Metformin alleviates cholestasis-associated nephropathy through regulating oxidative stress and mitochondrial function☆
2021-10-11MohmmMhiOmmtiHmirzMohmmiKhijhMousviNgrAzrpirOmiFrshRyhnhDhghniAsmNjibiSighhKmrnHossinNiknhRzHiri
Mohmm Mhi Ommti ,Hmirz Mohmmi ,Khijh Mousvi ,Ngr Azrpir ,Omi Frsh ,Ryhnh Dhghni ,Asm Njibi ,,Sighh Kmrn ,Hossin Niknh ,*,Rz Hiri
a.College of Life Sciences,Shanxi Agricultural University,Taigu,Shanxi,China
b.Pharmaceutical Sciences Research Center,Shiraz University of Medical Sciences,Shiraz,Iran
c.Department of Pharmacology and Toxicology,School of Pharmacy,Shiraz University of Medical Sciences,Shiraz,Iran
d.Transplant Research Center,Shiraz University of Medical Sciences,Shiraz,Iran
e.Department of Chemistry,Payame Noor University,Tehran,Iran
Keywords:Metformin Bile acids Cholestasis Cirrhosis Oxidative stress Mitochondrial function Energy crisis Cholemic nephropathy (CN)Renal failure
ABSTRACT Background and aim:Cholestasis-associated renal injury or cholemic nephropathy (CN) is a serious clinical problem.Previous studies mentioned that oxidative stress and mitochondrial impairment play a role in CN.There is no specific pharmacological intervention for CN.Metformin is an anti-diabetic drug administered for decades.On the other hand,novel pharmacological properties have emerged for this drug.The effect of metformin on oxidative stress parameters has been well-recognized in different experimental models.It has also been found that metformin positively affected mitochondrial function.The current study aimed to evaluate the effects of metformin in an animal model of CN.Methods:Rats underwent bile duct ligation (BDL) and were treated with metformin (250 and 500 mg/kg)for 14 consecutive days.Two weeks after the BDL operations,urine,serum,and kidney samples were collected and analyzed.Results:Markers of oxidative stress,including reactive oxygen species (ROS) formation,lipid peroxidation,protein carbonylation,depleted antioxidant capacity,and decreased glutathione (GSH) levels were detected in BDL animals.Moreover,mitochondrial indices,including adenosine triphosphate(ATP)level,dehydrogenase activity,mitochondrial membrane potential,and mitochondrial permeability,were impaired in the kidney of cholestatic rats.Renal histopathological alterations in cholestatic animals included tubular degeneration and interstitial inflammation,cast formation,and fibrosis.It was found that metformin significantly alleviated oxidative stress and improved mitochondrial indices in the kidney of cholestatic rats.Tissue histopathological alterations were also mitigated in metformin-treated groups.Conclusions:Metformin could be a candidate for managing CN.The nephroprotective role of metformin is primarily associated with its effects on oxidative stress parameters and mitochondrial function.
1.Introduction
Cholestasis could develop in response to xenobiotics (e.g.,alcohol or drugs) or diseases (e.g.,gall stones and hepatitis).1-4It has been found that kidneys are the most affected extrahepatic organs during cholestasis.5-7The accumulation of potentially cytotoxic chemicals (e.g.,hydrophobic bile acids),which are routinely excreted through the bile flow,seems to play a fundamental role in developing cholestasis-induced renal injury or cholemic nephropathy (CN).8-12Hydrophobic bile acids and bilirubin are the major suspected chemicals involved in the development of CN complications.8-12CN is a well-characterized complication in cholestatic patients.13-15The prevalence of CN in cirrhotic patients with acute kidney injury is approximately 18%.16On the other hand,bile cast nephropathy,as a severe complication of CN,could occur in up to 55% of cirrhotic patients.17
Although the histopathological characteristics and the prevalence of CN in cholestatic/cirrhotic patients is well-known,there is no precise mechanism for this disease so far.On the other hand,various studies mentioned oxidative stress in the pathogenesis of CN.8-12,18-21Defect in cellular enzymatic and non-enzymatic defense mechanisms,increased levels of reactive species,and damage of different targets such as proteins and lipids are documented in cholestasis-induced renal injury.10,11,18-21It has been found that mitochondrial impairment in the renal tissue could also play a role in the mechanism of CN.4Cellular mitochondria are also the primary source of reactive oxygen species (ROS).22Hence,impaired mitochondrial function in CN could enhance oxidative stress and its associated complications.22
Metformin is a guanidine anti-diabetic agent used for the management of blood glucose also for decades.23On the other hand,several other pharmacological properties have been attributed to metformin.24,25One of the most interesting features of metformin is its effects on oxidative stress parameters in biological systems.26It has been well-known that metformin significantly alleviated oxidative stress and its associated complications in various experimental models.25-27The positive effects of metformin on renal injury with different etiologies also have been investigated.28The administration of metformin in diabetic patients with renal complications is an excellent therapeutic option.28Diabetic nephropathy is a complication developed in approximately 20-40% of diabetic patients.It has been found that metformin could significantly mitigate diabetes-associated renal injury.29
The interaction of metformin with mitochondrial function is also an exciting feature of this drug.30It has been found that metformin could positively enhance mitochondrial function,decrease mitochondria-facilitated ROS formation,and improve cellular energy metabolism.30-32Metformin is also able to prevent the release of cell death mediators from mitochondria.33,34Several studies mentioned that metformin blunted mitochondria-mediated cell death and organ injury.33-35
In the current study,the potential nephroprotective effects of metformin have been investigated in cholestatic rats.Several oxidative stress biomarkers,tissue histopathological alterations,as well as mitochondrial indices,were evaluated.The results might represent a new therapeutic option in the management of cholestasis-induced renal injury.
