Mitigation of cholestasis-associated hepatic and renal injury by edaravone treatment:Evaluation of its effects on oxidative stress and mitochondrial function☆
2021-10-11MohmmMehiOmmtiHnieAttriAsmSivshpourMrziehShfghtNegrAzrpirHstiGhffriLeilMoeziRezHeiri
Mohmm Mehi Ommti ,Hnie Attri ,Asm Sivshpour ,Mrzieh Shfght ,Negr Azrpir ,Hsti Ghffri ,Leil Moezi ,Rez Heiri
a.College of Life Sciences,Shanxi Agricultural University,Taigu,Shanxi,China
b.Department of Pharmacology and Toxicology,School of Pharmacy,Shiraz University of Medical Sciences,Shiraz,Iran
c.Transplant Research Center,Shiraz University of Medical Sciences,Shiraz,Iran
d.Department of Veterinary Sciences,Islamic Azad University,Urmia Branch,Urmia,Iran
e.Pharmaceutical Sciences Research Center,Shiraz University of Medical Sciences,Shiraz,Iran
f.Department of Pharmacology,School of Medicine,Shiraz University of Medical Sciences,Shiraz,Iran
Keywords:Bile acids Cholestasis Cirrhosis Nephropathy Oxidative stress Renal failure
ABSTRACT Background and aim:The liver is the primary organ affected by cholestasis,and complications such as renal injury,renal failure,and the need for renal transplantation are associated with cholestatic liver disease.There is substantial evidence indicating that reactive oxygen species (ROS) and mitochondrial impairment are fundamental mechanisms underlying cholestasis-induced hepatic and renal injury.Edaravone (EDV) is a potent radical scavenger and antioxidant that may prevent oxidative stress and improve impaired mitochondrial function in various diseases.This study was performed to evaluate the effects and mechanisms of action of EDV on hepatic and renal injury in an animal model of cholestasis.Methods:Rats subjected to bile duct ligation (BDL) were treated with EDV 1 or 10 mg/kg/day intraperitoneally for 14 consecutive days.Biomarkers of oxidative stress and mitochondrial impairment in the liver and kidney were assessed in EDV-treated and untreated rats with cholestasis.Results:Significant increases in tissue ROS level,lipid peroxidation,protein carbonylation,and oxidized glutathione level were detected in rats subjected to BDL.Additionally,significant decreases in tissue glutathione level and antioxidant capacity were observed in the hepatic and renal tissues of rats with cholestasis.Markers of mitochondrial impairment,including mitochondrial depolarization,lipid peroxidation,mitochondrial permeabilization,depleted adenosine triphosphate content,and decreased dehydrogenase activity,were also detected in rats subjected to BDL.Furthermore,portal inflammation,necrosis,and tissue fibrosis were detected in the liver and significant tubular atrophy and interstitial inflammation,as well as fibrotic lesions,were detected in the kidneys of rats with cholestasis.EDV treatment significantly mitigated cholestasis-associated hepatic and renal injury.Conclusions:The antioxidative properties of EDV and its positive effects on the indices of mitochondrial function may be critical factors contributing to protection against cholestasis-associated hepatic and renal injury.
1.Introduction
Cholestatic liver disease is a severe clinical condition with a wide range of etiologies.1-3Infectious hepatic disorders,alcoholism,gallstones,and xenobiotics (drugs and toxins) are factors that promote cholestasis.1-3Regardless of its etiology,cholestasis is typically associated with the accumulation of potentially cytotoxic molecules such as hydrophobic bile acids in tissues and serum.1-3Inflammation,necrosis,changes in sinusoid morphology,and tissue fibrosis occur in the liver of patients with cholestasis.Prolonged cholestasis could lead to cirrhosis and liver failure.1-3Although the liver is the primary organ affected by cholestasis,other organs,including the kidneys,are severely affected.3-5Cholestasis-induced renal injury,also known as cholemic nephropathy (CN),4,6,7can result in renal failure and the need for organ transplantation.8-10Therefore,there is an urgent need for the development of effective therapeutic strategies against cholestasis-associated complications.
Previous investigations have revealed that oxidative stress plays a pathogenic role in cholestasis-induced organ injury.11,12As noted previously,the accumulation of cytotoxic molecules such as hydrophobic bile acids may be the critical event underlying the pathogenesis of organ injury associated with cholestasis/cirrhosis.13,14Hydrophobic bile acids can directly disrupt cellular targets such as membrane lipids because of their surfactant nature.13-15An elevated level of reactive oxygen species (ROS),disruption of membrane structures,oxidative damage of cellular proteins,and depletion of tissue antioxidant capacity have been reported in models of cholestatic liver and kidney disease.16-18In the present study,several biomarkers of oxidative stress were evaluated in hepatic and renal tissues of a rat model of cholestasis.
Mitochondrial impairment is another key mechanism of cellular injury identified in hepatic and renal tissues in experimental models of cholestasis.19-24Mitochondrial impairment can enhance oxidative stress.25Mitochondrial impairment is associated with the release of cytotoxic mediators,cell death,and ultimately,organ injury.In the present study,various indices of mitochondrial function were assessed in hepatic and renal tissues to reveal potential mechanisms of action of edaravone (EDV) in a rat model of cholestasis.
EDV (3-methyl-1-phenyl-2-pyrazoline-5-one;Radicava®) is a radical scavenger widely investigated for its pharmacological properties.26,27EDV can function as a potent antioxidant molecule in biological systems and has profound effects on cerebral ischemia,26,28,29with promising evidence of therapeutic efficacy in both animal models of stroke and human subjects.29-33Radicava®and Radicut®are brand names of EDV that are currently in clinical use for the treatment of amyotrophic lateral sclerosis and stroke.34,35
EDV has the capacity to protect organs against oxidative stress via several mechanisms.As noted previously,EDV is a potent radical scavenger that directly reacts with free radicals.36EDV has been found to effectively enhance cellular enzymatic antioxidant defense systems.37,38The positive effects of EDV on mitochondria and its role in suppressing mitochondria-mediated ROS formation have been reported previously.39-42EDV also significantly prevents mitochondria-mediated apoptosis and cell death and enhances cellular energy metabolism by regulating mitochondrial function.26,39-43Based on these findings,we hypothesized that EDV might provide protection against cholestasis-induced oxidative stress,mitochondrial impairment,and hepatic and renal injury.
In this study,rats subjected to bile duct ligation(BDL)were used as an animal model of cholestasis.44Rats underwent BDL and were treated with EDV (1 or 10 mg/kg/day via the intraperitoneal (i.p.)route) for 14 consecutive days.Serum and urine biomarkers of organ injury,biomarkers of oxidative stress in hepatic and renal tissue,indices of mitochondrial function,and tissue histopathology were monitored.
