Excellent electromagnetic wave absorption of MOF/SiBCN nanomaterials at high temperature
2021-10-25ChunjiaLUOPengMIAOYushengTANGJieKONG
Chunjia LUO,Peng MIAO,Yusheng TANG,Jie KONG
MOE Key Laboratory of Material Physics and Chemistry under Extraordinary,Shaanxi Key Laboratory of Macromolecular Science and Technology,School of Chemistry and Chemical Engineering,Northwestern Polytechnical University,Xi’an 710072,China
KEYWORDS Electromagnetic wave absorption;High temperature resistance;Metal-organic framework;Polymer-derived ceramics;Siliconboron carbonitride
Abstract Electromagnetic wave absorbing materials at high-temperature are urgently needed for stealth aircrafts or aero-engines worked in harsh environments.In this contribution,cobaltcontaining siliconboron carbonitride (MOF/SiBCN) nanomaterials were prepared by pyrolyzing metal–organic framework,i.e.cobalt 2-methylimidazole (ZIF-67),and hyperbranched polyborosilazane.The rhombic dodecahedral ZIF-67 and cobalt element promoted in situ formation of dielectric loss phases,including SiC nanocrystals,CoSi nanocrystals and turbostratic carbons.The ZIF-67/SiBCN nanomaterials showed excellent microwave absorption both at room and elevated temperature.The minimum reflection coefficient (RCmin) was -51.6 dB and effective absorption bandwidth (EAB) is 3.93 GHz at room temperature.At an elevated temperature of 600°C,the RCmin reached -30.29 dB and EAB covered almost the whole X-band (3.95 GHz,8.45–12.4 GHz).The ZIF-67/SiBCN nanocomposites are promising and useful platform for microwave absorbing materials at high-temperature.It may shed light on the downstream applications in designing next generation areo-engines and stealth aircrafts.
1.Introduction
With diffusion of Electromagnetic Wave (EMW) radiation,high-performance Microwave Absorbing Materials (MAMs)are the highly needed in the fields of information security,electronic safety,healthcare and defense security.1–5Nowadays,MAMs applied at ambient temperature have been widely investigated and used as bulk materials,composites or coatings,including traditional magnetic materials,carbon materials,ceramics,porous magnetite/carbon composites and two-dimensional MXenes or MoS2.6–15The stealth aircrafts or aero-engines with high speed are working in high temperature and oxidation environments,so the electromagnetic wave absorption and stealth performance at a high temperature more than 600°C is a cutting-edge topic.However,carbon-based MAM are not resistant to oxidation and high temperature.The Curie temperature of magnetic-loss MAMs is relatively low.When the environment temperature exceeds their Curie temperature,the magnetic materials are transformed into a paramagnetic material and lose EMW attenuation ability.Thus highly efficient MAMs under hightemperature and harsh environments need further attentions.
Ceramic matrix composites are widely used in harsh environment due to their high temperature resistance,good chemical,oxidation resistance and mechanical properties.15Polymer-Derived Ceramics (PDCs) has become a popular method to prepare ceramic-based MAMs due to designability of precursor structure and convenience of processing.16–18However,due to lack of high-dielectric nanocrystals,the EMW attenuation ability of pure PDCs is not desirable.PDCs-SiC by pyrolyzing polycarbosilane precursor only possesses average reflection coefficient (RC) of -9.9 dB in X-band(8.2–12.4 GHz).19The PDC-SiBCN ceramic pyrolyzed from polyborosilazane has a minimum RC with-15.8 dB and an effective absorption bandwidth (EAB,RC<-10 dB) of 2.6 GHz.20Nowadays,two methods can be used to improve absorption,i.e.adding high dielectric components or promoting in situ formation of nanocrystals.When high dielectric components (multi-walled carbon nanotubes,graphene or nano-SiC) are introduced in to the PDCs,the EMW absorption property will be significantly improved.21–23In previous researches,the carbon-rich benzene functional groups in precursor improved EMW absorption of pyrolyzed ceramics.16Another method is introducing transition metals of Fe,Co and Hf into precursors.18,24–26The transition metals can promote crystalline behavior of free carbons and Si-C-O components and subsequently tune the ceramic microstructure,dielectric property and EMW absorption.
