Concentration determination of gold nanoparticles by flame atomic absorption spectrophotometry
2021-09-10YuhongFuQuanWanZonghuaQinShanshanLiSenLiJiWangWeiZhang
Yuhong Fu• Quan Wan• Zonghua Qin• Shanshan Li• Sen Li•Ji Wang• Wei Zhang
Abstract While engineered nanoparticles are widely used and maybe eventually released into the environment,natural nanoparticles are also commonly found in the Earth system.Nanoparticles may critically affect the geochemical migration of associated elements and pose potential threats to the ecological environment.It is necessary to establish an accurate and reliable method for measuring the concentration of nanoparticles.AAS is one of the most commonly used methods for the concentration determination of nanoparticles.However,till now,there has been no systematic report on how experimental variables affect AAS measurements.In this study,we used gold nanoparticles(AuNPs)as an example and studied the influences of a list of factors on the concentration determination of AuNPs by AAS,including digestion method,ionization interference,acidic medium,background correction method,and organic matter.We demonstrate that all these factors may have varying degrees of influence on the measured gold concentrations.When the gold colloid is digested at room temperature for more than 8 h or at 60°C for more than 2 h,and the system contains a low concentration of organic matter,AAS can accurately measure the AuNP concentration at ppm-level.The deuterium lamp background deduction method is not recommended to use for samples with lower gold concentrations.
Keywords Gold nanoparticles · Atomic absorption spectrophotometry ·Quantitative analysis ·Organic matter
1 Introduction
Nanoparticles,including both natural nanoparticles and engineered nanoparticles,are abundant in the Earth system(Hochella et al.2008).By estimation,natural nanoparticles formed only by biogeochemical processes reached about 1012kg per year (Barnard and Guo 2012).Due to large specific surface area,as well as differences in surface and near-surface atomic structure,nanoparticles have extraordinary chemical and physical properties comparing with their bulk counterparts.Therefore,nanoparticles could influence many important geochemical processes,including geochemical migration,cycling of the associated elements,and mineral growth/solubility/weathering(Hochella et al.2008;Hochella,2019;Reich et al.2011).Despite their ubiquity throughout the 4.54 billion-year-old Earth system,nanoparticles and their impacts on the Earth system are not fully understood,and one of the most important reasons is the lack of analytical tools,standard method,and procedures to quantify the concentration,morphology,compositions,and reactivities of nanoparticles (Hochella et al.2019).
Among metallic nanoparticles,gold nanoparticles(AuNPs) have been found in several hydrothermal and supergene gold deposits with important economic value(Hong et al.1999;Hough et al.2011;Osovetsky 2016;Palenik et al.2004;Reich et al.2005).For example,in the Carlin-type deposits,AuNPs with a diameter of 5–10 nm have been demonstrated by transmission electron microscopy (TEM) (Palenik et al.2004;Reich et al.2005).Yet,despite decades of research,a thorough understanding of the metallogenesis of these gold deposits remains outstanding (Muntean and Cline 2018;Romanchenko et al.2007).In addition,due to their unique properties,engineered AuNPs are wildly used in the fields of catalysis,medical diagnosis,chemical sensing,etc.(Daniel and Astruc 2004).Because commercial products containing AuNPs will be eventually released and accumulated into the environment,potential risks of AuNPs to human health and their impacts on the environment have already raised widespread concerns (Davarpanah and Guilhermino 2019;Pacheco et al.2018;Unrine et al.2012;Wiwanitkit et al.2009).In order to assess the enrichment and ore formation mechanism,as well as the environmental implication of AuNPs,a large number of laboratory simulation studies have been carried out with synthesized AuNPs,including sorption/deposition of AuNPs on various minerals (e.g.,clay particles Fu et al.2020;Zhu et al.2009),sulfide minerals (Luo et al.2018;Mikhlin et al.2011),and hematite colloids(Smith et al.2015)),experimental studies on the migration of AuNPs in environmental media(Motellier et al.2019;Reith and Cornelis 2017),studies on toxicity and cellular uptake of AuNPs (Alkilany and Murphy 2010;Jiang et al.2013)etc.Undoubtedly,accurate and quantitative determination of AuNP concentrations is an essential prerequisite not only for the evaluation of their geochemical and environmental effects but also for the industrial production and quality control of AuNP products(Gillespie et al.2012;Godoy et al.2021;Jimenez et al.2021;Mankovskii and Pejovic-Milic 2020;Pan 2007).
