Gut microbiome in liver pathophysiology and cholestatic liver disease☆
2021-10-11ShengminYanXiaoMingYin
Shengmin Yan,Xiao-Ming Yin
Department of Pathology and Laboratory Medicine,Tulane University School of Medicine,New Orleans,LA,USA
Keywords:Gut microbiome Liver pathophysiology Cholestatic liver disease
ABSTRACT An increasing amount of evidence has shown critical roles of gut microbiome in host pathophysiology.The gut and the liver are anatomically and physiologically connected.Given the critical role of gut-liver axis in the homeostasis of the liver,gut microbiome interplays with a diverse spectrum of hepatic changes,including steatosis,inflammation,fibrosis,cholestasis,and tumorigenesis.In clinic,cholestasis manifests with fatigue,pruritus,and jaundice,caused by the impairment in bile formation or flow.Studies have shown that the gut microbiome is altered in cholestatic liver disease.In this review,we will explore the interaction between the gut microbiome and the liver with a focus on the alteration and the role of gut microbiome in cholestatic liver disease.We will also discuss the prospect of exploiting the gut microbiome in the development of novel therapies for cholestatic liver disease.
1.Introduction
Although the gut microbiota has been studied for decades,it has received an increasing level of attention in the past 20 years due to the availability of advanced genetic and metagenomic tools,1both of which enable the interrogation of the complexity of the microbiome.Indeed,the use of germ-free animal models and human microbiota-associated rodents makes it possible to experimentally prove functions of the gut microbiome in the host.2,3In humans,the gut microbiota includes 100 trillion bacteria that inhabit the gastrointestinal tract.4Gut microbiome composition and diversity are varied in different populations,indicating environment-dietmicrobe-host interactions.5-7
Accumulating evidence suggests that gut microbiome not only can be altered by host health conditions but also actively impacts host functions.Diets significantly affect the composition and functions of gut microbiota,8whereas microbial metabolites in turn impact host health.9,10For example,the microbial diversity confers resistance or susceptibility to cholera infection through altering the chemical environment of the gut,relying on the enzyme bile salt hydrolase.11The microbial metabolites can also alter enterocyte lipid metabolism.12Conversely,colonocyte metabolism leads to high epithelial oxygen consumption,and the consequent epithelial hypoxia helps to maintain a homeostatic gut microbiota dominated by obligate anaerobic bacteria.13Other factors like defective gut barrier and antibiotic use are also found to be involved in the interaction between gut microbiome and host metabolism.14Given its strong interplay with the host metabolism,gut microbiome is undoubtedly involved in the development of metabolic syndrome.In mice,maternal gut microbiota in pregnancy can affect the metabolic phenotype of the offspring.15In humans,a lower bacterium richness is associated with a higher level of overall adiposity,insulin resistance,and dyslipidemia.16Gut microbial metabolites derived from lipid,carbohydrate,and protein fermentation play important roles in the development of obesity,non-alcoholic fatty liver disease (NAFLD),and type 2 diabetes mellitus.17-20Interestingly,the gut microbiota can also regulate host digestion and absorption of dietary lipids,which may contribute to the host adaptability to dietary lipid variations.21
Besides the impact on the host metabolism,gut microbiota is also critical for the regulation of host immune system.22-26Evidence has shown that gut microbiome can alter sex hormone levels and contribute to the fate of autoimmune diseases,such as type 1 diabetes.27The gut microbiome has also been shown to have a control in the thymic development of mucosal-associated invariant T cells by production of 5-(2-oxopropylideneamino)-6-d-ribitylaminouracil (5-OP-RU).28Ketogenic diets low in carbohydrate but rich in fat can induce a pronounced shift in the gut microbiome,thereby resulting in the decrease of intestinal pro-inflammatory T helper 17 (Th17) cells.29Interestingly,host immune system can in turn regulate the gut microbiome.In a study based on ageassociated metabolic syndrome,T cell-dependent events are found to be associated with the expansion o.Desulfovibrioand loss o.Clostridia,key features that are associated with obesity in mouse models and metabolic syndrome in humans.30
The gut microbiome has also been found to be involved in oncogenesis,31,32cancer therapy,33-35drug metabolism,36-39and numerous other health conditions.40-43The gut and the liver are anatomically and physiologically connected.The “gut-liver axis” is critical for liver homeostasis and pathogenesis of various liver diseases.44-46In this review,we will discuss the interaction between the gut microbiome and hepatic pathophysiology in liver disease with a focus on the role of gut microbiome in cholestatic liver disease.
2.Gut microbiome and liver pathophysiology
2.1.Inflammation and liver injury
Gut-liver axis can contribute to liver inflammation by the regulation of microbial products.47One recent study showed that resident immune cells can display a spatial polarization in the liver,which was attributed to commensal microbiota and was crucial to the prevention of pathogen dissemination.48In this study,myeloid and lymphoid cells were found to concentrate around the periportal region.The observed asymmetric localization was not controlled by liver development,but related to sustained MYD88-dependent signaling in liver sinusoidal endothelial cells induced by commensal bacteria.Mechanistically,MYD88-dependent signaling formed chemokine gradients by regulating the composition of the pericellular matrix.
Evidence has also shown a potential interplay of gut microbiome with liver injury.Antibiotics pretreatment effectively reduces transplant injury in mice and humans.49In acetaminophen(APAP)-and thioacetamide (TAA)-induced acute liver failure (ALF),gut microbiome alters Toll-like receptor(TLR)signaling and contributes to a MYC-dependent transcriptional program,which orchestrate the activation of stellate,endothelial,and Kupffer cell during ALF.50These studies suggest that gut microbiome can contribute to the development of liver injury.However,protective roles of gut microbiome in liver injury have also been reported.Gut-residen.Lactobacillus rhamnosusGG (LGG) seems to be able to protect against oxidative liver injury in two models,APAP overdose and acute ethanol toxicity,by producing a small molecule activator of nuclear factor erythroid 2-related factor 2 (NRF2),5-methoxyindoleacetic acid.51In hepatic autophagy-deficient mice,the gut microbiota plays a protective role in liver injury via fibroblast growth factor 15 (FGF15)-fibroblast growth factor receptor 4(FGFR4)signaling.52These studies indicate that the functional role of gut microbiota in liver injury can vary in different contexts.
