Zinc,copper,and strontium isotopic variability in the Baiyangping Cu–Pb–Zn–Ag polymetallic ore field,Lanping Basin,Southwest China
2021-09-10CaixiaFengShenLiuGuoxiangChiXianwuBiRuizhongHuIanCoulson
Caixia Feng• Shen Liu• Guoxiang Chi• Xianwu Bi• Ruizhong Hu•Ian M.Coulson
Abstract The Baiyangping Cu–Ag polymetallic ore district is located in the northern part of the Lanping-Simao foreland fold belt,between the Jinshajiang-Ailaoshan and Lancangjiang faults,and the deposit can be divided into eastern and western ore zones.Based upon microscope observation of ore minerals and analysis of zinc,copper,and strontium isotope composition,we conclude that:(1)the zinc isotopic compositions of sphalerite from the eastern and western ore belt of the Baiyangping polymetallic ore deposits are enriched in both the heavy(-0.09‰ to+0.15‰) and light (-0.19‰ to -0.01‰)zinc isotopes.Rayleigh fractionation is likely the additional factor controlling the observed temporal and spatial variations in zinc isotopes in the two studied ore zones.The zinc isotopic composition in the Baiyangping polymetallic Pb–Zn deposits may have the same fractionation as that of magmatic-hydrothermal,VHMS,SEDEX,and MVT deposits,as demonstrated by geological and other geochemical evidence;(2) the range of δ65Cu in massive tetrahedrite is from -0.06‰ to+0.12 ‰ that relates to the early stages of ore-formation,which are higher than that of venial chalcopyrite (from -0.72‰ to -0.07‰)formed at a late ore-forming stage in the western ore belt.Different ore-forming stages and alteration or leaching processes are likely the main factors controlling the observed variations in copper isotopes in the western ore zone;(3) the 87Sr/86Sr value of hydrothermal calcite in eastern (0.7080–0.7093) and western (0.7085–0.7113) ore belt suggested that mineralization of early calcite,with 87Sr/86Sr values much higher than in ancient Late Triassic seawater,may be related to recrystallization from a radiogenic Sr-rich or silicifying fluid,either from the strata that the ore-forming fluid flows through or from other fluids.
Keywords Zn–Cu–Sr isotopic variation · Cu–Pb–Zn–Ag poly-metallic ore deposit · Baiyangping · Lanping Basin
1 Introduction
Fe,Cu,Zn,Mo,Li,and Mg are examples of non-traditional metal stable isotopes (Zhu et al.2001;Luck et al.2003,2005;Norman et al.2004;Johnson et al.2004a,b;Poitrasson et al.2005;Weyer et al.2005).Copper has two isotopes,63Cu,and65Cu,which in nature occur in the proportions:69.17% and 30.83%,respectively (Shields et al.1965).By contrast,zinc has five isotopes:64Zn,66Zn,67Zn,68Zn,and70Zn,naturally occurring in the following proportions:48.63%,27.92%,4.10%,18.75%,and 0.62%,respectively (Rosman 1972).With the advent of multicollector-inductively coupled plasma-mass spectrometry(MC-ICP-MS)(Mare´chal et al.1999),it is now possible to study the isotope geochemistry of transition metals in natural systems (Thomas et al.2005).Analytical protocols for Zn isotope analysis by MC-ICP-MS were developed in the late 1990s (Halliday et al.1995;Mare´chal et al.1999)and early 2000s (Rehkämper et al.2001;Mare´chal and Albare`de 2002;Archer and Vance 2004;Johnson et al.2004a,b;Chapman 2006),and analyses based upon these protocols show substantial natural variations.Zinc isotope ratios are expressed in the δ notation(δ66Zn=[(66Zn/64Zn)sample/ (66Zn/64Zn)standard-1] × 1000).In the case of copper,the isotopic ratio is commonly expressed in the following δ notation (δ65Cu=[Rsample/RNIST976--1] × 1000,where R=65Cu/63Cu) (Zhu et al.2000).MC-ICP-MS measurements of Cu isotopic variations in natural samples with small analytical errors (2σ=0.05‰)were first reported by Mare´chal et al.(1999).Subsequently,many precise isotopic compositions of Cu were measured by MC-ICP-MS with analytical errors of typically 0.03‰–0.04‰ (1σ),for both Zn doped and sample standard bracketing (SSB) techniques (Zhu et al.2000;Mare´chal and Albare`de 2002).
In this paper,we present new zinc-,copper-,and strontium-isotope data for sphalerite,tetrahedrite,chalcopyrite,and gangue minerals,collected from the eastern and western ore blocks in sediment-hosted,Cu–Ag–Pb–Zn polymetallic deposits of the Baiyangping ore district in western Yunnan,China.It’s located in the northern part of the Lanping-Simao foreland fold belt,between the Jinshajiang-Ailaoshan and Lancangjiang Faults.This area is divided into eastern and western ore zones (Xu and Li 2003;He et al.2005,2009;Hou et al.2007,2008;Feng et al.2011b),and is interpreted to have formed in a continental collision setting,controlled by thrust tectonics,in the strongly deformed Lanping sedimentary Basin (He et al.2005,2009;Hou et al.2007,2008;Xue et al.2007).The area contains important reserves of Ag (4150 t),Cu(32.07 Mt),Pb(22.78 Mt),Zn(17.74 Mt),and Co(1444 t)(Third Geology and Mineral Resources Survey 2003).Previous studies of the Baiyangping district have examined the formation of the sedimentary basin,the structural evolution of the area,and the sources of ore metals and hydrothermal fluids (Tian 1997,1998;Gong et al.2000;Xue et al.2002,2003,2007;Zhu et al.2002;Shao et al.2003;Wang and He 2003;Xu and Li 2003;Yang et al.2003;Chen et al.2004;He et al.2004,2005,2006,2009;Zhang 2005;Fan et al.2006;Liu et al.2010;Wang et al.2011;Feng et al.2011b),which demonstrates that these two ore blocks differ from common ore deposit models,such as magmatic-hydrothermal type,volcanic-hosted massive sulfide(VHMS),sedimentary exhalative(SEDEX)and typical Mississippi valley-type (MVT) deposits (He et al.2005,2009;Feng et al.2011b).
The availability of detailed information on the geology,mineralogy,and geochemistry of the study area allows for an investigation of the variability in zinc,copper,and strontium isotopes within these deposits,and to identify the primary controls on any identified isotopic variation.In documenting and interpreting the first zinc,copper,and strontium isotope data for the two ore blocks,we use these data,in combination with previous research results,to discuss and elucidate the possible controls on the zinc,copper,and strontium variations during Pb–Zn mineralization,and sources of the various metals and fluids in an ore-deposit generation.
