Defect Engineering in Two-Dimensional Graphitic Carbon Nitride and Application to Photocatalytic Air Purification
2021-09-01WeiWangYuHuangZhenyuWang
Wei Wang , Yu Huang , Zhenyu Wang
1 State Key Laboratory of Loess and Quaternary Geology, Key Laboratory of Aerosol Chemistry and Physics, Institute of Earth Environment, Chinese Academy of Sciences, Xi’an 710061, China.
2 University of Chinese Academy of Sciences, Beijing 100049, China.
Abstract: Since the pioneering work on polychlorinated biphenyl photodegradation by Carey in 1976, photocatalytic technology has emerged as a promising and sustainable strategy to overcome the significant challenges posed by energy crisis and environmental pollution. In photocatalysis, sunlight, which is an inexhaustible source of energy, is utilized to generate strongly active species on the surface of the photocatalyst for triggering photo-redox reactions toward the successful removal of environmental pollutants, or for water splitting. The photocatalytic performance is related to the photoabsorption, photoinduced carrier separation, and redox ability of the semiconductor employed as the photocatalyst. Apart from traditional and noble metal oxide semiconductors such as P25, bismuth-based compounds, and Pt-based compounds, 2D g-C3N4 is now identified to have enormous potential in photocatalysis owing to the special π-π conjugated bond in its structure. However,some inherent drawbacks of the conventional g-C3N4, including the insufficient visible-light absorption ability, fast recombination of photogenerated electron-hole pairs, and low quantum efficiency, decrease its photocatalytic activity and limit its application. To date, various strategies such as heterojunction fabrication, special morphology design, and element doping have been adopted to tune the physicochemical properties of g-C3N4. Recent studies have highlighted the potential of defect engineering for boosting the light harvesting, charge separation, and adsorption efficiency of g-C3N4 by tailoring the local surface microstructure, electronic structure, and carrier concentration. In this review, we summarize cutting-edge achievements related to g-C3N4 modified with classified non-external-caused defects (carbon vacancies, nitrogen vacancies, etc.) and external-caused defects (doping and functionalization) for optimizing the photocatalytic performance in water splitting, removal of contaminants in the gas phase and wastewater, nitrogen fixation, etc. The distinctive roles of various defects in the g-C3N4 skeleton in the photocatalytic process are also summarized. Moreover, the practical application of 2D g-C3N4 in air pollution control is highlighted. Finally, the ongoing challenges and perspectives of defective g-C3N4 are presented. The overarching aim of this article is to provide a useful scaffold for future research and application studies on defect-modulated g-C3N4.
Key Words: Graphitic carbon nitride; Defect engineering; Photocatalysis; Practical application
1 Introduction
Graphitic carbon nitride (g-C3N4), as a typical organic 2D polymer semiconductor material, has gradually become a research hotspot due to their novel physicochemical properties and advantages including ultrahigh specific surface area,tailorable band and electronic structure, quantum confinement effect, high chemical and thermal stability, low cost, nontoxicity, and earth abundance1-5. What’s more, a unique coordination ring surrounded by aπ-πconjugated bond in g-C3N4structure provides active sites for adsorption and reaction(Fig. 1a)6,7, and g-C3N4can be easily exfoliated into ultrathin layers to afford more sites8. Resultantly, significant achievements have been made both in their synthesis methods and practical applications. Various synthesis methods including thermal polymerization9, hydrothermal and solvothermal treatment10, and so forth to date have been applied for the preparation of g-C3N4. Thermal polymerization has been considered as the most promising one due to easy control of reaction conditions and the potential for large-scale synthesis. g-C3N4could be derived from pyrolysis of nitrogen-rich precursors, such as melamine (Fig. 1d)6, cyanamide, urea11,thiourea, dicyandiamide,etc. Nowadays, g-C3N4has found its applications in various industries including optoelectronics12,the environment13, and the energy14.
Photocatalytic technology is a green, efficient, sustainable strategy to overcome the tremendous challenges from energy and environment, as inexhaustible sunlight has been utilized to induce and generate strong active species on the surface of semiconductor materials, to motivate photo-redox reactions, and to achieve the removal of environmental pollutants or water splitting15,16. The photocatalytic activity of semiconductors depends on their light absorption, the generation/separation/migration of photo-induced electrons and holes, and photo-redox ability17,18. g-C3N4has become an attractive semiconductor with suitable band gap (~2.7 eV) for efficient photocatalytic reactions since it was firstly applied to produce hydrogen under visible light19. And, it is non-metallic and its large-scale synthesis can be easily achieved. Whereas, primitive g-C3N4obtained from pyrolysis is struggling with not only limited visible light absorption ability but also confined separation rate of charge carrier.
Therefore, various modification methods including heterojunction interface fabrication20, special morphology design7, and defect engineering construction21have been used to tune the physicochemical property of g-C3N4(Fig. 1e), in order to enhance its photocatalytic ability. For example, Wanget al.in situconstructed a series of Type-II g-C3N4-based carbonate heterojunctions (g-C3N4/Bi2O2CO3, g-C3N4/LaCO3OH, g-C3N4/SrCO3)viathe self-decomposition of g-C3N4in the hydrothermal process, which exhibited superior photocatalytic nitric oxide (NO) removal efficiency compared to monophase22,23.The reinforcement of NO removal performance has been mainly attributed to the fact that the g-C3N4-based carbonate heterojunctionsin situprepared have tight interface contact,which can improve the separation efficiency of photo-excited electron-hole. Compared with other modification strategies,defect engineering has been proved to regulate the electronic structure and band structure efficiently24.
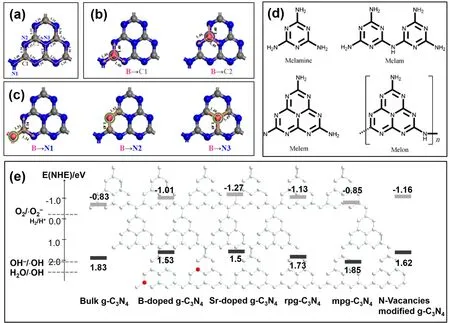
Fig. 1 (a) Pristine g-C3N4 mode. Boron atom substitution doping at (b) two inequivalent carbon sites or (c) three inequivalent nitrogen sites,reproduced with permission from Appl. Catal. B: Environ., Elsevier 7. (d) Carbon- and nitrogen-containing materials obtained from the thermolysis of mercury(II) thiocyanate, adapted from Angew. Chem. Int. Ed., Wiley publisher 6. (e) Electronic band structures of different g-C3N4.
