Development of Iron-Based Heterogeneous Cocatalysts for Photoelectrochemical Water Oxidation
2021-09-01YanLiXingshengHuJingweiHuangLeiWangHoudeSheQizhaoWang
Yan Li, Xingsheng Hu, Jingwei Huang , Lei Wang, Houde She, Qizhao Wang
College of Chemistry and Chemical Engineering, Gansu International Scientific and Technological Cooperation Base of Water-Retention Chemical Functional Materials, Research Center of Gansu Military and Civilian Integration Advanced Structural Materials, Northwest Normal University, Lanzhou 730070, China.
Abstract: The use of fossil fuels has caused serious environmental problems such as air pollution and the greenhouse effect. Moreover, because fossil fuels are a non-renewable energy source, they cannot meet the continuously increasing demand for energy. Therefore, the development of clean and renewable energy sources is necessitated. Hydrogen energy is a clean, non-polluting renewable energy source that can ease the energy pressure of the whole society. The sunlight received by the Earth is 1.7 × 1014 J in 1 s, which far exceeds the total energy consumption of humans in one year. Therefore, conversion of solar energy to valuable hydrogen energy is of significance for reducing the dependence on fossil fuels. Since Fujishima and Honda first reported on TiO2 in 1972, it has been discovered that semiconductors can generate clean, pollution-free hydrogen through water splitting driven by electricity or light. Hydrogen generated through this approach can not only replace fossil fuels but also provide environmentally friendly renewable hydrogen energy, which has attracted considerable attention. Photoelectrochemical (PEC) water splitting can use solar energy to produce clean,sustainable hydrogen energy. Because the oxygen evolution reaction (OER) over a photoanode is sluggish, the overall energy conversion efficiency is considerably low, limiting the practical application of PEC water splitting. A cocatalyst is,thus, necessary to improve PEC water splitting performance. So far, the synthesis of first-row transition-metal-based (e.g.,Fe, Co, Ni, and Mn) cocatalysts has been intensively studied. Iron is earth-abundant and less toxic than other transition metals, making it a good cocatalyst. In addition, iron-based compounds exhibit the properties of a semiconductor/metal and have unique electronic structures, which can improve electrical conductivity and water adsorption. Various iron-based catalysts with high activity have been designed to improve the efficiency of PEC water oxidation. This article briefly summarizes the research progress related to the structure, synthesis, and application of iron oxyhydroxides, iron-based layered double hydroxides, and iron-based perovskites and discusses the evaluation of the performance of these cocatalysts toward photoelectrochemical water oxidation.
Key Words: Photoelectrochemical water splitting; Oxygen evolution reaction; Ferric hydroxide;
1 Introduction
With the increasing population and the rapid development of society, the demand for energy is rising concomitantly. Fossil fuels are widely used as a non-renewable resource1,2. But the storage of fossil fuels such as coal, oil and natural gas is limited,and the use of traditional fossil fuels brings many problems, such as air pollution and the greenhouse effect3,4. Obtaining energy from renewable resources such as solar energy and biochemical energy has become an important hotspot in current research5.Hydrogen is a clean and non-polluting energy source with broad prospects in clean and sustainable energy6-9. Since Fujishima and Honda10first reported TiO2photoanodes in 1972, it has been found that solar energy can be directly obtained by semiconductors through electric or light-driven water splitting11,12, producing clean, pollution-free, and storable hydrogen, which is considered an effective method13,14. Not only can it replace fossil fuels, but also provide environmentally friendly renewable hydrogen energy, which has aroused great attention to solving energy and environmental problems. In PEC water splitting, with light illumination, electrons in the valence band of semiconductor material transfer to its conduction band,and holes generate in the valence band, where electrons have the ability to reduce water while holes have the ability to oxidize water. The whole water splitting reaction involves two half reactions: oxygen evolution reaction (OER)15,16and hydrogen evolution reaction (HER)17,18. For water splitting, the OER process involves four-electron transfer19, that is, removing four protons from water molecules to generate an oxygen molecule,leading to a large overpotential and slow kinetics20-22. Therefore,a higher overpotential is usually required to achieve a larger current density. This severely hinders the overall efficiency of water splitting and hinders industrial scale water splitting to produce hydrogen23. Therefore, it is still a challenge to find an excellent cocatalyst that can lower the energy barrier and accelerate the OER reaction with low cost, and environmental friendliness24,25.