2 Geological setting
2.1 Regional geology and tectonic setting
The Sanjiang area in southwest China(Fig.1a)is a tectonic belt located between the Tethyan-Alpine realm and the western Pacific and has experienced Proto-Tethyan,Paleo-Tethyan,and Meso-Tethyan phases of evolution (Li et al.1991;Zhong and Ding 1993).The complexity of the structure of the crust in this area has created favorable conditions for mineralization (Chen 1991;Luo et al.1994;Liao and Chen 2005;Hou et al.2006,2007,2008).
The Mesozoic-Cenozoic Lanping Basin is an important part of the Sanjiang tectonic belt;it lies between the Lancangjiang and Jinshajiang Fault zones (Fig.1b).The basin underwent a complex history of tectonic evolution,from Late Triassic rifting through Jurassic-Cretaceous subsidence and early Tertiary foreland development to final incorporation in the Lanping-Simao foreland fold belt(Wang et al.2001),as a consequence of crustal shortening in eastern Tibet related to Indo-Asian collisional tectonics(Wang and Burchfiel 1997).
The Lanping Basin lies at the triple junction between the Eurasian,Indian,and Pacific plates,which is a complex tectonic environment that generated conditions favorable for the formation of the renowned Sanjiang polymetallic ore belt that is exceptionally enriched in copper (Chen 1991;Hou et al.2008).The Baiyangping district is located in the northern part of the Lanping Basin.The eastern part of the basin is part of the Yangtze Plate,while the western part forms part of the Tibet-Yunnan Plate (Fig.1b;Xue et al.2007).Thrust-nappe systems are a significant deformation style in the Lanping fold belt,which resulted from Indo-Asian collision and subsequent oblique convergence during the Paleocene to Eocene.Two approximately E–Wstriking geological cross-sections across the northern Lanping Basin show that the main thrust Faults dip to the west in the western segment and to the east in the eastern segment of the basin (He et al.2009).The distributions of the eastern and western ore belts are governed by the thrust-napped system;the belts are associated with frequent tectonic and magmatic activity forming complex and diverse geological landscapes (Fig.1c).The district shows multi-stage mineralization and a variety of ore-forming elements and petrological characteristics (Yin et al.1990;Tian 1997,1998;Li et al.1999;Chen et al.2000;Yang et al.2003;Xue et al.2007;He et al.2009).
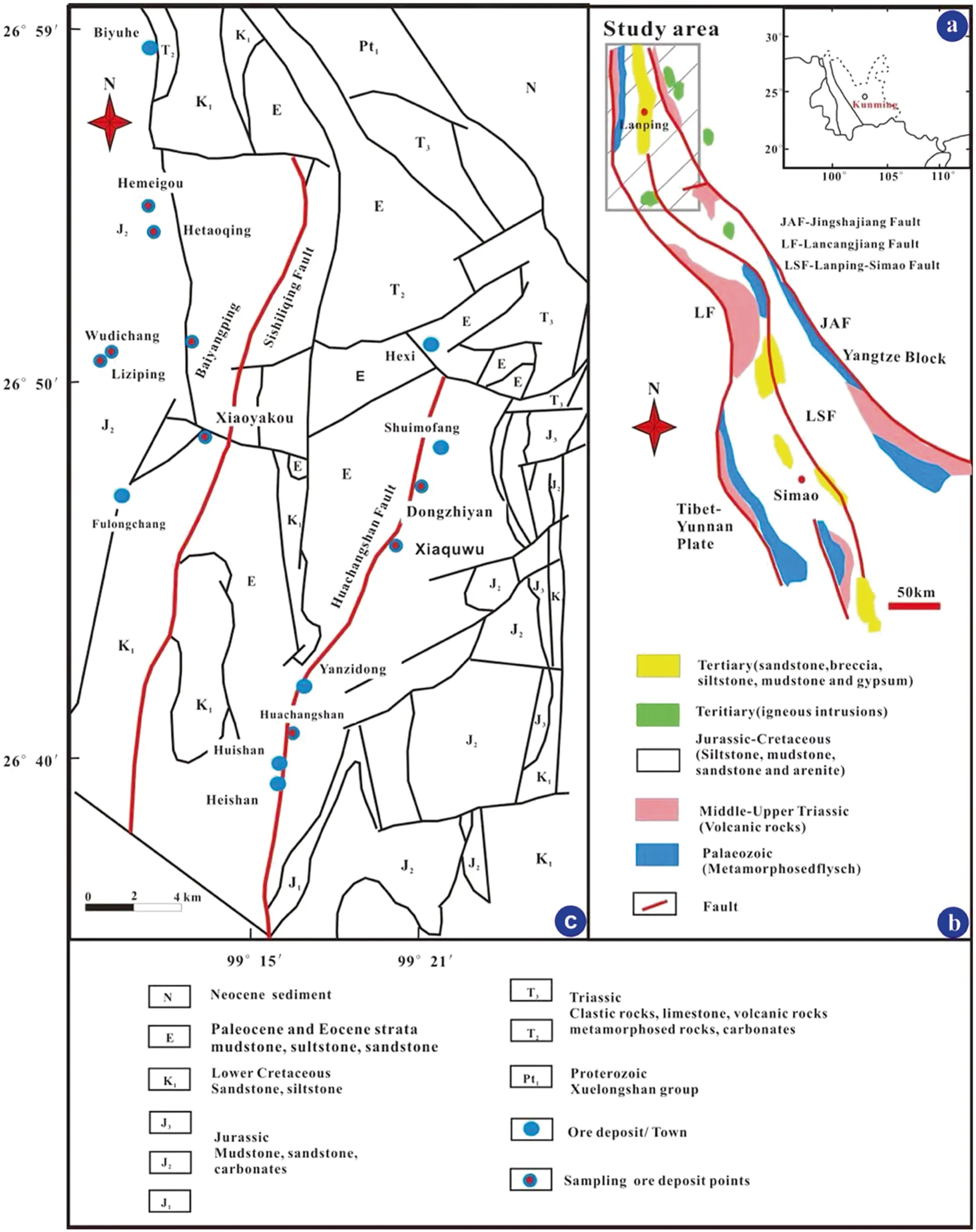
Fig.1 a Map illustrating the location of the study area in southwest China;b tectonic location and geological sketch map of the Lanping basin highlighting the strata and faults present; c geological sketch map of the Baiyangping Cu–Pb–Zn–Ag polymetallic ore deposit,Lanping basin,southwestern China indicating the strata,faults present,and distribution of ore deposits (Xue et al.2007;Feng et al.2011b)
2.2 Stratigraphy
The exposed strata in the Baiyangping mining district are Mesozoic and Cenozoic continental ‘‘red-bed’’ clastic formations.There are several sedimentary gaps in the stratigraphic column of the Lanping Basin (Qin and Zhu 1991;Xue et al.2002).The basin is filled with siliciclastic rocks,except for the lowest part of the sequence and the Late Triassic Sanhedong Formation (T3s),which consists mainly of fossiliferous marine limestone (Xue et al.2002,2006).The Mesozoic-Cenozoic strata that crop out in the ore district are summarized as follows (Fig.2;Xue et al.2002,2006;He et al.2009;Feng et al.2011b):E2g:Eocene Guolang Formation,comprising argillaceous sandstone,siltstone,and gypsum;E1y:Paleocene Yunlong Formation,comprising breccia,sandstone,and gypsum in the upper part,and siltstone,mudstone,and gypsum in the lower part;K2h:Middle Cretaceous Houtousi Formation,comprising quartz arenite and arkosic arenite;K1j:Lower Cretaceous Jingxing Formation,comprising coarse-grained sandstone and arenite with coal seams and plant fossils;J2h:Middle Jurassic Huakaizuo Formation,comprising siltstone and mudstone with abundant fossils;T3m:Late Triassic Maichuqing Formation,comprising continental gray to black shale,siltstone,and fine-grained sandstone with local coal seams and plant fossils;T3wl:Late Triassic Waluba Formation,comprising continental mudstone and siltstone;T3s:Late Triassic Sanhedong Formation,comprising marine limestone and dolomitic limestone,andT3w:Late Triassic Waigucun Formation,comprising conglomerate and sandy mudstone.TheN2s(Sanying Formation),N2j(Jianchuan Formation),E3,andJ1y(Yangjiang Formation) strata are absent within the Lanping Basin but occur in other areas in the Lanping-Simao fold belt (He et al.2009).