Nevertheless,quantitative analysis of nanoparticles is still of considerable challenges.Most quantitative analytical methods (including wet chemistry and instrumental analyses) are established mainly for dissolved organic/inorganic molecules/ions (with size smaller than 1 nm) in solutions.Comparatively,the mass or particle number of aerosol or colloidal particles (greater than 100 nm) can be usually obtained using analytical methods based on light scattering or particle movement.Nanoparticle dispersions are different from solutions or colloidal dispersions of larger particles.Each individual nanoparticle (size range 1–100 nm) is an independent solid phase in the liquid dispersion,which is often theoretically considered thermodynamically unstable due to large surface energy.The total mass concentration of nanoparticles in a typical dispersion is often very small,while the exact composition and structure of some nanoparticles (e.g.,AuNPs) is still not completely clear (Sardar et al.2009).Consequently,many traditional analytical methods especially those based on the colligative properties of solutions may not be suitable for quantitative analysis of nanoparticles (Gillespie et al.2012;Yu and Andriola 2010).Conversely,validation studies are usually necessary before any particular analytical method is attempted to quantitatively analyze nanoparticle dispersions (Godoy et al.2021).
For quantification of gold in AuNP dispersions,mass spectrometry,electrochemical methods,and spectroscopic methods have been usually applied (Hendel et al.2014;Meermann and Nischwitz 2018;Yu and Andriola 2010).Among them,atomic absorption spectrophotometry(AAS)is extensively used since it is a simple,fast,and low-cost technique suitable for a wide variety of elements(Gillespie et al.2012;Godoy et al.2021).However,till now,there is no systematic report on how operational parameters,as well as sample preparation procedures,affect the AAS determination of AuNP concentrations.For example,Gillespie et al.(2012) and Jao et al.(2011) showed that acetylene/air flame AAS could determine the total concentration of gold in AuNP dispersions without any digestion,while other studies normally used aqua regia for chemical digestion of AuNPs(Khlebtsov et al.2020;Yazid et al.2010).In this work,we experimentally evaluated a number of conditions that may influence the quantification of AuNPs by AAS.Based on our results,a viable method for AAS determination of AuNP concentrations is proposed.
2 Materials and methods
2.1 Reagents
Chloroauric acid tetrahydrate (HAuCl4•4H2O) (≥99.9%)was purchased from Shanghai Jiuyue Chemical Reagent Company,China.Sodium citrate dihydrate (≥99.0%) was purchased from Shanghai Shenbo Chemical Reagent Company,China.Hydrochloric acid (36~38%) and nitric acid (65~68%) were purchased from Sinopharm Chemical Reagent Company,China.Sodium chloride(≥99.5%) was purchased from Shanghai Guangnuo Chemical Technology Company,China.Potassium chloride (≥99.5%) was purchased from Shantou Xilong Chemical Company.All chemicals were of analytical or guaranteed reagent grade and were used without further purification.Before use,all glassware and magnetic stir bars were thoroughly soaked in aqua regia (HCl/HNO3-=3:1,V/V),and then rinsed with copious amounts of deionized water.Deionized water was obtained from a Millipore synergy UV system (resistivity,18.2 MΩ•cm).The gold standard solution (GSB G 62068-90,1000 μg/mL) was purchased from the National Iron and Steel Material Testing Center,China.A series of gold-standard solutions containing 1.0–18 mg/L gold were diluted from the 1000 μg/mL standard by 10%(V/V)HCl or 10%(V/V)aqua regia.
2.2 Synthesis of AuNPs
The citrate-based reduction method (also known as Frens method) is one of the most commonly used methods for preparing AuNPs,and was thus used to synthesize our AuNP samples (Frens 1973).Briefly,300 mL of chloroauric acid solution (HAuCl4,0.01%,w/w) was heated to boiling under reflux and vigorous magnetic stirring.Thereafter,11.2 mL of a freshly prepared sodium citrate solution(1%,w/w)was quickly added to the boiling solution and stirred for 15–20 min.A wine-red colloid was obtained and cooled down to ambient temperature.The asprepared gold colloid (~60 mg/L) was stored in a refrigerator at 4 °C and protected from light and was diluted to~12 mg/L for AAS measurements.The size and morphology of the prepared AuNPs were characterized using a transmission electron microscope (TEM;JEM-2000FXII,JEOL,Japan) operated at 160 kV.Particle size analysis was performed using ImageJ (US National Institutes of Health) software.
