Bile acids and metabolic surgery☆
2021-10-11HuiXueLuyoHungJuiTuLiliDingWendongHung
Hui Xue ,Luyo Hung ,Jui Tu ,Lili Ding ,**,Wendong Hung ,*
a.Shanghai Key Laboratory of Complex Prescriptions and MOE Key Laboratory for Standardization of Chinese Medicines,Institute of Chinese Materia Medica,Shanghai University of Traditional Chinese Medicine,Shanghai,China
b.Department of Diabetes Complications and Metabolism,Institute of Diabetes and Metabolism Research Center,Beckman Research Institute,City of Hope National Medical Center,Duarte,CA,USA
Keywords:Metabolic surgery Obesity Type 2 diabetes (T2D)Non-alcoholic fatty liver disease (NAFLD)Bile acid Farnesoid X receptor (FXR)Takeda G protein-coupled receptor 5(TGR5)
ABSTRACT The epidemic of obesity and its co-mortalities has reached an alarming level worldwide.Currently,metabolic surgeries,especially the Roux-en-Y gastric bypass and vertical sleeve gastrectomy,are the most effective and sustainable treatments for obesity,type 2 diabetes,non-alcoholic steatohepatitis,as well as other metabolic diseases.However,the invasive nature of the surgeries limits their broad applications to the general public.Therefore,developing alternative non-invasive approaches to mimic metabolic surgery is an important direction of the field.Recent studies have identified several potential metabolic surgery-induced downstream endocrine mediators,among which bile acids are key candidate signaling molecules.Bile acids are profoundly altered by metabolic surgery,which contributes to the metabolic effects of the surgery.In this review,we focus on the most recent studies on the roles of bile acids and bile acid receptors farnesoid X receptor and Takeda G protein-coupled receptor 5 in mediating the metabolic effects of metabolic surgery.We conclude that targeting bile acid pathways may be a promising pharmacological approach to mimic the beneficial effects of metabolic surgery.
1.Introduction
Metabolic surgery,also known as bariatric surgery,consistently leads to profound metabolic improvement such as weight loss,type 2 diabetes (T2D) remission,improvement of non-alcoholic fatty liver disease (NAFLD),and other metabolic complications.1-3Indeed,guidelines for the management of T2D from the 2nd Diabetes Surgery Summit(2015)have suggested metabolic surgery as a therapy option of T2D for the patients with body mass index(BMI) >35 kg/m2.This has been endorsed by a number of professional organizations,including the International Diabetes Federation and American Diabetes Association.4Among many different modifications of surgical procedures,adjustable gastric banding(AGB),duodenal-jejunal bypass (DJB),Roux-en-Y gastric bypass(RYGB),and vertical sleeve gastrectomy (VSG),have received the most validation in terms of metabolic benefits in both humans and rodent models.5-8The simplest bariatric surgery of the ones mentioned above is AGB.AGB is a typical restrictive procedure in which a saline-filled silicon band is fitted around the stomach near the esophageal junction.In contrast,DJB is more complicated but more effective than AGB.In DJB,the biliopancreatic secretions are diverted into the distal jejunum through an end-to-side anastomosis.5,9Among the metabolic surgeries,RYGB and VSG are currently the most effective,sustainable,and practical therapies in the clinic for morbid obesity and T2D.10,11Compared to VSG,RYGB is a more complex procedure in which the rearranged intestine is connected to the remaining small pouches of resected stomach.The purpose of RYGB is to reduce fat absorption and food intake by combining the reduction of stomach volume with the bypass of both the duodenum and proximal jejunum.On the contrary,VSG is a simpler procedure where approximately 80% of the stomach is resected along the greater curvature.12-15The remaining stomach is shaped like a banana.Recently,extensive mechanistic studies have provided enormous evidence that RYGB and VSG can no longer be considered purely restrictive surgical procedures,even though the primary objectives of those operations were designed to restrict food consumption.16,17For example,a dramatic early improvement of hyperglycemia prior to a significant loss of body weight strongly argues that the anatomical changes and the subsequent metabolic adaptation may account for the immediate systemic improvement of glucose homeostasis after RYGB or VSG.18,19
Despite the compelling overall improvement of systemic metabolism after metabolic surgeries,these clinical practices remain invasive and sometimes risky.The mortality rate of metabolic surgery has been reported to be between 0.3%and 2.0%.There are also significant risks of developing long-term side effects,including severe infection,anastomotic leaks,protein-calorie malnutrition,hypoglycemia,and deficiencies in iron and vitamins A,B12,D,and E.20,21Therefore,it is essential to gain a better understanding of the molecular and physiological mechanisms by which metabolic surgery confers beneficial effects.The long-term goal is to develop innovative pharmacological approaches to mimic metabolic surgery for the treatments of obesity,T2D,NAFLD,and other metabolic complications.In this review,we mainly focus on the most updated findings in the mechanistic studies of metabolic surgery.