Although numbers of review articles about g-C3N4have sprang up, focusing on element doping25, heterojunction26,27,core-shell structure28, active site29, photocatalytic hydrogen evolution30within the last few years, the knowledge regarding the defective g-C3N4in photocatalysis has not been summarized systematically. In this mini review, we classify the defects of g-C3N4into the non-external-caused defects including nitrogen vacancies, carbon vacancies, and fragments deficiencyetc., and external-caused defects such as doping, and functionalization.The state-of-art research progress of the defective g-C3N4has been summarized. The practical application of g-C3N4in photocatalytic decontamination of air pollutants has also been presented. In the end, we propose the challenges and perspectives for the defective g-C3N4.
2 Recent progress in defective g-C3N4 for photocatalysis
Generally, different coordinated atom groups own different bind energy, resultantly exhibiting different performance31. As shown in Fig. 1b,c, there are three inequivalent nitrogen sites(N1, N2, and N3) and two inequivalent carbon sites (C1 and C2)in g-C3N4framework. In other words, both C and N atoms deficiency in g-C3N4framework might have different regulating capacity for g-C3N4. Therefore, various defects have been classified and summarized in the following section.
2.1 Non-external-caused defect in g-C3N4
For photocatalysis process, the anionic vacancies in semiconductor are vital to the activity of semiconductors since they can influence the electronic configuration of semiconductors, and act as the adsorption-activation site for target molecules2, so are nitrogen vacancies (VN) in g-C3N4structure. VNcan be introduced into g-C3N4by calcining its precursors under different airflow (such as nitrogen atmosphere N2, hydrogen atmosphere H2,etc.)32-37, quick post-thermal treatment38-40, or heat treatment with a reducing agent41-43. For example, Zhao and coworkers introduced VNinto g-C3N4through heating pristine g-C3N4in the H2flow32. X-ray photoelectron spectroscopy (XPS) results showed that the introduced VNlocated at bicoordinated nitrogen atoms sites, in line with the density functional theory (DFT) calculations.Characteristic experiments showed that the modified g-C3N4owned more suitable bandgap, which was helpful for the migration of photo-generated hole-electron pairs, and resultantly produced more reactive oxygen species than pristine g-C3N4did.Moreover, they found that the NO+reaction intermediates that contributed to accelerated NO removal could be formed on VNsites. Consequently, NO removal efficiency over the VNmodified g-C3N4was 2.6 times that over the conventional one.
Notably, Xieet al.innovatively prepared modified g-C3N4with two types of cooperative nitrogen vacancies (NHxand N2Cvacancy) using a one-pot KOH-assisted calcination method, and used the VNfabricated g-C3N4for solar-driven H2O2evolution44.They found that NHxaccelerated the separation and transfer of photo-induced h+-e−pairs and N2Cvacancies were beneficial for oxygen activation in two-electron process (Fig. 2a). Liet al.obtained g-C3N4with intra- and inter-triazine VNviapolymerizing melamine under the atmosphere of H2and Ar mixture, or CCl4atmosphere, respectively37. Results revealed that the inter-triazine VNexhibited stronger electron localization,resulting in a 2.2 times higher normalized reaction rates than the intra-triazine VN. Therefore, the same type modified vacancies at different sites might show completely different effects on their photocatalytic activity, and different coordinated VNcan be introduced into g-C3N4framework according to target reaction needs.
It has been proved that specific morphologies and structures such as nanotube, nanofiber, porous structure had synergistic effect with VNto modulate the photocatalytic efficiency of g-C3N445,46. For instance, Xueet al.achieved VNmodified g-C3N4(PFL-g-C3N4) with porous structure for photocatalytic N2fixation47. Results evidenced that the superior photocatalytic performance of PFL-g-C3N4could be attributed to the broadened light absorption range originating from the narrowed band gap caused by the porous structure, and the more negative conduction band (CB) level due to the introduction of VN.
Especially, Wanget al.combined porous tubular micronanostructure with VNto fabricate VNmodified g-C3N4microtubes (Fig. 2b), which exhibited 1.8 times higher NO removal ratio than bulk one did under visible light irradiation, byin situsoft-chemical treatment. They also investigated the influence of VNconcentration and 1D tubular structure on the carrier behaviors, and adsorption-activation of O2and NO48.Experiments and DFT calculations results indicated that surface VNand the microtube structure were able to promote the separation and oriented transportation of photo-induced electron-hole pairs respectively (Fig. 2c). Furthermore,operando diffuse reflectance infrared Fourier-transform spectroscopy (in situDRIFTS) proved that VNwas the NO and O2adsorption-photoreactions site, instead of terminal N―H bonds as pristine g-C3N4participated in photocatalytic process(Fig. 2d). Other 1D architecture g-C3N4nanotubes or nanorods with tunable VNwere also fabricatedviathermal etching approach49, thermal polymerization of the pyridinium alkaline ionic liquid-modified hydrogen-bonding melaminecyanuric acid supramolecular (PHMCS) pre-aggregates50or a high pressure and high temperature (HPHT) process combined with hard template method51. Tanget al.used NH4Cl as gas template to partly disrupt theπ-πinteractions between adjacent g-C3N4layers, and successfully synthesized 3D bubbly nitrogen defective g-C3N4, which was assembled by surface VNmodified few-atomic-layers g-C3N4nanosheets8. The special few-atomiclayered g-C3N4, as typical 2D material, offered a larger exposed surface area for effective charge carrier transfer and reagent adsorption-activation. Moreover, the VNcould induce mid-gap state below CB, which was conducive to the absorptivity, and could act as a reservoir to capture charge electrons. Resultantly,the CH4evolution over 3D bubbly nitrogen defective g-C3N4was 3.16 and 5.14 higher than that over pristine ultrathin one and bulk one, respectively. Also, the VNdoped hierarchical macromesoporous g-C3N4(Nv MM CN) with an inverse opal structure was realized through combining NH4Cl with ordered SiO2colloidal crystal, and showed 7.5-fold H2evolution yield over bulk g-C3N4under visible light irradiation52.