2.Materials and methods
2.1.Ethical approval
Animal care and use were performed in conformity with the ethical guidelines,and all animal experiments were approved by the ethics committee of Shiraz University of Medical Sciences,Shiraz,Iran.
2.2.Chemicals
Trichloroacetic acid,p-dimethyl amino benzaldehyde,N-chloro tosylamide (chloramine-T),di-nitro-phenyl hydrazine,ethylenediamine tetra-acetic acid (EDTA),sodium citrate,citric acid,n-propanol,sodium acetate,2,4,6-tri(2-pyridyl)-s-triazine (TPTZ),mannitol,meta-phosphoric acid,thiobarbituric acid,calcium chloride anhydride,dimethyl sulfoxide,sucrose,and 2-amino-2-hydroxymethyl-propane-1,3-diol-hydrochloride (Tris-HCl) were obtained from Merck (Darmstadt,Germany).Dichlorodihydrofluorescein diacetate(DCF-DA),oxidized(GSSG)and reduced(GSH)glutathione,metformin hydrochloride,rhodamine 123,methanol HPLC grade,ethyl acetate,ethanol,and glacial acetic acid were purchased from Sigma-Aldrich (St.Louis,MO,USA).Kits for evaluating biomarkers of organ injury were purchased from Pars Azmoon®(Tehran,Iran).Serum and urine bile acids were assessed using the EnzyFluo™kit (BioAssay®Systems,Hayward,CA,USA)based on a fluorometric method.
2.3.Animals
Male Sprague-Dawley rats(N=32;weighing 250-300 g)were obtained from Shiraz University of Medical Sciences,Shiraz,Iran.Rats were housed in plastic cages in a standard environment(temperature of 23 ± 1°C,a 12:12 dark:light photoschedule,and 40%of relative humidity).Rats had access to a typical rodent’s diet(RoyanFeed®,Isfahan,Iran)and tap wate.ad libitum.
2.4.Cholestasis-induced surgery and experimental setup
Animals were anesthetized(10 mg/kg of xylazine and 70 mg/kg of ketamine,i.p.),a midline incision was made,and the common bile duct was identified and ligated.36The sham operation consisted of laparotomy and bile duct identification and manipulation.36Rats were allotted into four groups (8 rats/group).Animals were treated as follows:①sham-operated(vehicle-treated);②bile duct ligation(BDL);③BDL+metformin(250 mg/kg/day,oral);④BDL+metformin (500 mg/kg/day,oral).Two weeks after the BDL operations,urine,serum,and kidney samples were collected and analyzed.
2.5.Kidney histopathology and organ weight indices
Kidney samples were fixed in buffered formalin solution.Then,paraffin-embedded kidney tissue(5 μm)was prepared and stained with hematoxylin and eosin (H&E).Renal fibrotic changes were determined by Masson’s trichrome staining.37,38The organs (liver,spleen,and kidney)weight indices were measured as organ weight index=wet organ weight (g)/body weight (g) × 100%.