Meta-Organic Framework (MOF) and derived materials have been reported as a new kind of MAMs,such as Co/TiO2nanocomposites,Co/C nanocomposites,porous carbon-wrapped Ni composites and magnetic porous carbon nanorods.27–30The materials are usually possessing a high specific surface area and good electrical conductivity.Under electromagnetic field,they show both magnetic,dielectric attenuation and excellent EMW absorption property.But MOF-derived materials are not resistant to high temperature and oxidation.If PDC and MOF are combined to design a new type of MAMs,the advantages will be significant.Low dielectric pure PDC facilitates the incidence of WMW into material.The MOF can induce crystallization of PDC and interfaces,which are conducive to microwave attenuation.
In this contribution,we introduced cobalt based metaorganic framework,cobalt 2-methylimidazole (ZIF-67),to siliconboron carbonitride (SiBCN) ceramic matrix to prepare MAMs with excellent microwave absorption at both room and high temperature.The cobalts in ZIF-67 can promote the formation of SiC,CoSi nanocrystals and turbostratic carbons,which is beneficial to EMW attenuation.The ZIF-67/SiBCN nanocomposites possessed a minimum RC of-51.6 dB and an EAB of 3.93 GHz at room temperature.When the measuring temperature is elevated at 600°C,the minimum RC reached -30.29 dB and EAB was 3.95 GHz(8.45–12.4 GHz),almost covering the whole X-band.The ZIF-67/SiBCN nanomaterials with excellent microwave absorption at high temperature possess great potential in high temperature and harsh environments.
2.Experimental
2.1.Materials
The compounds of dichloromethylvinylsilane (DCMVS),dichloromethylsilane (DCMS),borane dimethylsulfide complex (2.0 M in tetrahydrofuran) and hexamethyldisilazane(HMDZ) were purchased from Alfa Aesar China (Tianjin,China).Cobalt nitrate hexahydrate(99.99%)was bought from Macklin Co.(Shanghai,China) and 2-methylimidazole (99%)was purchased from TCI Co.(Shanghai,China).Anhydrous tetrahydrofuran(THF)was freshly distilled under reflux using sodium/benzophenone.The other reagents were analytical grade and used without further purification.
2.2.Synthesis of hyperbranched polyborosilazane
The synthesis was conducted using standard Schlenk technique.The hyperbranched polyborosilazane (hb-PBSZ) was prepared as described in our previous work.31First,a 250 mL Schlenk flask charged with DCMVS (23.56 mL,175 mmol) was cooled down to 0°C.Borane dimethylsulfide solution (29 mL,58 mmol) was added with an argon-purged syringe and stirred at ambient temperature for 24 h.Second,the DCMS (6.33 mL,59 mmol) and HMDZ (70 mL) was added into the mixture in flask.The temperature was heated to 180°C and kept for 4 h under reduced pressure for reaction.The trichlorosilane,byproducts and solvent were removed under reduced pressure.Finally,the product of hb-PBSZ was obtained as yellow glassy solids at room temperature.
2.3.Preparation of ZIF-67
The zeolitic imidazolate framework ZIF-67 was synthesized according to references.32,33First,1.5 mmol of cobalt nitrate was dissolved in 12 mL deionized water and 67 mmol dimethylimidazole was dissolved in 80 mL deionized water,respectively.Second,the two solutions were mixed and stirred vigorously for 6 h.After stirring 24 h,the purple precipitates were collected by centrifugation.After three times wash using methanol as eluent and dry for 24 h,the as-prepared ZIF-67 was obtained as purple solid.
2.4.Preparation of ZIF-67/SiBCN ceramics
The hb-PBSZ was dissolved in anhydrous THF to form solution,subsequently,the ZIF-67 with a mass fraction of 0.5%,1%and 2%was added into the solution under an argon atmosphere,respectively.After stirring and evaporation of solvent,the mixture was cross-linked at 400°C for 2 h in an argon atmosphere.The cross-linked precursors were ball milled and passed through a 100 mesh sieve.The pure fine powders were pressed into a green body with a dimension of 70 mm×15 mm×4 mm under a pressure of 70 MPa.Then,the green bodies were transferred into tube furnace (GSL-1700X,Kejing New Mater,Ltd.,Hefei,China) for pyrolysis under an argon atmosphere without pressing.The pyrolysis was performed at 400°C(heating rate,2 K/min,holding time,2 h) and followed at 1000°C (heating rate,2 K/min,holding time,4 h).Finally,the formed ZIF-67/SiBCN ceramic specimen were annealed at 1100°C,1200°C,1300°C and 1400°C in argon atmosphere,respectively.And the pure monolithic ceramic was polished to the sizes of 22.86 mm×10.16 mm×3 mm before dielectric property measurement.
