Farnesoid X receptor and fibroblast growth factor 15/19 as pharmacological targets☆
2021-10-11SyedMlihGrceGuo
Syed Mlih ,Grce L.Guo,b,c,d,e,*
a.Department of Pharmacology and Toxicology,Ernest Mario School of Pharmacy,Rutgers University,Piscataway,NJ,USA
b.Environmental and Occupational Health Science Institute,Rutgers University,Piscataway,NJ,USA
c.Rutgers University Lipid Center,Rutgers University,Piscataway,NJ,USA
d.New Jersey Cancer Center,Rutgers University,Piscataway,NJ,USA
e.VA Medical Center,East Orange,NJ,USA
Keywords:Bile acids Farnesoid X receptor (FXR)Fibroblast growth factor 15 (FGF15)Fibroblast growth factor 19 (FGF19)Non-alcoholic steatohepatitis (NASH)
ABSTRACT The farnesoid X receptor (FXR) is a nuclear receptor and transcriptional regulator activated by bile acids or synthetic FXR agonists.FXR is expressed highly in the liver and intestine where modulation of FXR critically regulates the expression of genes involved in cholesterol and bile acid homeostasis,hepatic gluconeogenesis/lipogenesis,and inflammation.We review the roles of FXR and one of its intestinal target genes,fibroblast growth factor (FGF) 15 in mice/FGF19 in humans,play in regulating these important pathways in health and diseases.The main purpose of this review is to review therapeutics that target bile acid signaling to treat non-alcoholic steatohepatitis(NASH),a stage of disease within the spectrum of non-alcoholic fatty liver disease (NAFLD)with a focus on current preclinical studies in mice and clinical research.NASH is a huge medical burden and characterized by hepatic steatosis,inflammation,and progressive development of liver fibrosis.However,there is currently no Food and Drug Administration approved treatment option for NASH.While there are multiple factors contributing to NASH pathophysiology,bile acid regulation is proposed to have a major role in NASH pathogenesis.Synthetic FXR agonists and FGF19 protein may be promising agents to treat NASH,with obeticholic acid(OCA),cilofexor,tropifexor,nidufexor,EDP-305,and NGM282 currently in phase II or III clinical trials of NASH.FXR antagonism has also emerged,and antagonists like ursodeoxycholic acid(UDCA)and glycinebeta-muricholic acid (Gly-MCA) are in pre-clinical stage development for NASH treatment.This mini review seeks to evaluate and organize the literature available on FXR ligands and pathways for the treatment of NASH.
1.Introduction
A growing public health concern is the increasing prevalence of non-alcoholic fatty liver disease (NAFLD).This is a spectrum of diseases typically characterized by slow progression from simple steatosis to non-alcoholic steatohepatitis (NASH),a more severe subtype of NAFLD.1NASH is alarming because,left unattended,it will develop into more severe liver diseases including fibrosis,cirrhosis and even hepatocellular carcinoma (HCC).2The pathogenesis of NAFLD/NASH consists of multiple factors such as lipid accumulation,oxidative stress,inflammation,and bile acid dysregulation.3The farnesoid X receptor (FXR) regulates the transcription of many hepatic and intestinal genes,including mouse fibroblast growth factor (FGF) 15 with its human homologue,FGF19,to maintain cholesterol/bile acid homeostasis,reduce hepatic gluconeogenesis,and suppress inflammation.4There are no Food and Drug Administration (FDA) approved treatments for NAFLD/NASH except for lifestyle modification.This situation may be updated soon as there are a few clinical trials to test FXR or FGF19 as important drug targets for NASH treatment.FXR agonists,including obeticholic acid (OCA),cilofexor,tropifexor,nidufexor,and EDP-305,as well as modified FGF19 protein (NGM282),have displayed favorable effects on NAFLD/NASH though accompanied with unfavorable side effects,such as pruritus and/or elevation in low-density lipoprotein-cholesterol (LDL-C) and high-density lipoprotein-cholesterol(HDL-C)levels.5-9It is our hope that through whole-body or tissue-specific FXR modulation and understanding the effects of FGF15/19 on liver functions,better treatments for NAFLD/NASH could be developed.
2.Disease states
2.1.NAFLD
NAFLD is a spectrum of liver diseases unrelated to high alcohol intake and other liver disease etiologies.1NAFLD prevalence rates have been increasing and seen in patients who have other chronic health conditions,such as obesity and insulin resistance (and/or Type 2 diabetes).10The prevalence of NAFLD in Type 2 diabetics is 60%,resulting in increased mortality rates due to liver cirrhosis.11Over 90% of morbid obese patients undergoing bariatric surgery have NAFLD.12The prevalence of NAFLD in the world is rising with a current,approximate prevalence of 25%.The United States is estimated to have a NAFLD prevalence of 20-30%.13NAFLD is a slow progressive disease starting from simple steatosis in the liver defined as more than 5% macrovascular steatosis.However,these increasing prevalence rates are alarming,because the progressive form of NAFLD,NASH can develop.
