Hydrogeochemical characteristics and its role in controlling arsenic mobilization in a shallow aquifer
2021-11-04AyanDasAbhijitMandal
Ayan Das • Abhijit Mandal
Abstract The relevance of groundwater hydrogeochemistry to explain the occurrence and distribution of arsenic in groundwater is of great interest. The insightful discussions on the control of shallow groundwater (<50 m) hydrogeochemistry in arsenic mobilization are known to be a viable tool to explain the arsenic menace in shallow groundwater. The present investigation emphasizes the hydrogeochemical driver and/or control over the reductive dissolution of Fe-bearing host minerals and thereby releasing arsenic into the shallow groundwater of the study area. The study suggests that hydrogeochemical evolution is mainly governed by carbonate minerals dissolution, silicate weathering, and competitive ion-exchange processes in the shallow aquifers (<50 m). The present study also indicates the prevalence of carbonate minerals dissolution over silicate weathering. The emergence of Cl- concentration in the shallow groundwater founds the possibilities of anthropogenic inputs into the shallow aquifers (<50 m).The reducing environment in shallow aquifers (<50 m) of the study area is evident in the reductive dissolution of Febearing shallow aquifer minerals which absorb arsenic in the solid phase and mobilize arsenic onto shallow groundwater. The study opted for many statistical approaches to delineate the correlation among major and minor ionic constituents of the groundwater which are very helpful to understand the comprehensive mechanism of arsenic mobilization into shallow groundwater.
Keywords Arsenic mobilization ∙Groundwater hydrogeochemistry ∙Aquifer sediments ∙Shallow aquifers ∙Redox environment
1 Introduction
The presence of inorganic arsenic in groundwater of shallow aquifers (<50 m) of south-east Asia (especially in Bengal Basin, West Bengal part, India) has been reported by many researchers (Mukherjee and Bhattacharya 2001;Bhattacharya et al. 2002a, b; Bhattacharyya et al. 2003a;Ahmed et al. 2004; Nath et al. 2007, 2008a; Mukherjee et al. 2008; Mukherjee-Goswami et al. 2008; van Geen et al. 2008; Chatterjee et al. 2010; Fendrof et al. 2010). The scale of human exposure due to geogenic arsenic in the Holocene sedimentary aquifers of Bengal Basin has been considered as a mass poisoning in human history (Smith et al. 2000). The arsenic contamination in groundwater in Bengal Basin was firstly reported from West Bengal, India in the early 1980s (Saha 1984; Guha Mazumder et al.1988). The arsenic concentrations in groundwater from this part of the globe often exceed WHO guideline values(AsT<10 μg/L) as well as the national safe limit (AsT-<10 μg/L) (Bhattacharyya et al. 2003b; Ahmed et al.2004). About 45 million people from this area are suffering from groundwater arsenic contamination (RGNDWM Report 2001; Chatterjee et al. 2003). After the Green revolution, millions of tube-wells have indiscriminately been drilled in the alluvial deposits of Bengal Basin (West Bengal part, India) due to rapid growth in need of groundwater for agricultural purposes which results in the widespread use of groundwater without proper chemical testing, which eventually leads to arsenic exposure to population (PHED Report 1993; CGWB Report 1999;RGNDWM Report 2001; McLellan 2002). Thousands of shallow aquifers (<50 m) contaminated with geogenic arsenic (AsT>10 μg/L) were also reported in Bengal Basin (Bhattacharyya et al. 2003a).
The source of arsenic in shallow groundwater of the Bengal Basin has been considered to be geogenic and mostly limited to the Holocene aquifers (Das et al. 1995;Bhattacharya et al. 1997; BGS and DPHE 2001). Several arsenic mobilization models have been recommended (Das et al. 1995; Bhattacharya et al. 1997; Acharyya et al. 1999;Nickson et al. 2000; BGS and DPHE 2001; Smedley and Kinniburgh 2002; Chatterjee et al. 2003). Among all the proposed models, the ‘reduction model’ is most favored for the mobilization of arsenic in groundwater (Bhattacharya et al. 1997; Nickson et al. 2000; BGS and DPHE 2001;Smedley and Kinniburgh 2002; Chatterjee et al. 2003). Iron oxides and oxyhydroxides are identified to adsorb arsenic and they are common constituents of the sediments as grain coatings (Nickson et al. 2000).
Many investigators offered their contributions to understand as well as to decipher the problem of arsenic menace in groundwater (Mandal et al. 1996; Bhattacharya et al. 1997; McArthur et al. 2001; Chatterjee et al.2003, 2005; Nath et al. 2007). The chemistry of the solid phase (sediments, minerals, and underlying bedrocks) and their interaction with the aqueous phase (i.e., groundwater)plays a significant role in controlling the mobilization of arsenic into groundwater under different redox environments (Bhattacharya et al. 1995a, b). Several factors control the occurrence and origin of arsenic in groundwater such as solid-phase precipitation-dissolution of arsenicbearing minerals, adsorption–desorption, redox kinetics,the ion-exchange capacity of the aquifers, mineralogical characteristics, microbial activity, content of fresh organic matter, and anthropogenic influences (BGS and DPHE 2001; Smedley and Kinniburgh 2002; Oremland and Stolz 2003; Islam et al. 2004; Das et al. 2021; Das 2020; Das and Mondal 2021; Bhattacharya et al. 1997; McArthur et al.2004).
The present study deals with hydrogeochemical investigation within shallow depth (<50 m) to envisage groundwater evolution and arsenic mobility in sub-surface geology. Statistical approaches (piper diagram, factor analysis, and correlation mathematics) have been opted to explain the occurrence of arsenic in groundwater in terms of related covariance of the hydrogeochemical parameters.Finally, mechanisms of controlling groundwater hydrogeochemistry in shallow aquifers have been discussed in detail to understand the process of mobilization of arsenic into the shallow groundwater.