2.6.ROS formation
ROS in the kidney were measured based on a previously described method.36,39Briefly,a tissue homogenate(10%w/v)was prepared in ice-cooled Tris-HCl buffer (40 mM,pH=7.4,4°C,M=mol/L).Then samples of the tissue homogenate (100 μL) were mixed with 1 mL of Tris-HCl buffer (1 mL) and 2′,7′-dichlorofluorescein diacetate(final concentration 10 μM).The mixture was incubated at 37°C (30 min,in the dark).Finally,the fluorescence intensity was assessed using a fluorimeter (FLUOstar Omega®,fluorimeter,λex=485 nm and λem=525 nm).39,40
2.7.Lipid peroxidation
The thiobarbituric acid reactive substances (TBARS) were measured as a biomarker of oxidative stress-induced lipid peroxidation.36,41Briefly,the reaction mixture consisted of 500 μL of tissue homogenate (10% w/v in KCl,1.15% w/v),3 mL of metaphosphoric acid (1% w/v,pH=2),and 1 mL of thiobarbituric acid(0.375%,w/v).Samples were mixed well and heated in a water bath(100°C,45 min).42After the incubation period,the mixture was cooled,and then 2 mL of n-butanol was added.Samples were mixed and centrifuged(10,00.g,10 min).Finally,the absorbance of developed color in n-butanol phase was measured (λ=532 nm,EPOCH®plate reader,BioTek®,VT,USA).36,43
2.8.Renal glutathione content
The kidney glutathione content (oxidized and reduced;GSSG and GSH)was measured using an HPLC method.44,45Briefly,tissue samples were deproteinized and derivatized with iodoacetic acid and fluoro-2,4-dinitrobenzene.44,45Tissue samples (200 mg) were homogenized in 2 mL of Tris-HCl buffer(250 mM;pH=7.4;4°C),and 500 μL of TCA(50%w/v)was added.Samples were mixed well,incubated on ice(10 min),and centrifuged(15,00.g,15 min,4°C).Then,the supernatant was collected in a 5 mL tube and treated with the NaOH:NaHCO3(300 μL of a 2 M:2 M solution).Afterward,100 μL of iodoacetic acid (1.5% w/v in water) was added,and samples were incubated in the dark(1 h,25°C).After the incubation period,500 μL of fluoro-2,4-dinitrobenzene (1.5% v/v in absolute ethanol)was added and incubated in the dark(25°C,at least for 24 h).Finally,samples were centrifuged(17,00.g,30 min,4°C)and injected(20 μL)into an HPLC apparatus.44,45The HPLC system consisted of an NH2column as the stationary phase (25 cm,Bischoff®chromatography,Leonberg,Germany).A gradient method with a flow rate of 1 mL/min was used.45Buffer A(methanol:water;4:1 v/v)and buffer B(acetate buffer:buffer A;1:4 v/v)were used as mobile phases.45
2.9.Protein carbonylation
The oxidative damage of proteins in the kidney of cholestatic animals was assessed by evaluating carbonyl group formation based on the reaction with dinitrophenylhydrazine (DNPH).46Briefly,kidney samples (200 mg) were homogenized in 2 mL of the triton X-100(0.1%,v/v)-containing phosphate buffer(pH=7.5,4°C).Then the tissue homogenate was centrifuged(70.g,10 min,4°C),and the resulting supernatant was treated with 300 μL of DNPH;10 mM,dissolved in 6 M HCl).Samples were incubated at room temperature(1 h,in the dark)with vortexing every 10 min.46Afterward,100 μL trichloroacetic acid (20% w/v) was added and mixed.Tubes were centrifuged (10,00.g,5 min),and the supernatant discarded.The pellet was washed four times,with ethanol:ethyl acetate(1 mL of 1:1 v/v),and the precipitate was re-dissolved in guanidine (6 M solution,pH=2.3).Finally,samples were centrifuged (17,00.g,2 min),and absorbance of the supernatant was measured (λ=370 nm EPOCH®plate reader,BioTek®,VT,USA).46
2.10.Ferric reducing antioxidant power (FRAP) of kidney tissue
FRAP assay was used to assess kidney tissue total antioxidant capacity in cholestatic animals.36,47For this purpose,the FRAP reagent was prepared (freshly) by mixing 25 mL of acetate buffer(300 mM,pH=3.6),with 2.5 mL of TPTZ (10 mM in 40 mM HCl)and 2.5 mL of Fe2Cl3.6 H2O (20 mM).A 10% (w/v) of kidney homogenate was prepared in ice-cooled Tris-HCl buffer(250 mM Tris-HCl,pH=7.4).Then,100 μL of tissue homogenate was added to 0.15 mL of deionized water and 1.5 mL of the FRAP reagent.The reaction mixture was incubated at 37°C(5 min,in the dark).Finally,the absorbance of developed color was measured (λ=595 nm,EPOCH®plate reader,BioTek®,VT,USA).36,48
2.11.Kidney hydroxyproline content
Kidney hydroxyproline content was assessed as an index of tissue fibrosis.49Briefly,a 10%w/v of the kidney tissue homogenate was prepared in Tris-HCl buffer (250 mM,pH=7.4,4°C).Then,1 mL of the tissue homogenate was digested in HCl (6 M;120°C,18 h).An aliquot of digested homogenate (25 μL) was added to 25 μL of citrate-acetate buffer (pH=6) and dried at room temperature.Afterward,500 μL of 56 mM chloramine-T solution was added,and the mixture was incubated at room temperature(20 min).Then,500 μL of the Ehrlich’s reagent(15 g of p-Dimethyl amino benzaldehyde in n-propanol/perchloric acid;2:1 v/v) was added.The mixture was incubated at 65°C (15 min).Finally,the intensity of developed color was measured at λ=550 nm(EPOCH®plate reader,BioTek®,VT,USA).49
2.12.Kidney mitochondria isolation
Rat kidney was washed(NaCl 0.9%w/v,4°C)and minced in the mitochondria isolation buffer composed of 2 mM HEPES,70 mM mannitol,220 mM sucrose,0.5 mM EGTA and 0.1% bovine serum albumin(pH=7.4,4°C).Minced tissue was transported into fresh isolation buffer(5 mL buffer/g tissue)and homogenized.The whole kidney mitochondria were isolated by the differential centrifugation method.50,51First,the tissue homogenate was centrifuged at 100.g(20 min,4°C).Then,the supernatant was centrifuged at 10,00.g(20 min at 4°C) to pellet the mitochondrial fraction.The second centrifugation round was repeated at least four times using a fresh mitochondria isolation buffer medium.Finally,the mitochondrial pellet was re-suspended in a buffer(5 mL buffer/g tissue)containing 70 mM mannitol,220 mM sucrose,and 2 mM HEPES(pH=7.4).The mitochondria fractions used for assessing mitochondrial permeabilization and mitochondrial depolarization were re-suspended in permeabilization assay buffer (pH=7.2;10 mM HEPES,125 mM sucrose,65 mM KCl),and depolarization assay buffer(pH=7.2;10 mM KCl,50 μM EGTA,220 mM sucrose,68 mM mannitol,5 mM KH2PO4,2 mM MgCl2,and 10 mM HEPES).51Samples,protein content,were measured based on the Bradford method.