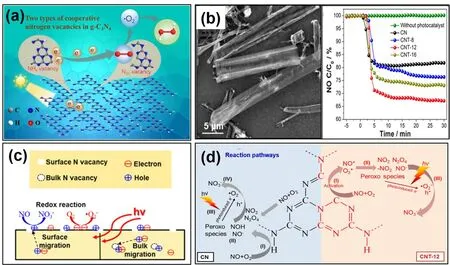
Fig. 2 (a) Schematic diagram of efficient solar-to-H2O2 conversion by polymeric carbon nitride with two types of cooperative nitrogen vacancies (NHx vacancy and N2C vacancy), adapted from Appl. Catal. B: Environ., Elsevier 43. (b) SEM images and the visible light photocatalytic NO removal activities of CNT-12, (c) schematic diagram depicting the roles of N-vacancies, (d) proposed reaction pathways for adsorption and the photocatalytic oxidation of NO over CN and CNT-12, reproduced with permission from ACS Appl. Mater. Interfaces,American Chemical Society 48.
In short, the introduction of VNin g-C3N4framework can alter the band gap and act as active sites to capture electrons, resulting in reinforced photocatalytic performance. Based on the facts that g-C3N4is a typical semiconductor owning more electrons than holes, and VNsite in g-C3N4can capture photo-induced electrons, leading to local area holes concentration greater than electrons, Caoet al.successfully fabricated VNmodified g-C3N4/g-C3N4p-nhomojunctions (PN-x) through postcalcination of conventional g-C3N4under H2atmospheres33.They found that the PN-xhomojunctions exhibited much higher activity than bulk one in terms of Rhodamine B degradation.
Carbon vacancies (VC) in g-C3N4have also been explored, but with much less attention than VN. Liuet al.prepared VCengineered g-C3N4nanosheets (Cv-CNNs)viathermally treating bulk g-C3N4with NH4Cl, and studied the influence of VCon bandgap structure of g-C3N453. Results revealed that the VCwas successfully, concomitantly introduced into g-C3N4framework during the formation of pores on nanosheets, leading to the increased oxidizing capacity of g-C3N4by altering its band structure. As a result, Cv-CNNs showed excellent photocatalytic ability in terms of sulfadiazine degradation and hydrogen generation. Lianget al.found that VCcould not only serve as piggy bank of photo-induced electrons to boost the separation of charge carries, but also act as capture site to transfer the trapped photo-excited electrons from vacancies to absorbed O2for the generation of reactive oxygen species (Fig. 3a)54. Up to now,various methods such as heat treatment in an ammonia atmosphere55, low boiling point solvent mediated56, hightemperature peeling57have also been used to introduce VCinto g-C3N4. Leiet al.combined inverse opal structure with VCto modify g-C3N4by NH4HF2liquor etch58. Wanget al.explored He+ion irradiation method to introduce VCand VNsimultaneously into g-C3N4nanosheets, and tuned the vacancy concentrations by He+ion fluence59. Both DFT calculations and experimental results showed that the presence of VCand VNenhanced visible light absorption ability, elongated photoexcited carriers lifetime, and resultantly improved the photocatalytic performance of g-C3N4.
In addition to the vacancies derived from nitrogen or carbon atoms loss, defects caused by fragments deficiency can also mediate the electronic structure and physicochemical properties of g-C3N4. Sunet al.modified g-C3N4with the tunable intrinsic structure defects (structure defect poor g-C3N4SDP-CN and structure defect rich g-C3N4SDR-CN), and uncovered the effect of structure defects on the charge carrier behaviors60. XPS identified that the structure defects originated from partial loss of heptazine units in g-C3N4skeleton (Fig. 3b). Both fluorescence and the ultrafast transient absorption results suggested that the introduced structure defects were advantageous for the charge electrons separation (Fig. 3c). As a consequence, structure defects rich g-C3N4displayed greatly improved photocatalytic H2evolution compared with structure defects poor one. Although many studies on defects fabricated g-C3N4have been reported, some disadvantages of vacancies engineering have been found. For example, excessive vacancies could be recombination centers for electrons and holes, which would reduce the separation efficiency of photo-generated carriers. Furthermore, the introduction of defects could decrease the crystallinity of g-C3N4.
Generally, the crystallinity of g-C3N4plays an important role in the photocatalytic performance, as increased crystallinity is favorable for the optimized electronical and structural properties, such as the narrowed band gap and the extendedπconjugated system and non-localizedπ-electrons61,62. Wanget al.used oxalyl dihydrazide (ODH) as a gas template agent to construct the VNfabricated g-C3N4with increased crystallinity63.They found the modified g-C3N4showed increased visible light harvesting, accelerated charge migration, and hence superior photocatalytic efficiency in terms of tetracycline hydrochloride(TC-HCl) and sulfamethoxazole (SMZ) degradation, due to the introduced VNand increased crystallinity (Fig. 3d)63.
2.2 External-caused defect in g-C3N4
Doping has been proved to be one of efficient strategies to optimize g-C3N4. Especially, metal ions have been extensively applied as dopants for g-C3N4for the optimization of photocatalytic performance, due to its larger atomic radius and more unoccupied valence orbitals. Zhouet al.intercalated g-C3N4with group IIA element ions (MCN) including Mg, Ca, Sr,and Ba64. Results suggested the doped alkaline earth ions could coordinate with the N atoms in the triazine rings of g-C3N4leading to distorted g-C3N4structure, and hence improved charge carrier separation ability (Fig. 4a). Moreover, the DFT calculation results further confirmed the formation of electron transport channels which were helpful to the electron transport between two layers, and the enlarged size of electron transport channels accompanying the increased size of incorporated atom.Furthermore, they found that the formation of structural distortions availed the generation of amino groups for the chemisorption sites of O2, leading to the generation of more reactive oxygen species for the oxidation of NO. Alkali metal including Li, Na, K and Rb also were successfully used to modify g-C3N4through water bath heating followed by high temperature calcination method65. DFT calculations and experiments results demonstrated that Rb doped g-C3N4owned the highest light absorption intensity and broadest light response range from 500 to 1000 nm, and showed the best CO2reduction performance with 3 times higher than bulk ones did. In another study, the bandgap of transition metal atoms doped g-C3N4monolayers (Mn, Fe, Co, Ni) was explored by first principles calculations66. Results indicated that g-C3N4with different doping levels showed different absorption spectra, and a large absorption peak which located at 1.5 eV appeared as the vacancy sites were fully occupied accompanying the optimum doping level. In general, the introduced metal can coordinate with the g-C3N4framework and create new energy levels in the band structure of g-C3N4, accordingly changing the physical and chemical property such as the charge densities and transition states to broaden the visible light response and suppress the electron-hole charge recombination rate. However, the newly formed energy levels caused by the introduction of metal may also serve as recombination centers, resulting in decreased quantum efficiencies67.