2.5.Characterization
Thermogravimetric analysis (TGA) and mass spectrometry(MS)analysis was performed on a simultaneous thermal device(STA,449C Jupiter,Netzsch,Gera¨tebau GmbH,Selb,Germany) coupled with a quadrupole mass spectrometer(QMS,403C Ae¨olos,Netzsch,Germany).The high resistance to temperature of ceramics was measured under a steady flow of argon and air (60 mL/min) with a heating rate of 10 K/min at a range from ambient temperature to 1400°C.Transmission electron microscopy (TEM,FEI Tecnai G2 F30) and energydispersive X-ray spectroscopy (EDS) was operated at 200 kV,coupled with electron diffraction analysis.Powder X-ray diffraction (XRD) measurement at room temperature was performed using a SmartLab-9 diffracmeter (Rikagu Corp.) with Cu Kα radiation from 10° to 80°.Raman Spectroscopy studies were performed using a Raman Microprobe Instrument(Invia,Renishaw,USA)with 514.5 nm Ar+laser excitation.Imaging X-ray photoelectron spectroscopy (XPS)measurements were conducted on a K-Alpha spectrometer(Axis Ultra,Kratos Analytical Ltd.,U.K.) and the core level spectra were measured using a monochromatic Al Kα X-ray source (hν=1486.7 eV).The analyzer was operated at 23.5 eV pass energy and the analyzed area was 200–800 μm in diameter.The lowest energy resolution is 0.48 eV (Ag 3d5/2).Binding energy was referenced to the adventitious hydrocarbon C1s line at 285.0 eV.The relative complex permittivity (ε=ε′-jε′′) of ceramic monoliths with a dimension of 22.86 mm×10.16 mm×2.6 mm was measured by a vector network analyzer (VNA,MS4644A,Anritsu,Atsugi,Japan)using wave-guide method in X-band.Based on the singlelayer plate,the power transmission coefficient was calculated.The Reflection Coefficient (RC) can be calculated using the measured relative complex permeability and permittivity from 25°C to 600°C in air atmosphere.During the measurement of complex permittivity and permeability,the sample was placed vertically in the center of test chamber,heating by an inner heater at 10°C/min.During the procedure,a period of 20 min was required for the system to stabilize when each set-point temperature was achieved in order to ensure the accuracy of measurement.
3.Results and discussion
3.1.Formation and microstructure of ZIF-67/SiBCN nanocomosites
The preparation route of ZIF-67/SiBCN nanomaterial is shown in Fig.1.The ZIF-67 was prepared from cobalt nitrate and 2-methylimidazole.As shown in Fig.2(a),the rhombic dodecahedral morphology was observed.The powder XRD pattern (Fig.2(b)) shows the pure ZIF-67 is a type of Cobased MOF with a unit cell containing two 2-methylimidazole (mIM) linkers per cobalt (Co(mIM)2).34The two-dimensional element image (Fig.2(c)–(e)) indicates the existence of cobalt,carbon and nitrogen uniformly dispersed in ZIF-67.Due to the usage of DCMVS and DCMS for synthesis of hb-PBSZ,the ZIF-67/ hb-PBSZ can be cross-linked via the dehydrogenation coupling of Si-H bonds and hydrosilylation addition of vinyl group and Si-H bond.Under pyrolysis at 1000°C,the thermolytic degradation of organic groups of both hb-PBSZ and ZIF-67 was accompanied by the evolution of H2,CxHy,NHx,H2O,COxand oligomer fragments (Fig.3).After annealing at 1100–1300°C,the ZIF-67/SiBCN nanomaterials were generated as shown in Table 1.According to the mass fraction of ZIF-67 and annealing temperature,the SiBCN monolithic ceramics with Co/C,SiC and graphitized carbons were labeled as C1-C7.

Fig.2 SEM image,TEM image,XRD patterns and element maps of ZIF-67.

Fig.3 TGA-mass spectrum curves of hb-PBSZ measured at a scanning rate of 10 K/min under an argon atmosphere.