2.13.Mitochondrial adenosine triphosphate (ATP) levels
Based on a previously reported method,mitochondrial ATP level was assessed by HPLC.52Briefly,isolated mitochondria (1 mg protein/mL)were treated with 100 μL ice-cooled meta-phosphoric acid(50% w/v,4°C),mixed well,and incubated on ice (10 min).Afterward,samples were centrifuged (30 min,17,00.g,4°C),and the supernatant (100 μL) was treated with 15 μL of ice-cooled KOH solution (1 M).Samples were centrifuged again (30 min,17,00.g,4°C),and 20 μL of the supernatant was injected into an HPLC system.The HPLC system consisted of an LC-18 column (25 cm μ-Bondapak®column).The mobile phase was composed of potassium phosphate buffer (100 mM KH2PO4,pH=7 adjusted with KOH),tetrabutylammonium hydroxide (1 mM),and acetonitrile (2.5% v/v).53An isocratic method with the flow rate was 1 mL/min was used.The UV detector was set at λ=254 nm.53
2.14.Mitochondrial membrane potential
Mitochondrial depolarization in isolated kidney mitochondria was estimated using rhodamine 123.54-56Briefly,the mitochondrial fractions (0.5 mg protein/mL;in the depolarization assay buffer) were treated with 10 μL of rhodamine 123 (final concentration of 10 μM) and incubated at 37°C (15 min,in the dark).Afterward,samples were centrifuged(17,00.g,2 min,4°C),and the fluorescence intensity of the supernatant was assessed by a fluorimeter(FLUOstar Omega®,BMG Labtech®,with λex=485 nm and λ em=525 nm).57,58
2.15.Mitochondria permeabilization
Based on a previously reported protocol,the decrease in optical density at λ=540 nm was used to assess mitochondria permeabilization.36,50,59Briefly,isolated mitochondria (0.5 mg protein/mL) were suspended in permeabilization assay buffer(10 mM HEPES,125 mM sucrose,65 mM KCl,pH=7.2).The absorbance was monitored (25°C,during 30 min of incubation),using an EPOCH®plate reader (BioTek®,VT,USA).50,59,60
2.16.Mitochondrial dehydrogenase activity
Mitochondrial dehydrogenase activity was estimated using 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide(MTT).61-63For this purpose,isolated kidney mitochondria samples(1 mg protein/mL) were incubated with 40 μL of MTT (5 mg/mL solution in mitochondria isolation buffer) and incubated at 37°C(30 min,in the dark).Then,samples were centrifuged (17,00.g,10 min),and the purple formazan pellet was dissolved in 1000 μL of dimethyl sulfoxide.Samples were centrifuged again (17,00.g,10 min),and the absorbance of the supernatant was determined at λ=570 nm (EPOCH®plate reader,VT,USA).62,64
2.17.Lipid peroxidation in isolated mitochondria
TBARS were measured in isolated kidney mitochondria of cholestatic rats.59,65First,mitochondria preparations were washed once in MOPS-KCl buffer(50 mM MOPS,100 mM KCl,4°C,pH=7.4)to remove sucrose.For this purpose,isolated kidney mitochondria were suspended in 5 mL of the MOPS-KCl buffer and centrifuged(15,00.g,15 min).Then,the pellet was re-suspended in MOPS-KCl buffer and used for TBARS measurement.The mitochondria preparations (1 mg protein/mL) were mixed with 1.5 mL of a solution containing 500 μL of HCl(0.24 mol/L),500 μL of thiobarbituric acid(0.375% w/v),500 μL of trichloroacetic acid (15% w/v),and Trolox(500 μM).Samples were heated for 15 min (100°C water bath).59Then,1 mL of n-butanol was added,and vortexed (5 min).Samples were centrifuged(17,00.g,5 min),and the absorbance of the upper phase was measured (λ=532 nm,EPOCH®plate reader,BioTek®,VT,USA).59
2.18.Statistical analysis
Data are given as mean±standard error of the mean(SEM).Data comparison was performed by one-way analysis of variance(ANOVA) test with Tukey’s multiple comparisons as the post hoc.The score of histopathological changes is given as median and quartiles for five random pictures in each group.The statistical analysis of histopathological scores was performed by the Kruskal-Wallis and the Mann Whitne.Utest.Values o.P<0.05 were considered as a statistically significant difference.