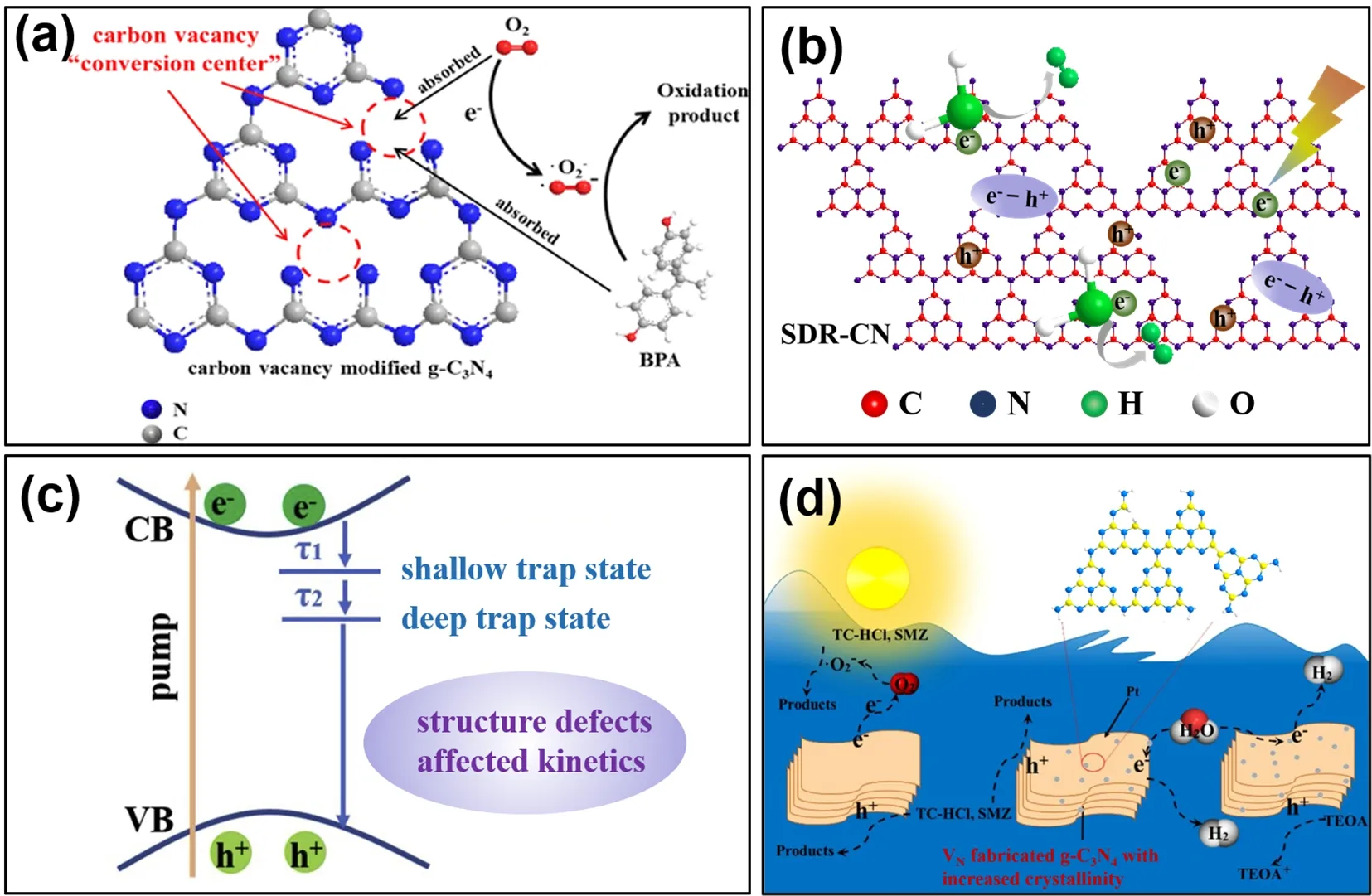
Fig. 3 (a) Reaction mechanism of carbon vacancies under visible-light irradiation, reproduced with permission from ACS Appl. Nano Mater., American Chemical Society 54. The schematic diagram of SDR-CN (b) and photophysical processes in SDR-CN (c), adapted from Appl. Catal. B: Environ., Elsevier 60. (d) Schematic diagram of TC-HCl and SMZ photodegradation by VN fabricated g-C3N4 with increased crystallinity, adapted from Appl. Surf. Sci., Elsevier 63.