Table 1 Composition of ZIF-67/SiBCN nanocomposites derived from preceramic precursors.
The atomic composition of ZIF-67/SiBCN ceramic was determined by XPS (Fig.4) and EDS (Fig.6(g)–(k)).The results reveal silicon,cobalt,boron,carbon,nitrogen,and oxygen elements in ceramic,which formula is listed in Table 1.It is obviously that the value of cobalt/silicon is increased with the increase of mass fraction of ZIF-67 and hb-PBSZ.As shown in Fig.4,for C5,the SiC phase with a Si 2p bonding energy of 101.4 eV and C 1s bonding energy of 282.5 eV and Si3N4phase with a Si 2p bonding energy of 102.5 eV and N 1s bonding energy of 397.5 eV can be detected.35The main peak at 284.4 eV in C 1s is the typical symbol for graphitic carbons.The B 1s peak can be deconvoluted into B-C and B-N peaks.The existence of oxygen and C-O,C═O,Si-Oxare attribute to the contaminants or absorption O2in hydrophilic ceramic powders before the measurement.36Thus,the typical atomic composition of ZIF-67/SiBCN ceramics is Si1B0.26-C3.34N0.62Co0.002with SiC,CoSi nanocrystals and graphitic carbons.

Fig.4 XPS imaging spectra for the ceramic material C5 produced from the precursor P2 and pyrolyzed at 1200°C under an argon atmosphere.

Fig.6 Schematic diagram and TEM test of the proposed ZIF-67/SiBCN nanocomposites (C5 and C7).
The powder XRD analysis in Fig.5(a) and (c) was further to identify the microstructure of ZIF-67/SiBCN ceramics.The pure SiBCN ceramic (C1) pyrolyzed from hb-PBSZ without addition of ZIF-67 possesses an amorphous structure.For ZIF-67/SiBCN ceramic,it begins to crystallize after the addition of ZIF-67 and annealing over 1200°C.For C2 with low mass fraction of ZIF-67 in precursor (0.5%),there are only three week peaks indexed as β-SiC at 35.9°,60.3° and 72.3°.When the mass fraction is increased to 2%(C7),the SiC peaks become sharp and strong.At the same time,the sp2-hybridized carbons’ broad peak appeared at 26.3°.The some crystallization peaks of cobalt silicide (CoSi) also appeared.The peaks at 2θ=28.3°,40.6°,45.6°,and 50.0° are indexed as (110),(200),(210),(211) crystal face of CoSi crystals (JCPDS 50-1337),respectively.In addition,the powder XRD patterns in Fig.5(c)of C3-C6(1%ZIF-67)indicated the important effect of annealing temperature.As the annealing temperature increase,the SiC,CoSi nanocrystals and graphitic carbons were also observed,especially at 1300°C.During the annealing,the Co atoms originated from ZIF-67 can react with Si-C phase and form eutectic liquid phase together.According to the solid-liquid-solid mechanism,the Co-Si bond will be the crystal nucleus to induce the formation CoSi and SiC nanocrystals and graphitic carbons.37
The graphitic carbons were verified by Raman spectra in Fig.5(b) and (d).Two typical peaks at 1350 cm-1(D band)and 1580 cm-1(G band)were observed.For the C7 with CoSi annealed at 1200°C,a broad peak is appeared at around 2750 cm-1,which can be separated into second-order G′peak and G+D combination peak.As well known,D band is related to defect/disordered carbons and G band represents stretching vibration of sp2graphitic carbons,where the integral intensity ratio(ID/IG)can be applied to evaluate graphitization degree of carbons.38,39After being fitted by Gaussian-Lorentzian curve,the detailed information includingID/IG,peak position and full width at half maximum (FWHM) is listed in Table 2.TheID/IGof C2,C5 and C7 are 2.78,2.62 and 2.34,respectively.TheID/IGof C4-C6 decreases from 2.76 to 2.49 (Fig.5(d)).It indicates that more amorphous carbons are converting to graphitic carbons because of the existence of transition metal cobalt,CoSi and increased annealing temperature,which is in good correspondence to XRD results.
