cu,Hg, Pb (Doshi et al. 2006; Prasher et al. 2004). The dissolved V is enormously found in the seawater. However,the hydrothermal activities could be a source of V. The massive amount of metal elements could accumulate in the algal remanents, which were reduced and fixed via bacterial actions.
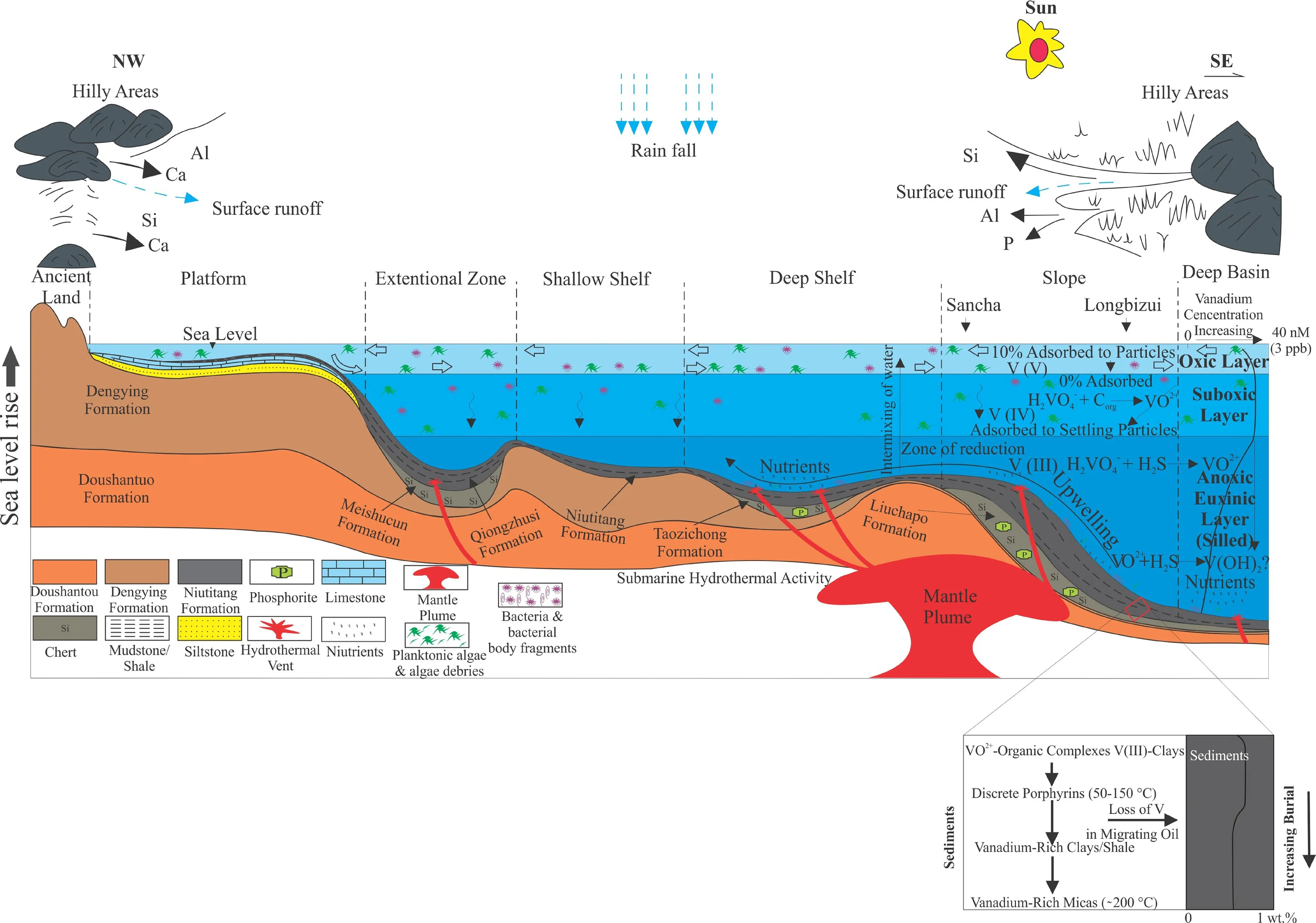
Fig. 12 Vanadium accumulation model in the Niutitang Formation
5 Conclusion
This article describes the occurrence of vanadium in nature-its biogeochemical cycling and its relationship with organic matter. The following are the conclusive facts that controlled the enrichment of OM in these sediments:
1. There are three naturally occurring Metallo-organic compounds of vanadium, based on the type of ligands,i.e., mixed ligands tetradendate, tetrapyrrole complexes, and humate complexes.
2. The black rocks of the Niutitang Formation deciphered a strong positive association of vanadium with the total organic carbon content. A similar trend is also observed in the various black sediments around the globe. The strong positive association of V with the OM might be due to a strong association of vanadium with the microbial shells.
3. Lithophile is the main geochemical characteristic of vanadium. However, it has a little tendency for siderophile and a biophilic tendency. As vanadium forms various complex ions and exists in three different states, + 5, + 4, and + 3, it has a diadochic replacement tendency of different elements within the mineral structure by vanadium.
4. Vanadium doesn’t form an autonomous mineral in igneous rocks, but it has a higher concentration in the basic igneous rocks. It resides in the other mineral structures in the form of V4+by replacing Al3+, Ti4+,and Fe3+. In the sedimentary rocks, the most common vanadium is V4+, and V5+, whereas the V3+is mainly distinctive to the sediments’ buried organic matter.
5. Clays and pyrite are the most favorable mineral for vanadium enrichment. However, it is suggested the quartz other than biogenic origin might be unfavorable for vanadium enrichment.
6. During the Early Cambrian period, the massive transgression in the sea level created a favorable environment for organisms to survive. Additionally,the hydrothermal activities brought massive nutrient supply in the form of vanadium and other metal elements from the deep Earth. These creatures consumed the vanadium-rich nutrients, which became a part of their bodies in the form of hard and soft parts.Later on, when these organisms died and were submerged in the sediments. After the diagenetic actions, this vanadium became a part of these black sediments along with organic carbon. Therefore, these black rocks in the Yangtze region are enriched in vanadium and organic carbon.
AcknowledgementsThe authors are grateful to the National Natural Science Foundation (NNCF) of China for awarding us pecuniary aid with Grant Numbers 41572099, and 41872127 to accomplish this scientific research.
Declarations
Conflict of interestThe authors declare no conflicts of interest.
杂志排行
Acta Geochimica的其它文章
- Interception, degradation and contributions of terrestrial organic carbon obtained from lignin analysis in Wujiang River, southwest China
- Mineralogy and geochemistry of fine-grained Dahab stream sediments,Southeastern Sinai,Egypt:emphasis on the intergrowths of Fe–Ti oxides
- Response of silicate chemical composition variation on thermal metamorphism of ordinary chondrites and classification of petrologic types: the case of L chondrites from Grove Mountains, Antarctica
- Hydrogeochemical characteristics and its role in controlling arsenic mobilization in a shallow aquifer
- Constraints on unconsolidated pyroclastic flow sediments related REE enrichments originated from potassic-alkaline Go¨lcu¨k stratovolcano: Darıdere-Direkli-Yakao¨ren (DDY) table 4deposits,southwestern Anatolia of Turkey
- Fluoride ions in groundwater of the Turkana County, Kenya, East Africa