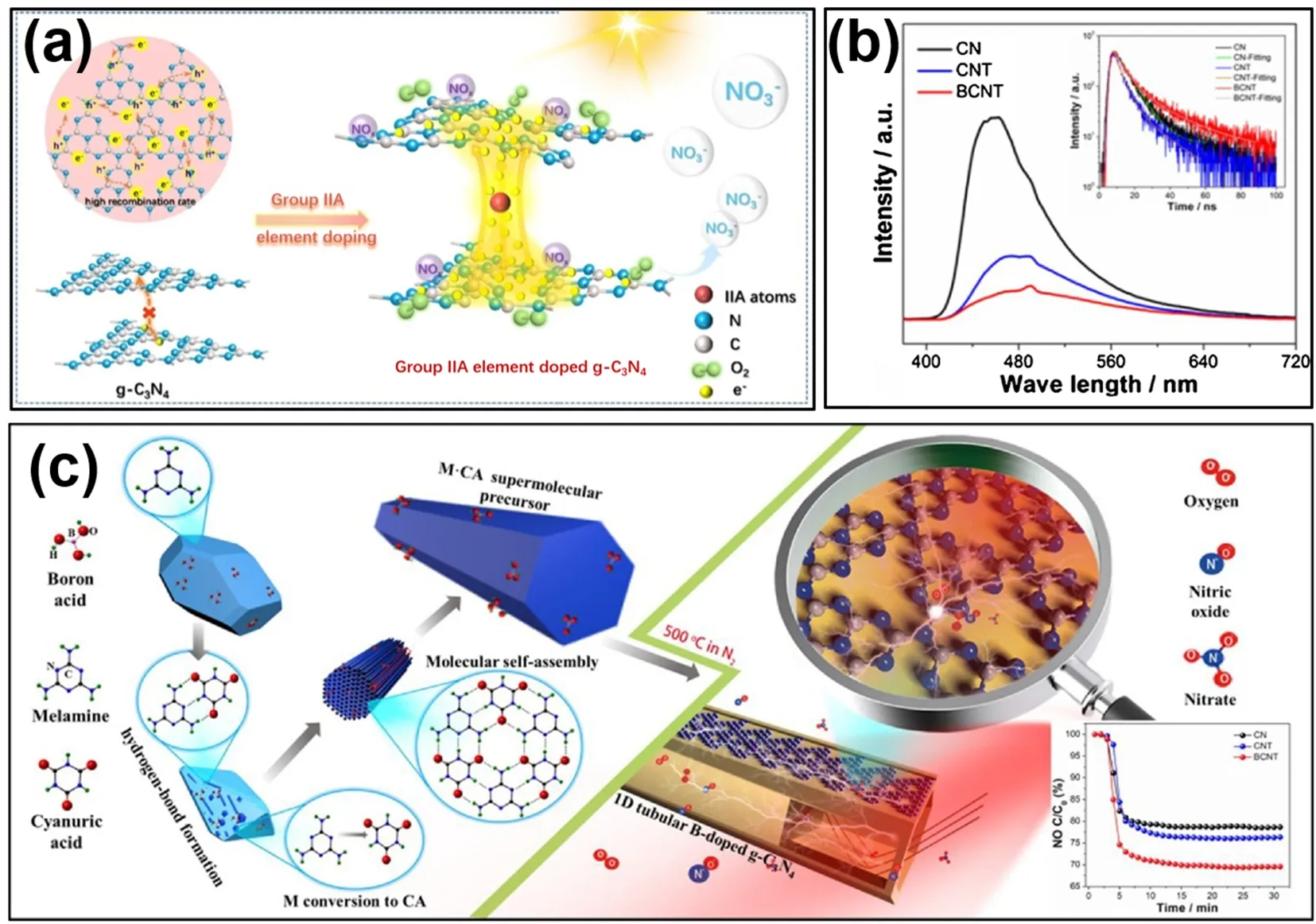
Fig. 4 (a) Schematic diagram of photocatalytic removal of NO by intercalated carbon nitride MCN, adapted from Appl. Catal. B: Environ., Elsevier 64. (b) PL spectra and (c) preparation and the photocatalytic NO removal mechanisms of BCNT,reproduced with permission from Appl. Catal. B: Environ., Elsevier 7.
Non-metal doping is an effective way to tune the electronical and optical property of semiconductors without the destruction of the metal-free property of g-C3N4. Notably, boron is easy to achieve substitution doping since both atomic radius and the chemical properties of boron are very similar to those of C.Additionally, the incorporation of B can govern the surface chemical properties of g-C3N4such as the formation of Lewis acid sites that play an important role in the photocatalytic process. Wanget al.predicted that C1 and C2 were more energyfavorable for B doping than N in the heptazine unitviaDFT calculation (Fig. 1b,c), and successfully prepared B-doped tubular g-C3N4by pyrocondensation polymerization of melamine cyanuric acid (M·CA) and boric acid (Fig. 4c)7.Hence, the substituted doping of B formed the N―B bonding,which in turn narrowed the band gap from 2.7 eV of g-C3N4to 2.54 eV. The photocatalytic NO removal ratio over B doped tubular g-C3N4was 1.5 times higher demonstrated about than that over the incipient g-C3N4(Fig. 4c).
Apart from B atoms, nitrogen and phosphorus have also been used to substitute C atoms of g-C3N4framework. N doped g-C3N4with different N content were obtained by one-pot thermal polymerization of urea in air (CN-S) and nitrogen atmosphere(CN-N), and employed for H2O2decomposition for MB degradation68. FTIR analysis showed the replacement of C by N could induce the conversion of C―C bond to C―N bond, and the formation of VC. XPS results indicated the atomic ratios of C/N in CN-N (0.43) was much lower than that of CN-S (0.53).Characterization of the structure and properties suggested that the newly formed C―N bonds was beneficial for the transfer of photo-induced carries, and the more positive valence bands. As a result, the degradation rate of MB over CN-N was much faster than that over CN-S. Huet al.fabricated P-doped g-C3N4(PCN)viathermal condensation of melamine and hexachlorotriphosphazene69. They found P atoms doping could not only substitute the C atoms of g-C3N4, but also covalently bond with the linked N of the heptazine rings to fill in the VCand repair the structural defect because of its much less electronegativity compared to C atoms. As a result, the separation of charge carries was accelerated, leading to enhanced photocatalytic activity. The P doped g-C3N4with VNwas also successfully obtained through a two-step calcination method70.
Sulfur has also been employed to tune the activity of g-C3N471-73. Lv and coworkers constructed S-doped 2D g-C3N4nanosheets (2D-SCN with a 4.0 nm thickness) by polycondensation of thiourea followed by thermal oxidative etching71. XPS showed that the S atoms were doped by substituting lattice nitrogen. They validated that S doping enlarged the specific surface area, introduced surface VNand O species. DFT calculation proved that the formation of S―C―N bond boosted the charge transfer, leading to enhanced photocatalytic ability for hydrogen evolution.