2.2.Alcohol-induced liver injury
Alcohol-associated liver disease (ALD) covers a spectrum of diseases ranging from mild reversible hepatic steatosis to severe inflammatory and cirrhotic status.53,54Alcohol misuse can alter the composition of gut microbiome and intestinal permeability and a significant amount of evidence has shown interplays of gut microbiota with the development of ALD.55,56One study transplanted human gut microbiota from alcoholic hepatitis (AH)patients into mice,and found that microbiota caused severe liver inflammation in mice following alcohol-feeding.57Another study investigated mice from two distinct animal facilities with different susceptibility to alcohol,and found that fecal microbiota transplantation (FMT) from alcohol-resistant mice to alcohol-sensitive mice prevented the latter from alcohol-induced liver injury and steatosis.58These two studies indicate that gut microbiota may contribute to individual susceptibility to ALD and can be a potential therapeutic target.Indeed,one study has shown that restoration of a particular type of bacteria.Akkermansia muciniphila,which is depleted by alcohol consumption can promote intestinal barrier integrity and ameliorate the pathogenesis of ALD in mouse models.59Another study also shows that alcohol-associated dysbiosis may cause the dysfunction of bile acid homeostasis,thereby reducing intestinal nuclear farnesoid X receptor(FXR) activity and FGF15 expression.60Restoring FXR activity or overexpressing a nontumorigenic FGF19 variant,a human FGF15 ortholog,in mice can be beneficial to reduce alcohol-induced liver pathology.
2.3.Fatty acids metabolism
The gut microbiome plays a critical role in metabolic homeostasis.Antibiotic-induced microbiome depletion in mice can shift colonocyte energy utilization from short-chain fatty acids (SCFAs)to glucose.61The gut microbiome ferments dietary fiber and produces acetate,thereby promoting hepatic fatty acid desaturation and elongation in mice.62Emerging evidence also shows a role of gut microbiota in amino acid metabolism.63,64
The important role of gut microbiome in host metabolism has shed a light on its impacts on NAFLD.NAFLD describes a fatty liver disease that occurs in the absence of significant alcohol consumption.Since the intimate connections between NAFLD and metabolic syndrome,a new nomenclature,metabolic associated fatty liver disease (MAFLD),has been proposed and used in some recent publications.65-67Results from multiple study cohorts have shown alterations of gut microbiome at different stages of NAFLD,and identified a few consistent microbiome signatures discriminating healthy individuals from those with different stages of NAFLD and other metabolic diseases.68In general,gut microbiota contributes to the progression of NAFLD by metabolites and the factors derived from them.68Gut microbiota-derived SCFAs,such as acetate,proprionate,and butyrate,prevent the progression of NAFLD.However,decrease in total bile acid pool size and gut microbiota-derived lipopolysaccharide (LPS),lactate,ethanol,and trimethylamine are considered as drivers of NAFLD progression.68Recently reported studies have identified some other microbial metabolites in NAFLD patients.In a cross-sectional study of well-characterized twins and families,one microbial metabolite,3-(4-hydroxyphenyl)-lactate is identified in the serum,which is associated with both hepatic steatosis and fibrosis.693-(4-hydroxyphenyl)-lactate is significantly correlated with the abundance of several gut microbiome species that are reported to be associated with advanced fibrosis,suggesting a potential link between the gut microbiome and its metabolites that shares gene-effect in hepatic steatosis and fibrosis.Another microbial metabolite,N,N,N-trimethyl-5-aminovaleric acid (TMAVA),is found to be increased in liver steatosis in patients and mouse models,which may be due to the change of the intestinal bacteri.Enterococcus faecalisan.Pseudomonas aeruginosa.70Further mechanical studies suggest that TMAVA promotes high-fat diet (HFD)-induced liver steatosis in mice,which can be reduced by carnitine supplementation.Knockout of gammabutyrobetaine hydroxylase,which causes carnitine deficiency and decreases fatty acid oxidation,also exacerbates HFD-induced fatty liver in mice.
2.4.Cirrhosis
Cirrhosis is the late stage of liver fibrosis seen in a wide range of liver diseases.Gut dysbiosis has been demonstrated in both animal models and patients with cirrhosis.71-76Early study suggested that gut dysbiosis in patients with cirrhosis was characterized by the overgrowth of potentially pathogenic bacteria,such as Enterobacteriaceae and Streptococcaceae,together with the decrease of beneficial populations such as Lachnospiraceae.76More recent studies have shown that the progression of cirrhosis is associated with the alteration of gut microbiome,especially in acute-onchronic liver failure (ACLF).73,77Changes in the profile of bile acids may link to the alteration of gut microbiome in liver cirrhosis.78,79These findings also lead to the exploration of gut microbiome as diagnostic and prognostic measures for cirrhotic patients.Indeed,gene markers in gut microbiome may have the potential to be used to identify patients with liver cirrhosis.75Discrete stool metagenomic and metabolomic signatures in NAFLD patients may offer universal utility as a non-invasive diagnostic test for cirrhosis when combined with age,serum albumin levels,and serum aspartate aminotransferase levels.72
2.5.Tumorigenesis
Patients with chronic cirrhosis may progress to develop hepatocellular carcinoma(HCC).The diversity of fecal microbiota can be increased from cirrhosis to early-stage HCC.80Several bacteria are found to be enriched in early HCC,including the phylum Actinobacteria and 13 other genera.Decrease of butyrate-producing genera and increase of LPS-producing genera are also observed in early HCC patients.Further analysis suggests that the characteristic microbiome signatures can be useful for the establishment of noninvasive tools for early diagnosis of HCC.Findings from patients with NAFLD-related HCC have also shown tha.Bacteroidesand Ruminococcaceae are enriched in the HCC group,while the abundance o.Bifidobacteriumis decreased.81Given the crucial role of bile acids in gut and liver interactions,the crosstalk between bile acid and gut microbiome inevitably contributes to the gastrointestinal inflammation and carcinogenesis.82Indeed,a gut microbiome-mediated bile acid change has been associated with a liver-selective anti-tumor effect in mice.83M.et al.83showed that CXCL16 expression of liver sinusoidal endothelial cells was correlated with primary bile acid levels,while inversely correlated with secondary bile acid levels.Expression of CXCL16 caused the accumulation of hepatic natural killer T (NKT) cells,thereby mediating the anti-tumor immunosurveillance effect in the liver.