3.Results
3.1.Organ weight indices in cholestatic rats
Significant changes in serum biomarkers of hepatic injury,as well as a high serum level of bile acids and bilirubin,were detected in the BDL model of cholestasis(Table 1).On the other hand,signs of hepatomegaly and splenomegaly were evident in cholestatic rats(Fig.1A).Histopathological changes included bile duct proliferation,necrosis,and fibrosis in the BDL rats (Fig.1B).These data confirm the appropriate induction of cholestasis in the current model.No significant changes in the renal weight index were detected in BDL animals(Fig.1A).It was found that metformin(250 and 500 mg/kg)mitigated serum biomarkers of hepatic injury and blunted hepatomegaly and splenomegaly in cholestatic animals (Table 1 and Fig.1).
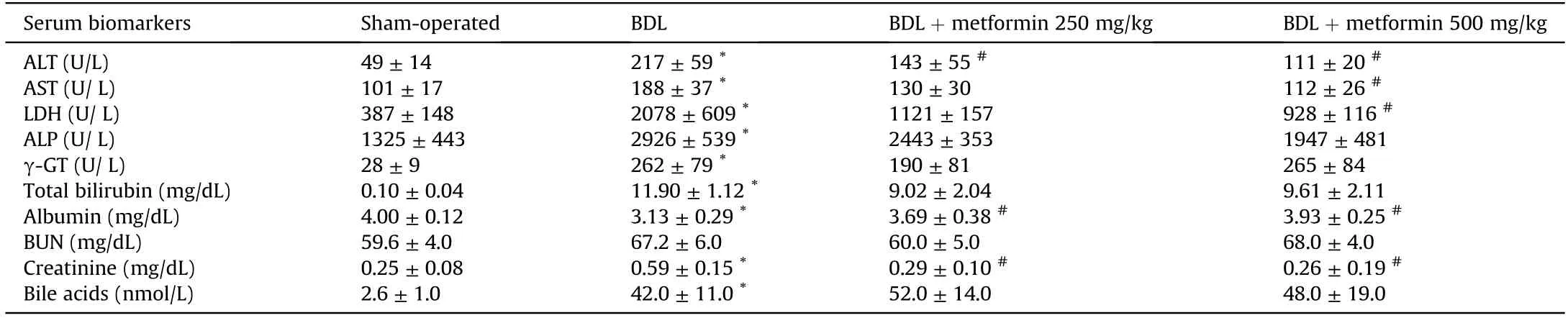
Table 1 Serum biochemical measurements in cholestatic rats.
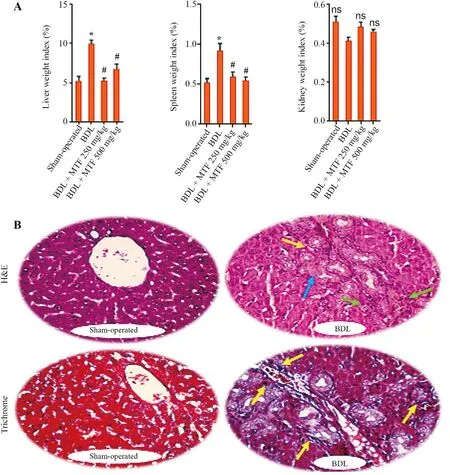
Fig.1.Organ weight index in BDL rats.
3.2.Urinalysis and serum biochemical measurements
Significant changes in serum and urine biomarkers of renal injury were also detected in the BDL group (Table 2).It was found that urine gamma-glutamyl transpeptidase (γ-GT),alkaline phosphatase (ALP),and protein levels were significantly increased in BDL rats(Table 2).On the other hand,metformin(250 and 500 mg/kg) significantly decreased renal injury biomarkers in the serum and urine of cholestatic animals (Tables 1 and 2).The effect of metformin on serum biochemistry,urine biomarkers,and organs weight indices was not dose-dependent in the current model(Fig.1 and Tables 1 and 2).
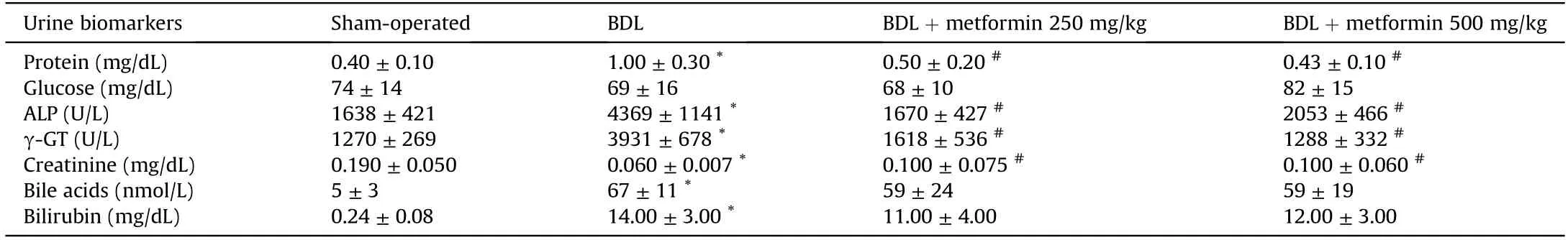
Table 2 Urine analysis in cholestatic animals treated with metformin.