2.2.NASH
NASH is defined as hepatic steatosis,inflammation,and hepatocyte ballooning-hepatocyte damage with or without hepatic fibrosis.14The pathophysiology of NASH is complex and multifactorial.Lipid accumulation,hepatocyte injury,and inflammatory cell infiltration are key characteristics of NASH.Lipid classes that have a role in hepatocellular toxicity and injury include,but are not limited to,saturated non-esterified fatty acids,palmitate,lysophosphatidylcholine,free cholesterol,sphingolipids (including ceramides),and sphingosine 1-phosphate.15NASH has an extensive recruitment of immune cells,which causes a pro-inflammatory reaction.This inflammatory process mainly involves innate immunity mechanisms,but recent studies propose that adaptive immunity is also involved in NASH.15Fibrosis progresses rapidly and NASH can result in more severe liver disease,such as cirrhosis and HCC.2NASH also became the leading liver disease etiology in adults registered to liver transplant waiting lists,especially for female patients.There was an increase among patients without HCC,and in the first quarter of 2019,NASH represented 33.9%(669 of 1974)of wait-listed registrants without HCC.16
2.3.Pathogenesis of NAFLD to NASH
The pathogenesis and disease progression of NAFLD has multiple factors such as bile acid dysregulation and insulin resistance.The two-hit theory is commonly used to explain the pathogenesis and disease progression of NAFLD.The initial “hit” is excess lipid accumulation in the hepatocytes following increase.de novolipogenesis and fatty acid uptake and/or reduced fatty acid oxidation and very low-density lipoprotein (VLDL) efflux.3Various second hit(s)causes hepatocyte damage,oxidative stress,and hepatic inflammation.3
2.4.FXR in NASH
FXR is a ligand-activated nuclear receptor and transcriptional factor with its endogenous ligands being bile acids.FXR regulates the expression of genes involved in maintaining cholesterol/bile acid homeostasis,reducing hepatic gluconeogenesis/lipogenesis,and suppressing inflammation.FXR is highly expressed in the liver and distal small intestine.4Research from our lab found that mice deficient in whole-body FXR developed more severe NASH.3The role of intestinal FXR in NASH and metabolic disease development is less clear because animal studies suggest that either the inhibition or activation of intestinal FXR have similar beneficial effects.17NAFLD patients are deficient in an FXR target gene,FGF19,which regulate.de novobile acid synthesis,lipogenesis,and energy homeostasis.18-20
3.FXR and FGF15/19
FXR is highly expressed in the liver and ileum and critical in regulating bile acid homeostasis,lipid metabolism,inflammation and fibrosis (Table 1).The effects of FGF15/19 in enterocytes and hepatocytes are summerized in Table 2.

Table 1 FXR modulation and effects on different cell types.

Table 2 Effects of FGF15/19 on enterocytes and hepatocytes.
3.1.FXR and FGF15/19 in (ileal) enterocytes
FXR is highly expressed in the ileum and the activation of FXR induces expression of genes encoding the ileal bile acid binding protein and organic solute transporter α/β,which are involved in regulating bile acid transport.21,22FXR activation induces gene expression o.Fgf15in mice o.FGF19in humans,a hormone secreted into portal circulation.23FGF15 or FGF19 reaches the liver to bind and activate a membrane tyrosine kinase receptor fibroblast growth factor receptor 4 (FGFR4),resulting in a signal cascade to inhibit bile acid synthesis by repressing the transcription of th.Cyp7a1/CYP7A1gene encoding cholesterol 7alpha-hydroxylase(CYP7A1),an enzyme that catalyzes the rate-limiting step of bile acid synthesis via the classic pathway.23,24This repression is mediated by the extracellular signal-regulated kinase in humans and extracellular signal-regulated kinase/Jun N-terminal kinase in mice.25In intestine-specifi.Fxr-knockout (KO) mice,specific FXR deficiency in intestine disrupts FXR-FGF15 mediated suppression o.Cyp7a1gene expression.22Similarly,FGF19 analogs,when administered in humans,had repressed bile acid synthesis in healthy volunteers.26
The intestinal FXR-FGF15/19 axis is also important in suppressing the expression of th.Cyp8b1/CYP8B1gene encoding sterol 12alpha-hydroxylase,CYP8B1.25CYP8B1 mediates the production of cholic acid (CA) and is important in regulation of the hydrophobicity of the bile acid pool by regulating the CA to chenodeoxycholic acid (CDCA) ratio.25This enzyme is needed for 12alphahydroxylation of 7alpha-hydroxy-4-cholesten-3-one,an intermediate and marker for the rate of bile acid synthesis.27