2 Study area settings
The study area (Part of Chakdah block of Nadia district,West Bengal, India; latitude: 23°00′20′′N–23°05′20′′N;longitude: 88°31′40′′E–88°49′00′′E) is an integral part of the world’s largest delta (Ganges–Brahmaputra-Meghna deltaic alluvium, GBM system), located in the eastern side of the river Hoogli, the main distributary of river Ganges(Fig. 1). The study area is present on the western wing of the Bengal Basin (West Bengal part, India). The area is comparatively flat and intersected with minor rivers, small streams, and swamps, backwaters (Nath et al. 2005). The river Hoogli to the northwestern side is merely the main drainage of the region. The common gradient is towards the southeast. The study area is bounded by the river Hoogli (distributaries of the main regional river the Ganges) on the west and river Ichhamati on the east side.The study area is situated approximately 60 km away from Calcutta on the north.

Fig. 1 Study area map of Nadia district, West Bengal, India (yellow coloured dots indicate sampling sites)
The climate of the study area is mainly tropical with three comprehensive seasonal distinctions, viz., hot and dry summer (March–May) with infrequent thunderstorms, wet monsoon (June–October), and cold and dry winter(November–February) with occasional rainfall (Statistical Handbook 2005). The yearly precipitation ranges from 1295 to 3945 mm, especially limited to the monsoon period. The average temperature varies between 10 and 42 °C.The average humidity fluctuates between >65% to 92%which often exceeds annual potential evaporation from 1500 to 2500 mm (Statistical Handbook 2005). The degree of evaporation reaches its maximum in the summer season(April–May) and reaches its minimum in the rainy season(August–September). The groundwater level fluctuated throughout the year and reaches its maximum in monsoon,also the low-lying area is inundated every year due to the rising of the water table above ground level (Hoque et al.2014). In this part of the Bengal Delta Plain (BDP) agriculture is the main source of income for the rural population and is practiced throughout the year using groundwater as cheap for irrigation (mainly during spring irrigation). It uses groundwater as high as 70%–80% of the total annual recharge (RGNDWM 2002). In the district of Nadia, it has been reported that 636 deep tube wells (DTW,>50 m bgl), 587 shallow tube wells (STW, <20 m bgl),and 245 medium-depth tube wells (MDTW, 20–50 m bgl)and 319 river kick pumps were used for irrigation in a calendar year (Statistical Handbook 2005). But now the district organizations are not inspiring the drilling of irrigation wells due to the resultant enrichment of groundwater with arsenic above the permissible limit.
The general slope of the study area is towards the south and south-east and is located within the flood plain of the river Hoogli. The study area has a general surface elevation ranging from 6 to 10 m mean sea level. This area is chiefly the Holocene sedimentary successions of fluvial deposits by the meandering river Hoogli with iron-coated micaceous sand, silt, and clay with upward fining sequences(PHED 1993; Bhattacharya et al. 1997; Pal et al. 2002).The shallow aquifers are mostly created by sandy concealed with surface silty clay. The shallow aquifer sediments (<50 m) are composed of calcite, dolomite,kaolinite, chlorite, feldspar, quartz, minor biotite,muscovite, illite, and iron oxides/oxyhydroxides (Nath et al. 2005, 2007,2008; Mukherjee et al. 2007a). The arseniferous belts are found in the abandoned meander channels and meander scrolls (Bhattacharya et al. 1997).The lithology of the shallow aquifers (<50 m) is most prevalent by intercalations of sand, silt, and clay of the Holocene age (Nath et al. 2005). The shallower aquifers(<20 m) consist of clay and silty clay forming the top of the stratigraphy and just below it (20–50 m) the aquifers are composed of fine to coarse sand with clayey intercalation. The deeper aquifers (>50 m) are having sand and gravel (Deshmukh and Goswami 1973). Geological backgrounds are also important to understand the menace of arsenic contamination in shallow groundwater (<50 m).Shallow palaeo-channels (<30 m bgl) contain grey sand that was raised by the alluvial deposition of Holocene age and late-Pleistocene brown sands capped the underlying palaeo-interfluvial sequences (Hoque et al. 2014). Arsenic contaminated groundwater in the palaeo-channel sands can annex the brown sands of palaeo-interfluves containing sedimentary Fe-hydroxides) than grey sands which adsorb arsenic (Horneman et al. 2004).
Many works have been documented from the present study area to understand the process of mobilization of arsenic in shallow groundwater and here we are also interested to envision a more perceptive study to understand and also to judge the present understandings. This research adopted statistical and hydrogeochemical tools to understand and/or to explain the multiple mechanistic pathways of arsenic mobilization functioning in the shallow aquifers (<50 m). It also compares different geochemical mechanisms (silicate weathering, carbonate dissolution, and evaporation-dissolution) which are responsible for arsenic mobilization. Furthermore, the present study depicts the ion-exchange kinetics during prolonged water–rock interaction which is evident to be responsible for arsenic poisoning.
3 Materials and methods
3.1 Groundwater sampling and analysis
The groundwater samples (n = 40) were taken with a frequent interval of time from March to May 2016. All the groundwater samples have been collected from both private and community tube wells with a varying range of depth with shallow range (<50 m). The sample water was taken three times from the same tube well to measure the onsite parameters precisely in the wellhead. The groundwater samples were taken in prewashed and acid-cleaned high-density poly-ethylene vials (Tarsons) after pumping the tube wells for few minutes (2–3 min depending on the depth of the wells) to ensure satisfactory flushing. At each sampling site, several field parameters [Eh, pH, and electrical conductivity (EC), TDS] were measured using a WTW multi-meter 100 (WTW, Germany). Alkalinity (reported as HCO3-) was also measured using the titrimetric method (as described in Bhattacharyya et al. 2003a). The DO (dissolved oxygen) of the sampled water was measured by the Winkler method. Each sampling process (including the collection of water, well parameters, and measurement of some on-site field parameters) required 1 to 2 h approximately. The hardness of the collected groundwater was estimated by EDTA titrimetric method using Eriochrome Black-T as an indicator. Water samples were filtered through 0.45 μm cellulose nitrate membrane filters(Sartorius AG, Germany) and collected in three separate bottles: (i) one for the analysis of total As (acidified with conc. HNO3, 1% v/v, Merck) (ii) for other major cations(acidified with conc. HNO3, 1% v/v, Merck) and (iii) one for the major anions were separated on-site (unacidified).The position (latitude and longitude) of each tube-wells were recorded by a handy GPS meter with proper additional information.