2.Potential mechanisms of bariatric surgery
Historically,the astonishing beneficial effects of metabolic surgery have been ascribed to the pure mechanical aspects of the operations,such as malabsorption and restriction of food consumption.22Later,two perspectives of the foregut and hindgut hypotheses are deemed to explain the potential mechanisms of metabolic surgery.The foregut hypothesis proposes that an unidentified diabetogenic factor is suppressed when the duodenum is not exposed to nutrients.Several reports support the foregut hypothesis,stating that the duodenal bypass in RYGB might be responsible for the weight loss and other benefits immediately after the operation.23-26However,there are conflicting reports.27The hindgut hypothesis proposes that the rapid delivery of nutrients to the distal intestine induces secretion of glucagon-like peptide 1 (GLP-1) from a specific intestinal neuroendocrine cell named L-cells,thereby augmenting the postprandial insulin release to decrease prandial blood glucose levels.Immediate and sustained significant increase in postprandial GLP-1 secretion has also been reported after RYGB.28-32
Since the emergence of foregut and hindgut hypotheses,additional potential mechanisms of metabolic surgery have been published.Some of them are:(i) the increase in intestinal gluconeogenesis;33(ii) the decrease in hepatic glucose production;34(iii) improvement of β-cell function;35,36(iv) insulin sensitivity;37,38and (v) alterations of incretin release.Among the hypotheses,the increase in incretins released from enteroendocrine cells (EECs) was proposed to be major contributor to overall metabolic improvements.35,39,40Data from animal models show that metabolic surgery can induce metabolic programming along the gastrointestinal(GI)tract and other organs.41-43And the increase in GLP-1,an incretin,at one-month post-surgery coincide with an elevation of total serum bile acid levels.44Similarly,the increased level of total serum bile acid is consistent with the rise of GLP-1 level after VSG in humans and mice.45Reports like these bring forth the significance of bile acids into the spotlight.A growing number of studies suggest that endogenous bile acids may play critical roles in improving obesity and T2D following metabolic surgery.45-47Currently,there is great interest in the specific mechanisms of how bile acids mediate bariatric surgery-associated metabolic improvement.