The pure SiBCN ceramic C1 is amorphous.For the ZIF-67/SiBCN ceramic,such as C5 in Fig.6(b)–(f),the SiC nanocrystals and turbostratic carbons can be found around CoSi in SiBCN matrix.For C7 with a high mass fraction of cobalt presented in Table 1,a large area of SiC nanocrystals and turbostratic carbons were formed as shown in Fig.6(l)–(n).As demonstrated in Fig.1,the introduction of ZIF-67 in hb-PBSZ really changed the composition and microstructure of pyrolyzed SiBCN ceramics.The rhombic dodecahedral provide enough toughing face with SiBCN,so only a low mass fraction of ZIF-67 can induce numerous SiC,CoSi nanocrystals and graphitic carbons in amorphous SiBCN matrix.It can image that these regular nanocrystals and graphitic car-bons will indeed affect dielectronic property and prefer EMW absorbing performance.
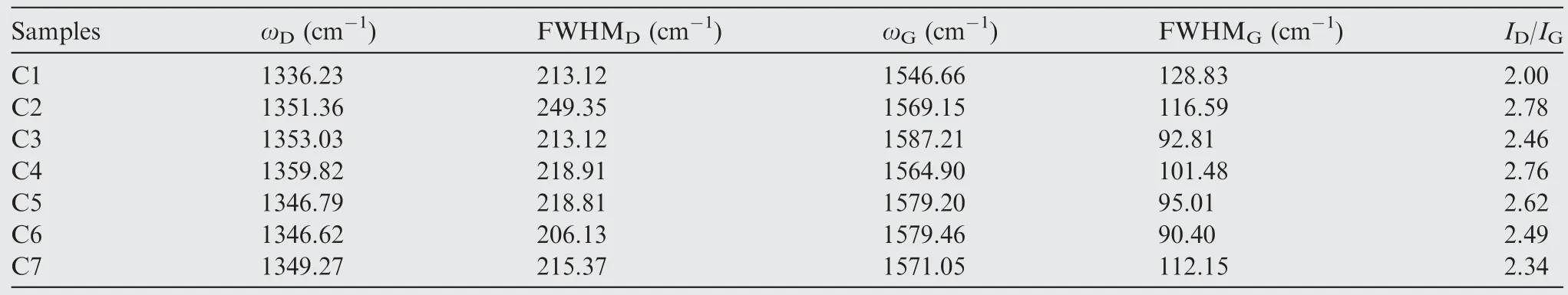
Table 2 Raman spectra parameters of ZIF-67@SiBCN ceramics derived from polymeric precursors.
3.2.Dielectric property and microwave absorption of ZIF-67/SiBCN nanocomosites
Since only very low amount of cobalt was among in ceramic,their magnetic permeability is very weak.So this ceramic is actually a dielectric-loss material.The complex permittivity (ε=ε′-ε′′) and dielectric loss tangentare two main parameters to characterize dielectric property and guide design.40The real permittivity (ε′),imaginary permittivity (ε′′) and dielectric loss tangent (tanδ) of ZIF-67/SiBCN ceramic monoliths (C1,C2,C5 and C7) is shown in Fig.7(a)–(c).

Fig.7 Dielectric properties and microwave absorption properties of ZIF-67/SiBCN nanocomposites(C1,C2,C5 and C7)with different contents of ZIF-67 annealed at 1200°C.
For these four ceramic monoliths with increased cobalt content,the ε′is in the range of 4.57–4.45,5.90–5.42,9.75–8.71 and 11.03–9.03 and the ε′′is in the range of 0.17–0.14,2.07–1.67,5.66–4.84 and 6.94–5.40,respectively.The pure amorphous SiBCN phase has a low complex permittivity,which facilitates the incidence of WMW into material.As a result,the tanδ is increased from 0.32 to 0.63.Based on the metal backplane model,the RC of can be can be calculated according to Eqs.(1)–(2):15

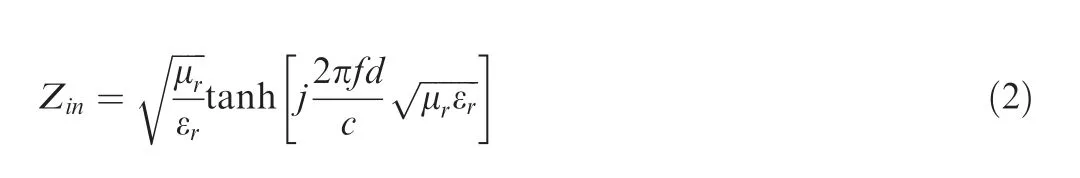
whereZinis normalized input impedance,μrand εrare permeability and permittivity of materials,f,dandcrepresent microwave frequency (Hz),thickness (m) and velocity (m/s) of EMW in vacuum,respectively.When RC is less than-10 dB,it means that at least 90% of EMW energy will be absorbed.