Major cations and anions were measured by UV–VIS spectrophotometry (Perkin Elmer Lambda-20) and 761-compact IC (Ion Chromatography, Metrohm). Total iron concentrations were measured colorimetrically in the laboratory following theo-phenanthroline method using the Perkin Elmer Lambda-20 spectrophotometer. In the beginning, required standard arsenic solutions were prepared from the stock solution of 1000 mg/L As solution(Merck, Germany). 1 mL of each standard and unknown groundwater sample was taken in 50 mL volumetric flasks.After that 4 mL of concentrated HCl was added to the standard, unknown, and blank solutions. Then, 5 mL KI and 5 mL ascorbic acid solution were added to all the solutions. Later than waiting for a minimum of 45 min, all the solutions were made up to the volume level of 50 mL and the corresponding absorbance was measured using HG-AAS mode (Varian AA-240, Australia, detection limit <1 μg/L) at 193.7 nm wavelength (Akhter et al.2005). Moreover, ten percent of the collected groundwater samples (n = 4) have been reanalyzed (except field parameters) to check the precision of the analysis and for all the elements it was >96%.
3.2 Statistical analysis and geophysical approach
A multivariate statistical analysis of the dataset was performed to test the hydrogeochemical eccentricity based on specific trace element distribution patterns. The software package XLSTAT-2015 was used to perform factor analysis (Table 3), which represents an excellent method to extract the most important factors related to the association of arsenic with other elements. To facilitate the interpretation of the factor analysis (loadings), the correlation coefficient matrix (Table 2) has been constructed with a critical ‘r’ value of 0.312 for 38 (n - 2) degrees of freedom. Also, Grapher-14 has been used to construct a piper diagram (Fig. 2) to understand the groundwater chemistry and to interpret geochemical process characteristics.

Fig. 2 Piper diagram illustrating the main hydro-geochemical features of the groundwater from the study area
The satellite image of the study area was acquired from Google Earth. Google Earth has been used to demarcate the study area as well as sampling sites.
4 Results and discussion
4.1 Groundwater chemistry
The statistical summaries of chemical analysis results for groundwater samples (n = 40) are presented in Table 1.The pH of the groundwater samples is near neutral or circum-neutral (6.88–7.6, Table 1) which indicates the well-buffered conditions of the groundwater. The electrical conductivities (EC) of the sampled groundwater are very high (up to 649 μScm-1, Table 1). The results show that the groundwater is fresh and often loaded with dissolved elements (TDS 38.72–415.36 mg/L, Table 1). The Ehvalues of the shallow groundwater samples are very low which indicates a highly reducing nature of the shallow aquifers (down to - 12.8 mV, Table 1). The dissolved oxygen value is very low (maximum 0.4 mg/L, Table 1)which further indicates the anoxic conditions of the shallow aquifers of the study area.
Among major cations, Ca2+is always dominating the groundwater composition followed by Mg2+, Na+, and K+.Bi-valent alkaline earth metals (both Ca2+and Mg2+) are released during the carbonate minerals dissolution (Nath et al. 2008a, b; Neidhardt et al. 2013). The study reveals that, Ca2+(207.08–323.3 mg/L, Table 1) is always predominated over Mg2+(55.0–145.14 mg/L, Table 1) and Na+(14.3–25.2 mg/L, Table 1) predominated over K+(3.2–11.3 mg/L, Table 1). Alike, monovalent alkali metals(both Na+and K+) are possibly released from silicates minerals dissolution which is very frequent in shallow aquifers (<50 m) of Bengal Basin (Chatterjee et al. 2004;Charlet et al. 2007).
Among the redox-sensitive elements (Fe and As), iron is the highest (0.9–4.6 mg/L, Table 1) and arsenic is the lowest (28.9–143.0 μg/L, Table1). The present investigation shows the anoxic nature of the shallow aquifers(<50 m) with the presence of dissolved redox-sensitive elements (Fe and As) (Bhattacharyya et al. 2003a; Ahmed et al. 2004; Bhowmick et al. 2013). Among these elements,inorganic arsenic is more poisonous in dissolved form and the lower oxidation state [As (III)] is more toxic than a high oxidation state [As (V)] (Hopenhayn-Rich et al. 2000;Guha Mazumder et al. 2010). The results show that arsenic concentration is often exceeding WHO guideline value(AsT<10 μg/L) as well as national safe limit (AsT-<10 μg/L) (WHO 2011a, b; BIS-10500 2012).
Among the anions, HCO3-is the highest(312.0–542.0 mg/L, Table 1) and phosphate is the lowest(0.4–3.06 mg/L, Table 1). The rest are in between (NO3-:0.05–38.0 mg/L; SO42-: 3.8–12.4 mg/L; Cl-:27.1–128.5 mg/L; Table 1). The study suggests that HCO3-always has a base-line value (312.0 mg/L, Table 1)which supports the carbonate dissolution in the shallow aquifers (<50 m). The large variation of HCO3-concentration values further predict the multiple origins of HCO3-(Bhattacharyya et al. 2003b; Harvey et al. 2006). Other than carbonate and silicate dissolution, HCO3-in groundwater can be originated from the vadose zone or biogenic CO2gas dissolution (Kumar et al. 2012). It delineates the microbial inputs in decomposing fresh organic matter and dissolution of iron-bearing host minerals (Fe-oxides and/or oxyhydroxides) thereby releasing arsenic into shallow groundwater.