Noble-metal oxides (IrO2and RuO2) have shown the most effective OER activity under acidic and basic conditions, with low overpotential and small Tafel slope. But the high cost and low reserves of these precious metal oxides on earth greatly limit their practical use26. Therefore, it is necessary to develop OER cocatalysts with high activity and low cost27. So far, the research on the synthesis of the first-row transition-metal-based (e.g., Fe,Co, Ni and Mn) cocatalysts is relatively concentrated, such as transition metal chalcogenide compounds28,29, phosphides30,hydroxide materials31,32, oxides and (oxygen) hydroxides33,34.These materials have been widely proven as promising cocatalysts in the direction of OER35. Iron is one of the most abundant elements in the earth's crust and is less toxic than other transition metals. At the same time, iron-based compounds have the properties of semiconductors/metals and unique electronic structures, which can increase the conductivity and adsorption of water. In order to improve the efficiency of PEC water oxidation,various iron-based catalysts with relatively high catalytic activity were designed by many researchers. And it is widely used to modify photoelectrodes for PEC water splitting such as α-Fe2O336,37, BiVO438-40, WO341,42.
This article briefly summarizes the recent research progress in the structure, synthesis and application of iron-based OER cocatalytic materials, including iron oxyhydroxides, iron-based layered double hydroxides and iron-based perovskitesetc., in PEC water splitting. Finally, the challenges and opportunities of future research are discussed. It is hoped that this review will provide readers with a unique perspective in the field of PEC water splitting.
2 Progress on iron-based OER cocatalysts for PEC water oxidation
2.1 Iron oxyhydroxides
Iron oxyhydroxides are one series of iron mineral widely presented in nature, most of which are wide bandgap (> 2 eV)semiconductors or insulators. It is reported that there are several types of stable phases, namely goethite (α-FeOOH)43,44,akaganeite (β-FeOOH)45and lepidocrocite (γ-FeOOH)46-52.These crystal structures are shown in Fig. 146. α-FeOOH is the most common and stable iron oxyhydroxides53-55, which has similar orthorhombic crystal structure to α-AlOOH. β-FeOOH is a hydrous Fe(III) oxyhydroxide species56. γ-FeOOH is an orthonormal structure and main components of rust57. It is the second most common phase of FeOOH after goethite. γ-FeOOH has multiple effects on the surface and interface, exhibiting smaller size effect and quantum size effect58.

Fig. 1 Structures of the FeOOH polymorphs, adapted from RSC Adv. The Royal Society of Chemistry 46.
In recent years, many transition-metal-based catalysts have been developed. Iron-based compounds, especially FeOOH, as an earth-rich and environmentally friendly material, are expected to be used for water oxidation due to their strong adsorption of hydroxyl groups. There are many methods for preparing FeOOH, such as chemical bath deposition, chemical conversion, hydrothermal method59, electrodeposition60etc., in which, hydrothermal method and electrodeposition method are widely used. Maet al. rationally designed and successfully prepared FeOOH nanosheet arrays on foamed nickel by surface hydrothermal method, and applied them to OER reaction61.Chowdhuryet al. used ferrocene as the precursor of metal ions and used an anode potential electrochemical deposition method to prepare iron oxyhydroxide films in the presence of nonaqueous solvents, which eliminated the interference of precipitation problems during the deposition process62. Yanget al.also successfully deposited iron oxyhydroxide (FeOOH) on the carbon fiber cloth substrate by electrodeposition26. FeOOH nanoparticles were successfully prepared by Leeet al.through a simple chemical conversion method. First, the mixture of ferric nitrate, ethylene glycol and oleic acid amine were heated to prepare iron alkoxide nanosheets. Then it was converted into FeOOH nanoparticles with alkali treatment in aqueous KOH solution, and finally FeOOH nanoparticles with the size smaller than 10 nm were obtained63. Liet al.used a simple chemical bath deposition method to deposit an ultrathin FeOOH layer on a three-dimensional core-shell WO3@α-Fe2O3photoanode14.