2.3 Eastern ore zone
The eastern (or Sanshan-Hexi) ore zone is the second-largest ore district in the Lanping Basin and contains significant reserves of Cu (~0.3 Mt),Ag (>3000 t),Pb+Zn(>0.5 Mt),and Sr,with grades of 1.6%–3.3%Zn,0.81%–3.55%Pb,and 23–220 g/t Ag(Third Geology and Mineral Resources Survey 2003;He et al.2009).Fifteen economic ore bodies are located in the eastern ore zone,and these occur in the form of veins,lenses,weakly-stratified and irregularly-shaped bodies.The dimensions of these ore bodies are between 200 and 1000 m in length and between 2 and 8 m wide (Third Geology and Mineral Resources Survey 2003;Liu et al.2010).
The eastern ore zone consists of the Hexi,Xiaquwu,Dongzhiyan,Yanzidong,Huishan,Heishan,and Huachangshan ore blocks that are distributed mainly along the Huachangshan thrust Fault(Fig.1c).The belt generally dips to the east but locally dips to the west in the vicinity of the Dongzhiyan-Xiaquwu block.A fractured zone occurs in this block,being 10–20 m in width and exhibitinglithological zoning,from an inner compressional schistose zone to an outer zone of fractured rock.Most of the ore blocks occur along the thrust fault and within zones of fracturing in the hanging-wall carbonate sequence (T3s).Approximately E–W-trending strike-slip faults in the eastern thrust system commonly truncate the ore bodies,indicating that the faults postdate mineralization.The host sequence of the eastern ore zone consists of carbonates of the Late Triassic Sanhedong Formation (T3s) and underlying sandstones of the Paleocene Yunlong (E1y) and Eocene Baoxiangsi formations (E2b).T3s carbonates,a significant ore horizon,are composed of thick gray brecciated limestone,dolomitic limestone,and dolomite in the lower part,and gray brecciated siliceous limestone and muddy limestone in the upper part (Fig.2).The upper carbonate sequence hosts ore bodies in the Huishan,Heishan,and Huachangshan blocks,whereas the lower carbonate sequence hosts ore bodies in the other ore blocks.

Fig.2 Stratigraphic column for the Baiyangping Cu–Pb–Zn–Ag polymetallic ore deposit,Lanping basin,southwestern China (He et al.2009)
Alteration of the host rocks is dominated by dolomitization,calcification,and silicification.Alteration mineral assemblages consist of pyrite,barite,fluorite,calcite,celestite,dolomite,and minor quartz.Ore minerals include sphalerite,galena,pyrite (marcasite),and Cu-sulfides (tetrahedrite,Ag-and As-tetrahedrite,chalcocite,chalcopyrite,and bornite) with minor tenorite,cerussite,smithsonite,azurite,and covellite.Gangue minerals consist of calcite,celestite,siderite,dolomite,barite,fluorite,and minor quartz (Chen et al.2000;He et al.2009).
2.4 Western ore zone
The western (or Baiyangping-Fulongchang) ore zone is a large ore district in the Lanping Basin.The Baiyangping ore block contains reserves of Cu(0.22%–5.0%),while the Fulongchang ore block contains reserves of Ag(0.0041%–0.065%),Pb (1.0%–6.5%),and Cu (0.43%–4.20%) (Third Geology and Mineral Resources Survey 2003;Liu et al.2010).
The western ore zone comprises the Baiyangping,Fulongchang,Wudichang,Liziping,Hetaoqing,Xiaoyakou,and Hemeigou ore blocks,which are distributed mainly along the Sishiliqing thrust Fault (Fig.1c).Approximately NE-striking second-order faults,related to an N–S-striking thrust fault and an E–W-striking strike-slip fault in the western thrust system,commonly truncate the ore bodies.The Fulongchang ore block is dominated by Cu–Ag and is locally rich in Pb,Zn,and Co.Deposits include the Fulongchang (Baiyangping) Cu–Ag–Pb–Zn deposit,the Hetaoqing Cu–Ag deposit,and the Wudichang Zn–Pb–Ag deposit (Fig.1b;He et al.2009).The host sequence of the western ore zone consists of quartz sandstone,siltstone,and mudstone of the Early Cretaceous Jinxing Formation (K1j),and mudstone,sandstone,and carbonate of the Middle Jurassic Huakaizuo Formation(J2h) (Fig.2).
Alteration of the host rocks is dominated by weak silicification and carbonatization.Alteration mineral assemblages are consisting of pyrite,barite,fluorite,calcite,celestite,dolomite,and minor quartz.Copper-bearing sulfides include tetrahedrite,arsenian tetrahedrite,Ag-bearing tetrahedrite,chalcopyrite,bornite,and chalcocite.Other sulfides are pyrite,pyrrhotite,sphalerite,and galena (He et al.2009).Gangue minerals are dominated by quartz,with minor calcite,ankerite,barite,siderite,chlorite,and rare bitumen and graphite (Zhao 2006;He et al.2009).