3.3.Effects of metformin on oxidative stress biomarkers in cholestatic rats
Biomarkers of oxidative stress,including ROS formation,lipid peroxidation,protein carbonylation,and increased levels of GSSG were detected in the renal tissue of cholestatic animals (Fig.2).Moreover,a significant decrease in tissue antioxidant capacity,GSH,and GSH/GSSG ratio were found in the kidney of BDL rats (Fig.2).Metformin (250 and 500 mg/kg,oral) significantly decreased biomarkers of oxidative stress in the renal tissue of cholestatic rats(Fig.2).The effect of metformin on oxidative stress parameters was not dose-dependent in the most biomarkers assessed in the current study (Fig.2).
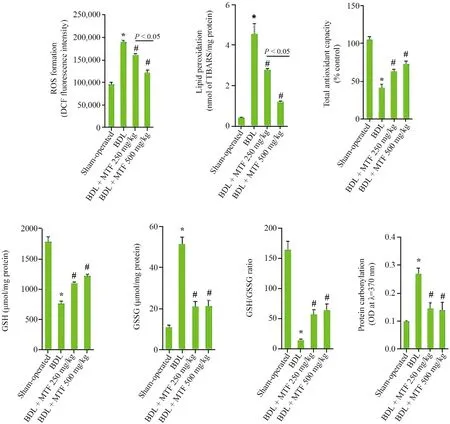
Fig.2.Kidney oxidative stress markers in BDL rats.
3.4.Impaired mitochondrial indices in the kidney of cholestatic animals
It was found that mitochondrial indices were hampered in the kidney of cholestatic animals (Fig.3).Significant decrease in mitochondrial dehydrogenase activity,depleted mitochondrial ATP,mitochondrial permeabilization,the collapse of mitochondrial membrane potential,and increased level of lipid peroxidation were detected in the mitochondria isolated from the kidney of cholestatic animals (Fig.3).On the other hand,metformin (250 and 500 mg/kg) significantly improved mitochondrial indices in cholestatic rats (Fig.3).The effect of metformin on mitochondrial parameters was not dose-dependent in the current investigation(Fig.3).
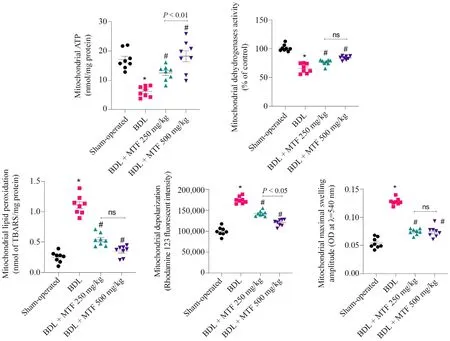
Fig.3.Mitochondrial indices in the kidney of cholestatic animals.
3.5.Effect of metformin on renal histopathological alterations during cholestasis
Tubular atrophy and interstitial inflammation were the prominent renal histopathological alterations in the BDL animals 14 days after the cholestasis induction (Table 3 and Fig.4).A significant increase in collagen deposition and renal tissue fibrosis was also detected in BDL rats (Fig.5).On the other hand,no significant changes in renal hydroxyproline content were detected 14 days after BDL surgery (Fig.5).It was found that metformin mitigated cholestasis-induced renal histopathological changes and tissue fibrosis (Table 3 and Fig.4).

Table 3 Grade of renal histopathological alterations in BDL rats.