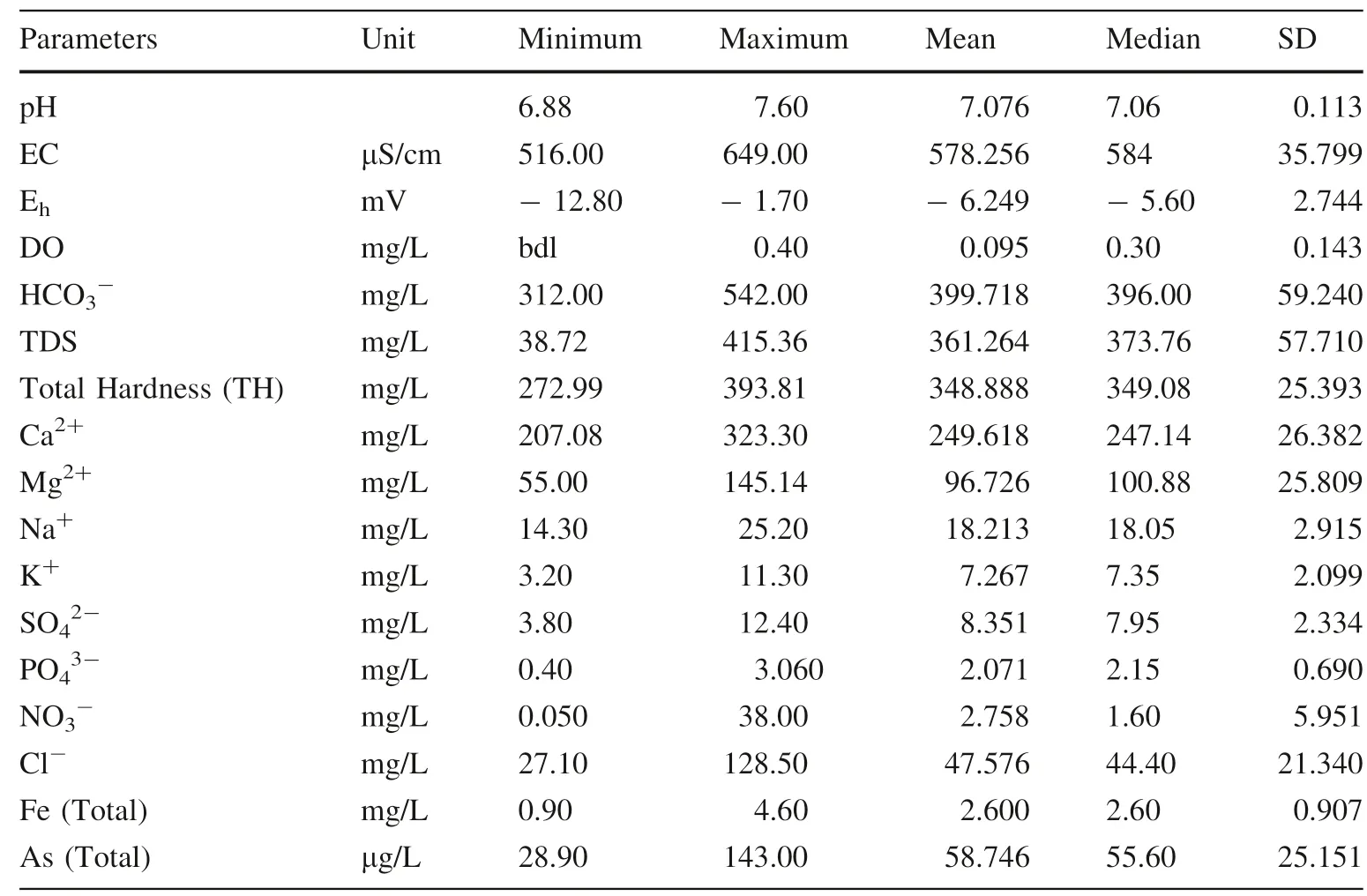
Table 1 Summary of the physico-chemical parameters of groundwater samples from the study area
All the points in the Piper diagram (Fig. 2) are clustered on the upper right-hand side in the central diamond which shows that the groundwater is of HCO3-–Cl-–Ca2+–Mg2+–Na+type. The evolution of groundwater chemistry is mainly associated with carbonate dissolution and silicate weathering and the connotation of chloride in shallow groundwater chemistry of the study area suggests the anthropogenic inputs (McArthur et al. 2012; Smedley and Kinniburgh 2002; BGS and DPHE 2001; Das 2020; Biswas et al. 2011; Bhowmick et al. 2013; Mukherjee and Fryar 2008; Das and Banerjee 2020).