2.Materials and methods
2.1.Materials
Trichloroacetic acid,sodium acetate,potassium chloride,thiobarbituric acid (TBA),dithiothreitol (DTT),sucrose,mannitol,ethylenediaminetetraacetic acid,calcium anhydride,phosphoric acid,3-(N-morpholino)-propanesulfonic acid(MOPS),glacial acetic acid,2,4,6-tri (2-pyridyl)-s-triazine (TPTZ),and 2-amino-2-hydroxymethyl-propane-1,3-diol-hydrochloride (Tris-HCl) were obtained from Merck(Darmstadt,Germany).EDV,rhodamine 123,dichlorodihydrofluorescein diacetate,acetonitrile (high performance liquid chromatography(HPLC) grade),oxidized glutathione(GSSG),methanol (HPLC grade),and reduced glutathione (GSH)were purchased from Sigma-Aldrich(St.Louis,MO,USA).Assay kits for determination of serum and urine biomarkers of organ injury,including alanine aminotransferase (ALT),lactate dehydrogenase,alkaline phosphatase (ALP),aspartate aminotransferase (AST),gamma-glutamyl transferase (γ-GT),protein,creatinine (Cr),glucose,blood urea nitrogen,and bilirubin,were obtained from Pars Azmoon®(Tehran,Iran).Urine and serum bile acid levels were determined using an EnzyFluo™kit (Product No.ABIN5691833).
2.2.Animals
Male Sprague Dawley rats(N=24;weighing 200-250 g)were obtained from Shiraz University of Medical Sciences,Shiraz,Iran.Rats were housed in cages with hardwood bedding in a standard environment ((23 ± 1)°C,12-h light:12-h dark photoperiod,and 40%relative humidity).Rats were allowed free access to a standard rodent chow diet (RoyanFeed®,Isfahan,Iran) and tap water.All experiments were performed in conformity with guidelines for the care and use of experimental animals approved by the ethics committee of Shiraz University of Medical Sciences,Shiraz,Iran(#97-01-36-18730).
2.3.BDL procedure and treatment with EDV
Animals were anesthetized using a mixture of xylazine 10 mg/kg and ketamine 80 mg/kg administere.i.p.;an abdominal midline incision was made through the linea alba;then,the common bile duct was located and doubly ligated.44Animals were then divided equally into four groups (n=6 rats/group) as follows:(i) Sham(vehicle-treated);(ii) BDL followed by vehicle treatment;(iii)BDL+EDV (1 mg/kg/day.i.p.);and (iv) BDL+EDV (10 mg/kg/day,i.p.).Biomarkers of organ injury were assessed 14 days after the BDL procedure.45
2.4.Urinalysis and serum biochemistry
Urine samples (200 μL) were collected during animal handling and were diluted with ice-cold normal saline (200 μL).Urine samples were centrifuged (17,000×g,20 min,4°C),and the clear supernatant was used for urinalysis.The rats were then deeply anesthetized (thiopental 80 mg/kg.i.p.),and blood samples were collected from the abdominal artery and transferred to standard tubes for serum preparation.Samples were centrifuged (8000×g,10 min,4°C),and the serum was collected.A Mindray BS-200®auto analyzer and commercial kits (Pars Azmoon®,Tehran,Iran) were used to measure serum and urine biomarkers of organ injury in rats with cholestasis.
2.5.Liver and kidney histopathological alterations and organ coefficient
Hepatic and renal tissue samples were fixed in a buffered formalin solution (0.64% sodium phosphate dibasic,0.4% sodium phosphate monobasic,and 10% formaldehyde in double-distilled water;pH=7.4).Paraffin-embedded sections (5 μm) were stained with hematoxylin and eosin (H&E).Kidney and liver fibrosis were determined using Masson trichrome staining.46Bile cast nephropathy was examined using periodic acid-Schiff staining.47Samples were analyzed by a pathologist who was blinded to the treatment protocol.The weight indices(organ coefficients(OC))for each of the organs evaluated (liver,spleen,and kidney) were determined using the formula OC=(wet organ weight (g)/body weight(g)) × 100%.
2.6.Detection of ROS
Tissue levels of ROS were estimated using 2′,7′-dichlorofluorescein diacetate (DCF-DA).44Briefly,tissue samples (200 mg)were homogenized in ice-cold 40 mM Tris-HCl buffer (5 mL,pH=7.4).Samples of the resulting tissue homogenate (100 μL)were mixed with Tris-HCl buffer (1 mL) and 5 μL of DCF-DA to a final concentration of 10 μM (M :mol/L).The mixture was incubated at 37°C for 10 min in the dark.44The fluorescence intensity(FI) of the samples was measured using a FLUOstar Omega®fluorimeter(BMG,Germany;λexcit=485 nm and λem=525 nm).44
2.7.Lipid peroxidation
The levels of thiobarbituric acid reactive-substances (TBARS)were measured in renal and hepatic tissues.44Briefly,the reaction mixture consisted of tissue homogenate (500 μL of 10% w/v tissue homogenate in KCl;1.15%w/v),1 mL of TBA(0.375%,w/v),and 3 mL o.meta-phosphoric acid (1% w/v,pH=2 adjusted with HCl).Samples were mixed well and heated (45 min,100°C) in a water bath.After incubation(100°C for 45 min),the mixture was cooled and 2 mL of n-butanol was added.Samples were then vortexed and centrifuged (15,000 ×g,10 min).The absorbance of the developed color in the upper phase (i.e.,the n-butanol phase) was measured(λ=532 nm)using an EPOCH®plate reader (BioTek Inc.,Highland Park,Winooski,VT,USA).44
2.8.Tissue and mitochondrial glutathione content
The levels of GSSG and GSH in renal and hepatic tissues of rats with cholestasis as well as in isolated mitochondrial preparations were measured based on a previously described HPLC method.48,49The HPLC system consisted of a stationary phase (a 25-cm NH2column;Bischoff Chromatography,Leonberg,Germany).Buffer A(methanol:water;4:1 v/v)and buffer B(acetate buffer:buffer A;1:4 v/v)were used as mobile phases.The flow rate of the mobile phase was 1 mL/min.A gradient method with a steady increase in buffer B to 95%in 25 min was applied,and the ultraviolet(UV)detector was set at λ=254 nm.49Tissue samples(200 mg)were homogenized in 2 mL of Tris-HCl buffer (250 mM,pH=7.4,4°C),and 500 μL o.meta-phosphoric acid (50% w/v,4°C) was added.Isolated mitochondrial preparations (1 mL,1 mg protein/mL) were also treated with 100 μL of ice-col.meta-phosphoric acid.Samples were mixed well,incubated on ice for 10 min,and centrifuged (17,000 ×g,30 min,4°C).Next,1 mL of the supernatant was collected and transferred to 5-mL tubes,and approximately 300 μL of NaOH:-NaHCO3(2 M:2 M)was added.Next,100 μL of iodoacetic acid(1.5%w/v in water) was added,and samples were incubated for 1 h at 4°C in the dark followed by the addition of fluoro-2,4-dinitrobenzene (500 μL of 1.5% w/v) in absolute ethanol and further incubation at 25°C for 24 h in the dark.Finally,25 μL of samples were injected into the HPLC system for evaluation.48,49
2.9.Hydroxyproline levels in hepatic and renal tissue
Hydroxyproline levels in hepatic and renal tissues were assessed as an index of renal fibrosis using the Ehrlich reagent.50For this,500 μL of hepatic tissue homogenate (prepared in Tris-HCl buffer 250 mM,pH=7.4) was digested in 1 mL of hydrochloric acid(120°C,24 h).A 25-μL aliquot of the digested homogenate was added to 25 μL of citrate-acetate buffer (pH=6) and dried at room temperature.Then,500 μL of 56 mM chloramine-T solution was added,and the mixture was incubated at room temperature for 20 min.Then,500 μL of freshly-prepared Ehrlich reagent(15 g of pdimethyl amino benzaldehyde in n-propanol/perchloric acid;2:1 v/v) was added,and the mixture was incubated at 65°C for 15 min.After cooling,the color intensity was measured spectrophotometrically at λ=550 nm (EPOCH®plate reader,Biotek Instruments,USA).50