Halogen atoms were also applied as dopants to optimize g-C3N4for photocatalysis. Huet al.obtained VCand I co-doped g-C3N4for photocatalytic N2fixation by co-condensation of dicyandiamide and KIO374. Iqbalet al.prepared VNand I codoped mesoporous g-C3N4by anin situdecomposition-thermal polymerization method with glucose and NH4I, for photocatalytic H2evolution75. F-doped g-C3N4(F-CNS) has also been prepared by Linet al.through fluorine-containing solvothermal treatment76. Characterization of the structure and properties revealed that the solvothermal treatment increased the crystallinity of g-C3N4, decreased the defects, and introduced VNas new active site in F-CNS. As a result, the modified F-CNS exhibited excellent photocatalytic activity compared with original g-C3N4. Generally, the introduction of element could optimize the band structure, prolong the light response, boost the separation efficiency of the photo-induced electron-hole pairs,thereby resulting in superior photocatalytic activity. However,excessive doping atoms would induce too much defects to form the recombination centers of electron-hole pairs charges, which is not conducive to photocatalysis.
Dual- and tri-doping of g-C3N4by non-metal or metal atoms has a synergistically positive impact on the band structure and optical properties, which has attracted widespread attention77.By thermal polymerization of dicyandiamide (DCDA) with KBH4, Wanget al.combined B-K co-doping with N vacancies to tailor the structural property of g-C3N4to optimize the photocatalytic CO2reduction78. Characterization of the structure and properties using X-ray powder diffraction (XRD), FTIR spectra, XPS,etc., showed that K was doped at the vacancy site,B atom preferred replacing bay site C in the heptazine structure,and the molar concentrations of B, K and VNwere nearly equal to each other in modified g-C3N4. Both experimental and DFT results evidenced that the superior CO2reduction efficiency were attributed to the electron-rich surface and optimized electronic structure resulting from the synergistic effects, where K could boost the electron transport as an electron donor, B could provide a suitable reduction potential, and the concomitant VNcontributed to the CO2adsorption. Apart from alkalis metal,transition metals also have been doped with nonmetal together to modify g-C3N4. Chenet al.successfully synthesized porous P-Mo co-doped g-C3N4with weak vacancies through polycondensation of melamine, P204, and H24Mo7N6O24·4H2O79. Results indicated that Mo6+ions dispersed in N conjugated aromatic ligands, and P substituted the C atoms in the g-C3N4framework. Yuet al.embedded Co atoms into the framework in the form of Co―N bonds, with the O doping at the N site in parent g-C3N4and generation of feeble VN,simultaneously80. Results proved the improved light response capability and the accelerated photo-induced charge separation and transport. In short words, g-C3N4co-doped by metal and non-metal has both advantages: the new generated energy levels in the band gap caused by metal doping, and the nitrogen vacancies (VN) caused by non-metal doping, without the individual drawbacks of monomer one.
Besides elemental doping, it has been verified that functionalization is one of effective strategies for the optimization of the optoelectronic properties of semiconductor6,81.Several techniques including coordination82-85, covalent bonding86,π-πinteractions87, and protonation88-90have been developed for g-C3N4functionalization. Zhaoet al.conducted the functionalization of g-C3N4with the interlayer coordination of Cu2+(CNCU1), along with the coexistence of surface deposited Cu0(CNCU2) and VN(CNCU3)83. The Cu2+ions decomposed H2O2into ·OH through a Fenton-like reaction, the VNand Cu0synergistically activated H2O2molecule, and thus promoted the NO removal efficiency of g-C3N4(Fig. 5a). Liuet al.also found that the coordinating of N 2plone electrons at the vacancies of tri-s-triazine polymer with Cu 3dorbits could extend theπ-delocalization structure of g-C3N4, resulting in broadened light absorption from Ultra Violet to Near Infrared Ray, effective charge transfer from N 2pof C3-N to O2molecular at active sites of linked C and Cu (namely, efficient evolution of reactive oxygen species including O2−and H2O2), and finally excellent and durable formaldehyde and NO removal efficiency84.Donget al.functionalized g-C3N4using celestine, since Sr2+ions could boost the separation of photo-induced carries, and Sr0were beneficial for the formation of ·OH82. In addition,polyoxometalates (PMO)85, Pt2+/Pt0hybrid nanodots91, Pt and Ru monomers92, Pt single atoms93, Au NPs94etc.were also coordinated with the g-C3N4to modify the electronic and band structure of g-C3N4to ultimately advance the photocatalytic performance including H2revolution, CO2reduction, nitrogen fixation, CO oxidation and so on.
Organic functionalization case is heat treatment of highly condensed lamellar melamine-cyanuric acid supramolecular(MCS) complex in argon flow and followed by air86. The resulting g-C3N4nanosheets owned in-plane nanoholes and high specific surface area, exhibited extremely enhanced light absorption in the visible region and excellent H2production.Results proved that thein situintroduced surface amino- and oxygen-containing groups could tune the electronic band structure of g-C3N4nanosheets to be more beneficial to H2production through the extended conjugated system of tri-striazine units of g-C3N4texture. Bellamkondaet al.used pyrimidine and benzene as different carbon content groups to modify g-C3N4(PD-CN, BD-CN) by copolymerization of 2,4,6-triamino pyrimidine or 1,3,5-triaminobenzene with cyanuramide, respectively87. Both the DFT calculations and experimental results verified that the introduction of benzene in g-C3N4framework has partial charge densities over valence band(VB) maxima and CB minima in different parts of heptazine rings, which was beneficial for the separation of charge carriers(Fig. 5b). Therefore, functionalization is one of good strategies for tuning the electronic band structure of g-C3N4to enhance light absorption and reinforce the separation of photo-generated charges.

Fig. 5 (a) The mechanisms of photocatalytic NO removal on CNCU1, CNCU2, and CNCU3, reproduced with permission from ACS Appl.Mater. Interfaces, American Chemical Society 83. (b) Schematic diagram of photocatalytic H2 evolution by benzene doped g-C3N4, reproduced with permission from J. Mater. Chem. A, Royal Society of Chemistry 87. (c) Schematic diagram of photocatalytic NO removal by pCN/TiO2 nanocomposite film, (d) optical photograph of pCN/TiO2 films, reproduced with permission from Appl. Catal. B: Environ., Elsevier 95.
Huanget al.successfully protonated g-C3N4(pCN) through modifying bulk g-C3N4with commercial concentrated HCl at room temperature95. Whereafter, TiO2sol has been further employed as chemical glue for the as-prepared pCN to overcome the low adherence of pCN and an easily scalable prepared pCN/TiO2nanocomposite film was fabricated with good adhesion (Fig. 5c,d). Results showed that NO removal ratio significantly increased with the enhancement of pCN loading mass, and the optimized film exhibited a considerably high and stable visible-light-driven deNO activity (~25%), excellent transmittance, and photo-excited hydrophilicity for potential self-cleaning ability, indicating the potential of the prepared catalysts in the practical application.