4.2 Factors controlling groundwater evolution
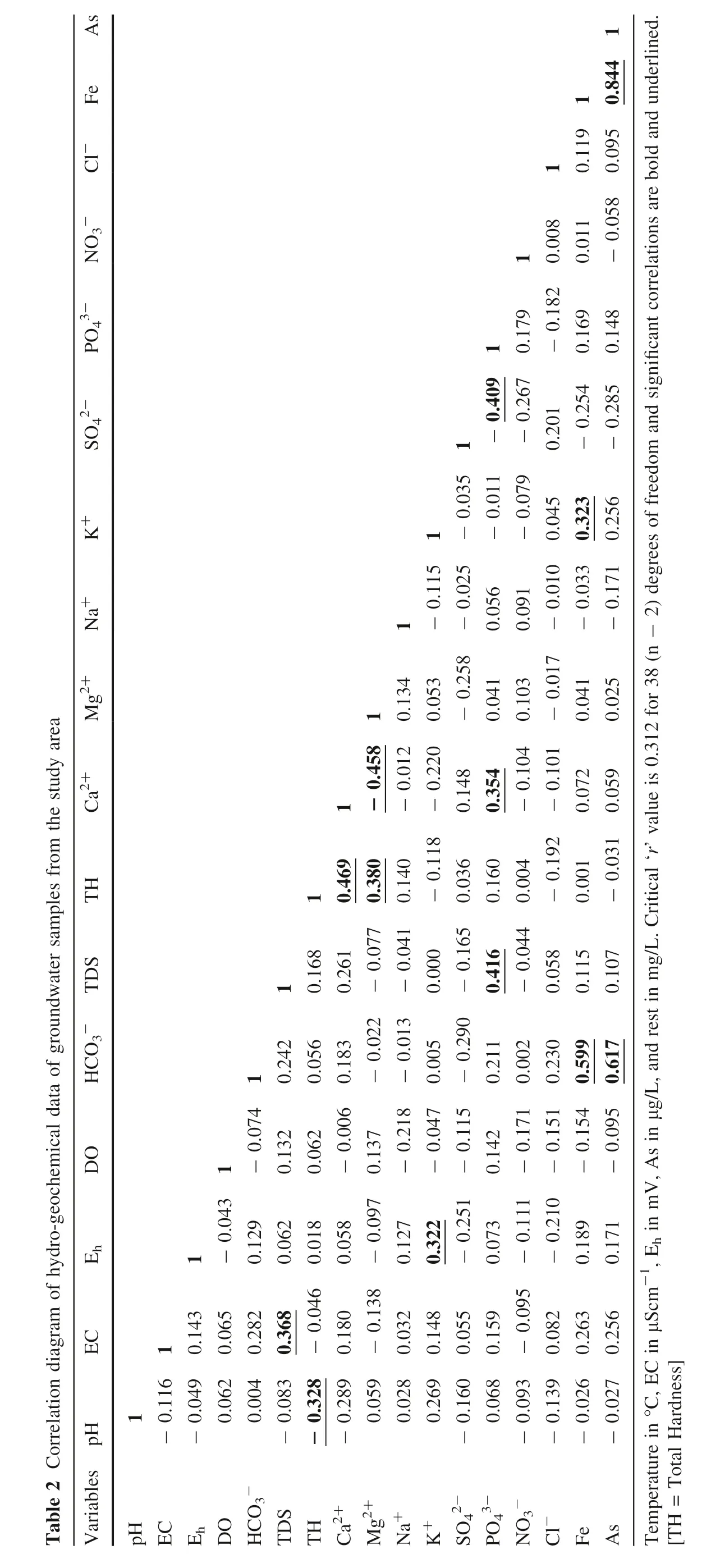
The mutual variance of the hydrogeochemical parameters(Table 2) specifies that the major ions (cations and anions)are certainly correlated to each other. Pearson correlation graph and factor analyses have been constructed to explain the associations among hydrogeochemical factors and groundwater chemistry. Strong positive correlation exists between EC–TDS (r = 0.368, Table 2) and Eh–K+(r = 0.322, Table 2). TDS shows correlation with PO43-(r = 0.416, Table 2) and total hardness (TH) with Ca2+(r = 0.469, Table 2) and Mg2+(r = 0.380, Table 2). Ca2+–PO43-and K+–Fe(II) exhibit strong positive correlation(r = 0.354 and 0.323 respectively).
HCO3-shows a very strong correlation with Fe(r = 0.599) and As (r = 0.617) suggesting carbonate dissolution pathways via microbial decomposition of organic matter (notably fresh organic matter). Furthermore, a very strong and significant association of arsenic with iron(r = 0.844) suggests microbial mediated decomposition of Fe-oxides and/or oxyhydroxides under different reducing environments of shallow aquifer sediments which mostly enhances the release of arsenic (Rowland et al. 2006).
e a a r udy s t the om f r e s mpl sa r te oundwa gr of ta da l c a m i he oc ge o-hydr of am agr di on t i la re 2 Cor e Tabl As Fe-C l-NO3 3-PO4 2-SO4 K+Na+Mg2+Ca2+TH TDS-HCO3 DO Eh EC pH e s abl r i Va 844 1 119 1 0.10..095.008 011 058 0 0.1 0.-.179 182 0 0.169 148 409 1-0.0.0.267 0 0.201 254 0.285 0.035 1--0.--0.011 0.079 0..045 323.256 115 1--0.0.025 -0.056 091 010 0 0.033 0.171 0 0.1-0.0.--.134.053 258 -0.041.103 017 -0.041 025 458 1-0.0.0.0.012 0 0.220 0 0.148 354 104 0 0.101 -0.072.059 1--0.0.-0.469 118 -0.192 -0.031 0 0.10.380 0..140 160.036 0.001.004--0.-168 077 0.261 041 0 0.165 0 0.044 0 0.10.0.000 0.416 0.058 115 0.107 0.0..183 242 022 -0.0.013 -290 --.005 056 0.211.002.230 599 617 074 1 0.0.-0.0.0.0.132 062 006 0 0..137 218 -0.047 0 0.115 -0.142 171 0 0.151 0 0.154 0.095 0.043 1-0.0.---0.--0.129 062.018 058 097 0 0.127 322 251 -0.073 111 -0.210 -0.189 171 1-0.0.0.0.0.-0.-0.0..143.065.282 368 046 0 0..180 138 -0..032.148.055.159 095 -0..082.263.256 116 1 0.10.049 0 0.062 0 0.004 0 0.083 0.328 -0.289 0 0.059 -0.028 0 0.269 0 0.160 0 0.068 0 0.093 -0.139 0 0.026 0 0.027 0 0.-----------2-3--pH EC Eh DO HCO3 TDS TH Ca2+Mg2+Na+K+SO4 PO4 NO3-C l Fe As d.ine r l unde and d bol e a r ons t i la re cor nt fic a gni s i and dom e e f r of s ee gr de 2)-(n 38 for 312 0.is lue va‘r’a l ic i t Cr L.mg/in s t re and L,μg/in As,mV in Eh m-1,μSc in EC ]s s,°C rdne in Ha e tur a l ra Tot mpe =Te[TH
Factor analysis of Physico-chemical parameters of the groundwater indicates 4 factors that explained 41.934% of total variability with eigenvalue>1.0 (Table 3). Factor 1(15.849% variance) has strong positive loadings EC, Eh,HCO3-and TDS (Table 3), which is generally a consequence of water–rock interaction and dissolution-weathering processes. Moreover, a positive loading between Fe and As in Factor 1 indicates that the reduction of Fe-oxides and/or oxyhydroxides were the predominant mechanism behind the arsenic release into the groundwater. This factor can be considered as an arsenic mobilization factor. Factor 2 (10.941%variance) with negative loadings for TH (total hardness) and Ca2+indicates the dissolution of calcium-bearing minerals(calcite) followed by carbonate dissolution. Factor 3 (9.033%variance) suggests magnesium-bearing minerals (dolomite)dissolution with negative loading for Mg2+whereas positive loading of SO42-indicates the sulphate reduction process.Factor 4 (6.111% variability) with negative loading for PO43-indicates the prevalence of arsenic mobilization processes.