3.Gut microbiome and cholestatic liver diseases
In clinic,cholestasis manifests with fatigue,pruritus,and jaundice that caused by the impairment in bile formation or flow.84Cholestatic liver disease may occur in a variety of clinical scenarios with etiologies varying in the anatomical location of the defect and in the acuity of presentation.84Early biochemical evidence of cholestasis includes increased serum alkaline phosphatase(ALP) and gamma-glutamyltranspeptidase (GGT),followed by the onset of hyperbilirubinemia.84The main acute causes of cholestasis include common bile duct stenosis and cholangitis,drug-induced liver injury,sepsis,pregnancy,and total parenteral nutrition.Causes of chronic cholestasis include primary sclerosing cholangitis(PSC),primary biliary cholangitis (PBC),secondary sclerosing cholangitis,biliary atresia,and genetic disorders.Notably,some acute cholestasis can develop into chronic presentation,while chronic cholestasis can manifest an acute flare-up.84,85
A number of animal models have been generated to explore the mechanisms of cholestatic liver disease with the goal to identify potential therapeutic targets.These models are developed based on following approaches:(i) bile duct ligation to create experimental biliary obstruction;(ii) chemical-induced cholestasis (e.g.,cholestasis caused by 3,5-diethoxycarbonyl-1,4-dihydrocollidine,DDC;alpha-naphthylisothiocyanate,ANIT;2,4,6-trinitrobenzenesulfonic acid,TNBS;lithocholic acid,LCA);(iii) viral infections;and (iv)genetic manipulation (e.g.,deletion or mutation of multidrug resistance 2 (Mdr2) or cystic fibrosis transmembrane conductance regulator (Cftr)).86,87Although the animal models recapitulate some of the main features of human cholestatic liver disease and provide valuable tools to investigate the fundamental mechanisms,they still have limited applicability in the human setting given the significant differences in the primary etiology and the metabolism pattern between rodents and humans.86
Despite cholestasis commonly occurs in various liver diseases,the therapeutic options are still limited and not effective in general.Treatments for cholestatic liver diseases caused by hereditary transporter defects are limited to the use of ursodeoxycholic acid(UDCA),primary bile acid replacement,biliary diversion,and liver transplantation.87A recent study showed that synthetic human adenosine triphosphate-binding cassette B4 (ABCB4.i.e.,MDR2)mRNA could be used to rescue cholestatic liver disease caused by ABCB4-deficiency in mice,suggesting gene therapy as a way for cholestatic liver disease.88Several types of drugs have shown potentials in improvement of PBC,including UDCA,obeticholic acid,and some immunosuppressive agents,with UDCA being the most effective treatment for PBC.89On the other hand,therapeutic effects of PSC are limited.89
3.1.Gut microbiome and bile acid metabolism
The gut microbiome plays a central role in the homeostasis of bile acid metabolism.Conjugated bile acids are deconjugated by bile salt hydrolases(BSH)produced by gut microbiota.Primary bile acids are mainly converted into secondary bile acids by microbial 7-dehydroxylation.82,90Bile acids in turn have direct antimicrobial effects on gut microbiota and indirect effects through FXR-induced antimicrobial peptides.91,92Decrease of bile acid levels in the gut favors the growth of Gram-negative bacteria,which can include potential pathogens and cause the production of potent LPS.92Increase of bile acid levels in the gut favors the growth of Grampositive members of the Firmicutes,which include bacteria with 7-dehydroxylation capability,and leads to a conversion of primary bile acids to toxic secondary bile acids.92
The transformation of bile acids by gut microbiome modulates the signaling properties of bile acids via two major bile acid receptors,the nuclear FXR and the Takeda G protein-coupled receptor 5 (TGR5).90In mice,gut microbiota deconjugates and reduces the levels of tauro-beta-muricholic acid,a naturally occurring FXR antagonist.93This microbial effect alleviates the suppression of FXR in the ileum,thereby leading to enhanced FXR-FGF15 signaling to reduce bile acid synthesis in the liver.The modification of the signaling properties of bile acids by gut microbiome has a great impact on the host.Evidence has shown that bile acid metabolites are linked to the development of host diseases,including NAFLD,94ALD,95short bowel syndrome-associated liver disease,96and gastrointestinal inflammation and carcinogenesis.82In a recent study,two distinct derivatives of LCA,3-oxoLCA and isoalloLCA,were identified to be T cell regulators in mice.97Mechanically,3-oxoLCA could inhibit the differentiation of Th17 cells through binding with a key transcription factor,retinoid-related orphan receptor-gammat(RORgammat),and isoalloLCA could increase the differentiation of regulatory T (Treg) cells by the production of mitochondrial reactive oxygen species(mitoROS),which resulted in an increased expression of forkhead box protein P3 (FOXP3).Administration of 3-oxoLCA and isoalloLCA can respectively reduce Th17 cell differentiation and increase Treg cell differentiation in the intestinal lamina propria.97
3.2.Changes of gut microbiome in patients with cholestatic liver disease
3.2.1.Gut microbiome and PBC
Gut microbiome is undoubtedly altered in patients with cholestatic liver disease and in such animal models.98PBC is an autoimmune cholestatic liver disease which represents the most common chronic cholestatic liver disease in the US.PBC can be attributed to a combination of genetic and environmental factors that trigger a T-lymphocyte-mediated destruction of intrahepatic bile ducts.84PBC is characterized by nonsuppurative cholangitis and destruction of small- and medium-sized bile ducts with a female proportion of nearly 90%.84PBC patients commonly have autoimmune diseases,such as Sjögren syndrome,hypothyroidism,cutaneous scleroderma,and rheumatoid arthritis.84Lipid abnormalities are common in PBC patients.The presence of antimitochondrial antibody (AMA) has been considered as the serologic hallmark of PBC,which is present in 95%of patients with PBC.84
Several pieces of studies have shown that gut microbiome is altered in PBC patients,99-101which can be partially restored following treatment.100Some potentially beneficial bacteria are depleted whereas other bacterial taxa containing opportunistic pathogens are enriched in the gut of PBC patients.99Although the diversity of gut microbiome seems varied in different studies,several bacteria are consistently altered in PBC patients.These changes include the depletion of genu.Bacteroides,and the enrichment of genu.Enterobacteriaceae.Veillonella.Lactobacillus,Haemophilus.Streptococcus,an.Klebsiella.99-101Potential interplays of gut microbiome with the metabolism and immunity were observed in PBC patients.99The alteration of gut microbiome has also been shown to be associated with bile acid compositions in serum and feces of PBC patients.102It is worth noting that UDCA therapy can partially restore the altered gut microbiome in PBC patients,100and the gut microbiome in turn is associated with the response of PBC patients to UDCA therapy.103Furukaw.et al.103found that PBC patients in the UDCA non-responder group tended to have lower abundance of the genu.Faecalibacterium,suggesting that gut microbiome could contribute to the efficacy of treatments in PBC patients.