3 Sampling and analytical methods
3.1 Zn and Cu isotope samples
Samples for Zn isotope analyses were collected from the Sanhedong Formation (T3s),in the Huangchangshan,Xiaquwu,and Dongzhiyan ore blocks in the eastern ore zone,and from the Huakaizuo (J2h) and Jingxing (K1j) formations in the Liziping,Wudichang,Hemeigou,Baiyangping,and Hetaoqing ore blocks,in the western ore zone of the Baiyangping ore district (Lanping,Yunnan Province,China) (Fig.3).Two distinct stages of sphalerite mineralization from the eastern and western ore belts have been identified,based upon mineral paragenesis,textural and structural characteristics,and cross-cutting relationships.These are (1) early sphalerite:automorphic,hypidiomorphic,and xenomorphic granular textures are the main ore textures,and this sphalerite occurs as a disseminated ore(Fig.3a,d) and;(2) late-stage sphalerite:sphalerite veins in calcite,automorphic granular texture,coarse-banded vein texture,and sphalerite cross-cutting earlier minerals(Fig.3b,e).Samples for Cu isotope analyses were collected from the Huakaizuo (J2h) and Jingxing (K1j) formations in the Liziping,Wudichang,Hemeigou,Baiyangping,and Hetaoqing ore blocks in the western ore zone of the Baiyangping ore district (Lanping,Yunnan Province,China)(Fig.4).Tetrahedrite and chalcopyrite are associated with ankerite and celestite veins.Automorphic/xenomorphic tetrahedrite occurs as disseminated ore and chalcopyrite occurs in veins cutting the tetrahedrite.
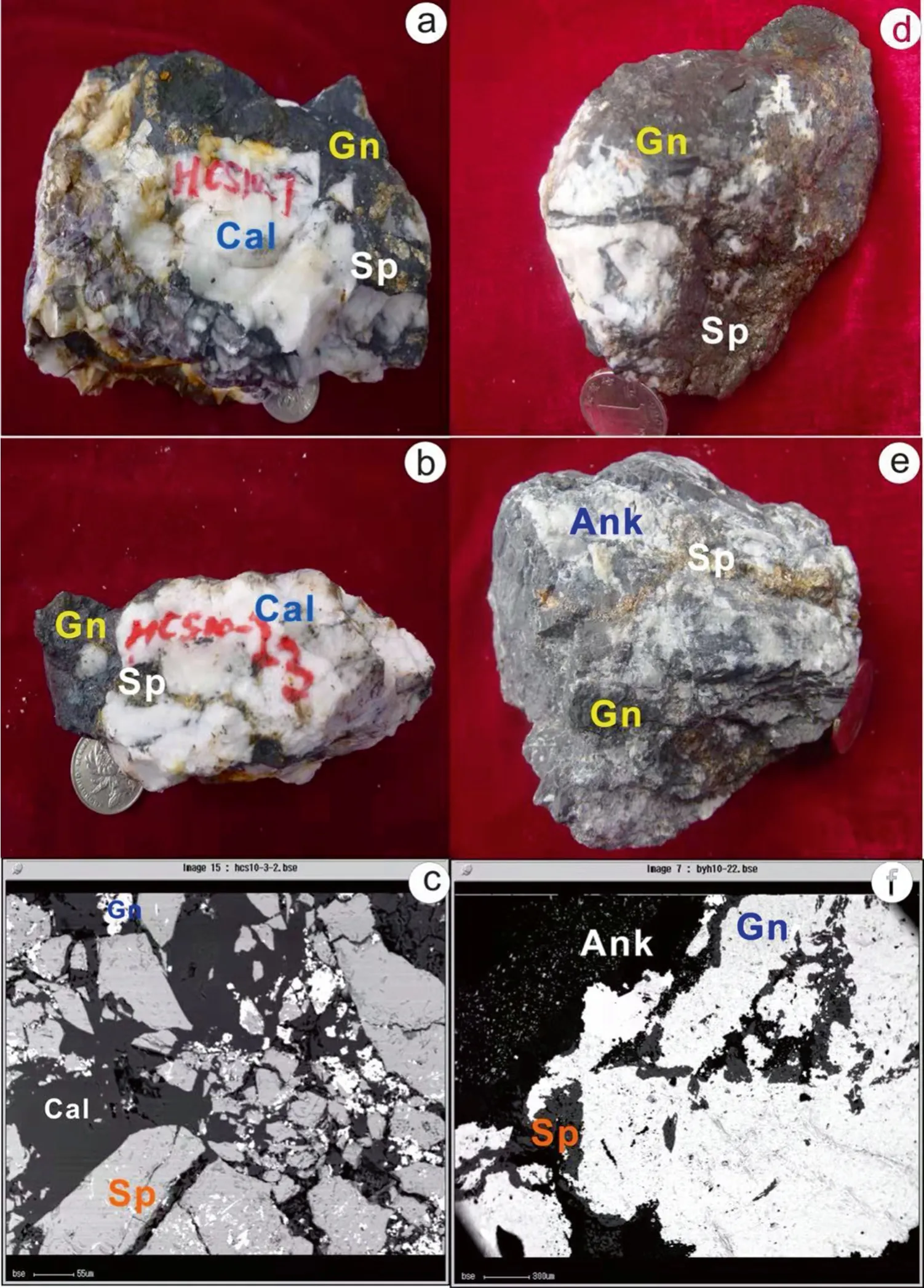
Fig.3 Photos of sphalerite and galena ore samples and backscattered electron (BSE)images of ore in eastern and western ore belts.Sp.Sphalerite,Gn. Galena, Cal. Calcite, Ank.ankerite, a–c represent ore samples in the eastern ore belt,d–f represent ore samples taken from the western ore belt.a Early-stage sphalerite-massive Gn.+disseminated Sp.+vein Cal.; b late-stage sphaleritemassive Gn.+vein Sp.+massive Cal.; c BSE image of Gn.+Sp.+Cal.;d early-stage sphaleritedisseminated and massive Sp.+massive Gn.+massive Cal.; e late-stage sphalerite,massive Gn.+vein Sp.+massive Ank; f BSE image of Gn.+Sp.+Ank
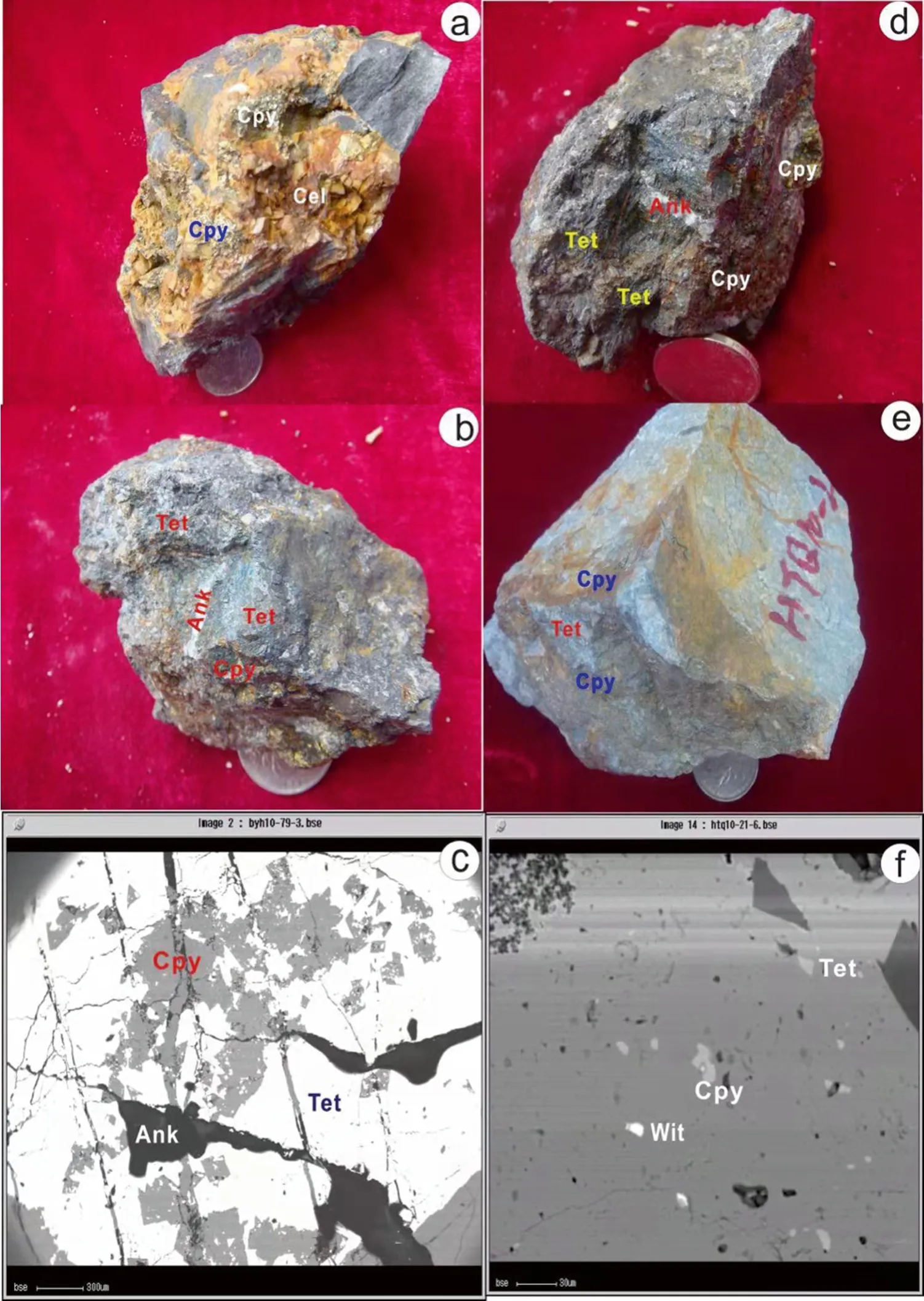
Fig.4 Photos of chalcopyrite and tetrahedrite ore samples and BSE images of ore in the western ore belt. Ccp.chalcopyrite, Cel. celestite, Tet.tetrahedrite, Cal. calcite, Ank.ankerite, Wit. wittichenite.
After petrographic observations,sulfide minerals(sphalerite,chalcopyrite,and tetrahedrite) from mineralization-stage rocks were trimmed to remove altered surfaces and then separated from their host rocks by crushing and handpicking.The picked samples were cleaned with deionized water and crushed and powdered in an agate mill.All samples were subsequently selected by handpicking under a binocular microscope.The identification of mineral phases was performed by electron probe microanalysis (Shimadzu EPMA-1600) at the State Key Laboratory of Ore Deposit Geochemistry,IGCAS.
3.2 Sr isotope samples