2.10.Protein carbonylation
Oxidative damage was evaluated by quantitative determination of protein carbonylation based on the reaction with 2,4-dinitrophenylhydrazine (DNPH).51Briefly,tissue homogenates(200 mg in a phosphate-buffered solution containing 0.1%Triton X-100,pH=7.5) were prepared and centrifuged (700×gfor 5 min).Then,500 μL aliquots of the resulting supernatants were treated with 300 μL of 10 mM DNPH dissolved in 2 M HCl.Samples were incubated for 1 h at room temperature and vortexed every 10 min.After incubation,10 μL of trichloroacetic acid(20%w/v)was added to each sample,followed by centrifugation (12,000×gfor 3 min).The supernatants were discarded,and the pellets were washed three times with 1 mL of ethanol:ethyl acetate (1:1 v/v).The samples were centrifuged after 10 min intervals,and the supernatants were discarded each time.The precipitates were then redissolved in 0.6 mL guanidine solution (6 M in 20 mM potassium phosphate adjusted to pH 2.3 with trifluoroacetic acid) for 15 min at 37°C.The solution was centrifuged at 7000×gfor 3 min.Then,2 M HCl was added to the supernatants in place of 2,4-dinitrophenylhydrazine to generate blank/controls.Absorbance was measured at λ=370 nm using an EPOCH®plate reader (Bio-Tek®Instruments,USA).51
2.11.Total tissue antioxidant capacity
Measurements of ferric reducing antioxidant power(FRAP)was used to estimate the tissue antioxidant capacity;44this assay involved the formation of a ferrous (Fe2+)-tripyridyltriazine complex (blue-colored) from the colorless oxidized ferric ion (Fe3+),which is used as an index of total antioxidant capacity.44The working FRAP reagent(freshly prepared)was composed of 25 mL of acetate buffer (300 mM,pH=3.6),2.5 mL of TPTZ (10 mM in 40 mmol/L HCl),and 2.5 mL of ferric chloride(FeCl3,20 mM).Tissue samples (200 mg) were homogenized in ice-cold Tris-HCl buffer(250 mM Tris-HCl,5 mM DTT,and 200 mM sucrose;pH=7.4).Then,100 μL of tissue homogenate was added to 900 μL of FRAP reagent.The reaction mixture was incubated at 37°C for 5 min in the dark.Samples were then centrifuged (17,000×gfor 2 min at 37°C).Absorbance was measured at λ=595 nm on an EPOCH®plate reader (BioTek®Instruments,USA).44
2.12.Isolation of mitochondria from rat hepatic and renal tissues
Samples of rat hepatic and renal tissues were washed with normal saline(0.9%w/v,4°C)and minced in ice-cold mitochondria isolation buffer (70 mM mannitol,2 mM 4-(2-Hydroxyethyl)piperazine-1-ethanesulfonic acid (HEPES),220 mM sucrose,0.5 mM EGTA,and 0.1% BSA;pH=7.4).Minced tissue was transferred into fresh isolation buffer(5 mL buffer for each 1 g of minced tissue) and homogenized.Isolated mitochondria were prepared using a differential centrifugation method.52First,unbroken cells and nuclei were pelleted at 1000×gfor 10 min at 4°C;after removal of the pellet,the supernatant was centrifuged again at 10,000×gfor 10 min at 4°C to generate a pellet enriched in mitochondria.This step was repeated at least three times by resuspending the pellet in fresh buffer.The final mitochondriacontaining pellets were re-suspended in buffer (5 mL buffer/g tissue) containing 70 mM mannitol,220 mM sucrose,and 2 mM HEPES at a pH of 7.4.The mitochondrial fractions to be used to assess mitochondrial swelling and depolarization were suspended in mitochondria swelling assay buffer(65 mM KCl,125 mM sucrose,and 10 mM HEPES;pH=7.2) or depolarization assay buffer(220 mM sucrose,10 mM KCl,5 mM KH2PO4,2 mM MgCl2,68 mM mannitol,50 μM EGTA,and 10 mM HEPES;pH=7.2),respectively.52Protein concentrations were determined using the Bradford method to standardize the obtained data.
2.13.Determination of mitochondrial ATP levels
Based on a previously reported protocol,the mitochondrial ATP level was assessed using an HPLC-based method.53Briefly,100 μL o.meta-phosphoric acid (50% w/v at 4°C) was added to isolated mitochondria(1 mg protein/mL);mitochondrial suspensions were incubated on ice for 5 min and centrifuged(30 min,17,000×g,4°C).Next,supernatants(100 μL)were treated with 15 μL of ice-cold KOH solution (1 M).Samples (25 μL) were then injected into an HPLC system that consisted of an LC-18 column(μ-Bondapak,25 cm).The mobile phase consisted of monobasic potassium hydrogen phosphate (100 mM KH2PO4;pH=7,adjusted with KOH),1 mM tetrabutylammonium hydroxide,and acetonitrile (2.5% v/v) at a flow rate of 1 mL/min;the UV detector was set at λ=254 nm.53
2.14.Lipid peroxidation in kidney mitochondria
TBARS were measured in isolated mitochondrial preparations.Previous studies reported that sucrose inhibits the lipid peroxidation test in isolated mitochondria samples.Therefore,mitochondrial preparations were washed once to remove sucrose using a MOPS-KCl solution (50 mM MOPS,100 mM KCl,4°C;pH=7.4).For this,isolated mitochondria were suspended in 5 mL of MOPSKCl buffer and centrifuged (15,000×g,20 min).The pellet was then re-suspended in MOPS-KCl buffer and evaluated using the TBARS assay.The mitochondrial suspension(1 mg protein/mL)was mixed with 1 mL of a solution containing trichloroacetic acid(15%w/v),HCl (0.24 N),500 μM Trolox,and TBA(0.375% w/v).Samples were heated in a water bath for 15 min at 100°C.Then 1 mL of nbutanol was added,and the samples were vortexed for 30 s.Samples were then centrifuged(15,000×g,10 min),and the absorbance of the n-butanol(upper)phase was measured(λ=532 nm,EPOCH®plate reader,BioTek®Instruments,USA).44
2.15.Mitochondrial depolarization
Mitochondrial uptake of the cationic dye rhodamine 123 was used as a measure of mitochondrial depolarization.54Rhodamine 123 accumulates in the mitochondrial matrix via facilitated diffusion;as this response is not observed in depolarized mitochondria,this condition will result in an increase in the amount of rhodamine 123 in the supernatant.Mitochondrial fractions(0.5 mg protein/mL in depolarization assay buffer) were incubated with rhodamine 123 (10 μM final concentration,10 min,37°C) in the dark.Samples were centrifuged (17,000×g,2 min,4°C) to precipitate the mitochondrial fraction.FI of the supernatant was measured using a multifunctional fluorimeter (FLUOstar Omega®,Germany) at λexcit=485 nm and λem=525 nm.54
2.16.Mitochondrial permeabilization and swelling
Mitochondrial swelling was assessed by monitoring changes in the absorbance of the samples at λ=540 nm.44Briefly,isolated mitochondria (0.5 mg protein/mL) were suspended in swelling buffer(125 mM sucrose,10 mM HEPES,and 65 mM KCl;pH=7.2);changes in absorbance were monitored at 25°C for 30 min using an EPOCH®plate reader (Biotek Instruments).A decrease in absorbance was indicative of an increase in mitochondrial swelling.The results are reported as maximal mitochondrial swelling amplitude(ΔOD at λ=540 nm).44
2.17.Mitochondrial dehydrogenase activity
The 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay was used for the measurement of mitochondrial dehydrogenase activity.Mitochondrial fractions (1 mg protein/mL)were incubated with 40 μL of MTT solution(5 mg/mL)and incubated at 37°C for 30 min in the dark.Samples were then centrifuged (17,000×g,10 min),and the reaction products (purple formazan crystals) were dissolved in dimethyl sulfoxide (DMSO;1 mL).Samples were centrifuged again (17,000×g,1 min),and the absorbance was measured at λ=570 nm using an EPOCH®plate reader (Biotek Instruments,USA).