In addition, Yawet al.reported a new type of V2O5/BiVO4photoanode material with rGO interlayer. By supporting FeOOH and NiOOH double cocatalysts, the photocurrent density of FTO/V2O5/rGO/BiVO4/FeOOH/NiOOH photoanode is 3.06 mA·cm−2at 1.5 Vvs.Ag/AgCl, which is 12 times and 1.5 times that of FTO/BiVO4and FTO/V2O5/rGO/BiVO4photoanodes.The initial potential of the anode dropped from 0.5 to 0.2 V. The introduction of FeOOH cocatalyst on the FTO/V2O5/rGO/BiVO4photoanode can suppress the accumulation of photogenerated holes by enhancing the transfer of photogenerated holes. When the rate of photogenerated hole transfer on the water oxidation interface is slower than the rate of injecting photogenerated holes into the FeOOH electrocatalyst film, it will result in the reorganization on the surface BiVO4, as well as partial accumulation of photogenerated holes at the interface between FeOOH and BiVO4/FeOOH. Adding a layer of NiOOH electrocatalyst film on the surface of FeOOH as the main water oxidation layer can overcome the slow water oxidation kinetics64.
α-Fe2O3/FeOOH nanoflakes grew on Au bottom layer have a photocurrent density of 3.1 mA·cm−2at 1.5 Vvs.RHE, and the initial potential is extremely low in 1 mol·L−1KOH under AM 1.5 G (100 mW·cm−2) simulated sunlight illumination. The improvement of PEC performance can be attributed to the synergy of FeOOH top decoration and Au bottom layer. FeOOH contributes to the hole transfer at the electrode/electrolyte interface, while the Au layer provides a groove for the transfer of electrons to the back contact: This makes the charge separation efficiency of the single crystal α-Fe2O3NFs photoanode greatly improved65.
Inspired by the formation of rust in nature, FeOOH quantum dots (QDs)66were prepared by metal isolation. The as-prepared FeOOH QDs have an average diameter of 3.5 nm and good crystallinity. In addition, FeOOH QDs were prepared on the ZnO nanorod film as a cocatalyst for water oxidation67. After the FeOOH QDs are loaded, the ZnO photoanode has higher surface charge injection efficiency (by a factor of ~2) and better longterm stability. The photocurrent density of FeOOH QDs/ZnO nanorod film is 2.1 times that of pure ZnO film. According to the analysis of Mott-Schottky plots, electrochemical impedance spectroscopy (EIS) and intensity-modulated photocurrent spectroscopy (IMPS)68(as shown in Fig. 2), it is found that the improvement of PEC performance is due to the passivation of the surface state, which causes a significant movement of the flat band potential. The significant increase in surface charge injection efficiency also has a certain effect on the improvement of PEC performance69.
Xiaoet al. successfully prepared two kinds of FeOOH by dipcoating method and hydrothermal method. The FeOOH obtained by the dip-coating method is an amorphous film (denoted as d-FeOOH), while the FeOOH obtained by the hydrothermal method is a granular crystalline β-FeOOH (denoted as h-FeOOH). As shown in Fig. 2d, OH terminal surface states (SS2)exist in the Fe2O3photoanode loaded with d-FeOOH, and hole transfer and recombination occur in SS2. However, since thepnjunction of the h-FeOOH/Fe2O3photoanode is formed, the transfer of photogenerated electrons and holes between h-FeOOH and Fe2O3is inhibited, making the photocurrent density of h-FeOOH/Fe2O3slightly higher than that of d-FeOOH/Fe2O3.Then Co-Pi thin film was prepared on the surface of the obtained FeOOH/Fe2O3photoanode. After loading the dual promoter, the initial voltage of the photoanode was significantly reduced, and the Fe2O3photoanode was highly improved70.