3.Bile acids and bile acid receptors
Bile acids are a group of endogenous metabolites with the cyclopentanoperhy or drophenanthrene structure.Bile acids are synthesized mainly in the liver from cholesterol through a series of enzyme-mediated catabolic metabolism.48,49There are two primary bile acids:cholic acid(CA)and chenodeoxycholic acid(CDCA).A certain amount of the primary bile acids is converted into secondary bile acids by intestinal microbial flora,mainly deoxycholic acid (DCA) and lithocholic acid (LCA).49A stable pool size of bile acids along the GI tract is maintained by enterohepatic circulation:in the ileum,almost 95%of bile acids are reabsorbed into the portal vein and then brought back to the liver,and only around 5%of bile acids are excreted in feces.49The well-known function of bile acid is emulsification of lipids for accelerating absorption by the intestine.However,we and other groups have demonstrated in the last two decades that bile acids can act as hormones to regulate a variety of metabolic processes,a break through finding in the field of bile acid research.49
The synthesis and enterohepatic circulation of bile acids are strictly controlled by the nuclear receptor farnesoid X receptor(FXR),which was discovered independently by multiple groups in 1999.50-52In the liver,FXR transcriptionally regulates the small heterodimer partner (Shp;NR0B2).Shpinactivates two other nuclear receptor transcription factors,liver receptor homolog 1(LRH1) and liver X receptor (LXR) α.The inactivation of LRH1 and LXRα reduces the expression level of cholesterol 7α-hydroxylase(Cyp7a1),the rate-limiting enzyme for bile acid production in the liver.49,53FXR also transcriptionally regulates a number of ATPbinding cassette (ABC) transporters,such as bile salt export pump(BSEP)and multidrug resistance protein 2(MRP2),to accelerate the transportation of bile acids across the bile canalicular membrane into the gallbladder.54In the intestine,atypical FXR target is fibroblast growth factor 15 (FGF15).After being released from the ileum into the portal vein,FGF15 is circulated to the liver where it binds to the hepatic fibroblast growth factor receptor 4 (FGFR4).A signaling transduction pathway initiated by FGFR4 leads to the activation of c-Jun N-terminal kinase(JNK)pathway to repress the gene transcription of bot.Cyp7a1and sterol 12α-hydroxylase(Cyp8b1).55In addition to FXR,another bile acid membrane receptor Takeda G protein-coupled receptor 5(TGR5)(also known as GPBAR1) was identified in 2003,and the functions of TGR5 on metabolic regulation were subsequently elucidated.56A major function of TGR5 is to increase energy expenditure in brown adipose tissue through a TGR5-cAMP-D2 signaling pathway.57TGR5 activation also improves glucose homeostasis by stimulating the release of GLP-1 via intestinal L cells,which in turn stimulates pancreatic β-cells to release insulin.58Although there are a number of other receptors that also bind to bile acids,49such as vitamin D receptor (VDR),pregnane X receptor (PXR),and sphingosine-1-phosphate receptor 2 (S1PR2),FXR and TGR5 are considered to be the two major receptors that provide the molecular links between bile acids and metabolic regulation.
4.Bile acids and metabolic surgery
Both human and animal models have confirmed that metabolic surgeries can significantly alter the serum bile acids levels and compositions,2indicating that bile acids might impart metabolic effects after the surgery.The interactions between bile acids,energy expenditure,and GI hormone signaling are attractive potential targets for metabolic surgery.2,59Human studies have shown that total bile acid levels are elevated after RYGB during either fasting or after a meal.60,61How total bile acid levels are altered by VSG is not uniform among reports;some studies showed that bile acid concentration in the plasma was increased after VSG,62and some showed that there was no change.63As expected,studies that examined AGB,an exclusively restrictive procedure,reported no significant changes in circulating bile acid concentration.7Because there seems to be a link between metabolic surgery and bile acid concentration,studies that are designed to reveal the temporal relationship between metabolic surgery and total bile acid concentration would bring valuable knowledgeable to the field.Unfortunately,most studies only report one preoperative and one postoperative time point;64only a few have reported longitudinal observations with multiple measurements.
Fortunately,animal models of bariatric surgery have been developed rapidly over the last few years.The advantage of animal models is that researchers can design experiments to investigate a wealth of information on the mechanisms of the surgery.The VSG and ileal interposition procedures have been studied mostly in rats and mice.Similar to clinical observations in human patients,VSG increases total serum levels of both conjugated and unconjugated bile acid species in rats and mice.43,59,65Studies of RYGB have also demonstrated increased bile acids in rats.66Although further studies are needed before a definitive conclusion can be reached,the data available thus far suggest that metabolic surgery seems to profoundly alter bile acids in terms of pool size,composition,and redistribution into the serum.Because most of the metabolic effects of bile acids are mediated by either FXR or TGR5,studies using genetic engineered animal models of these two bile acid receptors have provided keen insight into the potential roles of bile acids in mediating the metabolic effects of the surgery.