Table 3 Factor analysis of hydro-geochemical variables of groundwater samples
4.3 Mechanisms controlling groundwater hydrogeochemistry in shallow aquifers
The geochemical evolution of groundwater from shallow aquifers is mostly controlled by carbonate mineral dissolution, silicate weathering, and ion-exchange processes with aquifer minerals (Dowling et al. 2003; Mukherjee and Fryar 2008). The reasonable contributions of these processes to groundwater evolution solely be governed by the content of minerals in aquifer sediments and kinetics of the geochemical processes (Tardy 1971; Faure 1998). Extended flow path and prolonged groundwater residence time as a consequence of low hydraulic gradient are liable to silicate weathering and ion-exchange processes despite slow kinetics (Nath et al. 2005; Mukherjee et al. 2007a, 2009).In the present investigation, mass-balance strategies have also been employed to understand these geochemical processes occurring in aquifers of the study area (Mukherjee et al. 2009). As all the groundwater samples from the study area are clustered between the carbonate dissolution and silicate weathering zones in the bivariate plot (Fig. 3A, B)it delineates that carbonate dissolution and silicate weathering are simultaneously occurring in shallow aquifers(<50 m) of the study area. Moreover, the tendency of the groundwater samples to gather along (1:2) line in the bivariate plot of (Ca2++ Mg2+) versus HCO3-(Fig. 3C)is in great proportion than that in the bivariate plot between(Na++ K+) versus HCO3-(Fig. 3D), which recommends the carbonate minerals dissolution is more prevalent over silicate minerals weathering in shallow aquifers (<50 m)of the study area. The competent enrichment of PCO2in shallow aquifers compared to atmospheric PCO2and groundwater interaction kinetics in connection with calcite and dolomite minerals are the most predominant carbonate minerals dissolutions in the aquifers. The bivariate plots of Ca2+and (Ca2++ Mg2+) versus HCO3-vividly show that the groundwater samples are closer with calcite line than that with dolomite line which further suggests the higher Ca2+content than Mg2+in the groundwater of the study area (Fig. 4). Figure 5A undoubtedly explains the scope of active cation exchange in the aquifers. It has been reported that the slope of this bivariate plot would be less than - 1 for the aquifer with active cation exchange between(Ca2++ Mg2+) and Na+(Jankowski et al., 1998). Therefore, the slope of - 34.203 for the aquifer indicates that cation-exchange processes are responsible for the observed groundwater compositions (Fig. 5A).

Fig. 3 Bivariate plot of A Na+ normalized HCO3- and B Mg2+normalized Na+ versus Na+ normalized Ca2+ to recognize predominating minerals weathering in groundwater of the study area. Also the bivariate plot of C HCO3- versus (Ca2+ + Mg2+) and D HCO3-versus (Na+ + K+) to compare the extents of carbonate dissolution and silicate weathering in groundwater of the study area

Fig. 4 Bivariate plots of Ca2+ and (Ca2+ + Mg2+) versus HCO3-showing correlation of major ions to discriminate the chemical processes, i.e., carbonate dissolution (Calcite dissolution and Dolomite dissolution) in shallow aquifers of the study area
The higher scope of cation exchange in shallow aquifers might contribute to the relative enrichment of K+in the groundwater from the study area (Mukherjee et al. 2009)which is also supported by factor 2 (r = 0.345, Table 3).
In search of the source of Cl-in groundwater, a bivariate plot of Cl-versus Na+has been constructed(Fig. 5B). von BrO¨ mssen et al. (2007) reported that Clcontent in groundwater is possibly due to atmospheric deposition and mixing with connate seawater, but the present study rejects the probability of mixing with connate seawater. However, the bivariate plot suggests extensive cation exchange in the shallow aquifers (Fig. 5B). The mole ratio of Cl-to Na+is found more diverse (>1 and<1). The Cl-enrichment in shallow groundwater might be due to anthropogenic activities like agricultural practices, huge groundwater development for irrigation purposes, septic tank, and domestic wastewater (Nath et al.2008a). The above facts have been reported by many researchers (Charlet et al. 2007; Mukherjee et al. 2007b;Lawson et al. 2008).