The RC value of ZIF-67/SiBCN ceramic monoliths annealed at 1200°C (C1,C2,C5 and C7) is shown in Fig.7(d).As expected,the RC is near to zero in X-band for pure SiBCN ceramics pyrolyzed from hb-PBSZ.It is actually an EMW transmission material.After the introduction of cobalt in SiBCN by adding ZIF-67,the EM absorbing property was effectively enhanced.For C2 derived from hb-PBSZ with 0.5%ZIF-67,it possesses a RCminof -14.40 dB and an EAB of 2.96 GHz (9.44–12.4 GHz) at a thickness of 3.0 mm.When the addition of ZIF-67 is increased to 1% or 2%,C5 shows a RCminof -16.94 dB at a thickness of 2.6 mm and C7 possesses an enhanced EAB of 3.36 GHz (9.04–12.4 GHz) at a thickness of 2.3 mm.The EMW absorbing property of ZIF-67/SiBCN ceramics is closely related to microstructure.As mentioned above,the XRD,Raman and TEM results indicate that the introduction of ZIF-67 leads to the formation of SiC,CoSi nanocrystals and graphitic carbons.They are high dielectric phases to enhance dielectric constant.41When the material is placed in EMW field,the high dielectric phases are polarized centers and generate dipole polarization relaxation.In addition,the high dielectric phases are in situ formed in amorphous SiBCN matrix.The nanograin boundaries and interfaces between high dielectric phases and matrix will result in multiple reflections and interfacial polarization relaxation effect.42Thus,more EM energy was dissipated and EMW absorption was enhanced.
Besides EM energy dissipation,the impedance matching is also key factor to the incidence of EMW at interface.41The high ε′can cause reflection of EMW on the surface and too low ε′′leads to insufficient attenuation.In order to further improve EM absorption,the effect of annealing was studied.The complex permittivity and RC of ZIF-67/SiBCN ceramic monoliths annealed at 1100–1300°C (C3-C6) is shown in Fig.8.The ε′,ε′′and tanδ all increase with the increased annealing temperature.When the annealed temperature is 1150°C as shown in Fig.8(e)–(f),the EM absorbing property of C4 is excellent with a RCminof-51.6 dB(9.38 GHz)and an EAB of 3.93 GHz (8.47–12.4 GHz) at a thickness of 3.0 mm.When the annealing temperature is elevated,the ε′is high without benefit to impedance matching,so the EAB is no more than 2.9 GHz (8.2–11.1 GHz) for C5 or C6.As mentioned above,the annealing promoted the formation of SiC nanocrystals and turbostratic carbons.More nanograin boundaries between nanocrystals and amorphous phase result in interfacial polarization and electric dipole polarization.The polarization and relaxation greatly improve the EMW attenuate.41Both considering impedance matching at interface and EM energy dissipatin inner material,the C4 ceramic (1 % ZIF-67 addition &annealing at 1150°C) exhibits the best absorption performance,i.e.a RCminof -51.6 dB and an EAB of 3.93 GHz,covering almost the whole X-band.

Fig.8 Dielectric properties and microwave absorption properties of ZIF-67/SiBCN nanocomposites(C3-C6)with 1%ZIF-67 annealed in the temperature range of 1100–1300°C.
3.3.EMW absorption at high-temperature for ZIF-67/SiBCN nanocomosites
To achieve the high-temperature EMW absorbing materials,the high temperature resistance is first challenge.The thermogravimetric curves of C4 ceramic during 50–1400°C under argon or air atmosphere are shown in Fig.9.In argon atmosphere,there is no weight loss until 1400°C,indicating excellent thermal stability.In air atmosphere,it is stable before 1100°C.Between 1100–1400°C,only 3% weight increase was found because of the formation of silicon dioxide on the surface of ceramic.In turn,it can prevent the ceramic from being further oxidized.43,44So the ZIF-67/SiBCN ceramics possess excellent high-temperature resistance in either argon or air atmosphere.Next,their EMW absorption of representative C4 ceramic at an elevated temperature of 100–600°C was analyzed as presented in Figs.10 and 11.