2.18.Statistical methods
Data are expressed as the mean ± standard deviation (SD).Comparisons among the datasets were performed using one-way analysis of variance with Tukey’s multiple comparison.post hoctest.GraphPad Prism software(version 8.0,San Diego,CA,USA)was used for statistical analysis.Values o.P<0.05 were considered statistically significant.
3.Results
3.1.Liver,spleen,and kidney weight indices
The liver,kidney,and spleen weight indices were evaluated in the cholestasis and sham-operated groups (Fig.1).Hepatomegaly and splenomegaly were evident in rats subjected to BDL,although no significant change in the kidney weight index was observed 14 days after BDL.EDV treatment (1 and 10 mg/kg) significantly mitigated changes in the liver and spleen weight indices in rats with cholestasis,although the responses observed were not dosedependent responses to EDV.
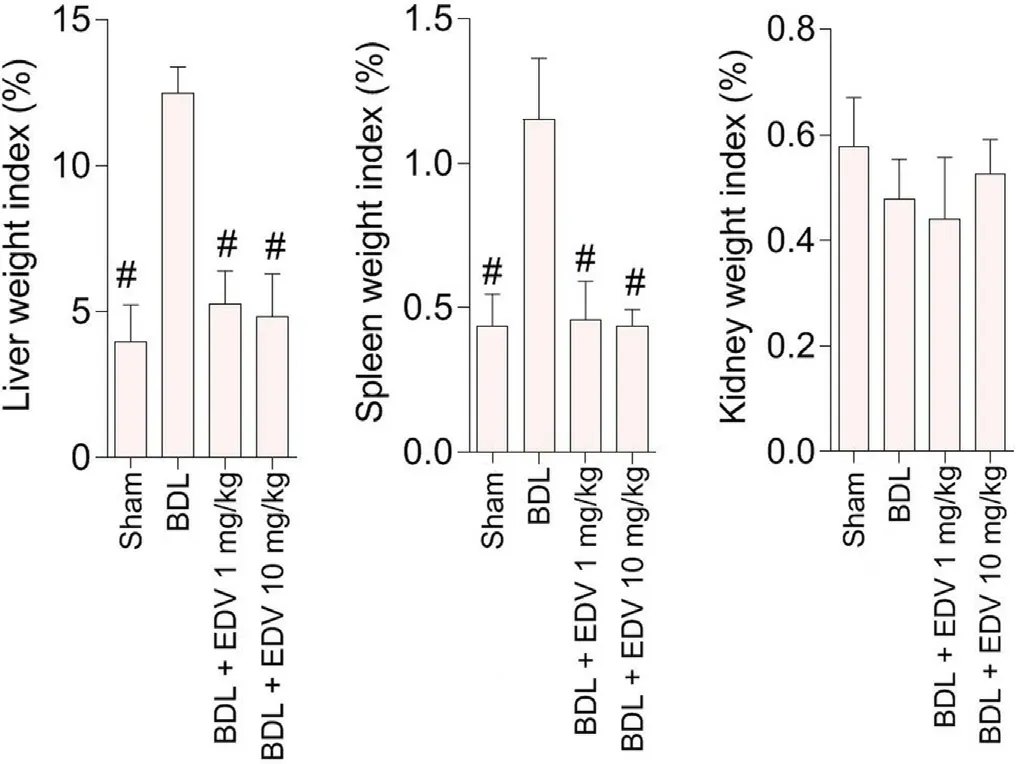
Fig.1.Organ weight indices in rats subjected to BDL.
3.2.Serum biochemistry and urinalysis
Biochemical measurements of serum biomarkers revealed significant bile duct injury,liver damage,and renal impairment in the BDL group(Fig.2).Biomarker levels of liver and kidney injury were significantly reduced by EDV treatment (1 or 10 mg/kg/day),although no significant changes in the levels of serum ALP and γ-GT were detected in EDV-treated rats.The effects of EDV treatment on serum biomarkers of organ injury were not dose-dependent.Urinalysis revealed significant increases in urine protein,glucose,γ-GT,and ALP in response to BDL together with a significant decrease in the urine Cr level (Fig.3).EDV treatment (1 or 10 mg/kg)improved renal function in rats subjected to BDL,although the effects were not dose-dependent (Fig.3).
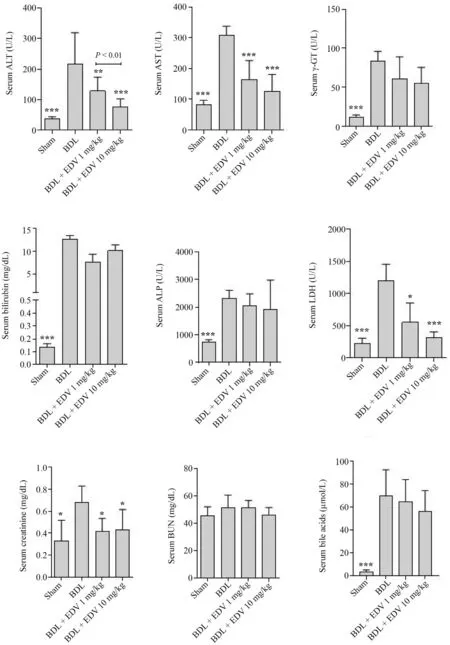
Fig.2.Serum biochemical measurements in rats subjected to BDL and treated with EDV.