3.2.2.Gut microbiome and PSC
PSC is a chronic cholestatic liver disease with an unknown etiology.It is characterized by inflammation and fibrosis of intra-and extrahepatic bile ducts and can cause multifocal stricture and dilation of bile ducts.84The male to female ratio of PSC patients is around 2:1.More than 70% of PSC patients are also complicated with inflammatory bowel disease (IBD).84
Compared to PBC,there is more evidence showing the alterations of gut microbiome in PSC patients (Table 1).99-101,104-115In general,the diversity of gut microbiome tends to be reduced in PSC patients compared to healthy controls (HC),and geography is contributable to the alteration of gut microbiome.In most of published reports,PSC and IBD had differential effects on gut microbiome,whereas no significant shifts were observed in PSC and PSC with IBD (PSC-IBD),suggesting that PSC is the main contributor to the alteration of gut microbiome.By revisiting a disease-association microbiome data set comprising 106 patients with PSC and/or IBD,Vieira-Silv.et al.116assessed quantitative taxon abundances and studied microbiome alterations.This study identified and validated a near-exclusion pattern between the inflammation-associate.Fusobacteriuman.Veillonellagenera,with the formal being detected only in Crohn's disease and Crohn's disease with PSC.Overall,evidence has indicated that the alteration of gut microbiome can be found in PSC patients and can potentially be used as a biomarker to distinguish PSC and IBD.
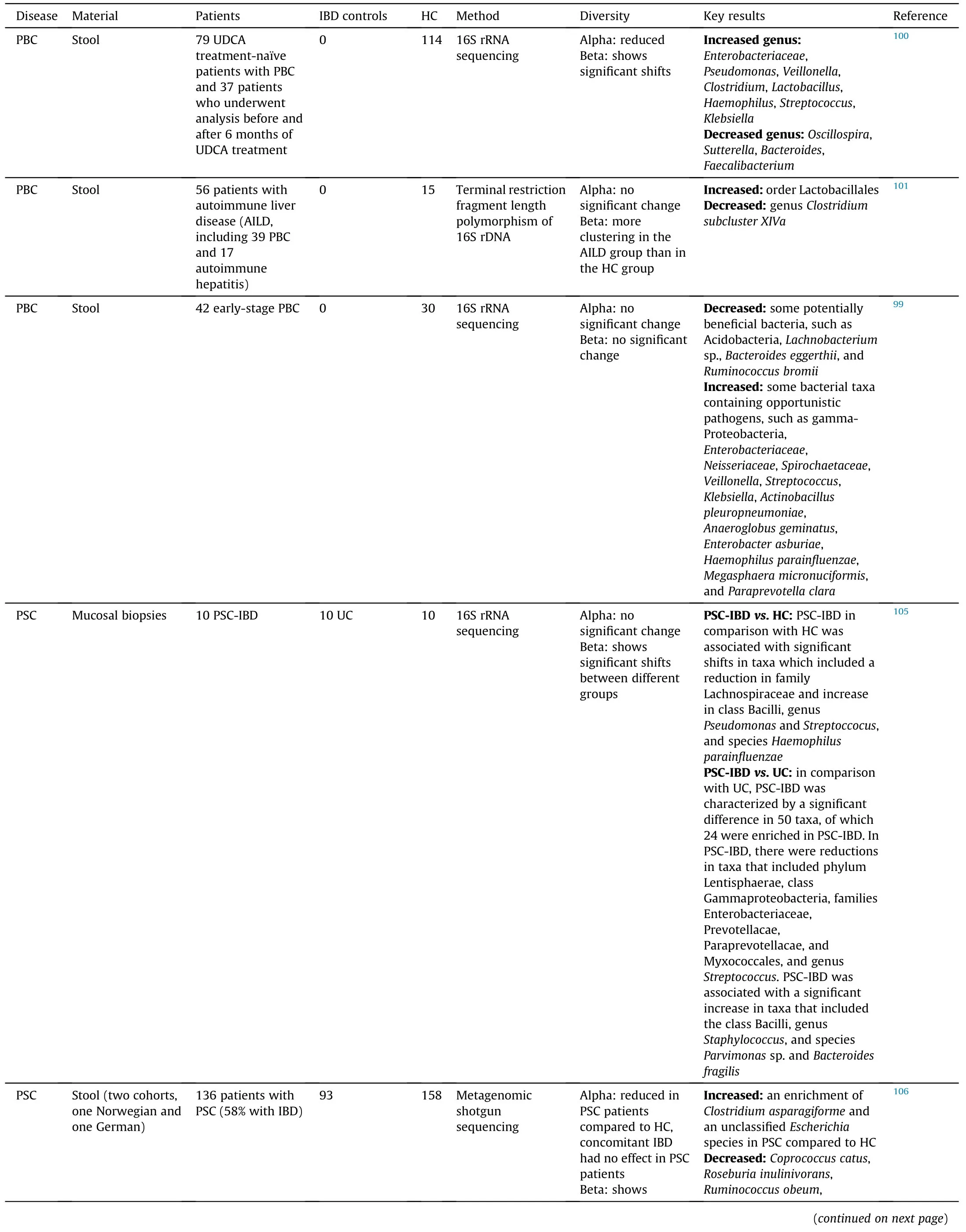
Table 1 Alteration of gut microbiome in PBC and PSC patients.