2.3 Concentration determination of AuNPs by AAS
Acetylene/air flame AAS (990SUPER,Persee,China)equipped with a gold hollow cathode lamp was used to analyze gold concentrations.A number of potential factors were selected to study their effects on the quantification of AuNPs including AuNP digestion method,ionization interference,acidic medium,organic matter,and background correction.
2.3.1 AuNP digestion method
Since the degree of AuNP digestion could affect the analysis results,we studied the influence of digestion time and digestion temperature.The main chemical digestion methods of gold colloids include aqua regia digestion and HBr/Br2digestion (Jao et al.2011).Due to the strong volatility and toxicity of HBr/Br2solution,aqua regia was chosen for the digestion of AuNPs.The chemical reaction equations of gold digestion by aqua regia are as follows:

In order to test the effect of different digestion time and temperature on the digestion of gold colloids (CAu),an aqueous solution of HAuCl4(SAu) with a known concentration (0.01%,w/w) was used as a reference.First,2 mL of SAuand CAurespectively was added into a 10 mL volumetric flask,then 1 mL of fresh-prepared aqua regia was added for digestion at room temperature or 60 °C for different times.After digestion,ultrapure water was added to make up the digested solution to a constant volume of 10 mL,which was then analyzed for the gold concentration with AAS.
2.3.2 Ionization interference
The flame is the energy source for the atomization of the measuring element and is also the carrier of the ground state atoms.For the limited types of commonly used flames,the optimal range of flame temperature is narrow.When the flame temperature is not high enough to completely atomize the element,the sensitivity of the measurement is reduced.On the other hand,if the flame temperature is too high,the ground state atoms will gain further energy to ionize into ions,and the resulting ionization interference will also reduce the sensitivity of the measurement.A method to remove ionization interference is to add an element such as an alkali or alkaline earth metal (also called an ionization inhibitor) with a lower ionization potential than that of the measured element.Under the same conditions,the ionization inhibitor preferentially ionizes,generating a large number of electrons,thereby inhibiting the ionization of the ground state atoms of the measured element.In order to test the influence of ionization interference,a certain concentration of potassium chloride or sodium chloride (as an ionization inhibitor) was added to the gold standard solution,and the linearity of the standard curve and the change in the measured gold concentration were investigated.
2.3.3 Acidic medium
The gold standard and sample solutions are usually prepared in an acidic medium.Different acidic media may interfere with the AAS test due to the introduction of different anions.The gold standard stock solution (1000 mg/L) was diluted with 10% (V/V) HCl aqueous solution and 10%(V/V)aqueous aqua regia solution respectively.Then the absorbance of the standard solutions was measured to test the influence of acidic medium on the linearity of the standard curve and the accuracy of measured gold concentration.
2.3.4 Background correction
For line source AAS,there are essentially three background subtraction methods including the deuterium lamp method,Zeeman method,and Smith-Hieftje method.Among them,the deuterium lamp method is the oldest but still most widely employed background correction technique,which uses the attenuation of the deuterium continuum radiation from broadband molecular absorption and light scattering as the background signal.It works in the ultraviolet region that covers the most sensitive absorption line of gold.We tested background correction using the deuterium lamp method.
2.3.5 Organic matter
Since an excessive amount of sodium citrate(~239 mg/L)existed in our gold colloid samples,we tested the influence of different sodium citrate concentrations on the AAS determination of gold concentration.Specifically,different amounts of sodium citrate were added to our gold colloid sample,a gold standard solution (5.0 mg/L gold),and water,respectively.After digestion and dilution,the gold concentrations were determined with AAS and were used to indicate the effect of sodium citrate.
3 Results and discussion
3.1 Morphology and size of AuNPs
Figure 1 presents the TEM image of our synthesized AuNPs.Most AuNPs display a spherical shape except a small fraction of ellipsoidal and pyramidal particles.The average diameter AuNPs is about 16.6 nm based on measurements of over 100 particles in the TEM images.