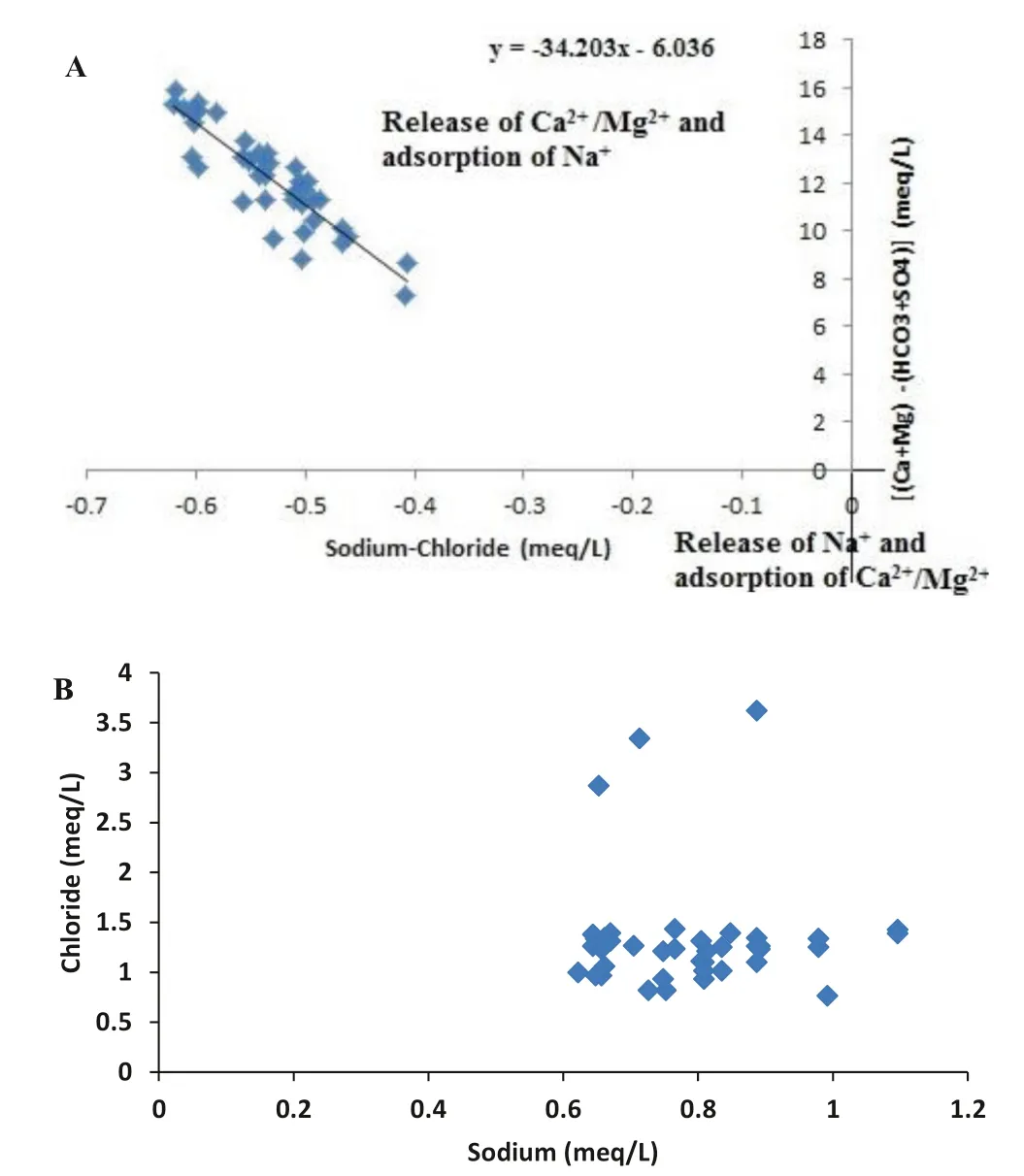
Fig. 5 Bivariate plot of A (HCO3- + SO42-) corrected (Ca2+-+ Mg2+) versus Cl- corrected Na+ to define the extent of the cation exchange process and B Cl- versus Na+ to trace the origin of Cl-in shallow aquifer (<50 m) of the study area
4.4 Arsenic speciation and mobilization of arsenic in shallow aquifers
The very low concentrations of NO3-and DO (even bdl)indicate the redox conditions in shallow aquifers (<50 m)which are often governed by the Fe (III)/Fe (II) redox equilibrium. Despite enrichment of SO42-over NO3-and PO43-is not so high (3.8–12.4 mg/L; mean 8.351 mg/L,Table 1). However, this low value may not correspond to sulphate reduction in shallow aquifers of the study area.The inadequacy of sulphidic minerals in the aquifer sediments might also limit the concentration of SO42-(Mukherjee and Fryar 2008). The concentration of arsenic ranges from 28.9 to 143.0 μg/L (Table 1). Relatively low Ehvalue (Down to - 12.8 mV) of the sampled groundwater defines the occurrence of a high concentration of groundwater arsenic. The As (III)/As (V) ratios are relatively higher in groundwater with low Ehvalues (Guo et al.2011). The enrichment of dissolved iron (mainly in Fe (II)state; 0.9–4.6 mg/L, mean 2.6 mg/L, Table 1) further suggests the fact behind the arsenic enrichment in groundwater of shallow aquifers of the study area. The most established hypothesis of arsenic mobilization is the reductive dissolution of iron oxides/oxyhydroxides under reducing condition, i.e., at low to very low Ehwhich indicate the redox potentiality of the groundwater where it comes in contact with the arsenic bearing minerals in the shallow aquifers (Bhattacharya et al. 1997; Das and Banerjee 2020; Das et al. 2021; Smedley and Kinniburgh 2002;Ahmed et al. 2004; McArthur et al. 2001; Nickson et al.2000; Chatterjee et al. 2018; Dowling et al. 2002). The surficial clay layer and/or clayey intercalations in the shallow aquifers of this part of the Bengal basin promotes the possibility of occurrence of reducing environment by restricting the inflow of oxygen into the aquifers and also consumption of dissolved oxygen for oxidation of accumulated organic materials aided by microbial respiration(Smedley and Kinniburgh 2002; Ravenscroft et al. 2005;BGS and DPHE 2001). It has also been reported that low Ehvalues are indicative of reducing environments which correspond to the reduction of As(V) and Fe(III) by releasing Fe(II) and As(III) into the shallow groundwater after the reductive dissolution of the Fe-oxides/oxyhydroxides and sorbed arsenic onto it (Mukherjee and Fryar 2008; Nickson et al. 2000). The positive correlation between iron and arsenic again suggests the reductive dissolution of Fe-oxides/oxyhydroxides followed by reductive desorption of As(V) to As(III) (Ahmed et al.2004; Harvey et al. 2005; Das and Banerjee 2020; McArthur et al. 2004). The strong positive correlation of HCO3-ion with iron and arsenic shows that regional biogeochemical process driven supply of HCO3-ion enhances the release of a whole load of arsenic on the aquifer minerals into groundwater which further promoted by the degradation of organic materials often present in the shallow aquifers (Ahmed et al. 2004; McArthur et al. 2004; Das 2020; Chatterjee et al. 2018). The high concentrations of arsenic in shallow groundwater of the Holocene aquifers may well be recognized with the complete reductive dissolution of Fe-oxides/oxyhydroxides motivated by microbial oxidation of accumulated organic materials,mobilizing the complete sorbed load of arsenic by maintaining a good correlation between arsenic and HCO3-ions(McArthur et al. 2004; Harvey et al. 2002; Das 2020). The Holocene aquifer materials with finer grain sizes experienced very poor oxidative weathering during transportation and got deposited with huge organic load under anoxic conditions which subsequently results in the reduction driven mobilization of arsenic into the shallow groundwater (Harvey et al. 2002; Smedley and Kinniburgh 2002;Islam et al. 2004).