Ni:FeOOH cocatalyst was loaded on the 3C-SiC photoanode by hydrothermal deposition of FeOOH and photoelectrodeposition of NiOOH by Jianet al.71. Under AM 1.5G 100 mW·m−2illumination, the 3C-SiC/Ni:FeOOH photoanode has a very low initial potential of 0.2 Vvs.RHE and photocurrent density of 1.15 mA·cm−2at 1.23 Vvs.RHE, significantly better than 3C-SiC and 3C-SiC/FeOOH (Fig. 3a). In addition, the maximum ABPE obtained at 0.63 Vvs.RHE is 0.20% (Fig. 3b).The results showed that the Ni:FeOOH nanostructure layer increases the photovoltage and promotes the transfer of charge to the electrolyte, thus significantly improving the water splitting performance. These results provide new insights for the development of photoanodes toward efficient solar-fuel generation.

Fig. 2 (a) Mott−Schottky plots, (b) EIS Nyquist plots, (c) IMPS plots, reproduced with permission from ACS Sustain. Chem. Eng.,American Chemical Society 69. (d) Schematics of photogenerated charge transfer pathways at electrode/electrolyte interface,adapted from Chin. J. Catal. Elsevier 70.

Fig. 3 (a) J–V curves, (b) ABPE at 1.0 V vs. RHE of the 3C-SiC, 3C-SiC/FeOOH and 3C-SiC/Ni:FeOOH photoanodes, reproduced with permission from Sol. RRL, Wiley 71.
Yueet al. used one-step hydrothermal deposition method to modify the BiVO4photoanode with F-doped FeOOH (F:FeOOH)catalyst. The photocurrent density of the composite photoanode(F:FeOOH/BiVO4) is 2.7 mA·cm−2(at 1.23 Vvs.RHE), and the initial potential shift is 150 mV negative compared with the pure BiVO4electrode. It may be related to the formation ofp-njunction between BiVO4and F:FeOOH. The photo-generated holes on the surface ofn-type BiVO4are trapped byp-type F:FeOOH, thereby reducing charge recombination72. According to the reports, the conduction and valence band positions of BiVO4and F:FeOOH are (0.30, 2.75 V) and (−0.35, 1.75 V),respectively, so their energy bands can be better matched,resulting in higher separation efficiency of the electrons and holes. Therefore, the photocatalytic water oxidation activity of F:FeOOH/BiVO4electrode is relatively high73.
In addition to these, the rGO-α-Fe2O3/β-FeOOH ternary heterostructure composite material was studied as a PEC water splitting catalyst. The photocurrent density of rGO-α-Fe2O3/β-FeOOH urchin-like structure reached 0.62 mA·cm−2. β-FeOOH acts as ap-type junction withn-type α-Fe2O374, which reduces the recombination rate of photogenerated carriers75.