Another potential beneficial effect of FGF15/19 on NAFLD is changing bile acid composition.For example,administering FGF19 protein in mice decreased bile acid synthesis and modified bile acid composition and pool size,which was found to protect the mice from intestinal inflammation.28An example of altered composition is increased tauro-beta-muricholic acid (TβMCA),which is one of the most hydrophilic bile acids,in the bile acid pool,so the overall hydrophobicity of hepatic bile acids was also decreased.17,29In the study where mice were maintained on high-fat,high-fructose,high-cholesterol diet and treated with FGF19 protein or an engineered nontumorigenic FGF19 modified protein,there was an increase in TβMCA.29TβMCA antagonizes FXR signaling in the intestine,which leads to increase in bile acid synthesis.30This antagonism demonstrates metabolic improvements via reduced biosynthesis of intestinal-derived ceramides.30Ceramides are mediators of cell death,inflammation,and insulin resistance,and its accumulation in the liver is associated with NASH.31
3.2.FXR in hepatocytes
In hepatocytes,FXR activation induces the transcription o.NR0B2/Nr0b2gene that encodes small heterodimer partner(SHP).25The liver FXR/SHP pathway is critical for suppressin.CYP8B1/Cyp8b1gene expression and has a minor role in suppressin.CYP7A1/Cyp7a1gene expression.25SHP does not bind to DNA but inhibits trans-activating activity of hepatocyte nuclear factor 4 and liver-related homologue-1.27Increased SHP protein inactivates these transcription factors through forming a heterodimeric complex that binds to gene promoter regions,thus inhibiting transcription.23,27,32SHP plays a minor role in the downregulation of CYP7A1 expression and SHP basal expression is relatively high in the liver.25FXR induction of SHP in mice aids i.Cyp7a1repression but is not necessary.23In wild-type mice,increased FGF15 levels led to decrease.Cyp7a1gene expression,but SHP expression did not significantly increase with enhanced FGF15 expression.23
Besides regulating bile acid homeostasis,FXR is also critical in the liver to regulate lipid metabolism and suppress inflammation.For example,induction of SHP by FXR also inhibits the expression of lipogenic enzymes via decreasing expression of transcription factor-sterol regulatory element binding protein-1c (SREBP-1c).33SREBP-1c is a key transcriptional regulator to enhanc.de novolipogenesis.33Lipogenesis is a metabolic pathway commonly dysregulated in NAFLD.33Thus,one of the mechanisms by which FXR activation regulates lipid metabolism is by modulating SHP expression,which reduces lipogenic enzymes expression via suppressing the expression of SREBP-1c.Hepatic triglyceride production is critically regulated b.de novofatty acid synthesis rate and fatty acid oxidation.Fatty acid oxidation is regulated by peroxisome proliferator activated receptor alpha(PPARα)and SREBP-1c.34Thus,another mechanism FXR regulates lipid metabolism is through upregulating PPARα which stimulates fatty acid β-oxidation.17PPARα is an FXR response gene in humans;however,this FXR dependent upregulation is not seen in mice.17The pathogenesis of NAFLD progression involves lipid accumulation in hepatocytes with increase.de novolipogenesis,reduced fatty acid oxidation,and/or VLDL efflux.3Therefore,FXR activation regulation of lipid metabolism would be beneficial in treating NAFLD progression.
FXR also has a role in glucose metabolism.A study evaluated the role of FXR in glucose metabolism using wild-type an.FxrKO mice.35.FxrKO mice showed diminished glucose tolerance,insulin sensitivity,and insulin responsiveness in the liver and skeletal muscle.35However,FXR activation suppressed expression of many genes involved in gluconeogenesis,such as peroxisome proliferator activated receptor γ coactivator-1α,phosphoenoylpyruvate kinase,and glucose-6-phosphotase,to collectively lower glucose level.35
3.3.FXR in hepatic stellate cells (HSCs)
Hepatic fibrosis is scarring of the liver with increased and altered disposition of extracellular matrix along with reduced breakdown of its components.36HSCs play a key role in the development of hepatic fibrosis which can progress to cirrhosis,liver failure,and HCC.37Activated HSCs produce higher amounts of extracellular matrix components that can lead to the fibrotic changes in cirrhosis.38HSCs are regulated by FXR,despite that FXR is expressed at a lower level in HSCs than in hepatocytes.17As mentioned above,in enterocytes and hepatocytes,FXR induces the expression of genes leading to the down-regulation of bile acid synthesis.In HSCs,activation of FXR and deficiency of FGF15 may lead to effects that could potentially improve hepatic fibrosis.
In HSCs,FXR activation in HSCs has anti-fibrotic effects;thus,this can be a potential therapeutic pathway for patients with hepatic fibrosis.Studies showed that HSCs treated with FXR ligands decrease α1(I)collagen synthesis.This was evidenced i.in vitroan.in vivorodent models with nontoxic and noncholestatic liver fibrosis,but the pathophysiologic mechanism is not clear.36In the bile duct ligation rodent model,which induces cholestasis,extensive hepatocyte necrosis,and bile duct proliferation,FXR activation was effective in promoting liver fibrosis resolution.36The experiment used 6alpha-ethyl-chenodeoxycholic acid(6-ECDCA or OCA),which,as a FXR ligand,is about 100-fold more potent than CDCA.37Similarly,SHP is induced in HSCs when treated with FXR ligands,resulting in inhibition of the expression of α1(I) collagen.36Thus,activation of FXR displayed anti-fibrotic effects that could be utilized as a therapeutic outcome.