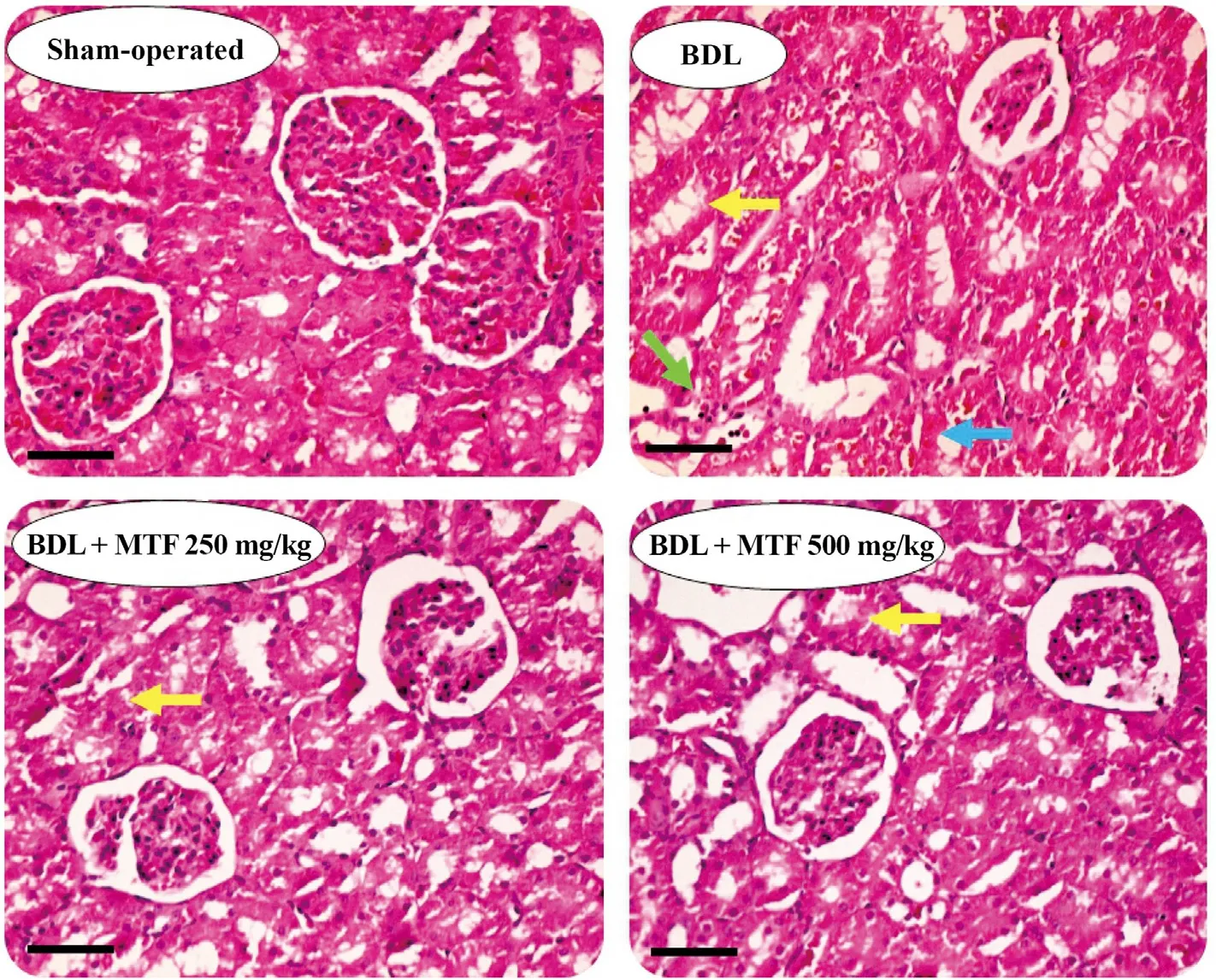
Fig.4.Effect of metformin on kidney histopathological alterations in BDL rats.
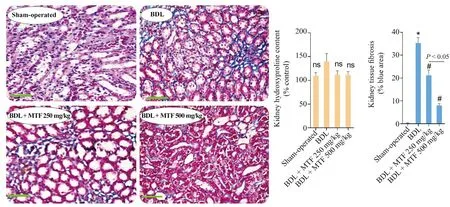
Fig.5.Kidney fibrosis and hydroxyproline content in BDL rats.
4.Discussion
Cholestasis-induced renal injury or CN is a severe clinical complication with a high rate of need for organ transplantation or even patients’ death.However,there is no specific therapeutic intervention against CN so far.Various investigations mentioned the primary role of oxidative stress and mitochondrial impairment in the pathogenesis of CN.Hence,compounds with antioxidant and mitochondria protecting properties might play a role in mitigating CN.In the current investigation,we found that metformin(250 and 500 mg/kg)significantly blunted CN in cholestatic rats.The effects of metformin in CN might be attributed to its role in counteracting oxidative stress and regulating mitochondrial function.
Metformin is administered as a first-line drug in the management of type 2 diabetes.23,25On the other hand,the effect of metformin in a wide range of other diseases has been investigated.23,25It has been found that metformin treatment could alleviate obesity,non-alcoholic fatty liver disease (NAFLD),polycystic ovary,and metabolic syndrome.The anti-aging property of metformin is another attractive characteristic of this drug.32All these properties seem to be connected with the effects of metformin on oxidative stress parameters and mitochondrial function.66,67
The effect of metformin against oxidative stress-induced injury toward biological systems is the subject of various studies.66,67It has been found that metformin significantly decreased oxidative stress and its associated complications in animal models or different clinical trials.68,69On the other hand,various studies mentioned the positive effects of metformin on renal function in different experimental models of human diseases or against xenobiotics-induced renal injury.29These studies mentioned the impact of metformin on oxidative stress parameters as a fundamental mechanism for the nephroprotective properties of this drug.70Metformin is known as a carbonyl trap agent.71,72This drug can interact chemically with reactive agents such as lipid peroxidation by-products (aldehydes) and prevent cellular injury induced by these metabolites.72,73In the current study,it was found that the amount of lipid peroxidation and protein carbonylation in the renal tissue of cholestatic animals was significantly suppressed by metformin.Based on these data,the effects of metformin on oxidative stress parameters could also serve as a fundamental mechanism for the renoprotective properties of this drug during cholestasis.