A comparison of the performances, including photocurrent density and the onset potential, is presented in Table 114,64,65,69-73,75-88.
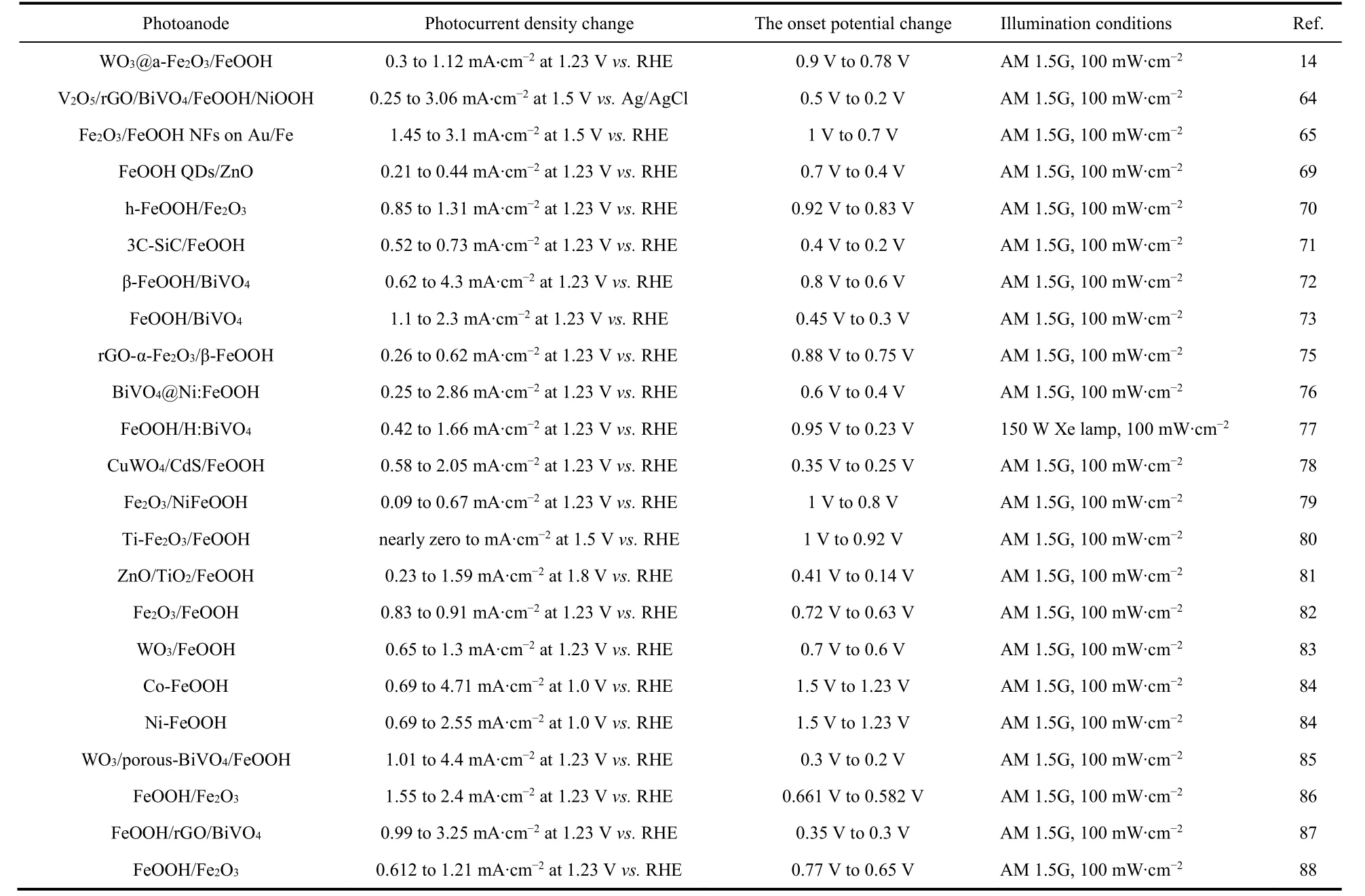
Table 1 Comparison of iron-based oxyhydroxides for PEC water splitting
2.2 Iron-based layered double hydroxides

In particular, the layered structure of NiFe-LDHs is relatively open, which can promote the diffusion of reactants and products,and even accelerate the electron transfer during the water oxidation reaction. Zhouet al. synthesized ultrathin TiO2/BiVO4heterojunction by silanization method. Then, the TiO2/BiVO4/NiFe-LDH composite material was constructed by using the hydrothermal method to support the NiFe-LDH cocatalyst on the TiO2/BiVO4photoanode. The photocurrent of TiO2/BiVO4/NiFe-LDH photoanode is about 2.5 times that of TiO2/BiVO4. This is caused by the synergistic effect of heterojunction and cocatalyst. The ultrathin TiO2nanosheets have a relatively large surface area coupled with BiVO4to construct a heterojunction, which effectively inhibits the recombination of photogenerated electron hole pairs. The NiFe-LDH cocatalyst accelerates the kinetics of water oxidation101.Younet al. used a simple solvothermal method to synthesize NiFe-LDH/rGO composite material in one pot, the process is shown in Fig. 4b. NiFe-LDH was deposited on the rGO layer uniformly, and the conductivity of the catalyst increased significantly due to the close contact. In addition, NiFe-LDH/rGO was first reported as a photocatalyst supported on hematite photoanode to participate in oxygen evolution reaction.The photocurrent of hematite supported by NiFe-LDH/rGO cocatalyst increased more than two times at 1.23 Vvs.RHE, and the cathode displacement also shifted significantly, showing good PEC water oxidation catalytic performance. Therefore,NiFe-LDH/rGO is considered to be an OER catalyst with high activity, low price and good stability102. Wanget al. prepared NiFe-LDH on BiVO4by hydrothermal method. The photocurrent density of NiFe-LDH/BiVO4composite photoanode is 2.17 times that of pure BiVO4. In addition, the stability of NiFe-LDH/BiVO4photoanode has also been improved103.