Fig.9 TG curves of C4 measured at a scanning rate of 10 K/min under argon atmosphere and air atmosphere.
For C4 ceramic,when the test temperature increases from 100°C to 600°C,the RCminis decreased from -15.51 dB to-30.29 dB and the EAB is increased from 2.73 GHz to 3.95 GHz.At 600°C,the thickness for best absorption is also decreased from 3.2 mm to 2.6 mm.The best EMW absorption performance is that RCminreaches-30.29 dB and EAB covering the whole X-band (3.95 GHz,8.45–12.4 GHz) at a thickness of 2.6 mm.It is unexpected that the EMW absorption is becoming better when the test temperature is elevated.It is depended on the dielectric property variation with temperature.The dependence of real permittivity,imaginary permittivity and loss tangent on temperature is described in Fig.12.When the test temperature increases from 100°C to 600°C,the ε′increases from 6.78-6.37 to 8.02–7.49 and the ε′′increases from 2.37-2.10 to 3.77–3.51.In another word,for every 100°C increases,the ε′and ε′′is increased as about 0.25 and 0.27,respectively.The dependence of complex permittivity on temperature can be explicated by the Debye theory45:

Fig.12 Dielectric properties of SiBCN nanocomposite (C4) measured at elevated temperature of 100–600°C.

where ω is the angular frequency,τ(T) is temperature dependent relaxation time,εsis static permittivity,ε∞is the relative dielectric permittivity at high frequency limit,σ(T) is temperature-dependent electrical conductivity and ε0is the dielectric constant in vacuum.
In ZIF-67/SiBCN ceramic,the SiBCN matrix shows semiconductor behavior and its σ(T) is increased with the rise of temperature.46So the ε′′will be increased.On the other hand,since various nanocrystals and graphitic carbons in situ formed in SiBCN,positive interfacial charges and dipoles can generate on nanoboundaries or in nanocrystals under alternating electromagnetic field.It leads to interfacial polarization,associated relaxation and electric dipole polarization.At a high temperature,the positive charges and dipoles will shorten τ(T)and lead to the increase of ε′.47Although the ε′is enhanced at 600°C,it is not high enough to harm the impedance matching between ceramic and air.However,the increased ε′′is really helpful for the energy dissipation of incident EMW inner ceramic.So the synergic effects of impedance matching and energy dissipation induced the highly efficient EMW absorbing property even at high temperature.