Samples for Sr isotope analyses were collected from the Sanhedong Formation (T3s) in the Huachangshan,Xiaquwu,and Dongzhiyan ore blocks within the eastern ore zone,and in the Liziping and Baiyangping ore blocks in the western ore zone of the Baiyangping ore district,Lanping,Yunnan Province,China.Samples were petrographically examined before gangue (calcite and dolomite),ore,and wall-rock samples were trimmed to remove altered surfaces,cleaned with deionized water,and crushed and powdered in an agate mill.Doubly polished calcite and dolomite thin sections were prepared from the selected samples and examined by standard microscopy before further analysis.The contents of Rb–Sr and Sr isotopic compositions were undertaken using thermal ionization mass spectrometry at the IGCAS.
3.3 Copper,zinc,and strontium isotope measurements
Complete separation of Cu and Zn in sulfide samples was achieved using anion exchange chromatography.Resin AGMP-1 was used as the anion exchange resin,and 7 mol/L HCl for Cu and 0.5 mol/L HNO3for Zn were used as eluents.In this case,Cu and Zn can be well separated from Co and other elements,as sulfide samples have low contents of Na+,Ca2+,Mg2+,and Al3+(Tang et al.2006b).The basic principle,the method of chemical separation,and the separation results of multi-element standard solutions and geological standard samples have been discussed previously (Tang et al.2006b;Tang et al.2006a).The copper and zinc isotope measurements were undertaken using a Nu Plasma (HR) MC-ICP-MS at the Isotope Geological Laboratory,Institute of Geology,Chinese Academy of Geological Sciences (IGCAGS),Beijing,China.The factors affecting mass discrimination are essential for mass bias correction during isotopic measurements using MCICP-MS.We used the SSB (sample-standard bracketing)method relative to the NIST976 (63Cu/65-Cu=2.24 ± 0.0021) copper standard for instrumental mass discrimination (Zhu et al.2000;Cai et al.2006;Li et al.2008a,b).Due to the lack of reference standard of JMC-Lyon,IRMM-3702 is increasingly used as the Zn reference standard.And the difference can be calculated byδ66ZnJMC-Lyon=δ66ZnIRMM-3702+0.28‰(Archer et al.2017).So we used the δ66ZnJMC-Lyon=0.37441 (Rosman 1972;Loss and Lu 1990),got the standard value of δ66ZnIRMM-3702 is 0.09441.It is proposed that HCl is a better medium than HNO3for sample introduction for Cu and Zn isotope measurements using MC-ICP-MS.The repeatability of65Cu/63Cu and66Zn/64Zn measurements in 0.1 mol/L HCl solution is 0.00008 (2σ),which is better than that for HNO3(Cai et al.2006;Li et al.2008a,b).
Strontium was extracted using cation exchange chromatography with 3 mol/L HNO3.Strontium was leached from the crushed sample powders using a procedure modified by Simonetti (2008).Approximately 100 mg of powder was leached overnight in 4 mL of cold 0.6 N HCl in clean Savillex 15-mL beakers.The sample was transferred into a centrifuge tube and diluted to 10 mL with doubly distilled water and centrifuged for 10 min at 1000 rpm.The supernatant was then filtered through Qualitative No.4 filter paper,evaporated to dryness and then re-dissolved in 1 mL of 2.5 N HCl.Samples were analyzed for strontium isotopic composition on a thermal ionization mass spectrometer.The total procedural blanks were 2–4 pg Sr and measurement of standard sample NBS987 yielded87Sr/86Sr=0.710262 ± 7.