3.2 AAS calibration curve
The calibration curve of absorbance as a function of gold concentration was obtained from measurements of several standard gold solutions,which were diluted from the concentrated gold standard by 10% (V/V) aqueous aqua regia solution.An excellent linear relationship (R2>0.999) is shown in Fig.2.Such linearity was also demonstrated in a higher concentration range(>15 mg/L).In order to test the reliability of AAS,10 repeated tests were performed on the standard gold solution with a concentration of 8.00 mg/L(Fig.3).The average concentration value was determined to be 7.99 mg/L with an RSD of 1.18%,indicating good accuracy and precision of the AAS method.Also,the radiation source (a gold hollow-cathode lamp) showed good stability after prolonged use (>30 min).

Fig.1 TEM micrograph of AuNPs
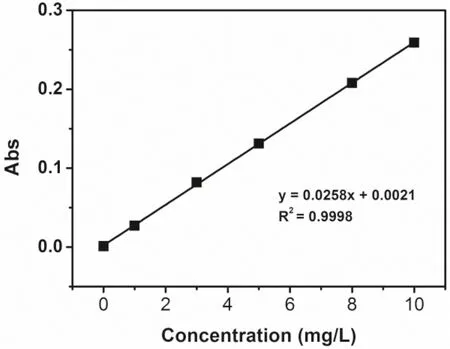
Fig.2 Standard curve of Au measured by AAS
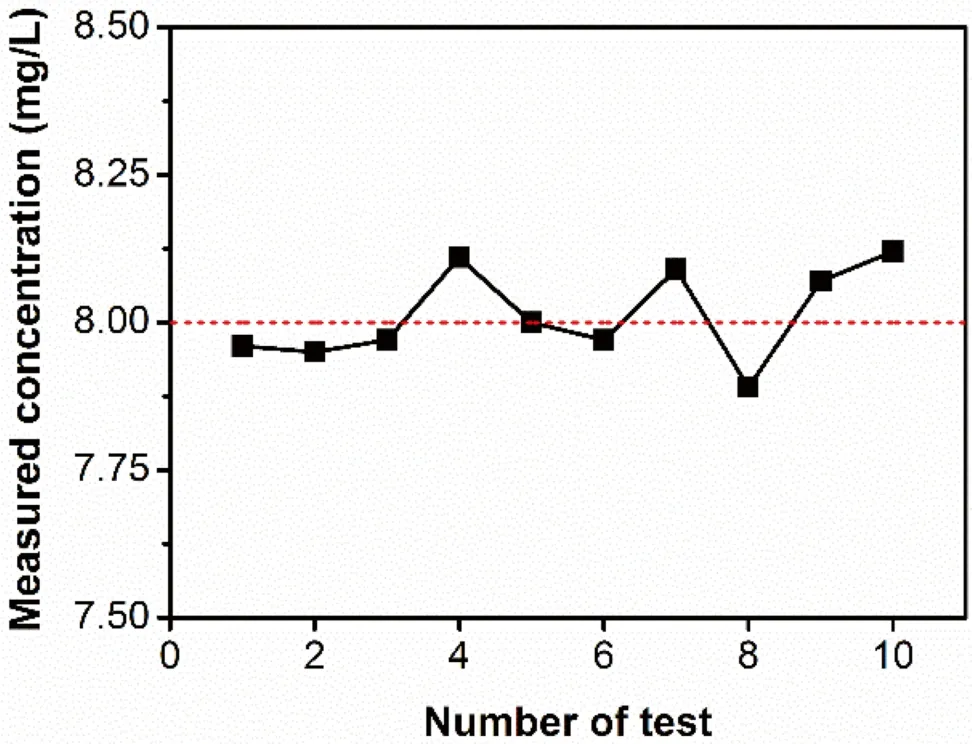
Fig.3 Repeated concentration measurements of Au standard solution(8.00 mg/L) by AAS
3.3 The influence of the gold colloid digestion method
In order to ensure complete digestion of AuNPs and thus to improve the atomization efficiency,the influence of the digestion temperature and time on the measured concentration of gold colloids was evaluated (Table 1).Chloroauric acid solution(i.e.,SAu)was used as the control group to represent the completely digested sample.Because our gold colloids(i.e.,CAu)were synthesized with chloroauric acid,the theoretical gold concentration of CAucan be calculated from that of SAubased on the dilution relations(shown in 2.2 and 2.3.1).While the measured and calculated concentrations of CAushould be very close inthe case of the complete digestion of AuNPs in CAusamples,the difference between measured and calculated concentrations of CAucan be used to evaluate the efficiency of each digestion method.