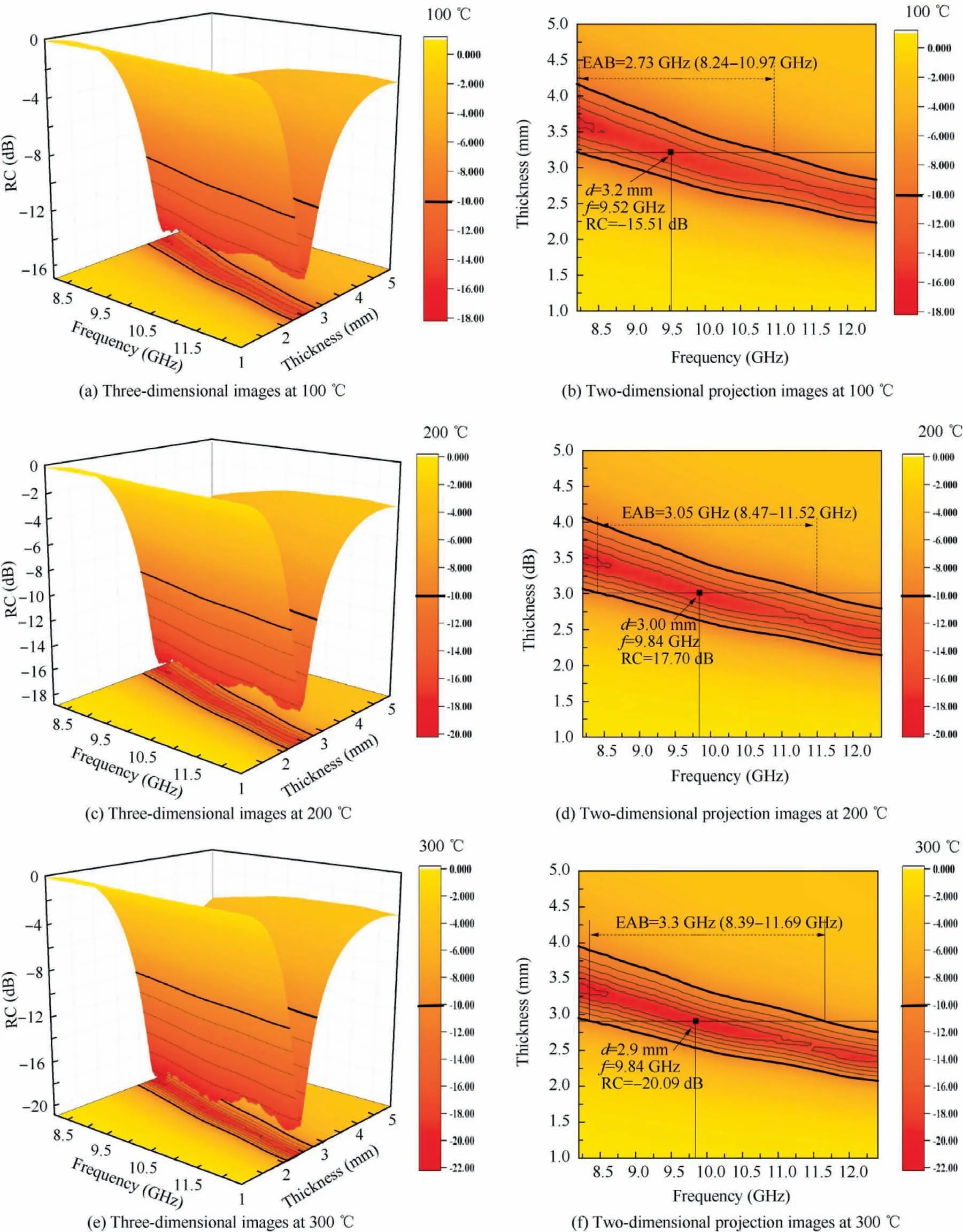
Fig.10 Three-dimensional images and two-dimensional projection image of calculated theoretical RC value of sample C4 measured at 100,200 and 300°C,respectively.
4.Conclusions

Fig.11 Three-dimensional images and two-dimensional projection image of calculated theoretical RC value of sample C4 measured at 400,500 and 600°C,respectively.
(1) ZIF-67/SiBCN nanocomposites containing cobalts were prepared using PDC method.The introduction of ZIF-67 into preceramic precursor of hyperbranched polyborosilazane promoted in situ formation of dielectric loss phases under high temperature pryrolysis,including SiC nanocrystals,CoSi nanocrystals and turbostratic carbons.
(2) The ZIF-67/SiBCN nanocomposite with 1 wt% ZIF-67 annealed at 1150°C exhibited excellent EM absorption property both at room and elevated temperature.The minimum RC was -51.6 dB and EAB is 3.93 GHz at room temperature.
(3) At an elevated temperature from 100°C to 600°C,the EMW absorption property was becoming better.At 600°C,the RCminreached-30.29 dB and EAB covered almost the whole X-band (3.95 GHz,8.45–12.4 GHz).
Overall,the ZIF-67/SiBCN nanocomposites with excellent absorption property both at room and elevated temperature can give a promising and useful platform for microwave absorbing materials with high-temperature resistance,possessing great potential in aero-engines and stealth aircrafts in harsh environment.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgement
The authors thank the grant from the National Natural Science Foundation of China (No.21875190),Innovation Team of Shaanxi Sanqin Scholars and the Natural Science Basic Research Plan for Distinguished Young Scholar in Shaanxi Province of China (No.2018JC-008).
杂志排行
CHINESE JOURNAL OF AERONAUTICS的其它文章
- Parameter effects on high-speed UAV ground directional stability using bifurcation analysis
- Supersonic flutter control and optimization of metamaterial plate
- Review of in-space assembly technologies
- Utilisation of turboelectric distribution propulsion in commercial aviation:A review on NASA’s TeDP concept
- The influence of inlet swirl intensity and hot-streak on aerodynamics and thermal characteristics of a high pressure turbine vane
- Full blended blade and endwall design of a compressor cascade