4 Results
Zinc isotope data in samples from the western and eastern ore zones of the Baiyangping polymetallic ore district reveal the following.1.The δ66Zn value of sphalerite samples from the eastern ore zone ranged from -0.09‰to+0.15‰,with a mean value of+0.01‰.Sphalerite,which precipitated in the early and late mineralization stages of the eastern ore zone,yields δ66Zn values of -0.09‰ to -0.02‰ and+0.03‰ to+0.15‰,respectively (Table 1).2.The δ66Zn value of sphalerite samples from the western ore zone (Liziping ore block) ranges from -0.21‰ to -0.01‰,with a mean value of -0.12‰;only one sample gave a high value (+0.05‰).Two sphalerite samples from the Wudichang ore block gave δ66Zn values of -0.09‰and+0.04‰,with a meanvalue of -0.03 ‰ (Table 1).Sphalerite,which precipitated during the early and late stages of mineralization in the western ore zone,yields δ66Zn values of -0.21‰to -0.09‰ and -0.04‰ to+0.05 ‰,respectively(Table 1).

Table 1 Zn isotopic composition of sphalerite in Baiyangping Cu–Pb–Zn–Ag polymetallic ore deposit,Lanping basin,southwestern China
Copper isotope data of the western ore zone of the Baiyangping polymetallic ore district yielded the following results.(1) The δ65Cu value of chalcopyrite and tetrahedrite samples from the Hemeigou ore block ranged from -0.20‰to -0.07‰and from 0.00‰to+0.12‰,respectively.Only one tetrahedrite sample from the Wudichang ore block had a δ65Cu value of+0.05‰.(2)The δ65Cu value of tetrahedrite and chalcopyrite samples from the Baiyangping ore block ranged from -0.08‰to -0.06‰ and from -0.72‰ to -0.29‰,respectively;the δ65Cu value of tetrahedrite samples from the Hetaoqing ore block ranged from -0.02‰ to+0.06‰(Table 2).Of note,in the western ore zone,tetrahedrite precipitated in the early mineralization stage,whereas chalcopyrite precipitated in the late mineralization stage.

Table 2 Cu isotopic composition of chalcopyrite and tetrahedrite in Baiyangping Cu–Pb–Zn–Ag polymetallic ore deposit,Lanping basin,southwestern China

Table 3 Rb–Sr data and 87Sr/86Sr ratios analysis conducted on the calcite in Baiyangping Cu–Pb–Zn–Ag polymetallic ore deposit,Lanping basin,southwestern China
The strontium isotopic compositions of eight samples(calcite and dolomitized calcite) from the eastern and western ore zones are listed in Table 3.The value of87Sr/86Sr in the eastern ore zone ranges from 0.7080 to 0.7093;the data show a trend of87Sr/86SrHuachangshanore block>87Sr/86SrXiaquwuoreblock>87Sr/86-SrDongzhiyanoreblock,the sole exception being dolomitized calcite sample HCS10-19 (87Sr/86Sr=0.7080).The value of87Sr/86Sr in the western ore zone ranges from 0.7085 to 0.7113,showing a greater spread than the range in the eastern ore zone.
5 Discussion
5.1 Zinc isotopes
Zinc isotopes have been used as tracers of (1) Biogeochemical and chemical processes (Mare´chal et al.2000;Zhu et al.2002;Pichat et al.2003;Pokrovsky et al.2005;Bermin et al.2006;Gelabert et al.2006;Vance et al.2006;Weiss et al.2005);(2) The terrestrial rocks (Ben Othman et al.2001,2003,2006);(3) Different sources of zinc(Mare´chal et al.1999,2000;Helal et al.2002) and (4)Climate change (Pichat et al.2003).In addition,Zn isotopes have increasingly been used over the last decade in studies of different types of ore deposits and their genesis:e.g.,the Alexandrinka volcanic-hosted massive sulfide deposit (Mason et al.2005);the carbonate-hosted Zn–Pb deposits of the Irish Midlands ore field (Wilkinson et al.2005;Crowther 2007);several modern submarine hydrothermal systems (John et al.2008);the Red Dog shale-hosted base metal deposit (Kelley et al.2009);several deposits in the Tongling area of Anhui,China (Wang and Zhu 2010);the world-class Navan Zn–Pb orebody,Ireland (Gagnevin et al.2012);the Jinding Zn–Pb deposit of Yunnan,China (Tang 2013);Mississippi Valley-type carbonate-hosted zinc deposits in Cantabria,Spain(Pasˇava et al.2014),and carbonate-hosted Pb–Zn sulfide deposits in southwest China (Zhou et al.2014).
Based upon previous results,it is suggested that variations in zinc isotope compositions are controlled by three main factors:the temperature gradient(Mason et al.2005),kinetic Rayleigh fractionation (John et al.2008;Kelley et al.2009;Mare´chal and Sheppard 2002;Wilkinson et al.2005;Gagnevin et al.2012),and the mixing of multiple zinc sources (Wilkinson et al.2005).
Sphalerite from the Baiyangping polymetallic ore district displays distinctly different Zn isotopic characteristics between the two ore zones.Samples from the eastern ore zone are enriched in heavy isotopic zinc,whereas samples from the western ore zone are enriched in light zinc(Table 1;Fig.3,5).Two types of aqueous fluids in these two ore zones have been identified from fluid inclusion studies (Xu and Li 2003;Chen et al.2004;He et al.2009;Feng et al.2014).The range in homogenization temperature (Th) values for these fluids is 92 to 283 °C (mean 152 °C,peak values at 130–140 °C) for the western ore zone,and 101 to 295 °C (with averages of 158.6 °C for calcite,176.8 °C for dolomite,162.2 °C for dolomitized limestone,151.9 °C for Celestine,172.3 °C for quartz,and 145.6°C for sphalerite,peak values at 120–200 °C)for the eastern ore zone (Feng et al.2014).The mean Th values generally display an increase from the western to the eastern ore zones.Pasˇava et al.(2014)concluded that high Zn isotope values are consistent with the rapid precipitation of sphalerite from Zn–Cl species at higher temperatures,while low Zn isotope values result from the fractionation of aqueous Zn sulfide species at lower temperature and higher pH.It is noted that the high Zn isotope values have not resulted from temperature but the Zn-complexes.Just like Fujii et al.(2011)suggested that sulfides precipitating from hydrothermal solutions should be isotopically lighter than the solution,but the extent of isotope fractionation decreases with temperature.Mare´chal and Sheppard(2002)conducted zinc isotope fractionation experiments at different temperatures.The α (fractionation factor) values of ZnCl2and Zn (NO3)2are nearly equal to 1.00004 and 1.00011(the reaction of ZnCl2and Zn (NO3)2with calcite at 30 °C and 50 °C,respectively),indicating that the change of temperature has no obvious effect on the fractionation of Zn isotopes.Therefore,although there is a temperature gradient variation between the western and eastern ore zones,whether the temperature has an effect on Zn isotope fractionation needs to be further studied in this paper.