Table 1 Different digestion methods of Au colloids
The results show that the digestion parameters are important factors affecting the accuracy of AuNPs concentration determination by AAS.Different digestion methods have no significant effect on the measured gold concentrations of SAusolutions (Fig.4).In contrast,the measured concentrations of CAucolloids vary appreciably,suggesting the significant influence of temperature and time on the digestion of AuNPs.As shown in Fig.5,the large negative relative errors between measured and calculated concentrations of CAufor D1 and D3 suggest that insufficient temperature or time will lead the AuNPs not to be completely dissolved,and will cause low test results.Conversely,the relatively low error for digestion at room temperature for more than 8 h (D2) or digestion at 60 °C for more than 2 h (D4) is within the test error range,indicating adequate digestion of AuNPs.Therefore,in view of the disagreement on whether AuNPs need to be digested in previous related studies(Gillespie et al.2012;Hendel et al.2014;Jao et al.2011),we believe that the direct determination of AuNPs by AAS without digestion will bring great errors.When using air-acetylene flame AAS to test the concentration of AuNPs,it is necessary to fully digest AuNPs under higher temperatures and/or sufficient time.
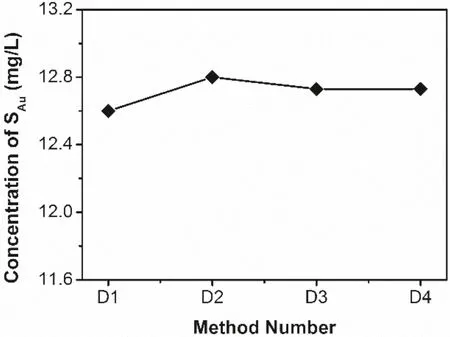
Fig.4 Measured concentrations of SAu under different digestion methods

Fig.5 Differences between measured and calculated concentrations of Au colloids under different digestion methods

3.4 Influences of ionization interference,acidic medium,and background correction
Four sets of experiments were set up to assess the effects of the background correction method,acidic medium of the standard solution,and ionization inhibitor (Table 2).Gold standard solutions with 10% aqua regia or hydrochloric acid were prepared respectively.Different types of ionizing inhibitors (KCl or NaCl) were added to the gold standard solutions with aqua regia.In one test,the deuterium lamp method was used to subtract the background.

Table 2 Media of gold standard solutions and measurement conditions
In order to showcase the impact of the experimental parameters,standard solutions with the highest (10 mg/L)and lowest(1.0 mg/L)concentrations in the standard curve were used as samples to test the accuracy of the concentration determination.The linearity of the standard curves of the 4 groups of experiments is all greater than 0.999.While the absorbance of the standard solution at the same concentration (using 5.0 and 10 mL/L as examples)without background subtraction(M1–M3)is very close,the absorbance of the standard solution in M4 is significantly smaller (Fig.6).As shown in Fig.7,the addition of different ionizing inhibitors and different acidic media appears to have insignificant improvement on the accuracy of the results,implying negligible gold ionization in the AAS flame and undetectable influence to the solution properties due to change of the acidic medium.It also shows that the gold concentration test is almost not affected by the types and concentrations of these coexisting salts and anions.The deuterium lamp background correction method significantly reduces the absorbance,which may result in a larger error in low-concentration samples with relatively low absorbance (e.g.,1.0 mg/L,Fig.7a).Conversely,for high-concentration samples,the deuterium lamp background correction method could reduce the relatively high absorbance to make it more consistent with the linear range of Lambert Beer’s law,which may improve the accuracy of concentration measurement (Fig.7b).