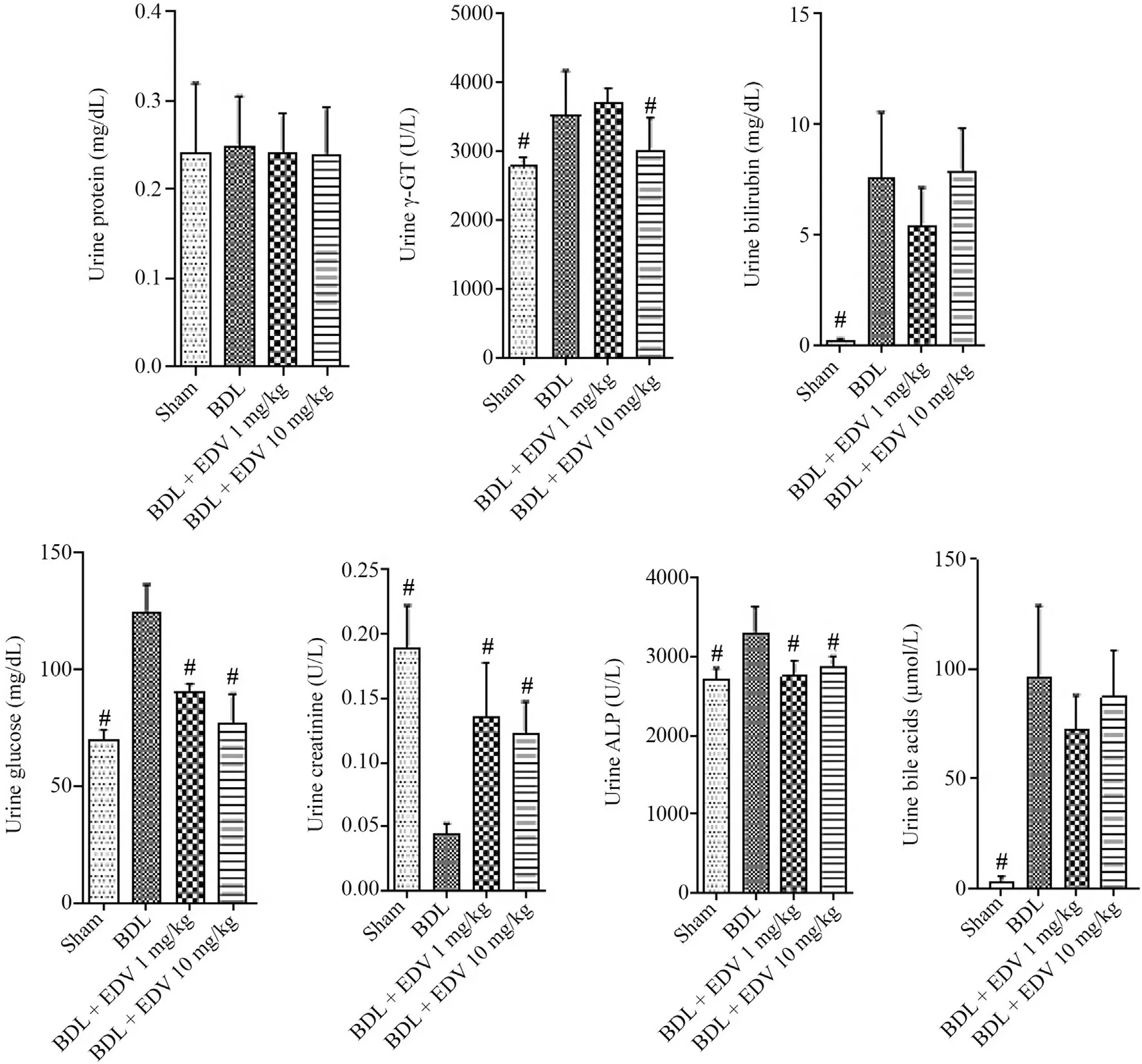
Fig.3.Urine biochemical measurements in rats subjected to BDL and treated with EDV.
3.3.Markers of oxidative stress in hepatic and renal tissues
Markers of oxidative stress were evaluated in the hepatic and renal tissues in rats subjected to BDL with or without EDV treatment(Table 1).Significant levels of ROS,lipid peroxidation,protein carbonylation,and increased GSSG content were evident in hepatic and renal tissues of rats at 14 days after BDL.Hepatic and renal stores of GSH as well as antioxidant capacity were significantly decreased in the BDL group.EDV treatment mitigated the biomarkers of hepatic and renal oxidative stress in rats subjected to BDL.
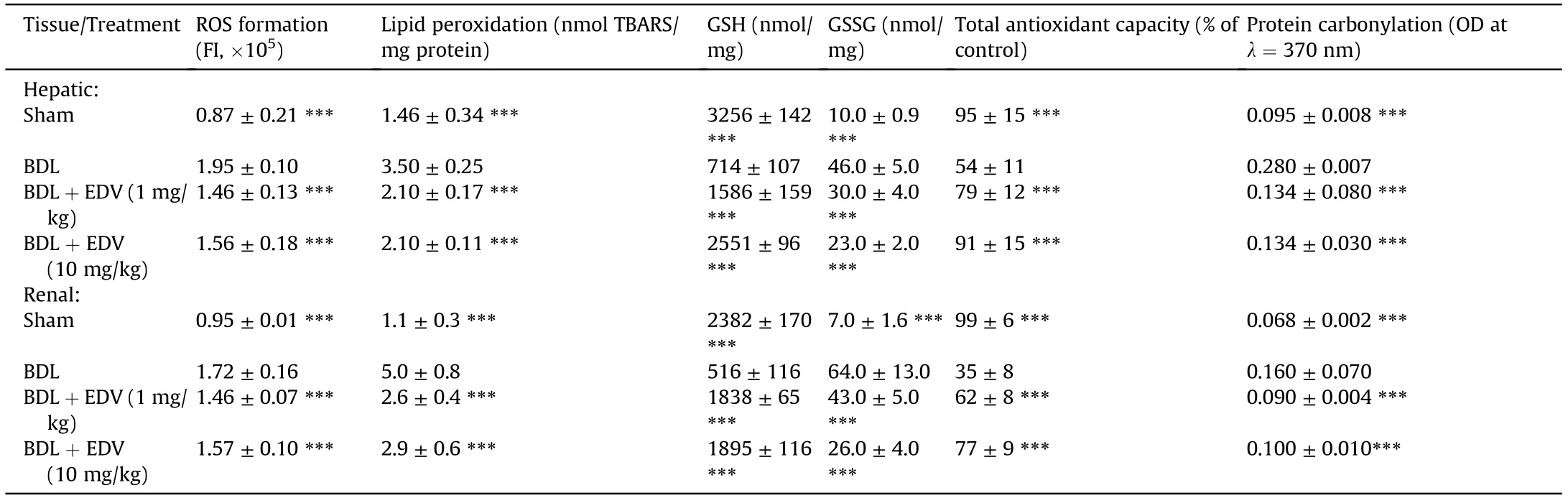
Table 1 Biomarkers of oxidative stress in hepatic and renal tissue.
3.4.Indices of hepatic and renal mitochondrial function in rats subjected to BDL
Significant decreases in mitochondrial ATP stores,dehydrogenase activity,GSH content,and GSH/GSSG ratio were noted in rats subjected to BDL(Figs.4 and 5).On the other hand,mitochondrial permeabilization,lipid peroxidation,and collapse of mitochondrial membrane potential were detected in mitochondria isolated from the livers of rats subjected to BDL(Fig.4).Mitochondrial indices in renal tissue were also significantly altered in rats subjected to BDL(Fig.5).EDV treatment improved mitochondrial indices in both hepatic and renal tissues of rats subjected to BDL.