3 Practical applications of g-C3N4 in photocatalytic air purification
As a competitive and promising technology, the ultimate goal of photocatalysis is to be applied to commercial or industrial application. Regarding the practical application of photocatalysis in semi-closed/open spaces, numbers of crucial problems need to be concerned such as large-scale-preparation,adherence, durability and effectiveness of photocatalysts, and the interference of environmental factorsetc., even for g-C3N4which is easy to be macro-prepared.
For this purpose, Huanget al.have developed the g-C3N4/TiO2hetero-hydrosol at room temperature according to the well-dispersion ability of 2D materials in solutions, and fabricated a photocatalytic air-purifying pavement which locates in the Baqiao District (Xi’an, China) for the removal of atmospheric NOx(Fig. 6), recently96. As shown in Fig. 6a, the experimental area includes the deNOxroad covered with the g-C3N4/TiO2(3.06 g·m−2), and the blank control one which was 60 meters away from deNOxone. The adhesion tests of the g-C3N4/TiO2film confirmed that the film was firmly bonded to the surface of the substrate because of the formed Ti―O―Si bond between the composite sol and the glass substrate. Results of outfield experiment showed that the daily average local-area NOxconcentration of the pavement decreased by 27.8% after coating. Furthermore, the average deNOxrate was up to 35.7%between 7:00-10:00 and 17:00-19:00. The solar irradiance was lower (< 5000 W·m−2) and the traffic volume was larger (> 70 vehicles/10 min) during the two periods. The remarkable sunlight utilization of the g-C3N4/TiO2made the traffic volume the main factor affecting the deNOxrate of the photocatalyst.Moreover, experimental results demonstrated that the g-C3N4/TiO2film owned photo-induced hydrophilicity which make it self-cleaning (Fig. 6c).
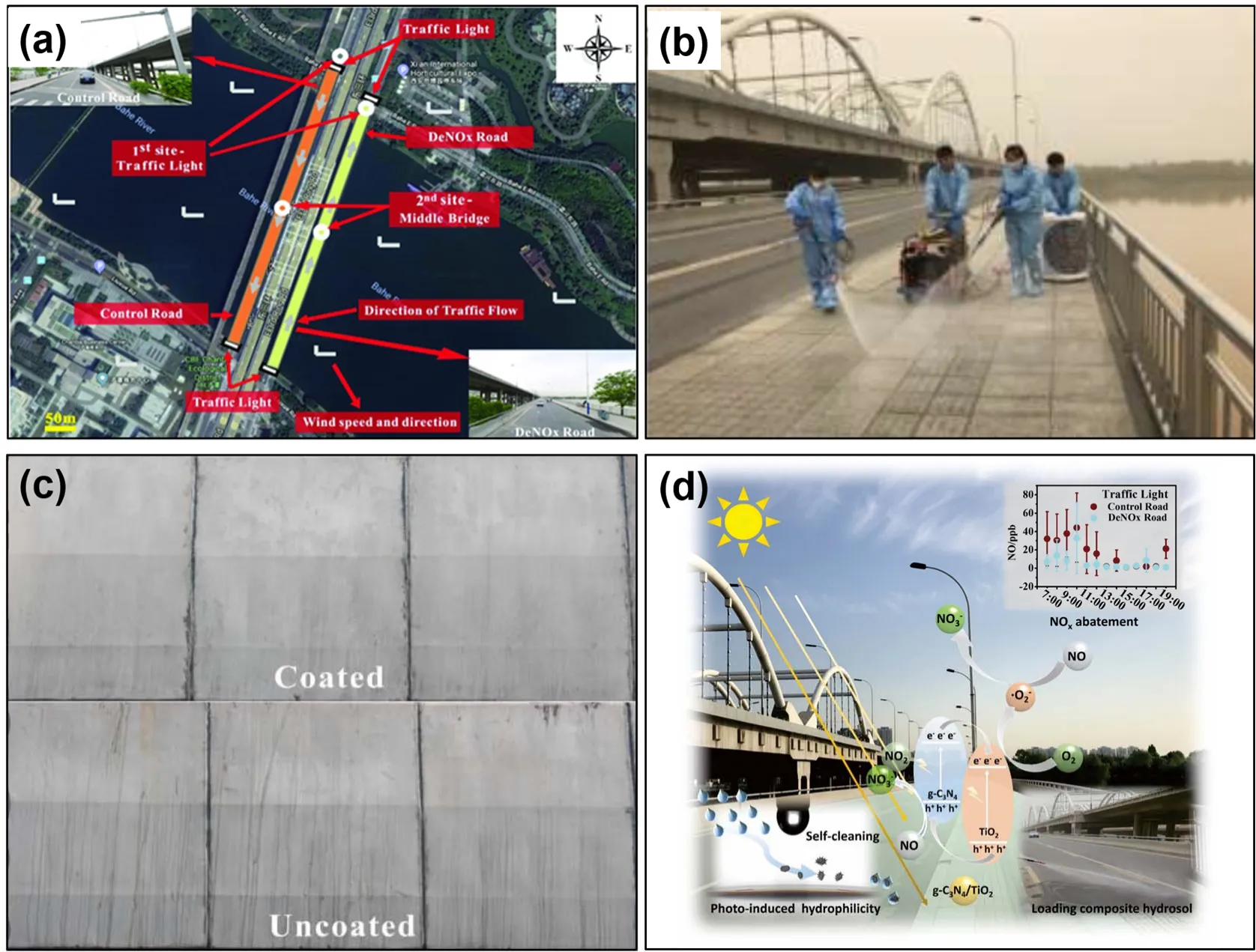
Fig. 6 (a) Aerial view of the coating district and sampling sites, (b) loading of g-C3N4/TiO2 composite sol onto the road via spray method,(c) surface of the concrete barrier coated (upward) with and (nether) without g-C3N4/TiO2 after 4 months, (d) schematic diagram of photocatalytic air-purifying pavement, reproduced with permission from Sol. RRL, Wiley96.