The increased δ66Zn values from the early to late-stage resulted from the Rayleigh fractionation of zinc isotopes.During fluid evolution in a hydrothermal system,the fluid becomes enriched in heavier Zn isotopes with the precipitation of minerals,and the Zn isotope composition of latestage minerals is heavier than that of early-stage minerals(Archer et al.2004;Mason et al.2005;Wilkinson et al.2005;John et al.2008;Kelley et al.2009;Wang and Zhu 2010;Gagnevin et al.2012).Sphalerites from both ore zones show such a trend in zinc isotope evolution(Fig.5),indicating that Rayleigh fractionation likely occurred.This process has been used to explain the variations in zinc isotopes in several deposits,including the Alexandrinka deposit (VHMS-type:-0.03‰ to+0.23‰;Mason et al.2005),the Irish Midlands deposit (Irish-type:-0.18‰to+0.64‰;Wilkinson et al.2005),the Red Dog deposit(SEDEX-type:0‰ to+0.60‰;Kelley et al.2009),the Navan deposit (Irish-type:-0.32‰ to+0.23‰;Gagnevin et al.2012),the Jinding Zn–Pb ore deposit in Yunnan,China (-0.85‰ to+0.05‰;Tang 2013),and carbon-hosted Pb–Zn sulfide deposits in southwest China(-0.26‰ to+0.58 ‰ and+0.07‰ to+0.71‰,respectively;Zhou et al.2014) (Fig.5).Thus,Rayleigh fractionation is likely to have been the additional factor controlling the observed temporal and spatial (Fig.5)variations in zinc isotopes in the two studied ore zones.Due to the obvious overlap of isotope data between different types of deposit,it is further suggested that the zinc isotopic composition (Table 1;Fig.5) in the Baiyangping polymetallic Pb–Zn deposits may have the same fractionation as that of magmatic-hydrothermal,VHMS,SEDEX,and MVT deposits,as demonstrated by geological and other geochemical evidence (He et al.2009;Feng et al.2014).

Fig.5 Zn isotopic compositions for the various types of the ore deposit and for those from the study area(Mare´chal et al.1999,2000;Helal et al.2002;Pichats et al.2003;Albare`de 2004;Mason et al.2005;Kelley et al.2009;Gagnevin et al.2012;Tang 2013)
5.2 Copper isotopes
Copper isotopes have been used to study significant Cu isotopic variations in soils (Bigalke et al.2010,2011;Liu et al.2014);bacteria-metal interactions in natural waters,soils,and rocks as well as isotope fractionation during adsorption onto metal oxy-hydroxides and kaolinite (Balistrieri et al.2008;Pokrovsky et al.2008;Navarrete et al.2011;Liu et al.2014;Li et al.2015);modern black smokers(Zhu et al.2000;Rouxel et al.2004;Berkenbosch et al.2015);redox weathering of Cu-bearing sulfides(Mathur et al.2012);massive sulfide deposits(Mason et al.2005);porphyry copper deposits (Graham et al.2004;Mathur et al.2005,2009;Li et al.2010);skarns (Graham et al.2004;Maher and Larson 2007);sedimentary copper mineralization (Asael et al.2007),and other hydrothermal ore deposits (Jiang et al.2002;Larson et al.2003;Markl et al.2006).
The natural variations in copper isotope ratios have been attributed to a number of processes,including liquid–vapor separation,multi-step equilibrium processes,redox reactions,physicochemical parameters (Eh,pH,andtemperature),and the involvement of organisms(Mare´chal et al.1999;Zhu et al.2000,2002;Jiang et al.2002;Larson et al.2003;Ehrlich et al.2004;Graham et al.2004;Rouxel et al.2004;Asael et al.2007;Mathur et al.2009).Different types of deposit show distinct changes in the isotopic composition of copper:for example,high-temperature hydrothermal deposits generally show a narrow range of δ65Cu values (Zhu et al.2000;Jiang et al.2001;Jiang 2003;Albare`de 2004;Markl et al.2006);epithermal and sedimentary deposits are characterized by low values of δ65Cu and a somewhat restricted range (-3.7‰ to +0.5‰) (Jiang et al.2001,2002;Jiang 2003;Qian et al.2006;Asael et al.2007);and the oxidation of ore leads to a wide range of δ65Cu values (-3.4‰to+2.41‰)(Markl et al.2006;Asael et al.2007).
Massive tetrahedrite from the western ore zone,which precipitated during the early ore-forming stages,shows δ65Cu values(-0.06‰to+0.12‰)that are heavier than those of vein chalcopyrite (-0.72‰ to -0.07‰),which formed during the late ore-forming stages.In Fig.6,we can see the most typical range of δ65CuNBS978values for hypogene chalcopyrite from magmatic sulphide ores,intrusion-related hydrothermal systems (e.g.,porphyry copper and base metal skarn ores),granite-related and nonmagmatic polymetallic vein deposits,and sediment-hosted copper ores.Determined Cu isotope values for samples from the study area fall within the field for sediment-hosted copper ores (Fig.7).Cu(0),Cu(I),and Cu(II) minerals occur across the globe.Although the valence of Cu in chalcopyrite has been the subject of much debate,the most recent mineralogical studies suggest that the valence state of most Cu in chalcopyrite is Cu(I)(Goh et al.2006;Pearce et al.2006).Fractionation between Cu(I) in minerals (e.g.,chalcopyrite)and aqueous Cu(II)in leachate can be as high as 2.7‰(Zhu et al.2002;Ehrlich et al.2004;Mathur et al.2005;Kimball et al.2009).Given that the samples of the present study are for chalcopyrite and tetrahedrite,which are both Cu(I) minerals (Fig.6),and δ65Cu values of tetrahedrite(-0.06‰to+0.12‰)are heavier than those of vein chalcopyrite (-0.72‰ to -0.07‰),our data suggests that different minerals with the same valence can display evidence for isotopic fractionation.In addition,previous studies have shown that the fractionation of copper isotopes between different coexisting sulfide minerals can be up to 0.4‰ in moderate-to high-temperature porphyry deposits(Larson et al.2003;Graham et al.2004).The influence of fractionation between different copperbearing minerals should be considered for the sake of rigorous logic in the discussion,even though the values may not be large.