In HSCs,the development of hepatic fibrosis can be reduced by activation of FXR,which is related to the expression of FGF15.In this pathway,deficiency of FGF15 in mice improved hepatic fibrosis in models of NASH and carbon tetrachloride-induced fibrosis is likely due to increasing in bile acid levels,which subsequently enhances FXR signaling in HSCs.38FGF15 is also hypothesized to act as a direct profibrotic stimulator to HSCs,bu.in vivomouse an.in vitroHSC cell line models did not demonstrate the actions FGF15/19 as such in HSCs.39Instead,the studies in these models suggest that FGF15 deficiency protects against fibrosis in mice potentially through increased bile acids that leads to activation of FXR in HSCs.39
4.FXR agonists and clinical effectiveness
Due to the known beneficial effects of FXR in regulating bile acid homeostasis,lipid metabolism and anti-inflammation,FXR modulators are now in clinical trials for the treatment of NASH.The structures and clinical effects of FXR modulators are summarized in Table 3.5-9,32,40-42The status of FXR modulators in the clinical trials are summarized in Table 4.43-47
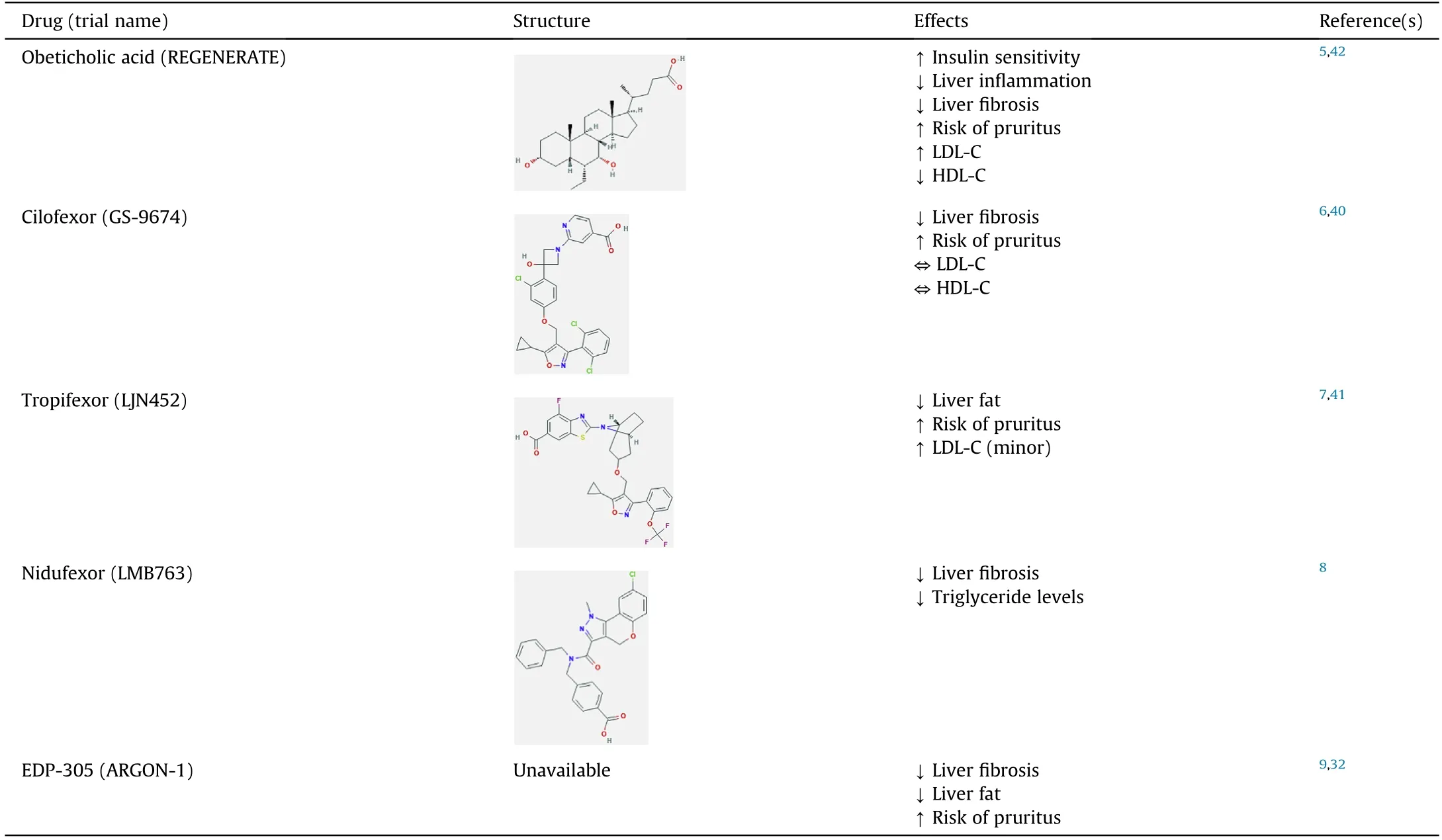
Table 3 Structures and clinical effects of FXR modulators.