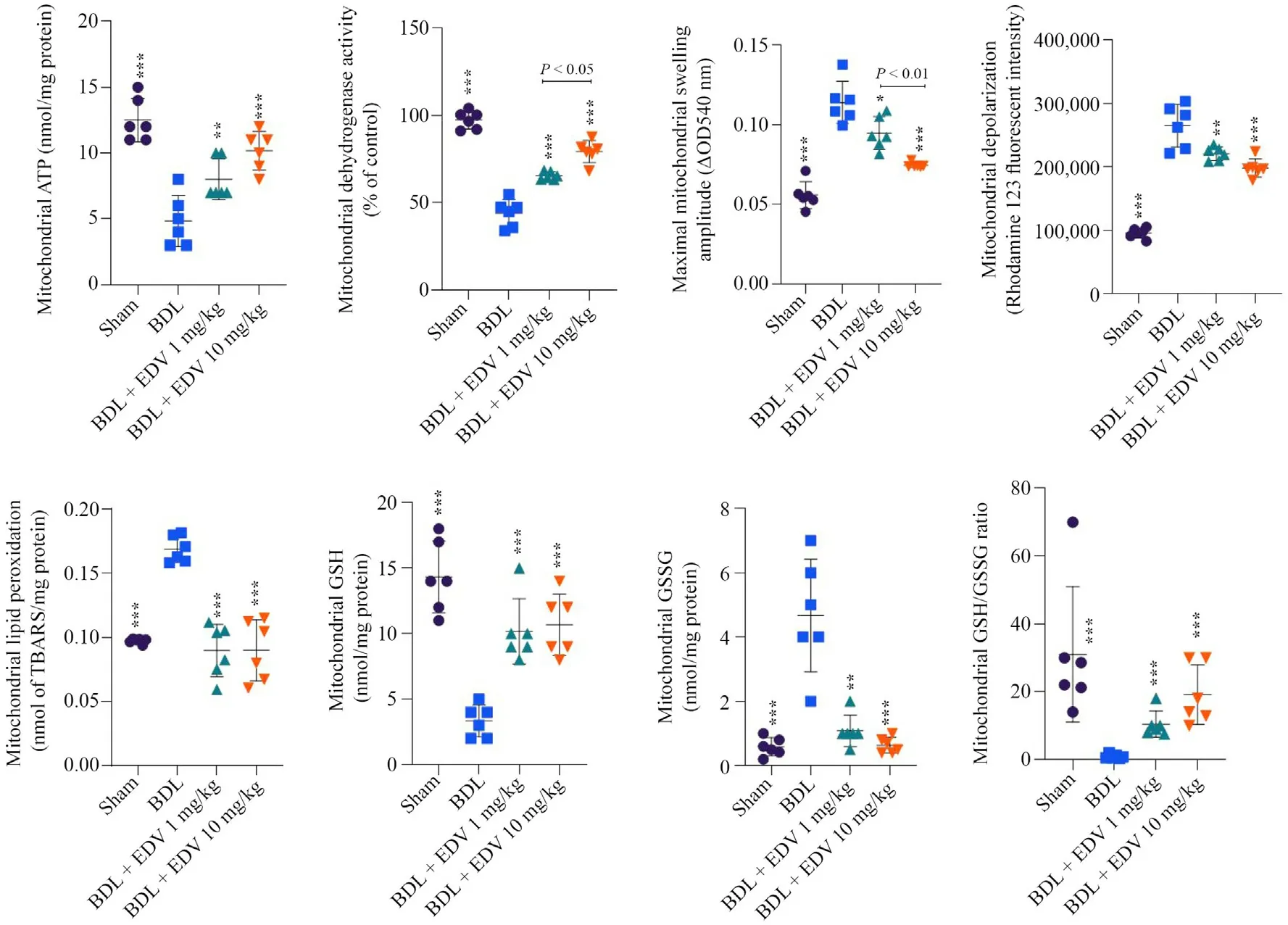
Fig.4.Liver mitochondrial indices in rats subjected to BDL and treated with EDV.
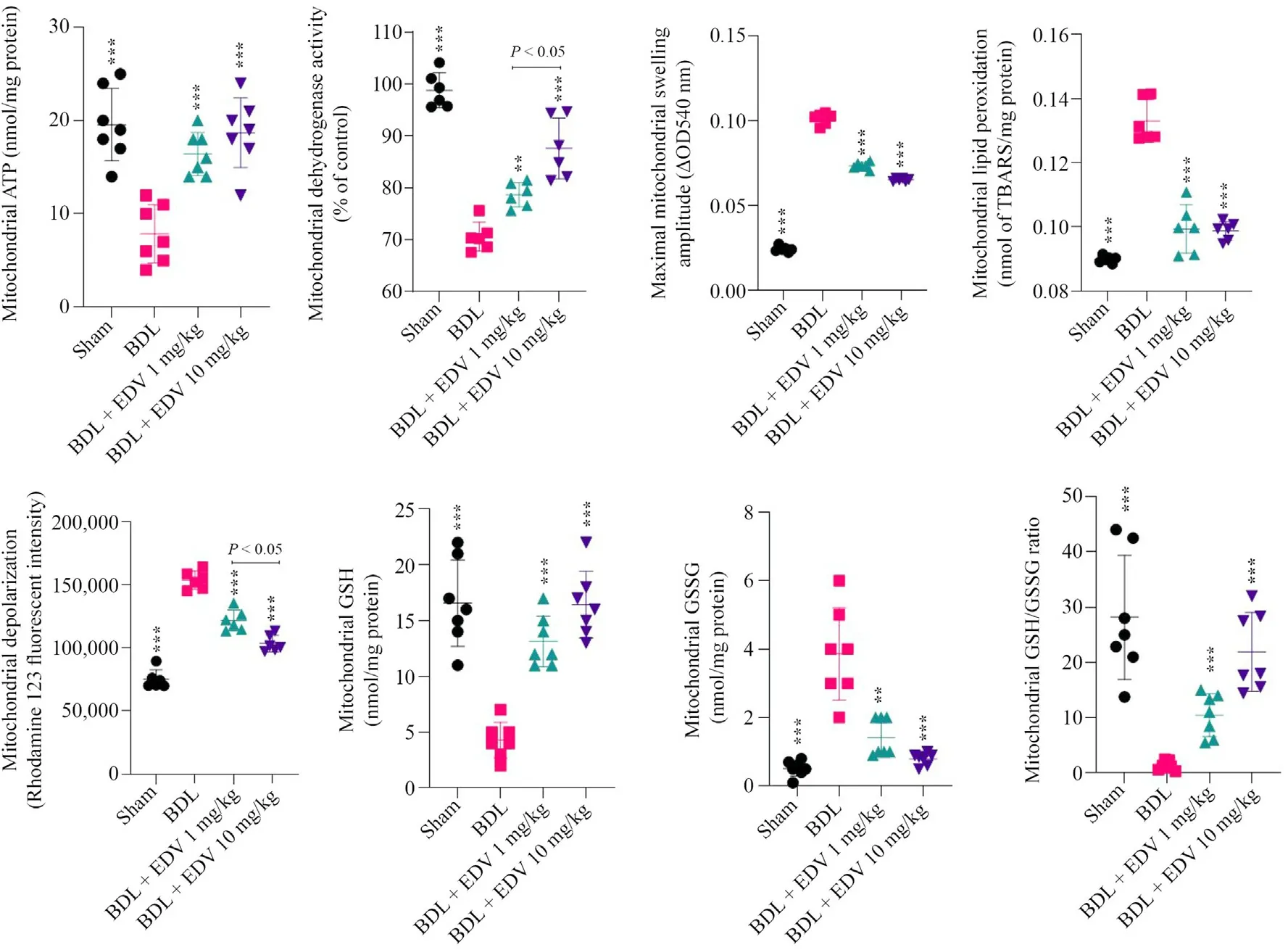
Fig.5.Kidney mitochondrial indices in rats subjected to BDL and treated with EDV.