Fig. 4 (a) The idealized structure of carbonate-intercalated LDHs with different M2+/M3+molar ratios, reproduced with permission from Chem. Soc. Rev., The Royal Society of Chemistry 98. (b) The synthetic method and formed NiFe LDH on RGO support for oxygen evolution,adapted from J. Power Sources, Elsevier 102.
Similarly, Lvet al. improved the efficiency of photoelectrodes by loading NiFe-LDH nanosheets on BiVO4particles. The current density of the NiFe-LDH/BiVO4photoanode at 1.23 Vvs.RHE is 1.93 mA·cm−2, and a slight cathodic shift can be observed. The results show that NiFe-LDH is an efficacious catalyst that can accelerate the kinetics of oxygen evolution reaction104. Zhuet al. successfully prepared BiVO4/iron-based LDH including Ni1−xFexand Co1−xFex. The photocurrent ofBiVO4/Ni0.5Fe0.5-LDH photoanode is 4 times higher than that of pure BiVO4at 1.23 Vvs.RHE, and the initial potential also shifts significantly (320 mV). A similar situation was shown on the BiVO4/Co0.5Fe0.5-LDH photoelectrode. Through theoretical calculations, iron-based LDH with narrow forbidden increases light absorption and improves the photocatalytic performance of BiVO4/iron-based LDH. In addition, the metallic properties of the BiVO4/iron-based LDH phase interface can promote charge separation105.
NiFe-LDH modified WO3/Fe2O3heterojunction photoanode was prepared by Baiet al. using a two-step hydrothermal method.At 1.8 Vvs.RHE, the photocurrent density of WO3/Fe2O3/LDH reached 3.0 mA·cm−2, which is 5 and 7 times that of WO3and α-Fe2O3, respectively. The photogenerated holes are transferred from VB of α-Fe2O3to the cocatalyst layer and Ni2+is oxidized to Ni3+and Ni4+. High valence nickel oxidizes water through holes to release oxygen. Nife-LDH can effectively separate photogenerated electron hole pairs and accelerate the water oxidation kinetics, thus improving the catalytic performance.The bandgap of the heterojunction narrows, the directional flow of electrons, and the holes accumulated on the electrode surface are consumed in time to expand the absorption of visible light and the separation of photogenerated carriers, thus promoting the water oxidation reaction106.
Chenet al. prepared a novel ternary photoanode by electrodeposition of NiFe-LDH on BiVO4/rGO. The photocurrent density of the BiVO4/rGO/NiFe-LDH electrode is significantly higher than that of the original BiVO4, BiVO4/rGO and BiVO4/NiFe-LDH electrode. The photocurrent density of the BiVO4/rGO/NiFe-LDH photoanode is 3.26 mA·cm−2at 1.23 Vvs.RHE, and the stability is also good. NiFe-LDH as a water oxidation catalyst can promote the transport of photogenerated holes from the BiVO4photoanode to the electrolyte, thereby improving the water oxidation performance107. Guoet al.prepared NiFe layered double hydroxide (LDH) nanosheets on the surface of molybdenum-doped BiVO4by electrodeposition.The photocurrent density of Mo:BiVO4/NiFe-LDH photoanode at 1.23 Vvs.RHE is 3.16 times that of BiVO4photoanode. IPCE reached 64%, which is 6.4 times that of the undoped BiVO4photoanode. The initial potential was reduced from 750 mV to 430 mV, a significant cathodic shift occurred, and the stability was also improved compared to the pure BiVO4photoanode97.Ninget al. electrodeposited NiFe-LDH nanosheets on graphene coated TiO2nanoarrays to obtain nanorod photoanodes. NiFe-LDH as a cocatalyst can accelerate the water oxidation reaction.Under the synergistic effect of rGO and NiFe-LDH, the photoelectric conversion efficiency is 2.6 times higher than that of pure TiO2, and the stability is also good. This is better than the photocurrent density of TiO2-based photoanodes in neutral media reported in the past. In addition, the method can also be extended to the preparation of other high-performance photoanodes (WO3/rGO/NiFe-LDH and α-Fe2O3/rGO/NiFe-LDH) high-performance photoanodes108. CoFe-LDH was also prepared by Guoet al.on the reduced titanium dioxide photoanode by electrochemical deposition method, and the current density at 1.23 Vvs.RHE was 0.78 mA·cm−2. In addition,they used density functional theory (DFT) to study the energy band structure, bandgap and band edge position of CoFe-LDH.The calculated bandgap and work function are 1.668 eV and 4.988 eV, CB minimum and VB maximum are −4.154 eV and−5.822 eV relative to vacuum. The results show that the energy band structure of CoFe-LDH is in good agreement with the reduced titanium dioxide. The relative VB maximum position of the photogenerated holes and the energy difference between them can make it easier for the holes to migrate from the VB of titanium dioxide to the VB of LDH, resultantly accelerating the transport and promoting charge separation, and suppress charge recombination, which leads to water oxidation performance improvement109.