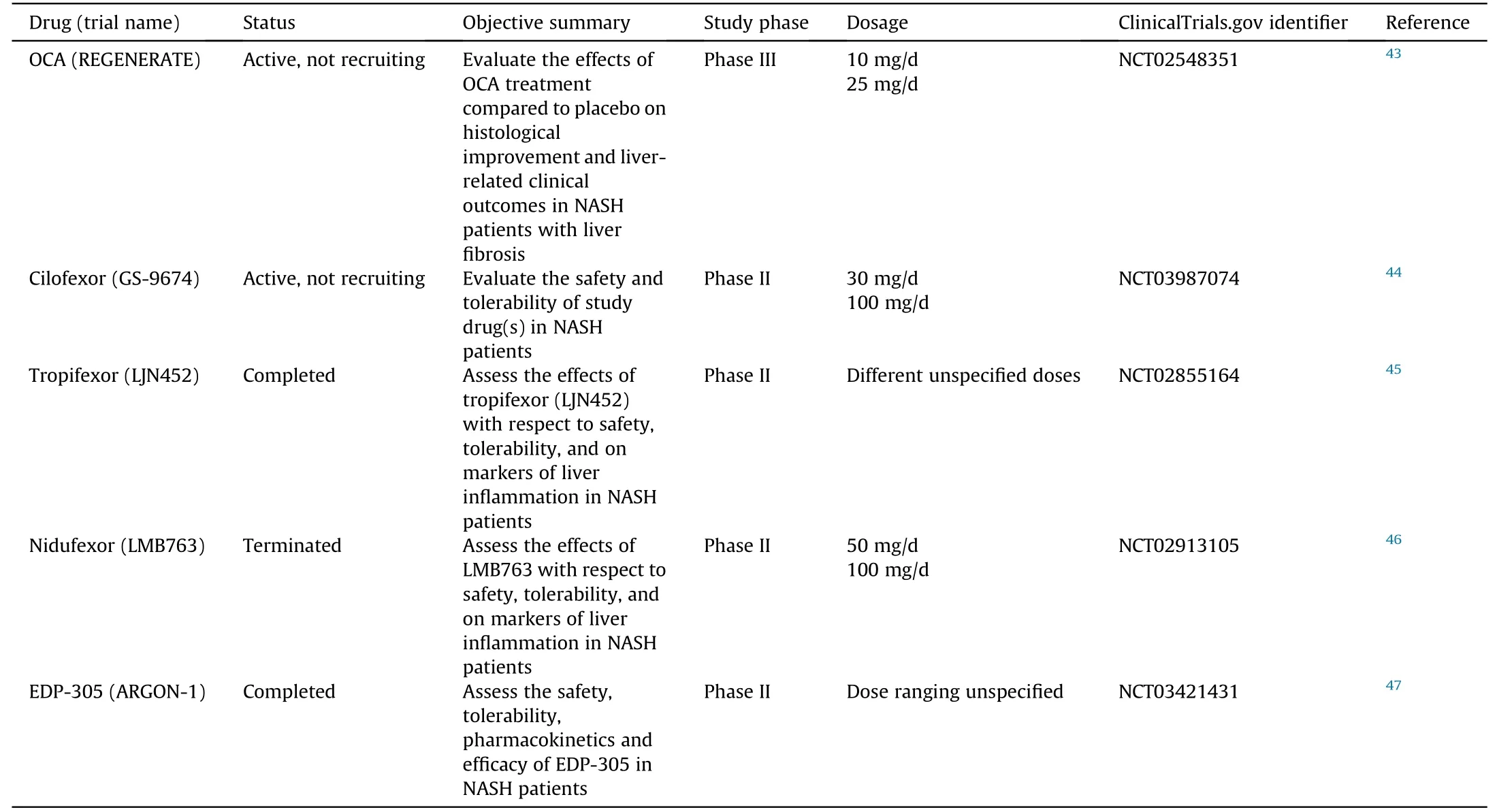
Table 4 Status of FXR agonists in clinical trials on NASH.
4.1.OCA
The first FDA-approved drug for FXR activation is OCA,also known as 6-ECDCA or INT-747,for primary biliary cholangitis.48OCA systemically agonizes FXR and undergoes enterohepatic circulation.49It has been investigated in phase II and phase III clinical studies and showed promising results related to management of cholestatic liver diseases and metabolic syndrome.49OCA is based on the bile acid scaffold of CDCA,the most potent endogenous ligand of FXR.49OCA demonstrates insulin sensitizing,antisteatotic,anti-inflammatory and anti-fibrotic effects in preclinical data in NAFLD animal models.42From Mar 16,2011 to Dec 3,2012,patients were randomly assigned to OCA or placebo.5
This phase IIb clinical trial compared the effect of OCA-25 m.vs.placebo on hepatic histology in NASH patients without cirrhosis showed promising results.5There was a decrease in NAFLD activity score (NAS) >2 points without worsening of fibrosis,in more patients on OCA(45%)than on placebo(21%).5Other results regarding OC.vs.placebo included improvement from hepatic steatosis(61%vs.38%),reduced lobular inflammation (53.vs.35%),less hepatocellular ballooning(46%vs.31%)and reduced fibrosis(35%vs.19%).5NAFLD pathophysiology involves increased bile acid concentrations.OCA maybe a promising drug candidate for NAFLD as OCA decreases bile acid levels.5However,OCA administration also caused side effects such as increased rates of pruritis in patients on higher dosages,an increase in LDL-C,and a decrease in HDL-C in the OCA groups compared to the placebo group.5The overall positive clinical results led to a phase III trial of OCA with a primary objective of evaluating the effects of OCA treatment against placebo on histological improvement and liver-related clinical outcomes in patients with non-cirrhotic NASH with liver fibrosis.50The preliminary results of this phase III trial demonstrate continuous positive clinical outcomes of decreasing hepatic fibrosis.However,in the summer of 2020,the FDA determined that the predicted benefit of OCA based on a surrogate histopathologic endpoint is uncertain and its benefits does not outweigh the potential risks to support accelerated approval for the indication of liver fibrosis due to NASH.51Currently,the FDA recommends additional submission post-interim analysis efficacy and safety data from the ongoing REGENERATE study and continuation long-term outcomes phase.51
4.2.GS-9674
GS-9674,also known as cilofexor,is a small-molecule,nonsteroidal FXR agonist that has been evaluated for safety and efficacy in NASH patients.GS-9674 decreases circulating bile acid levels via intestinal FXR agonism which induces the release of FGF19.40GS-9674 resulted in reduced levels of biomarkers indicating liver damage measured by serum activities of alanine aminotransferase(ALT) and aspartate aminotransferase (AST),hepatic steatosis and fibrosis.40GS-9674 differs from OCA because it does not undergoenterohepatic circulation,while OCA does.40In a randomized,double-blind,placebo-controlled phase II trial in non-cirrhotic patients with NASH,treatment with GS-9674 led to reduced hepatic fat content,inhibited bile acid synthesis,and decreased serum activities of ALT and AST (to a lesser extent than OCA).6Like OCA,pruritis was associated with higher dosage;but unlike OCA,there were no significant changes in LDL-C and HDL-C as levels were comparable to the placebo group.6This was attributed to the fact that GS-9674 is less available systemically as it agonizes FXR more locally in the intestine.6Studies on GS-9674 in monotherapy and combination are still ongoing.