The effects of metformin on cellular mitochondrial function,energy metabolism,and mitochondria-mediated cell death are an exciting feature of this drug.32,70The roles of metformin in mitochondria-mediated fatty acids metabolism,mitochondrial biogenesis,and mitochondria-facilitated ROS formation are also the subject of various experiments in this field.32,70It has been found that metformin significantly enhanced cellular energy(ATP)levels,prevented mitochondrial depolarization,and mitigated mitochondrial release of cell death mediators.70,74As mentioned,mitochondrial impairment could serve as a pivotal mechanism for CN.4,75The kidney is a high energy demand organ and contains numerous mitochondria.Enough ATP levels guarantee the proper reabsorption of different chemicals in the kidney nephron.75Actually,impaired reabsorption of chemicals (the Fanconi’s syndrome)have been reported in human cases of cholestasis-induced renal injury.76-78The adverse effects of potentially cytotoxic molecules such as hydrophobic bile acids or bilirubin on cellular mitochondria could be involved in mitochondrial impairment during CN.In the current study,renal mitochondrial parameters were hampered in cholestatic rats.It was found that metformin(250 and 500 mg/kg)significantly improved mitochondrial indices in the kidney of BDL animals.Higher ATP levels,decreased mitochondrial swelling,and preservation of mitochondrial membrane potential were detected in metformin-treated groups.These data mention the effects of metformin on cellular mitochondria as a primary mechanism involved in its nephroprotective properties in CN.
Another important finding of the positive effects of metformin in cholestatic rats was associated with the anti-fibrotic effects of this drug in the kidney.Previous investigations also mentioned the anti-fibrotic effects of metformin in other experimental models.79,80In line with these studies,we found that metformin significantly decreased collagen deposition in the kidney of cholestatic rats.On the other hand,it has been well-recognized that there is a robust connection between oxidative stress and tissue fibrosis.81Hence,the effects of metformin on oxidative stress parameters could play a role in its anti-fibrotic properties in the current model.It has also been found that metformin could significantly suppress the expression and activity of fibrosis-related mediators (e.g.,TGF-β).80,82,83Further studies are warranted to reveal the precise anti-fibrotic effects of metformin in CN.It is also important to mention that the limitation of the Erlich technique might be involved in detecting no significant renal hydroxyproline levels in the current study.On the other hand,the trichrome staining might be a better technique for detecting low levels of tissue fibrosis,especially at the earlier stage of cholestasis.
5.Conclusions
Metformin is a safe pharmaceutical that could be administered in patients with hepatic and renal disorders.23Therefore,this drug might find a place in the pharmacotherapy of cholestasis-induced organ injury.Our data indicate the nephroprotective properties of metformin in an animal BDL model of CN.The effects of metformin on oxidative stress parameters and its positive effects on mitochondrial function could play as pivotal mechanisms for the nephroprotective properties of this drug.Indeed,further investigations are required to identify other mechanisms involved in metformin nephroprotective properties and the clinical significance of the current data.
Authors’ contributions
M.M.Ommati and H.Mohammadi contributed equally to this work.H.Mohammadi,O.Farshad,R.Dehghani,S.Kamran,and A.Najibi were involved in the data collection,data analysis,writing the manuscript draft,and confirmation of the final manuscript.R.Heidari,M.M.Ommati,and H.Niknahad were involved in visualization of the data,writing the manuscript draft,and confirmation of the final manuscript.N.Azarpira was involved in analyzing the histopathological alterations,visualization of data,and confirmation of the final manuscript version.H.Niknahad and R.Heidari were involved in project supervision and study conceptualization.
Declaration of competing interest
The authors declare that they have no conflict of interest.
Acknowledgements
This work was financially supported by the Vice-Chancellor of Research Affairs of Shiraz University of Medical Sciences,Shiraz,Iran(Grant No.19444).Authors thank the Pharmaceutical Sciences Research Center of Shiraz University of Medical Sciences for providing appropriate technical and financial facilities to carry out this study.
杂志排行
Liver Research的其它文章
- Bile acid metabolism and bile acid receptor signaling in metabolic diseases and therapy☆
- Gut microbiome in liver pathophysiology and cholestatic liver disease☆
- Mitigation of cholestasis-associated hepatic and renal injury by edaravone treatment:Evaluation of its effects on oxidative stress and mitochondrial function☆
- Bile acids and metabolic surgery☆
- Farnesoid X receptor and fibroblast growth factor 15/19 as pharmacological targets☆
- Bile acid activated receptors:Integrating immune and metabolic regulation in non-alcoholic fatty liver disease☆