Fig.6 Effects of media of gold standard solutions and measurement conditions on the absorbance of some standard solution
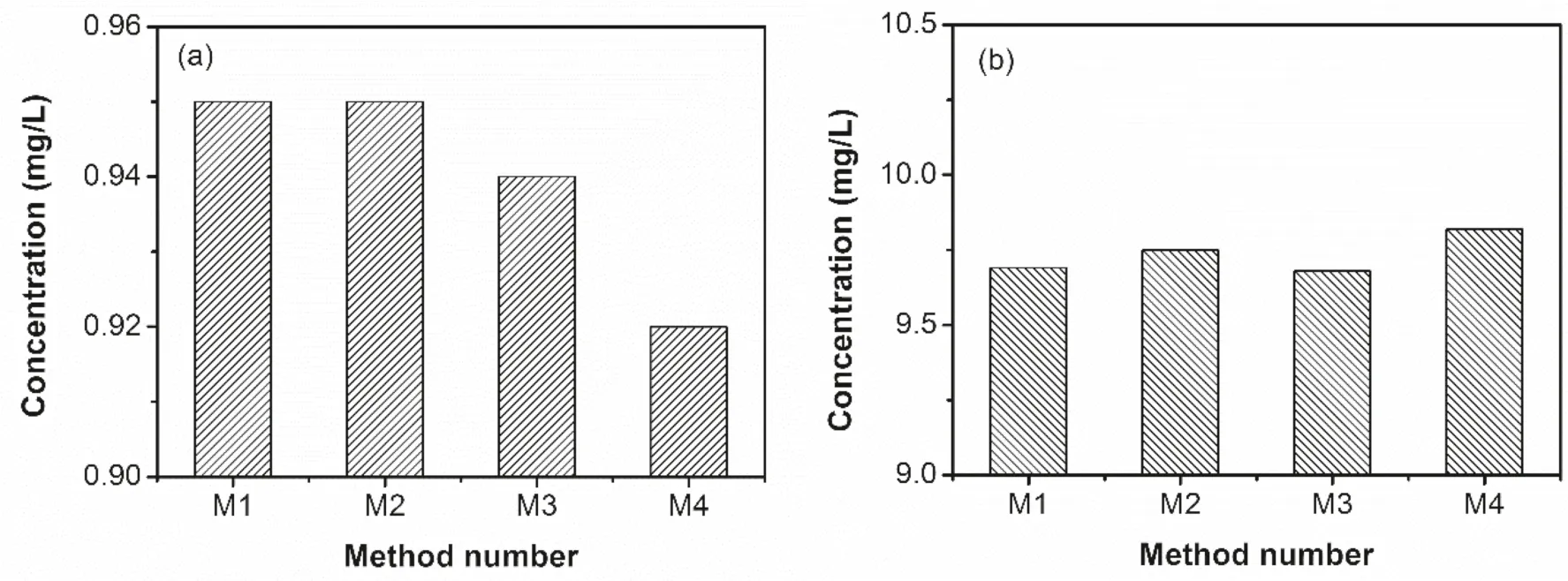
Fig.7 Effects of media of gold standard solutions and measurement conditions on concentrations of Au samples a 1.0 mg/L Au sample;b 10 mg/L Au sample
3.5 Influence of organic matter
The influence of organic matter on the gold concentration measured by AAS is shown in Figs.8 and 9.Basically,the absorbance becomes larger as the concentration of sodium citrate increases.Compared with the impact on the gold colloid,sodium citrate has a more obvious effect on the gold standard solution with a concentration of 5.0 mg/L.This may be because the gold standard solution itself does not contain any sodium citrate,while the diluted gold colloid sample contains~48 mg/L sodium citrate.On the other hand,compared with the gold concentration in the gold colloid (~12 mg/L),the gold concentration in the gold standard solution is lower (5.0 mg/L) and correspondingly the absorbance is smaller.Therefore,the interference due to the same amount of sodium citrate is greater on the gold standard solution (5.0 mg/L).It is also worth noting that the absorbance increase for the gold colloid and the 5 mg/L gold standard solution due to theaddition of 500 mg/L sodium citrate is 0.8% and 7.2%respectively,which is a positive but not very significant interference,especially to the gold colloids.For 7.3–239.6 mg/L of sodium citrate aqueous solution (without gold),the measured absorbance is almost zero,suggesting that sodium citrate itself does not significantly absorb the radiation from the gold element lamp.