Besides, cobalt-iron layered double hydroxides were modified on the surface of titanate nanowires by Sayedet al. LDH expands the absorption range of TiO2nanowires, and the bandgap is reduced to 2.57 eV. The initial potential also shifted significantly by 220 mV. In alkaline media, the photocurrent of photoanodes with mass ratios of 0.25 : 1, 0.5 : 1 and 1 : 1 increased by 9.4 times, 23.45 times and 34.32 times under 1 Vvs.AgCl,respectively. The increase of the specific surface area of LDH and the excellent catalytic performance improve the PEC performance of TiO2nanowires. In the PEC water splitting reaction of alkaline medium, CoFe-LDH as a catalyst should be paid attention to110.
A comparison of the performances, including photocurrent density and the onset potential, is presented in Table 297,101,102,104-114.

Table 2 Comparison of iron-based layered double hydroxides for PEC water splitting.
2.3 Iron-based perovskites
Perovskite-type composite oxide is a new inorganic nonmetallic material with unique physical and chemical properties.It has a highly symmetrical close-packed structure and its molecular formula is ABO3115-119. The A site is generally a rare earth or alkaline earth element ion (Li, Na, Ba, Sr, La, Pr,etc.).The ion radius is large, and it coordinates with 12 oxygens to form a dense cubic accumulation. Its function is mainly to stabilize the structure of perovskite117,120. The B site is generally a transition element ion (Ti, Fe, Co, Mn, Ta,etc.)121,122. The structure is shown in Fig. 5a. The ion radius is small, and it is coordinated with 6 oxygens, forming a cubic close-packed, and occupying the center of the octahedron. Because its valence state changes a lot, it usually plays a big role in determining the properties of perovskite materials123,124. The A-site and B-site can be partially replaced by other metal ions with similar radius,and the crystal structure is basically unchanged118. This material has stable crystal structure, special electromagnetic properties,and good redox and catalysis activities. As a new type of functional material, it has great potential in the fields of environment and catalysis. Due to the adjustable volume and surface composition of the perovskite oxide, it is convenient to adjust the physical and chemical properties, which have an effect on the photoexcitation and water oxidation process in the PEC water splitting process, and improve the PEC performance and efficiency125. Most of the perovskite-type oxides currently explored have a wide bandgap and are not suitable as photoactive layers. However, some single component perovskite oxides such as BiFeO3(BFO) can absorb visible light.

Fig. 5 (a) ABO3 perovskite structure: “A” site is occupied by Bi3+or La3+and “B” site is occupied by Fe3+, adapted from Appl. Surf. Sci Elsevier 116. (b) Current-potential plots for WO3, BVO, BFO, WO3/BVO, and WO3/BVO/BFO photoanodes under AM 1.5 G irradiation,Current-potential plots under light illumination and dark mode for (c) WO3/BVO and (d) WO3/BVO/BFO photoanodes, reproduced with permission from ACS Appl. Energy Mater. American Chemical Society 128.