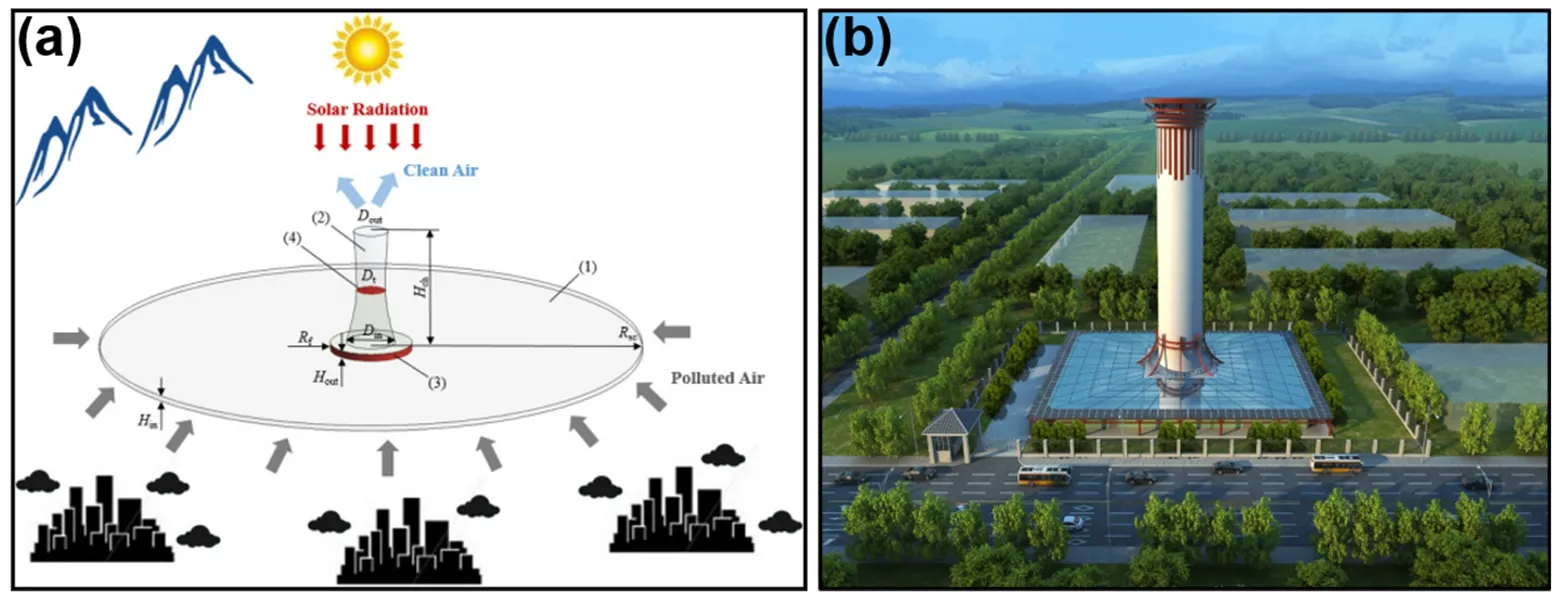
Fig. 7 (a) Design concept and (b) rendering of HSALSCS, adapted from Sci. Technol. Rev, Science and technology and review publishing house 97.
The developed g-C3N4/TiO2hetero-hydrosol has also been used in the hybrid solar assisted large-scale cleaning system(HSALSCS) (Fig. 7), which was first proposed for urban air purificationviathe heated air underneath the glass shelter driving the air flow through a 60 × 43 m2chimney deposited with photoactive materials, in 201597,98. Preliminary outfield experiment results show that the HSALSCS achieved a ~30%NOxremoval efficiency and remarkable self-cleaning.
4 Conclusions and perspective
g-C3N4has emerged as a research hotspot due to its special advantages such as the unique structure with coordination ring surrounded by aπ-πconjugated bond to afford active sites, and has been used for photocatalysis including NOxremoval, CO2reduction, N2photofixation, organic degradation, H2revolutionetc. However, the pristine g-C3N4intrinsically suffers from low light quantum efficiency, and fast recombination of charge carries, resulting in unsatisfactory photocatalytic activity.Among various modification strategies, defect engineering has been proved to be one of efficient strategies to modulate the electronical and structural property of g-C3N4, which could considerably increase the light adsorption capacity and boost the charge separation efficiency.
In this minireview paper, the latest progresses and advancements in defective g-C3N4have been outlined. Typically,non-external-caused defects such as N vacancies, C vacancies and fragments deficiency, and external-caused one derived from elemental doping, functionalization and so on have been developed for the reinforced photocatalytic activity which is related to the broadened light absorption, the improved charge transfer, the prolonged charge carrier lifetime, and optimized redox ability. Generally, vacancies could be a reservoir of photoinduced electrons to boost the separation of charge carries as well as a capture site to transfer the trapped photo-excited electrons to dissociative O2for the generation of reactive oxygen species. And, excessive defects would lead to the recombination of charge carries, which is disadvantageous for photocatalysis.
Overall, defect engineering of 2D g-C3N4have been taken seriously for their extensive and potential applications.However, the development of defective g-C3N4in photocatalysis is still at laboratory scale and a few directions deserve further study: (1) The development of innovative strategies for defect engineering, especially preparation methods that can fine-tune defect site and concentration; (2) Combining defect engineering with specific morphologies to optimize the electronical and band structure; (3) Combining theoretical calculation and experiments to obtain high redox potential for photocatalysis; (4) The development of a low cost, easy to operate, and large-scale preparation process for practical applications.
杂志排行
物理化学学报的其它文章
- 基于密度泛函理论下H2S 在单原子催化剂V/Ti2CO2 上的分解机理研究
- 理论计算评价光催化剂VOCs 降解性能:g-C3N4 量子点/石墨烯
- 富含缺陷的2D/2D 异质结促进光催化清洁能源转化
- Development of Iron-Based Heterogeneous Cocatalysts for Photoelectrochemical Water Oxidation
- 二维光催化材料电子结构和性能调控策略研究进展
- Non-Noble-Metallic Cocatalyst Ni2P Nanoparticles Modified Graphite-Like Carbonitride with Enhanced Photocatalytic Hydrogen Evolution under Visible Light Irradiation