4.3.LJN452
LJN452,also known as tropifexor,is a potent agonist of FXR as demonstrated in rodent models and has moved into phase II clinical trials in patients with NASH.In mouse models,LJN452 treated mice had lower NAS,presented a dose-dependent reduction in ALT/AST activities,and reduced hepatic inflammation,steatosis,and fibrosis.7A phase IIb study showed patients with biopsy-confirmed stage 2-3 fibrotic NASH,when treated with LJN452,displayed dose-dependent reductions in hepatic fat and ALT activity compared to the placebo group at 12 weeks.41
4.4.LMB763
LMB763,also known as nidufexor,is an FXR ligand currently in human clinical trials.It has agonistic behavior on FX.in vivo.LMB763 partially activates FXR target gene.in vitroand acts as a potent and specific gene modulato.in vivo.8Treatment with LMB763 demonstrated a significant decrease in NAS,triglyceride levels,and liver fibrosis.8In rats treated with LMB763,FXR target genes were upregulated and showed a rightward shift in potency compared to tropifexor.8LMB763 has a lack of drug-exacerbated pruritus as it was not observed at any dose in both single ascending dose and multiple ascending dose studies.8Nidufexor is currently in phase II human clinical trials in patients with NASH.8
4.5.EDP-305
EDP-305 is a very selective and potent FXR agonist with notable anti-fibrotic activity.The effects of EDP-305 on fibrosis were tested via treatment of human hepatic stellate cell.in vitroand of mic.in vivowith induced steatohepatitis and peri-sinusoidal fibrosis.The HSCs and mice treated with EDP-305 showed marked decreases in expression of important fibrogenic genes including smooth muscle actin,collagen type I α2,collagen type III α1,metallopeptidase inhibitor 1 and metallopeptidase inhibitor 2.32The anti-fibrotic mechanism of EDP-305 may involve an upregulation in microRNA29a,a vital post-transcriptional regulator of pro-fibrotic genes.32EDP-305 demonstrated positive results for NASH treatment in a 12-week,randomized,double-blind,placebo-controlled Phase IIa study.9Endpoints of statistically significant reduction in ALT activity and liver fat levels were observed in patients with EDP-305.9However,like other FXR agonists,EDP-305 had pruritis as a common treatment-emergent adverse event.9
4.6.INT-767
INT-767 is a semisynthetic bile acid derivative.INT-767 as a dual FXR and Takeda G protein-coupled receptor 5 (TGR5)-a membrane bile acid receptor agonist was recently developed to inhibit liver injury and metabolic disorders.49INT-767 led to hepatoprotection i.Mdr2knockout(KO)mice,evidenced by decreased serum ALT activity,hepatic inflammation,and fibrosis,in addition,significantly increased bile flow and HCO-3output.52In a rat model of NASH,INT-767 had restored the lipid,glucose metabolism to normal level,reduced insulin resistance through FXR-mediated regulation and reverting the dysregulation of FXR target genes involved in liver metabolism,53studies in these animal models suggest INT-767 as a potential therapeutic candidate for liver disease and metabolic disorders.
5.FXR antagonists and clinical status
5.1.Ursodeoxycholic acid (UDCA)
UDCA is a secondary or tertiary bile acid and a metabolic byproduct produced by intestinal bacteria in humans.54UDCA impacts cholesterol synthesis,bile acid synthesis,and lipid homeostasis.55,56One mechanism proposed by which UDCA affects such processes is by exerting antagonistic effects on FXR.55A study in which morbidly obese patients undergone a short-term treatment of UDCA showed a reduction in FGF19 and FXR activation,55,56which led to induction of CYP7A1 and increased bile acid synthesis,and subsequently decreased hepatic and LDL-cholesterol.55UDCA also activated cholesterol synthesis 3-hydroxy-3-methylglutaryl-CoA reductase,a key enzyme in cholesterol synthesis.55UDCA also induced neutral lipid accumulation in the liver as it shifted lipid metabolism to less toxic and more lipogenic fatty acids.55Furthermore,a randomized controlled trial of high dose UDCA for NASH showed beneficial impact on liver function as those with UDCA treatment had a decrease in ALT activity levels and serum markers of fibrosis and hepatic inflammation.57
5.2.Glycine-beta-muricholic acid (Gly-MCA)
Gly-MCA,similar to TβMCA,is a bile acid species found in rodents and antagonizes intestinal FXR.However,Gly-MCA differs from TβMCA as it is not hydrolyzed by gut bacterial derived bile salt hydrolase,suggesting better stability.58Oral administration of Gly-MCA leads to a decrease in levels o.Shpan.Fgf15(FGF19in human)mRNAs in ileum but does not exhibit a dose-dependent manner of inhibiting FXR signaling in ileum.58The significantly decreased ALT and AST activity levels in Gly-MCA treatment group compared with vehicle treatment group demonstrates Gly-MCA does not lead to liver toxicity.58Gly-MCA did not significantly accumulate in liver and mainly stays in ileum,functioning as an intestine specific FXR signaling inhibitor.A study in mice using high-fat diet (HFD)-induced fatty liver disease was performed for proof of concept of Gly-MCA’s therapeutic potential in metabolic symptoms and fatty liver disease.Gly-MCA treatment of mice fed a HFD for 9 weeks had lower liver weights and upon further examination,the liver histology indicated an apparent reduction in hepatic steatosis with no cholestasis,inflammation and necrosis.58A reduction in hepatic triglyceride levels was shown (decreased to 51%).58These results suggest that,at least in mice,Gly-MCA exerts a protective effect from HFD-induced hepatic steatosis.Therefore,although in preclinical trial phase,Gly-MCA may have therapeutic potential for fatty liver disease.