4.1.TGR5 and metabolic surgery
Among the molecular pathways interrogated thus far in metabolic surgery,bile acid signaling appears to be a major one.The roles of TGR5 in mediating the effects of metabolic surgery are clear,especially in VSG.Two independent studies,including one from our group,demonstrate that TGR5 is required to mediate the alleviation of hyperglycemia and fatty liver after VSG in mice.43,67In TGR5 knockout mice,the beneficial outcomes of VSG in mice are significantly compromised.Moreover,VSG strongly alters both serum and fecal bile acid levels and composition in mice,resulting in activation of TGR5 in the ileum and brown adipose tissues.The activation of TGR5 leads to improved glucose control and increased energy expenditure.43In concert with other pathways,VSGmediated activation of TGR5 is required to maintain the metabolic improvement after surgery.Therefore,TGR5 could be one of the molecular targets and mediators of VSG.
TGR5 is also essential in promoting GLP-1 secretion after bariatric surgery.68,69It is worth noting that although circulating GLP-2 levels also increase significantly after metabolic surgery,70little attention has been paid to whether the relationship between GLP-2 and TGR5 contributes to the improvement of metabolism after RYGB or VSG.In a human study,skeletal muscle gene expression of downstream targets of TGR5(mitochondrial COX IV and Kir6.2)were upregulated after RYGB.However,the TGR5 activation in the muscle was not found to be associated with higher resting energy expenditure.7But in other studies,energy expenditure after metabolic surgery-induced weight loss was increased.71,72A recent study reported that after VSG,bile acids in the intestine were converted into LCA by intestinal bacteria.Then,LCA increased the expression of bile acid transporters apical sodium-dependent bile acid transporter(Asbt)and organic solute transporter(Ost)α in the intestine,thereby promoting the transport of LCA to the liver via the portal vein.In the liver,LCA activates the vitamin D receptor to induce hydroxysteroid sulfotransferase (Sult2A),which in turn promotes the conversion of CA to cholic acid-7-sulfate (CA7S).46Subsequent study from the same group has determined that after VSG,CA7S is increased in the GI tract of mice and humans.When CA7S was administered to diet-induced obese mice,it can activate TGR5 receptor and enhance its expression to induce GLP-1 secretion in the colon L cells,thereby improving systemic glucose clearance.45
Although the reports mentioned above support the notion that TGR5 mediates the metabolic improvement associated with VSG,a study claims that TGR5 is not required for RYGB in mice.73Therefore,it seems likely that different mechanisms are employed by VSG and RYGB to bring about metabolic improvement.73Currently,most studies provide evidence that bile acids may mediate various metabolic processes after metabolic surgery.The altered bile acid composition leads to activation of TGR5 in various tissues,such as the liver and adipose tissues.As a result,glucose and lipid metabolism,energy consumption,and immune response are modulated after metabolic surgery.