The increase of arsenic concentration in shallow groundwater of the Bengal Basin runs parallel to the high content of arsenic in aquifer sediments (Harvey et al. 2002;Chatterjee et al. 2003; Nath et al. 2005). It explains the enrichment of arsenic concentration in groundwater which is a fact of groundwater evolution controlled by various hydrogeochemical interfaces (Bhattacharya et al.1997, 2002a, b; Nickson et al. 1998; Acharyya et al. 1999;Gault et al. 2005; Nath et al. 2008b). As a consequence of microbial decomposition of organic matter (notably fresh organic matter) DO (dissolved oxygen) and NO3-content is decreased and then redox equilibrium goes through Feoxides and/or oxyhydroxides reduction kinetics followed by SO42-reduction processes (Stuman and Morgan 1981).The source of organic matter in shallow aquifers is still yet to be understood (Harvey et al. 2002; Rowland et al. 2006;Neumann et al. 2010). The dissolution and/or desorption of iron and arsenic from its minerals sources has been controlled by the reductive dissolution of Fe-oxides/oxyhydroxides (Smedley and Kinniburgh 2002; Das 2020). The biogeochemical cycling of iron and arsenic within the aquifers acts as the principal process governing the arsenic mobilization dynamics (Chatterjee et al. 2018; Mukherjee and Fryar 2008; Mukherjee et al. 2008). So, it is found that groundwater is often enriched with both Fe and As. The considerable carbonate minerals dissolution from aquifer sediments of the Bengal Basin poses a significant role to mobilize arsenic into groundwater (Ahmed et al. 2004).
Low Ehvalue indicates the aquifer redox environment which is very susceptible to the reductive dissolution of Febearing minerals and to mobilize arsenic into the groundwater. It is further supported by factor 1 where high HCO3-concentration plays a crucial role in groundwater hydrogeochemistry. Very low concentrations of NO3-and PO43-point that redox kinetics is limited to the reduction of Fe-bearing minerals. Reductive dissolution of Fe-bearing minerals coupled with the microbial decomposition of organic matter helps to release arsenic into the groundwater from the aquifer sediments. Furthermore, enrichment of arsenic in groundwater may be due to anthropogenic inputs which are very much evident from the high Cl-the content of the groundwater.
5 Conclusion
The present study suggests that the geochemical evolution of groundwater from shallow aquifers is mainly governed by carbonate minerals dissolution, silicate weathering, and ion-exchange processes with aquifer sediments. Mass-balance strategies have been opted to explain the geochemical processes in shallow aquifers of the study area which indicates the prevalence of carbonate minerals dissolution rather than silicate weathering. The study recommends that the enrichment of monovalent alkali metals (Na+and K+)is due to the higher extent of cation-exchange processes in shallow aquifers (<50 m). The varied concentration of Cl-as well as mole-ratio of Na+to Cl-disseminates the anthropogenic inputs into the shallow groundwater compositions.
Low Ehvalue advises the aquifers’ redox condition which is very viable for reductive dissolution of Fe-bearing minerals (Fe-oxides and/or oxyhydroxides) and to release arsenic into groundwater. The factor analysis (Factor 1)helps to explain the strong and very close correlation of HCO3-with groundwater hydrogeochemistry and arsenic concentration in shallow groundwater (<50 m). The correlation among the major ions (cations and anions) also shows that hydrogeochemistry of the shallow groundwater is playing a vital role in the arsenic release mechanism.Reductive dissolution of Fe-bearing host minerals, as well as microbial decomposition, poses a suitable pathway for arsenic mobilization into shallow groundwater of the study area. Moreover, it has been found that anthropogenic activities may have a significant role in the enrichment of Cl-as well as arsenic in shallow groundwater.
AcknowledgementsThe authors want to thank the sampling team for their hard work and the local villagers for their hospitality during the field campaign.
Authors’ contributionsAyan Das: Conceptualization, original draft writing, formal analysis, review, and editing. Abhijit Mandal: Original draft writing and review and editing.
FundingNo funding has been received for this particular research.
Availability of data and materials The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Declarations
Conflict of interestThe piece of research does not have any known competing interest of conflict which may hinder the interpretation of the results. The current piece of research had no financial support from any agency and others so the authors declare that they have no competing interests.
杂志排行
Acta Geochimica的其它文章
- Interception, degradation and contributions of terrestrial organic carbon obtained from lignin analysis in Wujiang River, southwest China
- Mineralogy and geochemistry of fine-grained Dahab stream sediments,Southeastern Sinai,Egypt:emphasis on the intergrowths of Fe–Ti oxides
- Response of silicate chemical composition variation on thermal metamorphism of ordinary chondrites and classification of petrologic types: the case of L chondrites from Grove Mountains, Antarctica
- Constraints on unconsolidated pyroclastic flow sediments related REE enrichments originated from potassic-alkaline Go¨lcu¨k stratovolcano: Darıdere-Direkli-Yakao¨ren (DDY) table 4deposits,southwestern Anatolia of Turkey
- Fluoride ions in groundwater of the Turkana County, Kenya, East Africa
- Distribution of functional microorganisms and its significance for iron, sulphur, and nitrogen cycles in reservoir sediments