6.FXR induction of FGF19-FGFR4 pathway is associated with HCC in humans
FGF19 is associated with HCC development because of its mitogenic activity.FGF19transgenic mice expression with FGF19 overexpression in skeleton muscles have shown increased hepatocyte proliferation and developed HCC tumors.59Acute treatment of mice with recombinant FGF19 protein altered protein levels for a variety of genes in the livers,including pathways for cell proliferation and cell survival.60FGF19 levels were also increased in HCC patients.61FGF19 inhibition via anti-FGF19 monoclonal antibodies in HCC cells inhibited FGF19 stimulation of FGFR4-mediated events including FGFR4,fibroblast growth factor receptor substrate 2(FRS2) and mitogen-activated protein kinase (MAPK) phosphorylation,an.CYP7A1gene repression.FGF19-FGFR4 signaling is implicated in hepatocellular tumorigenesis.62FGF19 may be important in HCC development because FGF19-blocking antibody 1A6 also effectively inhibited FGF19 chemotactic activity in xenograft tumor models in mice and i.FGF19transgenic mice.63FGFR4 is most discussed compared to the other FGFRs (1,2,3) in HCC development.FGF19 (humans) and FGF15 (mice) activate FGFR4.FGFR4 is important in HCC development as the progeny o.FGF19transgenic mice bred wit.Fgfr4KO mice did not develop liver tumors.64
There are efforts made to eliminate the tumorigenic property of FGF19.Among these efforts,utilizing neutralizing anti-FGF19 monoclonal antibody has demonstrated anti-tumor activity;however,it was accompanied with adverse effects such as dose-related liver toxicity and severe diarrhea in monkeys.65Adverse effects occurred due to the disruption of endogenous FGF19 and its function of reducing bile acid synthesis by anti-FGF19 monoclonal antibodies.These variants prevented the FGF19-FGFR4 binding but impeded the intrinsic metabolic effects of FGF19.65There are studies and clinical trials also specifically targeting FGFR4 in the FGFR4-FGF19 pathway for the treatment of HCC.The development of these FGFR4 inhibitors ranges from preclinical stages to phase II clinical trials and the inhibitors demonstrate anti-tumor activity to varying extents.66
Another study reported an engineered FGF19 variant NGM282(also known as M70)that maintains the role of FGF19 in regulating bile acid homeostasis and lacks tumorigenic activity.In this study,animals were injected with adenovirus vectors (AAV) either containing the negative control,FGF19,or M52 non-tumorigenic variant of FGF19,FGF19-M52,which differs from wild-type FGF19 by five amino acid substitutions.67The plasma levels of transgene expression were considerably higher in FGF19-M52-treated mice compared to green fluorescent protein control (AAV-GFP(8.38±6.28)pg/m.vs.AAV-FGF19-M52(434.50±83.65)pg/mL).67There was no change in relative mRNA levels o.Cyp7a1between the FGF19 variants,suggesting no substantial change in the variants’ability to suppressing bile acid synthesis.67However,notably,the M52 variant did not display any tumorigenic activity while FGF19 administered mice had increased number of tumors per liver,increased liver weight and liver/body weight ratio compared to controls.67
FXR activation and expression of FGF19 could be potential therapeutic options,but carcinogenicity concerns can limit its clinical utility.FGF19 variants that maintain their roles in bile acid metabolism and express no tumorigenicity could be a solution to this problem.However,it is unclear whether an increase in FGF19 is a compensatory effect to reduce toxic bile acid levels or is .bonafideHCC promoter.Furthermore,there are studies showing that human FGF19 did not activate mouse FGFR4,and mouse FGF15 did not activate human FGFR4,suggesting the roles of FGF19 determined in mouse studies are less clear.68,69Therefore,long-term human epidemiological studies are urgently needed to clarify the human relevance.