In view of the outcomes of metabolic surgery,as well as the fact that TGR5 agonists are effective in increasing GLP-1 secretion and reducing blood glucose,targeting TGR5 could be an effective approach to at least partially mimic some of the beneficial effects of metabolic surgery.74,75Indeed,several pharmaceutical companies are actively developing novel TGR5 agonists as drug candidates for the treatment of T2D.76,77
4.2.FXR and metabolic surgery
In contrast to TGR5,the exact roles of FXR in metabolic surgery are still unclear.As a primary bile acid receptor,FXR regulates bile acids in the liver and intestine by transcriptionally modulating genes of key enzymes in bile acid synthesis,transporters,and other related targets.An earlier study showed that VSG brought about little weight loss and improvement in glucose control in FXR knockout mice,suggesting a potential role for FXR activation in the mechanism of VSG.41,78However,a recent study conducted by our group with four different genetically modified mouse lines(Fxr-/-,FxrΔL,FxrΔIN,and Cyp27a1-/-) showed that FXR was not activated after VSG in mice.We did not observe FXR activation in the liver or small intestine.79Instead,the downstream targets of FXR were found to be either suppressed or unchanged in the liver and ileum in response to VSG.These results suggest that VSG may not work through FXR activation,and that FXR deficiency and VSG may similarly improve metabolism by an alternative pathway(s).Further investigation indicates that VSG reduces intestinal bile acids levels;as a result,the fat absorption in the intestine is decreased,implicating that reduced bile acid levels in the small intestine and decreased lipid absorption could contribute to beneficial metabolic changes conferred by VSG.79
The roles of FXR in mediating RYGB-associated metabolic improvements have also been investigated.Ya.et al.80showed that RYGB increased the expression o.Fxrand its target transcription facto.Shpin the liver,which was correlated to an enhanced liver bile acid signaling.In another study by Kon.et al.,81RYGB increased the expression of transient receptor potential ankyrin 1 (Trpa1)through FXR-mediated elevation of acetylated histone H3 (ACH3)binding on the TRPA1 promoter.RYGB restored the expression o.Trpa1in the β-cells of diabetic Goto-Kakizaki (GK) rats,thereby enhancing the first stage of glucose-stimulated insulin secretion,and improving hyperglycemia of the rats.In order to determine whether or not metabolic surgery improved glucose metabolism and lipid metabolism via the FXR-GLP-1 axis,Albaug.et al.82conducted the bile diversion to the ileum (GB-IL) shunt experiment.GB-IL transfers bile from the gallbladder to the ileum without resecting the stomach,similar to RYGB.Following GB-IL,the intestine-specific FXR null (FxrΔ/E) mice showed no obvious metabolic improvements,which indicates that GB-IL may require intestinal FXR.By performing RYGB on high-fat diet fe.Fxr-/-an.Fxr+/+mice,L.et al.83found that RYGB surgery reduced the body weight and fat mass of bot.Fxr-/-an.Fxr+/+mice to a similar extent.This finding suggests that the effects on diet and energy expenditure may be unrelated to the FXR genotype.In addition,it is speculated that FXR signaling promotes the improvement in blood glucose control in a weight-independent manner after RYGB surgery,and that FXR-GLP-1 axis might be the main mediator of such improvement during the early postoperative period.Elevated serum bile acids and enhanced intestinal bile acid signals promote the release of GLP-1.In clinical trials,subjects undergoing cholecystectomy are more likely to experience changes in bile acids dynamics after RYGB,and the subsequent intestinal GLP-1 secretion.84
Besides VSG and RYGB,there are other bariatric surgeries that can alter bile acid circulation and signaling.For example,DJB can increase plasma bile acid levels,and DJB-mediated elevation i.Fxrand its downstream targe.Shpleads to weight loss,insulin resistance improvement,and non-alcoholic steatohepatitis (NASH) in mice.85
In summary,FXR may play different roles in RYGB and VSG.While RYGB seems to activate FXR and its target genes,VSG shows no clear evidence of FXR activation in both the liver and intestine.These results may indicate that different metabolic surgery could elicit distinct mechanisms to achieve final metabolic improvement.