Single-phase BiFeO3thin films were prepared by Wuet al. on TiO2photoanode by sol-gel method. The photocurrent density of BFO-5/TiO2composite material is 11.25 mA·cm−2, which is about 20 times that of pure TiO2photoanode. In addition, the photocurrent density of the electrode increased to 28.75 mA·cm−2at 1.5 Vvs.SCE under AM 1.5G through the positive polarization by them. The formation of BFO and TiO2heterojunction and the BFO produces a ferroelectric effect,which may bend the electronic band on the interface, and promote the separation and transmission of photogenerated carriers for a greatly improved PEC performance. This is a good strategy for improving the performance of optoelectronic and electrochemical materials126. BiFeO3is used as a passivation layer and catalyst to modify the BiVO4photoanode. Xieet al.prepared BiVO4/BiFeO3by surface passivation. The current density of the composite photoanode is 0.63 mA·cm−2at 0.6 Vvs.Ag/AgCl, which is 4.4 times that of bare BiVO4, and the initial potential also produces a negative shift of ~400 mV. In addition, the charge recombination rate of BiVO4/BiFeO3is reduced to 0.6 s−1, which is 28 times that of pure BiVO4. As a buffer layer, BiFeO3passivation reduces charge recombination,thereby improving PEC performance, and is a good catalyst for promoting charge separation and transfer127.
BiFeO3was also prepared by Singhet al.116by microwaveassisted (MW) and sol-gel (SG) methods, and the photoelectric properties of the materials were discussed. The results show that although the purity of BF-MW crystals is higher than that of BFSG, the performance of BF-SG is better than that of BF-SG during water decomposition. This may be due to impurities such as Bi2O3in BF-MW crystals. WO3/BiVO4(BVO)/BiFeO3(BFO)porous photoanode was synthesized by Khoomortezaeiet al.through sol-gel method. In the presence of the WO3layer, the performance of the BVO film was significantly improved. The highest photocurrent density of the triple heterojunction was 46.9 mA·cm−2at 2.53 Vvs.RHE (as shown in Fig. 5b). As shown in Fig. 5c and d, under dark conditions, even at an applied potential of 1.23 Vvs.RHE, the photocurrent density of WO3/BVO and WO3/BVO/BFO photoanodes can be almost ignored128.
3 Conclusion and perspectives
This article briefly summarizes the structure, synthesis and application of iron oxyhydroxides, layered double hydroxide and iron-based perovskite cocatalyst, and introduces its catalytic performance. The activity of the iron-based cocatalyst is related to the morphology, crystal structure, electronic structure and preparation method of the catalyst. The catalytic activity can be improved by adjusting these influencing factors. Surface passivation by dielectric layer can also improve the efficiency of photoanodes by enhancing charge separation and transfer at semiconductor/electrolyte interfaces.
Despite the reviewed extensive research, few important breakthroughs are achieved for practice use. To improve the performance of iron-based heterogeneous cocatalysts for use in practical application, the following tasks should be performed:First, a comprehensive understanding the roles of each component in iron-based heterogeneous cocatalysts should be gained byin situtechniques. Second, it should pay attention to the reason of changes in electronic properties and structure with introduction of alien element in iron-based catalysts, which significantly increase the performance for PEC water splitting.Third, some theoretical calculations are also necessary, which can provide effective guidance for catalyst optimization. In short,the design of high-performance iron-based catalysts is a very promising and challenging subject.
杂志排行
物理化学学报的其它文章
- 基于密度泛函理论下H2S 在单原子催化剂V/Ti2CO2 上的分解机理研究
- 理论计算评价光催化剂VOCs 降解性能:g-C3N4 量子点/石墨烯
- 富含缺陷的2D/2D 异质结促进光催化清洁能源转化
- 二维光催化材料电子结构和性能调控策略研究进展
- Defect Engineering in Two-Dimensional Graphitic Carbon Nitride and Application to Photocatalytic Air Purification
- Non-Noble-Metallic Cocatalyst Ni2P Nanoparticles Modified Graphite-Like Carbonitride with Enhanced Photocatalytic Hydrogen Evolution under Visible Light Irradiation