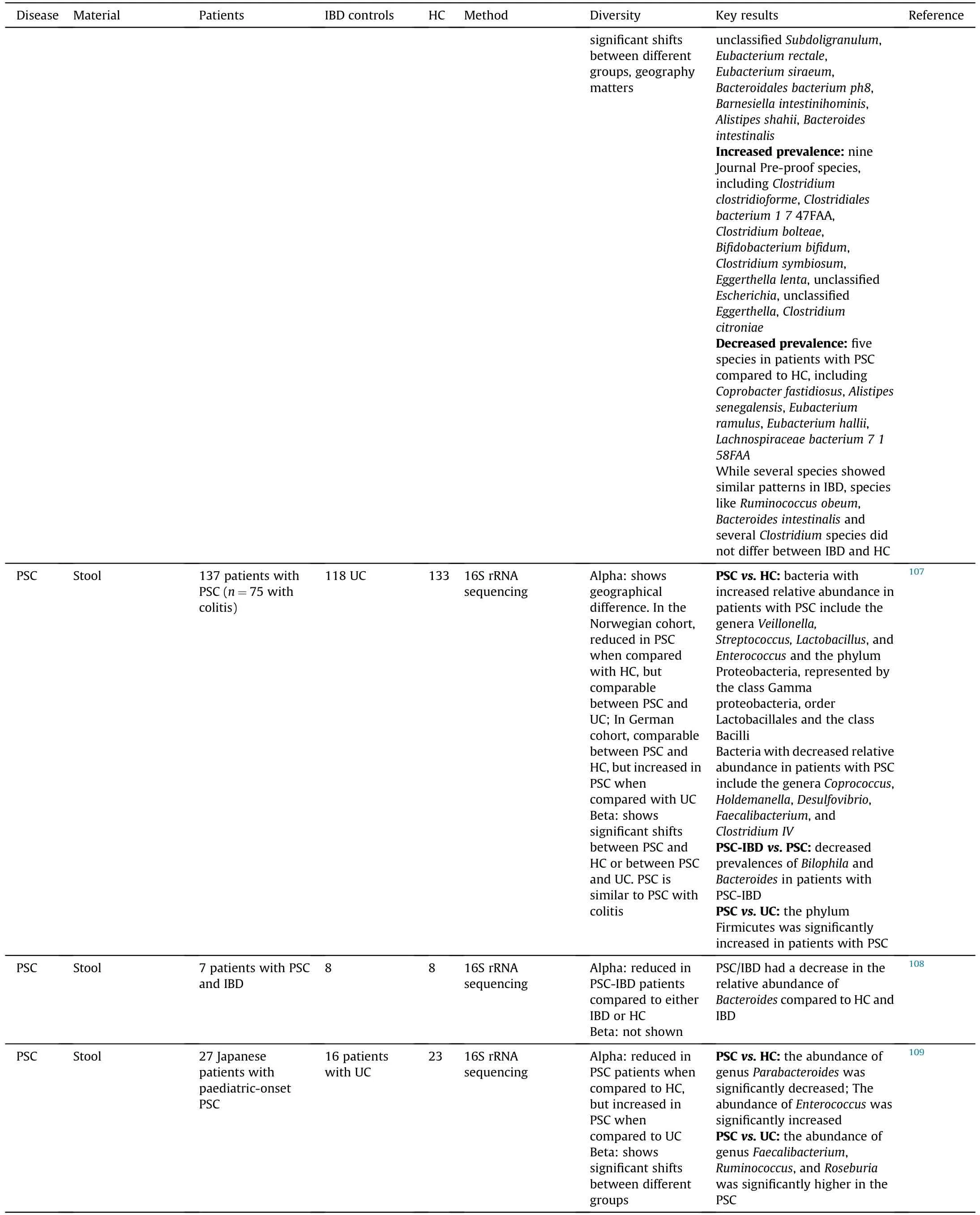
Table 1(continued)
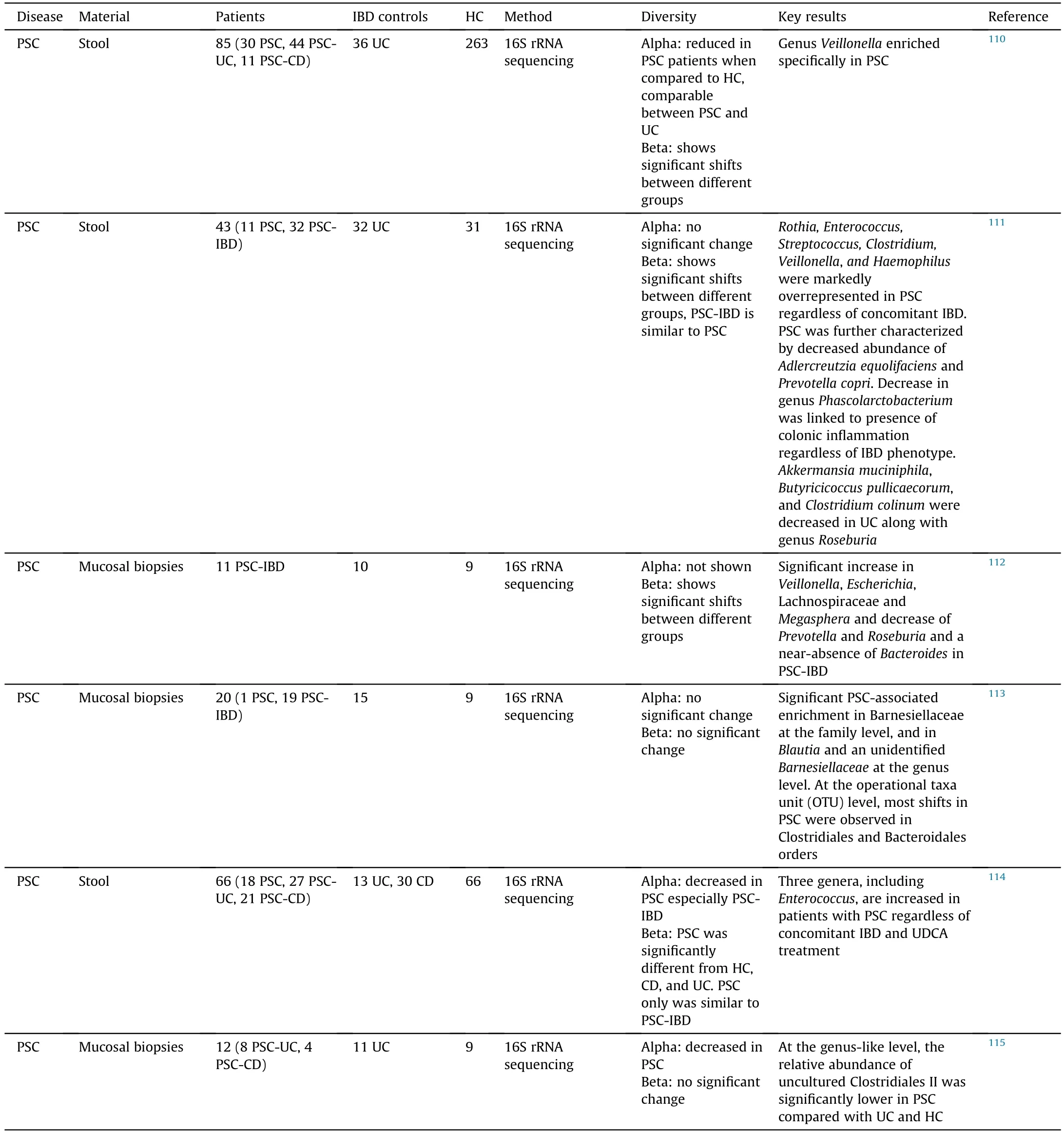
Table 1(continued)
Interestingly,several bacteria are consistently enriched in both PBC and PSC patients,includin.Veillonella.Lactobacillus,Streptococcus.Enterococcus,an.Haemophilus,whereas the proportion of some other bacteria are decreased in these patients,such a.Bacteroidesan.Faecalibacterium(Table 2).99-101,105-115These findings indicate that some gut microbiota can be altered by cholangitis regardless the etiology.