7.NGM282 in clinical trials
NGM282 is an engineered FGF19 analogue that has shown therapeutic potential in treating cholestasis and NASH.NGM282 has been shown to be effective in the prevention of progression from simple steatosis,NASH,and to advanced “burnt-out” NASH with HCC in a mouse model.29Not only for the reduction of liver injury index,a reduction in inflammation was observed as well as mRNA levels for markers of monocytes/macrophages were decreased in mice with NGM282 treatment,suggesting immune cell infiltration into hepatocytes was decreased.29A 12-week treatment with NGM282 has yielded similar beneficial results such as anti-fibrotic,anti-steatotic,and anti-inflammatory effects in NASH patients.Though like OCA,NGM282 treatment showed side effects including increased cholesterol levels.The coadministration of rosuvastatin in the NGM282-treated patients improved lipid profiles.70,71The mechanism of action of NGM282 involves activating FGFR4 to inhibi.CYP7A1gene expression;the study proposes the altered bile acid pool may add to antiinflammatory and anti-fibrotic benefits beyond those associated with liver fat reduction.70Regarding clinical potential further studies are needed;a phase IIb,randomized,double-blind,placebocontrolled,24-week paired biopsy study in patients with NASH is enrolling patients.70
8.Conclusions
There are multiple factors contributing to NASH pathophysiology,bile acid regulation is proposed to have a major role in simple steatosis-to-NASH progression.Currently,there is no FDA approved treatment,but rather,lifestyle modifications are recommended for NASH patients.FXR is a potential drug target,and FXR modulators are promising candidates in clinical trials,which have displayed positive improvement effects on NASH development and fibrosis(Fig.1).We think that further research should be conducted to determine the mechanisms by which FXR functions in a cellspecific manner,FXR antagonism plays in liver diseases,and FXR agonists cause pruritis.
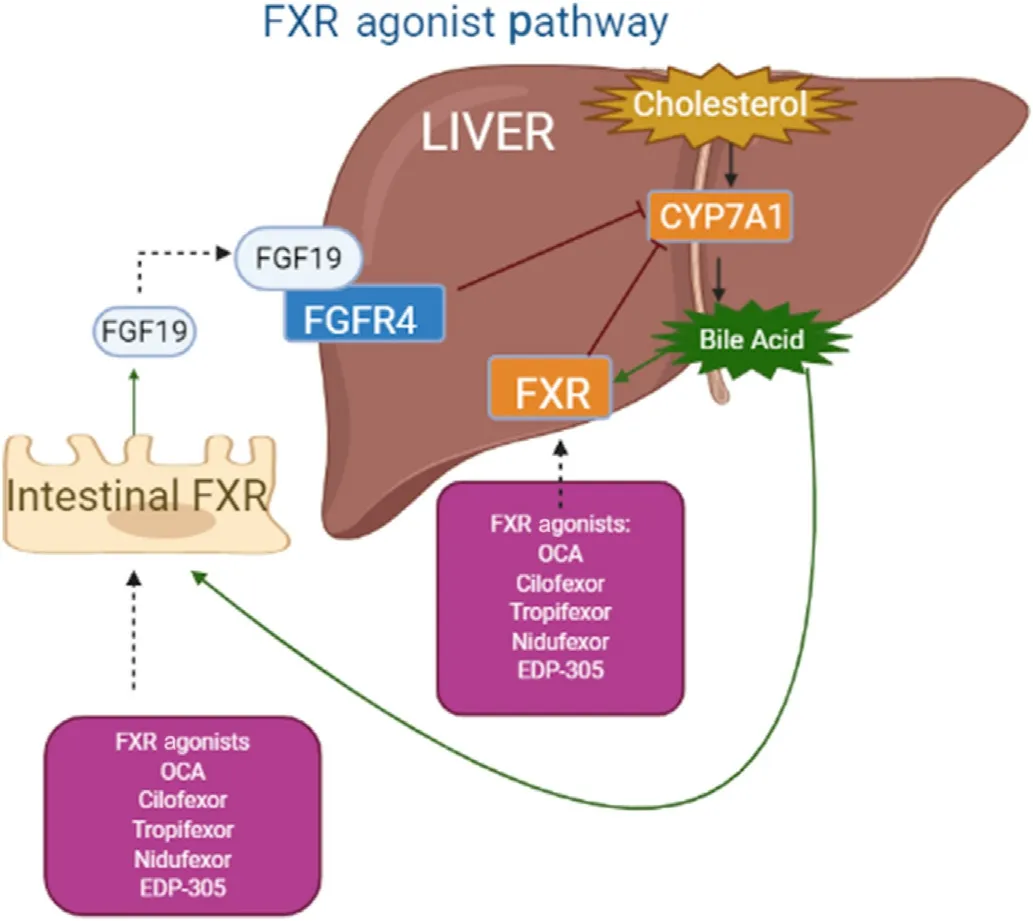
Fig.1.Intestinal FXR activation induces FGF19 expression in humans,which is considered a major mechanism in suppressing bile acid synthesis in the liver through the inhibition of gene expression of CYP7A1 and CYP8B1.
Authors’ contributions
S.Maliha wrote the manuscript,G.L.Guo wrote and revised the manuscript.
Declaration of competing interest
The authors declare that they have no conflict of interest.
Acknowledgements
Grant funding (USA National Institutes of Health:R01GM135258-GLG,R21ES029258-GLG,Veteran Administration:BX002741-GLG).We thank Dr.Curtis D.Klaassen for critically reviewing the manuscript.
杂志排行
Liver Research的其它文章
- Bile acid metabolism and bile acid receptor signaling in metabolic diseases and therapy☆
- Gut microbiome in liver pathophysiology and cholestatic liver disease☆
- Mitigation of cholestasis-associated hepatic and renal injury by edaravone treatment:Evaluation of its effects on oxidative stress and mitochondrial function☆
- Metformin alleviates cholestasis-associated nephropathy through regulating oxidative stress and mitochondrial function☆
- Bile acids and metabolic surgery☆
- Bile acid activated receptors:Integrating immune and metabolic regulation in non-alcoholic fatty liver disease☆