3.5.Hepatic and renal tissue histopathology
Portal inflammation,bile duct proliferation,and tissue necrosis were evident in hepatic tissues of rats with cholestasis(Table 2 and Fig.6).Hepatic tissue fibrosis was also significantly increased in rats subjected to BDL(Table 2 and Fig.6).EDV treatment(1 or 10 mg/kg/day) for 14 consecutive days significantly mitigated hepatic tissue histopathological changes and fibrosis in rats subjected to BDL(Table 2 and Fig.6).
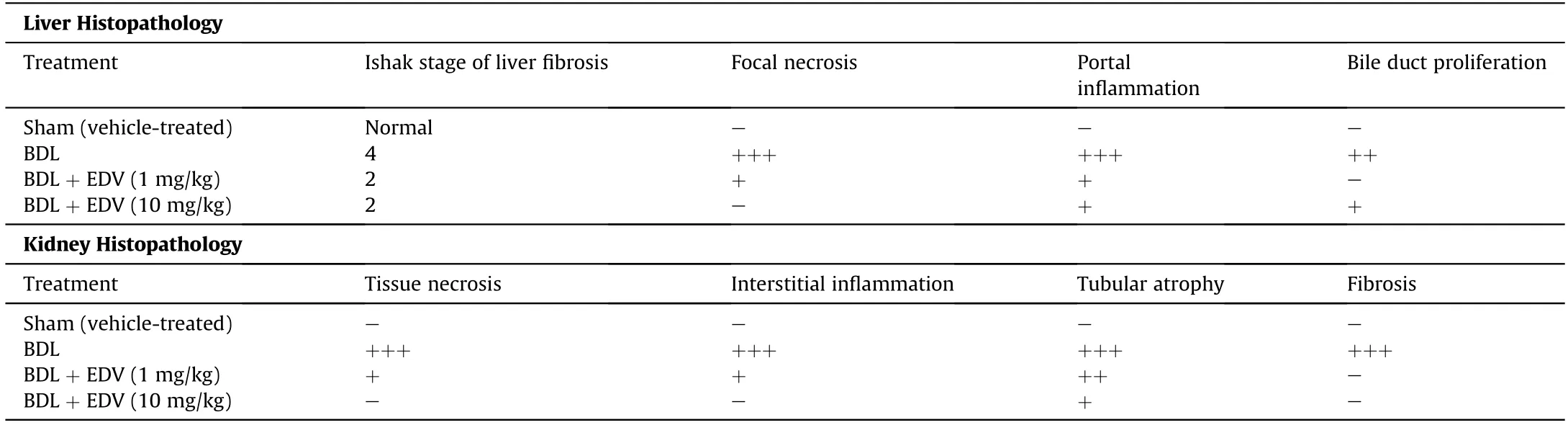
Table 2 Effect of EDV treatment on liver and kidney histopathology in rats subjected to BDL.
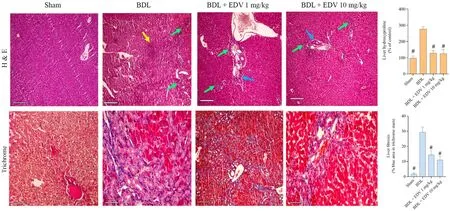
Fig.6.Histopathological changes(H&E staining) in hepatic tissue of rats subjected to BDL.
Histopathological alterations in renal tissue included interstitial inflammation,necrosis,and tubular atrophy (Fig.7 and Table 2).Moreover,significant renal tissue fibrosis was detected in the BDL group(Fig.7 and Table 2).Bile cast nephropathy was also detected in hepatic tissues of rats with cholestasis(Fig.8).Histopathological alterations in renal tissue and bile cast nephropathy were significantly mitigated following EDV treatment (Figs.7 and 8).
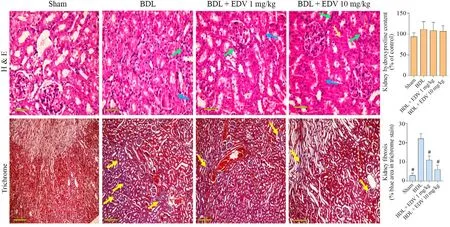
Fig.7.Histopathology(H&E staining)and fibrotic changes(Masson’s trichrome staining)in renal tissues of rats subjected to BDL and treated with EDV.
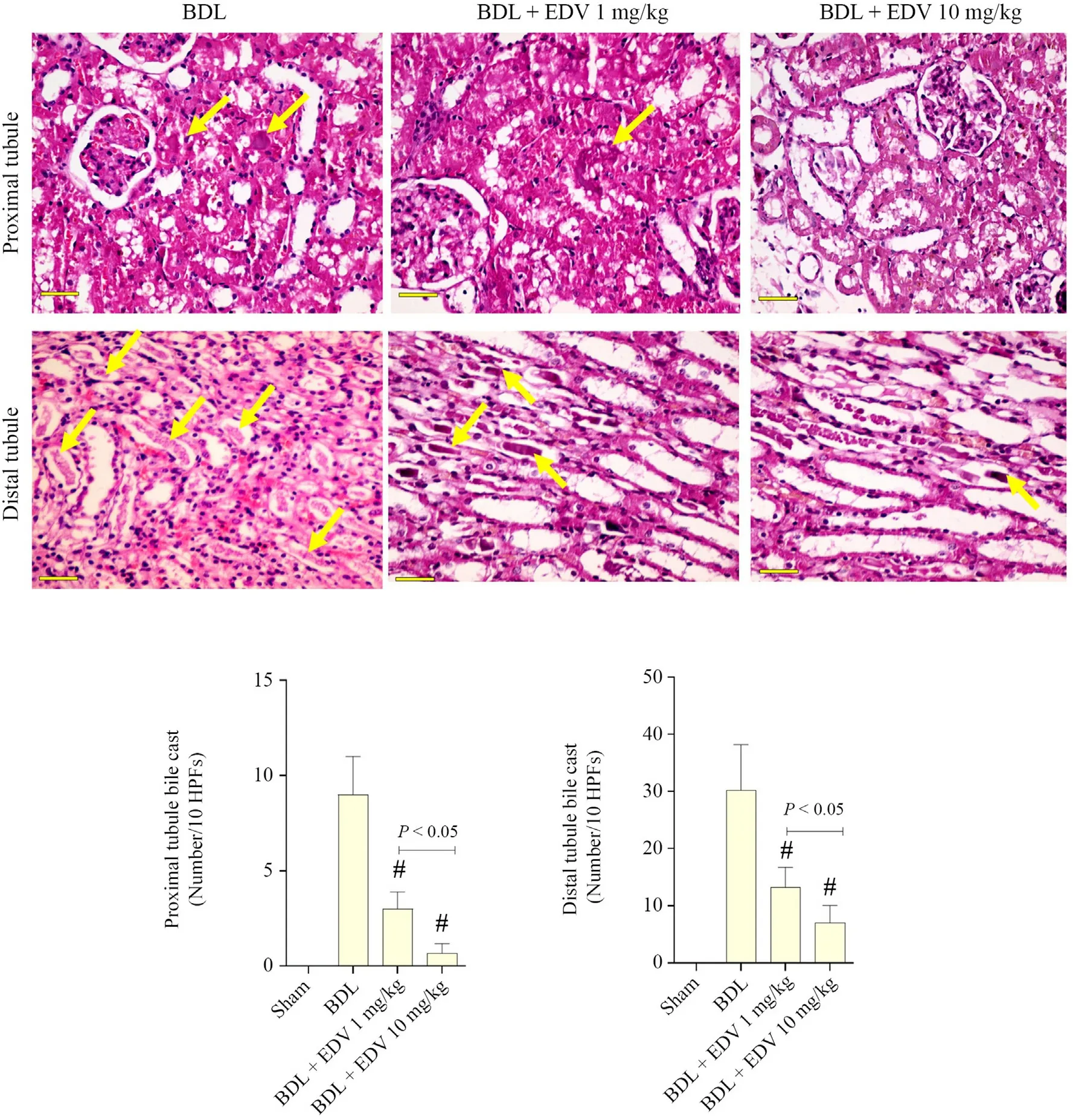
Fig.8.Administration of EDV prevented the formation of renal casts.