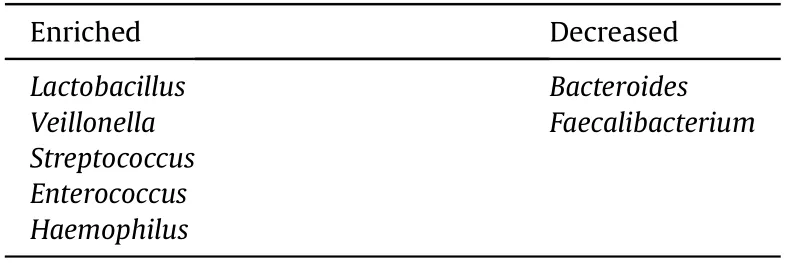
Table 2 Bacteria reported to be altered in both PBC and PSC patients.
3.3.Changes and roles of gut microbiome in animal models for cholestatic liver disease
The alteration of gut microbiome in patients with cholestatic liver disease can be associated with the changes of metabolism and inflammation in these patients.99,105However,causative roles of gut microbiome in the pathogenesis of cholestatic liver disease remain unclear in humans.Studies have been performed in animal models to investigate roles of gut microbiome in cholestatic liver disease.
3.3.1.Genetic models
Mdr2.i.e..Abcb4,is critical for phosphatidylcholine transportation and thereby plays an important role in bile formation.Disruption o.Mdr2in mice (Mdr2-/-) causes the inability of the liver to secrete phospholipid into the bile,which increases the toxic detergent activity of bile acids for hepatocytes and cholangiocytes.117.Mdr2-/-mice have been widely used as a model for PSC.Gut dysbiosis and increased gut permeability have been observed i.Mdr2-/-mice by several groups.118,119In the study by Tedesc.et al.,118serum levels of interleukin(IL)-17 were increased i.Mdr2-/-mice.Mechanically,gut dysbiosis caused the enrichment o.Lactobacillus gasseri(L.gasseri),which translocated to the liver due to increased gut permeability i.Mdr2-/-mice.L.gasseriactivated γδ TCR+cells to produce IL-17 in the liver,which contributed to the development of liver fibrosis and inflammation i.Mdr2-/-mice.In another study,Lia.et al.119showed that increased bacterial translocation amplified the innate immune response via hepatic NLR family pyrin domain containing 3(NLRP3).Transfer of microbiota fro.Mdr2-/-mice into healthy wild-type mice caused liver injury in recipient mice,whereas a pan-caspase inhibitor,IDN-7314,dampened inflammasome activation and rescued represented cholestasis-associated liver injury,serum bile acid changes,and gut dysbiosis.Furthermore,gut microbiome were shown to deteriorate the pathology of autoimmune cholangitis in dnTGFbetaRII mice and NOD.c3c4 mice.120,121These studies indicate that gut dysbiosis contributes to the pathogenesis of cholestatic liver disease and appreciation of gut dysbiosis is a potential therapeutic approach.
Interestingly,different from the findings in conventiona.Mdr2-/-mice,germ-fre.Mdr2-/-mice exhibited exacerbated biochemical and histological features of PSC,suggesting that gut microbiome may also protect mice against biliary injury.122Consistently,our recent findings from hepatic autophagy-deficient mice also indicate an adaptive protection against liver injury from gut microbiome.52Loss of autophagy-related gen.Atg5o.Atg7in the liver leads to autophagy deficiency and causes cholestatic liver injury characterized by hepatomegaly,fibrosis,and ductular reaction.Gut microbiome is altered in hepatic autophagy-deficient mice with an enrichment of bile acidmetabolizing bacteria.The profile of intestinal bile acids is altered and becomes more capable of activating FXR in hepatic autophagydeficient mice.Unexpectedly,antibiotics treatment exacerbates cholestatic liver injury i.Atg5-knockout livers.Further mechanistic studies suggest that altered gut microbiome provides an adaptive protection against the liver injury through the FGF15-FGFR4 signaling in hepatic autophagy-deficient mice.
3.3.2.Chemical-induction models
Studies are limited on the alteration of gut microbiome in chemical-induced mouse cholestasis models.Furuy.et al.123analyzed gut microbiome in mice fed with 0.1% DDC-containing diet for 8-week,followed by a 6-week recovery.Although the diversity of gut microbiome was not altered in DDC-fed mice,the authors observed a significant enrichment of Gram-negative bacteria in these mice compared to control mice,suggesting that DDC may cause the alteration of gut microbiome even after a 6-week recovery.
Role of gut microbiome has been studied in ANIT- and DDCinduced cholestatic liver disease using germ-free mice.ANIT administration or 0.1% DDC diet-feeding induced cholestatic liver injury in germ-free mice and germ-free mice conventionalized with the microbiome from wile-type specific pathogen free (SPF)animals.124Compared to the conventionalized mice,germ-free mice were resistant to liver injury,hepatic inflammatory changes,and fibrosis following either ANIT treatment or 0.1% DDC dietfeeding,despite the increase of hepatic bile acid levels in both types of mice.In addition,endotoxin was found to sensitize hepatocytes to bile acid-induced cell death.ANIT-treatment caused shift of gut microbiome in conventionalized germ-free mice and exacerbated intestinal permeability,which seemed to be related to macrophages and inflammasome.Indeed,inhibiting macrophage activation by clodronate-liposomes and NLRP3 inhibitors could alleviate chemical-induced cholestatic liver pathology.The evidence suggests that gut microbiome is critical for the development of chemical-induced cholestatic liver disease.
3.3.3.Human microbiota-associated models
The utilization of germ-free mice has led to the development of murine models carrying disease-associated gut microbiota from human patients.Aiming to further understand the role of gut microbiome in the pathogenesis of PSC,Nakamot.et al.125studied germ-free mice inoculated with microbiota derived from HC and patients with ulcerative colitis (UC) or with PSC-UC (HC mice,UC mice,and PSC-UC mice respectively).Th17 priming was only induced in the livers from PSC-UC mice,whereas it was induced in the colons from both HC and PSC-UC mice,indicating that gut microbiota derived from PSC-UC patients caused differential hepatic immune response.Interestingly,PSC-UC mice did not show obvious histological or serological changes in the liver even after a long colonization period of 90 days.The authors therefore gave these mice DDC-containing diet.DDC diet failed to cause significant hepatobiliary injury in germ-free mice as shown in another study mentioned above,124but both PSC-UC and HC mice displayed significant cholestatic phenotype with PSC-UC mice being more susceptible to DDC-induced hepatobiliary damage.Intriguingly,a dramatic enhancement of Th17 response was also observed in PSCUC mice following DDC-feeding.In order to study the potential mechanisms,the authors cultured bacteria recovered from the liver,mesenteric lymph nodes (MLNs),and spleens,and successfully isolated only bacterial clones from the MLNs of PSC-UC mice.Bacteri.Klebsiella pneumoniae.Proteus mirabilis,an.Enterococcus gallinarum,were identified by 16S rRNA sequencing.These bacteria were also prevalent in PSC-UC patients,especiall.Klebsiella pneumoniae.Further mechanistic studies showed that PSC-derive.Klebsiella pneumoniaewas responsible for epithelial damage,which may be associated with bacterial translocation and susceptibility to Th17-mediated hepatobiliary damages.Finally,antibiotics treatment ameliorated the Th17 response in livers of PSC-UC mice.Overall,this study delineates the role of gut microbiome in hepatic immune response,which is associated with the pathogenesis of PSC.The results provide preclinical evidence for a therapeutic strategy for PSC by targeting gut microbiota.