Fig.6 Ranges of copper isotope data for different valence copper minerals (Markl et al.2006)

Fig.7 Ranges of copper isotope data for global reservoirs,as well as for different types of ore deposits and those of the study area(Larson et al.2003;Markl et al.2006;Maher et al.2007;Asael et al.2007;Li et al.2010;Ferenc et al.2016)
Different stages of mineralization are characterized by dissimilar Cu isotopic contents(Zhu et al.2000;Jiang et al.2001;Harson et al.2003;Rouxel et al.2004;Mason et al.2005).Copper sulfides that formed in the early stages are rich in65Cu whereas those that precipitated from late-stage fluids are commonly65Cu-depleted.Supergene Cu minerals yield a wide range of δ65Cu values (-8.4‰ to +9.1‰),with a general shift towards isotopically heavy compositions,as compared with the inferred precursor minerals (Mason et al.2005).The Cu isotope contents of chalcopyrite and tetrahedrite from the western ore zone show such a trend in copper isotope evolution (Fig.7),indicating that the difference in Cu isotopes could have developed in different ore-forming stages.Experimental studies show that65Cu tends to be transferred into minerals from solution (Pekala et al.2011;Gregory and Mathur 2017);therefore,the Cu isotopic composition of the solution and minerals will increase as the reaction proceeds.However,the δ65Cu values decrease from the early stage to the late stage in the western ore belt,which may be caused by the mixture of ore-forming fluids with different δ65Cu values.This process has been used to explain variations in copper isotopes in several deposits,such as black smoker sulfide chimneys on the ocean floor (Zhu et al.2000);the large isotopic variations in Cu (δ65Cu from -3.70‰to+0.30‰) observed in a sediment-hosted hydrothermal vein-type deposit from Jinman,China (Jiang et al.2002);variations in copper isotopes in magmatic and hydrothermal ore-forming environments (Larson et al.2003);and variations in copper isotopes in the Alexandrinka volcanichosted massive sulfide deposit (Mason et al.2005).In addition,it has been shown that post-mineralization leaching can be significantly modifying the Cu isotopic data (Larson et al.2003;Graham et al.2004).Hence,alteration or leaching process should also be involved,same as to the description of microscopical photographs(Fig.4c,f).Thus;different ore-forming stages and alteration or leaching process are likely the main factors controlling the observed (Fig.7) variations in copper isotopes in the western ore zone.
5.3 Strontium isotopes
Numerous studies have shown that Sr-isotope ratios (e.g.,87Sr/86Sr)can be used to constrain the source of ore fluids.Studies of the isotopic composition of Sr in Sr-bearing minerals(e.g.,calcite,fluorite,and dolomite)not only may help to determine the source of ore elements but also can provide important constraints on the genesis of hydrothermal deposits (Kesler et al.1983;Lange et al.1983;Norman and Landis 1983;Barbieri et al.1987;Galino et al.1994;Fanlo et al.1998;Savard et al.2000;Peng et al.2001).
Rb cannot substitute for Ca2+in the calcite lattice,in contrast to Sr,which shows limited substitution that leads to very small Rb/Sr ratios in calcite (Deer et al.1966).Consequently,the composition of87Sr (decayed from Rb)has little impact on the initial Sr isotopic composition.It is then reasonable to assume that the87Sr/86Sr ratio in calcite is the initial Sr isotopic composition of the ore-forming fluid from which the calcite precipitated (Kesler et al.1983;Lange et al.1983;Norman and Landis 1983;Barbieri et al.1987;Galino et al.1994;Fanlo et al.1998;Savard et al.2000;Peng et al.2001).
Since the residence time of strontium in seawater(2–4 Myr)is much longer than the mixing time of seawater(~1.5 × 103a),it is considered that the distribution of strontium isotopes in seawater is uniform (Brannon et al.1991).The precipitated carbonate minerals in seawater show no significant Sr isotopic fractionation,and the Sr isotopic composition of carbonate minerals is consistent with that of seawater (Viezer 1989).Therefore,the87Sr/86Sr ratio in the Late Triassic Sanhedong Formation carbonate rocks should be similar to that of Late Triassic seawater(0.7076–0.7078)(Korte et al.2003).The87Sr/86Sr values of hydrothermal calcite from both the eastern and western ore zones are nevertheless much higher than the value of Late Triassic seawater but are consistent with those of hydrothermal calcite from the Jinding Pb–Zn deposit (Fig.8a,0.7078–0.7119,Luo et al.1994;0.7097–0.7104,Tang 2013).The high87Sr/86Sr value of hydrothermal calcite may,therefore,be related to recrystallization(Fig.8b,c,d,e)from a radiogenic Sr-rich or silicifying fluid,either from the strata that the oreforming fluid flows through or from other fluids (Savard et al.2000).

Fig.8 a Sr isotopic composition of calcite from the Baiyangping Cu–Pb–Zn–Ag polymetallic ore deposit,Lanping Basin,southwestern China; b massive Cal.+Sp.(Huachangshan ore block,eastern ore belt); c massive Cal.+Gn.(Liziping ore block,western ore belt);d wide-vein Cal.microscopic image,symbiosis with Sp.+Gn.+Qrz. e massive Cal.Microscopic image,symbiosis with Gn. Cal.Calcite, Sp. Sphalerite, Gn. Galena, Qrz. quartz
6 Conclusions
1.The zinc isotopic composition in the Baiyangping polymetallic Pb–Zn deposits may have the same fractionation as that of magmatic-hydrothermal,VHMS,SEDEX,and MVT deposits,as demonstrated by geological and other geochemical evidence.
2.The δ65Cu values of early-stage massive tetrahedrite from the western ore zone are from -0.06‰ to+0.12‰,heavier than those of vein chalcopyrite(from -0.72‰ to -0.07‰) that formed during the late ore-forming stage.Different ore-forming stages and alteration or leaching process are likely the main factor controlling the observed variations in copper isotopes in the western ore zone.
3.The87Sr/86Sr values of hydrothermal calcite in both the eastern and western ore zones suggest that the initial87Sr/86Sr values of early calcite were much higher than the value of Late Triassic seawater,which may be related to recrystallization from a radiogenic Sr-rich or silicifying fluid,either from the strata that the ore-forming fluid flows through or from other fluids.
AcknowledgementsWe are grateful to Prof.Zhu XK for guidance and help during Cu,Zn isotope experiments.This research was financially supported by General Project of Natural Science Foundation of Shaanxi Province (2020JM-423).
杂志排行
Acta Geochimica的其它文章
- Variations of methane stable isotopic values from an Alpine peatland on the eastern Qinghai-Tibetan Plateau
- Quantifying aluminosilicate manganese release and dissolution rates across organic ligand treatments for rocks,minerals,and soils
- Concentration determination of gold nanoparticles by flame atomic absorption spectrophotometry
- Evaluating soil erosion by water in a small alpine catchment in Northern Italy:comparison of empirical models
- Pressure calibration and sound velocity measurement to 12 GPa in multi-anvil apparatus
- Olivine and Cr-spinel as indicators of the petrogenesis and partial melting conditions of the high-MgO ultramafic volcanic rocks from NW Ad Dhala Province—Yemen