4.Discussion
Cholestasis and its associated complications are significant clinical challenges.2,3If left untreated,cholestasis can lead to severe fibrosis,cirrhosis,and liver failure.2,3Although the only promising therapeutic option is the identification and removal of etiologic agents,such as gallstones,several pharmacological interventions can delay organ injury associated with this condition.The liver is the primary organ affected by cholestasis.However,bile constituents that accumulate in the liver are finally secreted into the bloodstream instead of bile.Hydrophobic bile acids and bilirubin are among the biochemicals most closely associated with cholestasis-associated complications.The kidneys are the most vulnerable extrahepatic organ influenced by cholestasis.Bile acids and bilirubin can damage the kidneys due to their detergent-like properties and their propensity to form renal casts.Furthermore,several lines of evidence indicated that oxidative stress and mitochondrial impairment play pivotal role in the pathogenesis of cholestasis-induced hepatic and renal injury.22,24,44,55-57Hence,the administration of compounds with antioxidant properties and with the capacity to protect mitochondria might counteract cholestasis-induced organ injury.
EDV is a potent radical scavenger that ameliorates various oxidative stress-associated complications in both experimental models and in human disease.58,59The effects of EDV on conditions such as diabetes mellitus,renal injury,Alzheimer’s disease,and cardiovascular disorders have been investigated in detail.26,60-64Interestingly,EDV (under the trade name Radicava®) is approved for the treatment of cerebral ischemia and disorders such as embolic stroke.26,28,65,66Some studies also reported the nephroprotective properties of EDV.67
Although the mechanisms used by EDV to promote cytoprotection are far from clarified,many studies have reported its potent radical scavenging properties.58,59The effects of EDV and its role in promoting the host defense system have also been reported.38,68,69At the molecular level,EDV promotes the expression of antioxidant enzymes via its impact on the nuclear receptor nuclear factor erythroid-derived 2-like 2 (Nrf2).70-73Nrf2 is a transcriptional factor that modulates the expression of many antioxidant defense mechanisms that are activated in response to oxidative stress.74,75As EDV also mediated robust suppression of the ROS level and decreased the adverse effects of oxidative stress in both liver and kidney in the present study,one of its mechanisms might be mediated through its effect on Nrf2 receptors.The effect of EDV on Nrf2 in cholestasis should be examined in future studies.
Cellular mitochondria are dynamic organelles that are responsible for several biochemical processes,such as energy metabolism;they are also involved in cell death and survival.76,77Several lines of evidence show that mitochondria are important mediators of hepatic and renal injury during cholestasis.22-24,55,78-81Depleted cellular ATP levels,mitochondrial depolarization,and mitochondria-mediated cell death have been implicated in various experimental models of cholestasis.22-24,44,78-81Mitochondria are also a primary source of ROS.25Therefore,mitochondrial impairment could enhance oxidative stress.25On the other hand,excess ROS can lead to deterioration in mitochondrial function.25
The effects of EDV on cellular mitochondria are among the most remarkable features of this compound.39,82-85Previous studies revealed that EDV can enhance mitochondrial ATP production,prevent mitochondrial depolarization,and significantly block mitochondria-mediated cell death.39,82-85EDV also prevented the release of cell death mediators from mitochondria and thus blocked mitochondria-mediated cell death.62,84-86In the present study,we found that EDV significantly prevented mitochondrial permeabilization and lipid peroxidation;it also enhanced mitochondrial membrane potential and increased ATP levels in both hepatic and renal tissue in a rat model of cholestasis.Based on these findings,we conclude that cellular mitochondria may constitute a significant target for EDV in cholestasis-induced hepatic and renal injury.
The association between oxidative stress and tissue fibrosis has been reported in previous studies.87,88Regulation of Kupffer and stellate cell function is a pivotal factor with respect to tissue fibrosis in response to oxidative stress.87,88The anti-fibrotic properties of EDV have been documented in previous studies.89-91In the present study,we found that EDV treatment resulted in a significant decrease in both hepatic and renal fibrosis in rats with cholestasis(Figs.6 and 7).Although we did not evaluate the EDV-mediated mechanism of anti-fibrotic action,these may be directly related to its antioxidant effects.The molecular mechanisms associated with the anti-inflammatory properties of EDV in the liver and kidney of animal models of cholestasis should be the subject of future studies.
No major side effects have been reported in response to EDV treatment in human subjects.92EDV was used in clinical trials focused on stroke.29-32The present study findings revealed that EDV effectively mitigated cholestasis-induced hepatic and renal injury.The antioxidative properties of EDV are critical features of its protective properties.Further studies are warranted to evaluate the effects of EDV against cholestasis-induced organ injury in clinical settings.
Authors’ contributions
M.M.Ommati was involved in the data collection,data analysis,writing the manuscript draft,and confirmation of the final manuscript.H.Attari,A.Siavashpour,M.Shafaghat,and H.Ghaffari were involved in collecting research data,visualization of the data,writing the manuscript draft,and confirmation of the final manuscript.N.Azarpira was involved in histopathological alterations data analysis and visualization,and confirmation of the final manuscript version.L.Moezi and R.Heidari were involved in project supervision,study conceptualization,visualization and analysis of data,writing the manuscript draft,and confirmation of the final manuscript version.
Declaration of competing interest
The authors declare that they have no conflict of interest.
Acknowledgements
The authors thank the Pharmaceutical Sciences Research Center of Shiraz University of Medical Sciences,Shiraz,Iran and Life Sciences department of Shanxi Agricultural University,Shanxi,China for providing technical facilities to carry out this investigation.This study was financially supported by the Vice-Chancellor of Research Affairs of Shiraz University of Medical Sciences(Grant number:97-01-36-18730) and Shanxi Agricultural University (Grant numbers:2018YJ33 and K271999031 for National Natural Science Foundation of China and Outstanding Doctors Volunteering to Work in Shanxi Province,respectively).
杂志排行
Liver Research的其它文章
- Bile acid metabolism and bile acid receptor signaling in metabolic diseases and therapy☆
- Gut microbiome in liver pathophysiology and cholestatic liver disease☆
- Metformin alleviates cholestasis-associated nephropathy through regulating oxidative stress and mitochondrial function☆
- Bile acids and metabolic surgery☆
- Farnesoid X receptor and fibroblast growth factor 15/19 as pharmacological targets☆
- Bile acid activated receptors:Integrating immune and metabolic regulation in non-alcoholic fatty liver disease☆