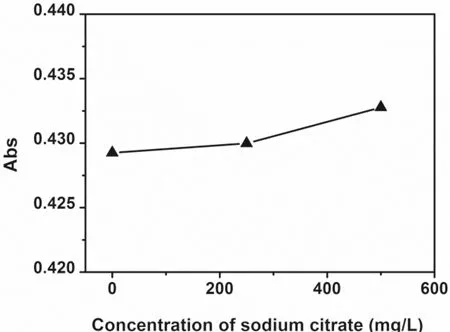
Fig.8 Effect of sodium citrate on the absorbance of Au colloids measured by AAS

Fig.9 Effect of sodium citrate on the absorbance of Au standard solutions (5.0 mg/L) measured by AAS
The flame of AAS can be divided into 4 zones,from bottom to top:the preheating zone,primary combustion zone,interzonal region,and secondary combustion zone.Among them,the interzonal region has the highest temperature and the largest density of free atoms and is therefore generally used for AAS analysis.Organic matter entering the atomizer may affect the flame height and thus the relative position of the interzonal region.Therefore,the interference of sodium citrate is likely due to the alterations of flame height.Since organic matter not only acts as a reducing agent or stabilizer in the preparation of nanoparticles,it also exists widely in nature,when using AAS to detect the concentration of natural or engineered nanoparticles,the content of organic matter should be minimized to reduce the positive interference.
4 Conclusions
To study the impact of nanoparticles on geochemical processes and also their environmental effects,it is necessary to establish accurate quantitative analysis methods.In this work,the influencing factors in the AAS determination of gold concentration in AuNP samples were systematically investigated.We have found that experimental variables such as digestion temperature,digestion time,background correction method,and organic matter all may impact the measurement.Different digestion methods of AuNPs have obvious effects on the concentration of AuNPs.Digestion at room temperature for more than 8 h and heating at 60°C for more than 2 h are demonstrated to promote the almost complete digestion of AuNPs.The digestion method obtained in this study is also applicable to other concentration testing techniques that need to dissolve AuNPs.The deuterium lamp background correction will reduce the absorbance of the samples,which may improve the accuracy for high-concentration samples,but reduce the accuracy for low-concentration samples.Organic matter such as citrate has a positive interference in the absorbance of gold.The acidic medium and the ionizing inhibitor have no significant influence on the absorbance of the standard solutions,with insignificant changes to the linearity of the standard curve and the accuracy of the measured concentrations.This also means the introduction of high-concentration inorganic ions(such as 2000 mg/L KCl,NaCl,etc.)do not have a significant impact on the AuNPs concentration measurement.Therefore,in the quantitative analysis of nanoparticles,in addition to sufficient digestion (for a long time or at a high temperature),appropriate instrument and experimental parameters need to be selected.For example,due to the low content of nanoparticles in the natural environment,it is not recommended to use the deuterium lamp method to subtract background absorption when testing the concentration of natural nanoparticles or engineered nanoparticles in simulated experiments.For research systems containing organic matter,by increasing the concentration of nanoparticles or reducing the content of organic matter,the positive interference of organic matter on the concentration determination of nanoparticles could be reduced.
AcknowledgementsThis work was supported by Guizhou Provincial Science and Technology Foundation (Qian Sci.Co.ZK[2021] No.198),Doctoral Research Startup Project in 2017 of Guizhou Normal University in China,the B-type Strategic Priority Program of the Chinese Academy of Sciences (Grant No.XDB41000000),the National Natural Science Foundation of China (41872046,41173074 and 42063008).We also thank Dr.Shirong Liu and Hongwen Ling(Senior Engineer)at the Institute of Geochemistry,Chinese Academy of Sciences,for lab assistance.
Author contributionsAll authors contributed to the conception and design study.Data collection and analysis:Yuhong Fu,Quan Wan and Sen Li;Methodology:Zonghua Qin,Shanshan Li and Ji Wang;Writing—original draft preparation:Yuhong Fu;Writing—review and editing:Quan Wan.All authors read and approved the final manuscript.
Availability of data and materialsThe datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Declarations
Conflict of interestOn behalf of all authors,the corresponding author states that there is no conflict of interest.
杂志排行
Acta Geochimica的其它文章
- Variations of methane stable isotopic values from an Alpine peatland on the eastern Qinghai-Tibetan Plateau
- Quantifying aluminosilicate manganese release and dissolution rates across organic ligand treatments for rocks,minerals,and soils
- Evaluating soil erosion by water in a small alpine catchment in Northern Italy:comparison of empirical models
- Pressure calibration and sound velocity measurement to 12 GPa in multi-anvil apparatus
- Olivine and Cr-spinel as indicators of the petrogenesis and partial melting conditions of the high-MgO ultramafic volcanic rocks from NW Ad Dhala Province—Yemen
- Zinc,copper,and strontium isotopic variability in the Baiyangping Cu–Pb–Zn–Ag polymetallic ore field,Lanping Basin,Southwest China