4.Gut microbiome as a potential therapeutic target for cholestatic liver disease
Given the critical role of gut microbiome in liver disease and beyond,gut microbiome-based therapeutic strategies are encouraging as shown by the evidence from pre-clinical studies in animal models and clinical trials.44,126,127These therapeutics address the following three aspects:(i) maintaining gut barrier integrity;(ii)modulating intestinal dysbiosis by antibiotics,prebiotics,probiotics,synbiotics,FMT,and bacteriophage-based therapy;and(iii)targeting gut microbiota metabolism.126Accumulating evidence has shown that therapies targeting to gut microbiota can improve PBC or PSC (Table 3).100,103,128-133Here,we will discuss some potential therapeutic approaches for cholestatic liver disease by targeting gut dysbiosis and its consequential effects (Fig.1).
4.1.FMT
As the most straightforward approach,transplantation of gut microbiota from healthy donors to patients seems effective based on several clinical trials.Clostridioides difficileinfection(CDI)causes symptoms ranging from diarrhea to life-threatening inflammation in the colon.FMT has been shown as a promising second-line treatment for CDI,especially recurrent CDI,although the treatment is so far still difficult to access with debatable safety concerns.134-138
According to USA National Institutes of Health (NIH) clinical trial database,numerous clinical trials of FMT for patients with liver diseases are on-going with some of them having been reported.FMT has been reported to improve hepatic encephalopathy,139,140alcohol use disorder,141and antibiotic-associated disruption of gut microbiome in cirrhosis patients.142One pilot clinical trial has shown the role of FMT in PSC patients.In this study,Allegrett.et al.129recruited 10 patients with PSC-IBD and gave them a single FMT by colonoscopy.Their report showed that FMT in PSC-IBD patients was safe and could improve gut microbiome diversity with no significant change in fecal bile acid profile.Although further trials are required to determine whether FMT has a role to play in PSC treatment,the result from this study is encouraging.
4.2.Antibiotics
Other than directly replacing gut microbiome in patients through FMT,controlling gut dysbiosis,particularly the growth of pathogenic bacteria,using antibiotics can be effective and more feasible.Indeed,several antibiotics have shown significant improvement in serological liver injury markers of PSC patients.130Among antibiotics that have been studied in PSC patients,vancomycin shows a remarkable efficacy in ameliorating symptoms of PSC patients in several clinical trials.131
Although therapies based on bacteriophage have not been reported in patients with cholestatic liver disease,a recent study has found that bacteriophage targeting to cytolyti.Enterococcus faecaliscan decrease cytolysin in the liver and improve alcohol-induced liver damage in humanized mice.143This study provides encouraging evidence that bacteriophage-based treatment may be an approach to precisely modify gut microbiota.Indeed,several bacteria are shown to be enriched in both PBC and PSC patients(Table 2),and PSC-derive.Klebsiella pneumoniaeseems critical to the development of cholestatic liver damage in murine models.125Thus,a precise editing of gut microbiota can be a potential therapy for cholestatic liver disease.
4.3.Probiotics,prebiotics,and specific bacteria recovery
The recovery from gut dysbiosis can also be achieved by giving probiotics and/or prebiotics to facilitate the growth of bacteria beneficial to host health,which has shown beneficial effects on multiple diseases.144-146In mice,probiotics modulate gut microbiome and suppress HCC growth by regulating the T-cell differentiation in the gut.32Impacts of probiotics on PSC have been studied in clinic.Probiotics (containing fou.Lactobacillusand tw.Bifidobacteriumstrains) did not show significant beneficial effects on symptoms,liver biochemistry or liver function in PSC patients.132A case study suggests that a combination of prednisolone,salazosulfapyridine,and a probiotic(Lactobacillus caseiShirota)treatment significantly improved a 13 years old PSC patient.133Although effects of probiotics on PSC are still controversial,probiotics may enhance the efficacy of other frontline therapies for PSC.
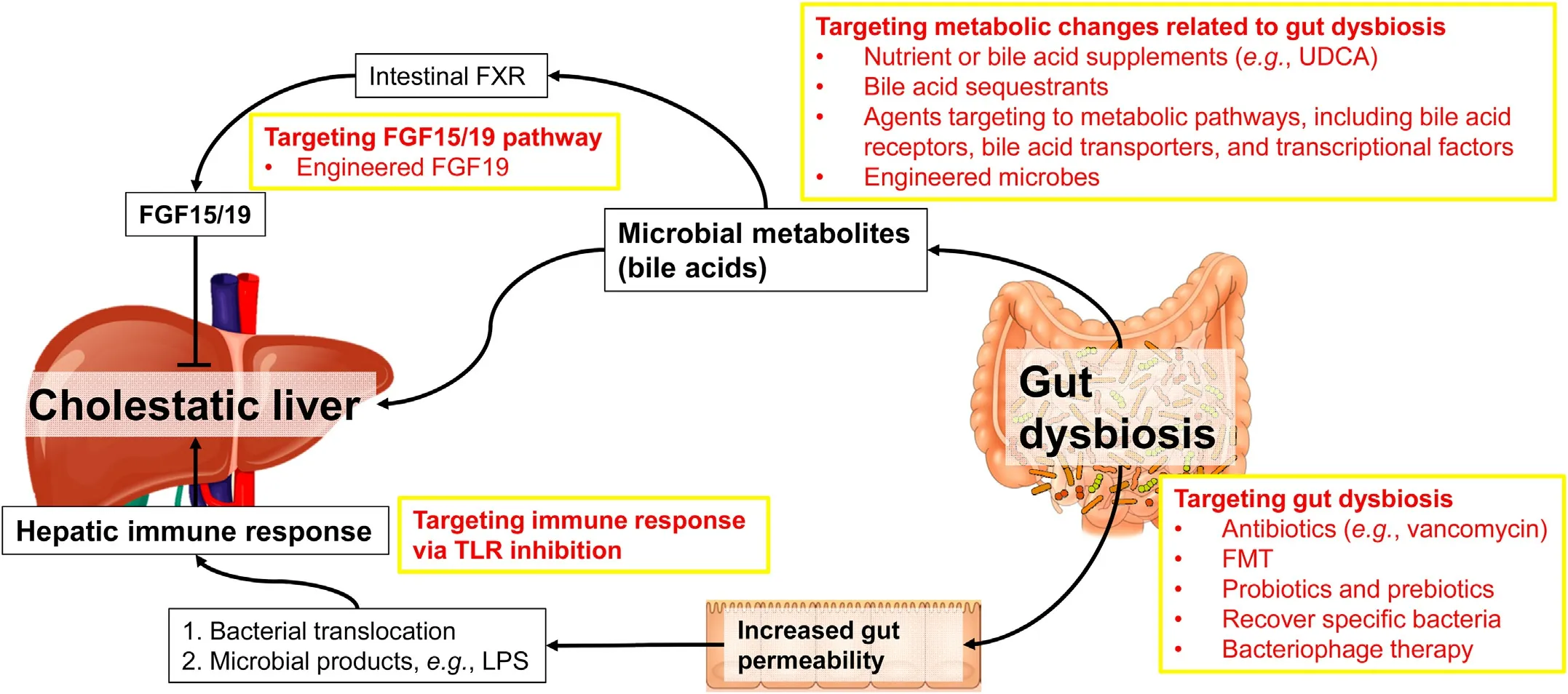
Fig.1.Roles of gut microbiome in cholestatic liver disease and potential therapies.