5.A model of bile acid functions in metabolic surgery
Bile acid circulation is an evolutionally conserved mechanism that is essential for nutrient absorption and other systemic metabolic processes.The primary physiological function of bile acids is to facilitate the intestinal absorption of dietary fats and fat-soluble vitamins.49VSG generates significant changes in the production,secretion,and redistribution of bile acids.86TGR5 is activated by the increased bile acid levels in the blood circulation after VSG.In parallel,VSG decreases bile acid levels in the intestine,leading to impaired intestinal lipid absorption.Therefore,VSG leads to a new dynamic of the enterohepatic circulation of bile acids,which may profoundly impact the systemic metabolism (Fig.1).86The anatomical modifications in RYGB and VSG are very different;intestinal anatomy is intact after VSG,but the duodenum is rerouted to avoid nutritional influx in RYGB so that the intestinal absorption of fats and other nutrients are restricted.87,88Although it is likely that RYGB and VSG have distinct mechanisms,available evidence suggests that the decrease in total nutrient uptake could be a shared mechanism underlying at least some of the metabolic effects of both surgery types.2,6
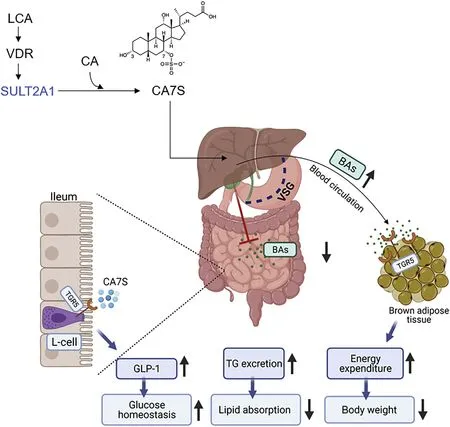
Fig.1.Bile acids as metabolic mediators following VSG.
Lastly,the longer-term studies outlined here are associated with significant body weight changes but do not address the mechanisms or pathways related to the acute metabolic benefits of metabolic surgery prior to substantial weight loss.However,the decreased caloric uptake implied by the decreased lipid absorption in our studies is consistent with a recent human study that showed that metabolic surgery and calorie restriction led to indistinguishable metabolic benefits.89
6.Summary and perspectives
The field of metabolic surgery research has made significant advances in the last decade at elucidating the mechanisms of these highly effective operations.Metabolic surgery alters several physiologic and molecular processes;whether or not all these processes are driven or modified by bile acids is still unknown,but this area of study remains promising for identifying novel and better targets for drug development to mimic metabolic surgery.
It should be noted that metabolic surgery is also highly associated with gut microbiota shifts.The mutual interactions between bile acids and gut microbiota may also significantly contribute to the metabolic effects of surgery,although the underlying mechanisms still remain elusive.
Although bile acids are best known for their important lipidsolubilizing role in the assimilation and absorption of fat and other nutrients in the intestine,they are now considered as bile hormones in modulating a variety of metabolic effects on lipid metabolism,glucose homeostasis,and gut microbiota alterations after metabolic surgery.Given their robust downstream effects in different metabolic tissues,both FXR and TGR5 may mediate some of the positive effects of metabolic surgery.Therefore,further understanding the roles of FXR and TGR5 in regulating metabolism will provide novel insights into the molecular mechanisms underlying the metabolic effects of metabolic surgery.At the same time,small molecules targeting bile acid receptors,as well as bile acid analogs and bile acid sequestrants,may provide new drug candidates in fighting against the current epidemic of obesity and its related metabolic syndrome.
Authors’ contributions
H.Xue,L.Huang,L.Ding and W.Huang configured and wrote the manuscript.J.Tu helped prepare the figure and revised the manuscript.All authors reviewed and approved the final version of the manuscript.
Declaration of competing interest
The authors declare that they have no conflict of interest.
Acknowledgements
This work was supported by the National Natural Science Foundation of China(81773961)to L.Ding,along with grants from John Hench foundation,George Schaeffer foundation and National Institute of Diabetes and Digestive and Kidney Diseases(R01DK124627) to W.Huang.
杂志排行
Liver Research的其它文章
- Bile acid metabolism and bile acid receptor signaling in metabolic diseases and therapy☆
- Gut microbiome in liver pathophysiology and cholestatic liver disease☆
- Mitigation of cholestasis-associated hepatic and renal injury by edaravone treatment:Evaluation of its effects on oxidative stress and mitochondrial function☆
- Metformin alleviates cholestasis-associated nephropathy through regulating oxidative stress and mitochondrial function☆
- Farnesoid X receptor and fibroblast growth factor 15/19 as pharmacological targets☆
- Bile acid activated receptors:Integrating immune and metabolic regulation in non-alcoholic fatty liver disease☆