Giving specific bacteria supplement is another way to recover the loss of certain bacteria owing to a disease condition.Akkermansia muciniphilais a Gram-negative intestinal commensal,which can promote barrier function partly by enhancing mucus production.Interestingly,one study showed that alcohol consumption caused depletion o.Akkermansia muciniphilain both murine models and human patients.59.Akkermansia muciniphilacould be recovered by oral supplementation in mouse models for ALD,and its recovery promoted intestinal barrier integrity and improved experimental ALD.One recent study reported that daily dosing SER-287,an oral formulation of Firmicutes spores,improved UC particularly in patients following vancomycin preconditioning.147Although these studies were not done in patients with cholestatic liver disease,identification and supplementation of specific bacteria can be one future direction to find novel therapies for cholestatic liver disease.
4.4.Therapeutic targets attributable to gut microbiome
Metabolic changes caused by gut dysbiosis play a crucial role in the pathogenesis of liver disease.As mentioned above,bile acid metabolism mediated by gut microbiome is a critical part of gutliver axis.Given the nature of cholestatic liver disease,targeting bile acid metabolism has shown promising therapeutical potential for cholestatic liver disease.Agents like UDCA and some bile acid sequestrants are widely used in clinic for cholestatic liver disease with high efficacy.130Several agents targeting bile acid receptors(e.g.,FXR,TGR5),bile acid transporters (e.g.,apical sodiumdependent bile acid transporter,ASBT;sodium/taurocholate cotransporting polypeptide,NTCP),and other transcriptional factors like peroxisome proliferator-activated receptor (PPAR) have been studied and shown to be hopeful as therapies of cholestatic liver disease.130A nontumorigenic variant of FGF19 also shows the potential to improve cholestatic liver diseases.148,149It is worth noting that UDCA therapy can partially restore the altered gut microbiome in PBC patients,100and the gut microbiome in turn is associated with the response of PBC patients to UDCA therapy,103suggesting therapies targeting to bile acid homeostasis may improve gut dysbiosis as well.
Besides pharmacological approaches,engineered microbes have been shedding a light on remedying host diseases by engulfing bacteria,which can express factors functioning in metabolism or immune response,in the gut.In a chronic-binge ethanol feeding mouse model,engineere.Lactobacillus reuteri,which can produce IL-22,is able to recover the expression of antimicrobial C-type lectin regenerating islet-derived 3 gamma (REG3G) in the small intestine,and thereby improves ethanol-induced liver damage.150The secondary bile acids deoxycholic acid (DCA) and LCA are produced by gut microbiome and play an important role in regulating host physiology.One recent study has delineated the metabolic pathway for bile acid dihydroxylation by identifying a set of six enzymes that are necessary and sufficient for the conversion of cholic acid to DCA.151The authors engineered the pathway int.Clostridium sporogenes,and successfully conferred production of DCA and LCA on a nonproducing commensal,indicating that this pathway can be expressed and controlled heterologously.This study not only establishes a complete pathway to produce secondary bile acids,but also provides a potential application of engineered microbes as a therapy.Future studies should be carried on developing engineered microbes that modulate bile acid composition in patients with cholestatic liver disease.
5.Conclusion and future perspectives
Role of gut microbiome in gut-liver axis and other physiological functions in the host has been extensively recognized in the past two decades.Interplays of gut microbiome with the liver have been elucidated with critical roles in host metabolic homeostasis and immune response.Gut dysbiosis contributes to various types of liver disease.In cholestatic liver disease,gut microbiome is altered and can be recovered following remedies.Gut microbiome in turn is attributed to the pathogenesis of cholestatic liver disease by its metabolites and its effects in immune regulation.Clinical trials have been started to determine the therapies for cholestatic liver disease by targeting gut microbiome,and FMT has shown encouraging efficacy in mitigating PSC.
Despite a large amount of evidence from animal models showing the functions of gut microbiome in cholestatic liver disease,it is still unclear whether those mechanisms are comparable to the situation in human patients.A better understanding of the interplay of gut microbiome with cholestatic liver disease in human may lead to novel therapies for cholestatic liver disease.
Authors' contributions
S.Yan and X.-M.Yin drafted,revised,and approved this manuscript.
Declaration of competing interest
The authors declare that they have no conflict of interest.
Acknowledgements
This work was supported in part by the USA National Institutes of Health (NIH) grants DK116605 (to X.-M.Yin) and LA CaTS Pilot Grant U54 GM104940 (to S.Yan).
杂志排行
Liver Research的其它文章
- Bile acid metabolism and bile acid receptor signaling in metabolic diseases and therapy☆
- Mitigation of cholestasis-associated hepatic and renal injury by edaravone treatment:Evaluation of its effects on oxidative stress and mitochondrial function☆
- Metformin alleviates cholestasis-associated nephropathy through regulating oxidative stress and mitochondrial function☆
- Bile acids and metabolic surgery☆
- Farnesoid X receptor and fibroblast growth factor 15/19 as pharmacological targets☆
- Bile acid activated receptors:Integrating immune and metabolic regulation in non-alcoholic fatty liver disease☆