Non-Noble-Metallic Cocatalyst Ni2P Nanoparticles Modified Graphite-Like Carbonitride with Enhanced Photocatalytic Hydrogen Evolution under Visible Light Irradiation
2021-09-01PengZhangJiquanWangYuanLiLishaJiangZhuangzhuangWangGaokeZhang
Peng Zhang , Jiquan Wang , Yuan Li , Lisha Jiang , Zhuangzhuang Wang , Gaoke Zhang ,*
1 Hubei Province Engineering Consulting Co, Ltd., Wuhan 430061, China.
2 School of Resources and Environmental Engineering, Wuhan University of Technology, Wuhan 430070, China.
Abstract: Energy crisis has become a serious global issue due to the increasing depletion of fossil fuels; therefore, it is crucial to develop environmentally friendly and renewable energy resources, such as hydrogen (H2), to replace fossil fuels. From this viewpoint,photocatalytic H2 production is considered as one of the most promising technologies. Noble metal platinum (Pt) can be applied as an efficient cocatalyst for improving the H2 production performance of photocatalytic systems; however, its high cost limits its further application. Thus, the development of novel, high-activity, and low-cost cocatalysts for replacing noble metal cocatalysts is of great significance for use in photocatalytic H2 evolution techniques. Herein, we successfully synthesized a Ni2P/graphite-like carbonitride photocatalyst (Ni2P/CN) using a conjugated polymer (SCN)n as precursor for enhanced photocatalytic H2 production under visible light illumination. Various characterization techniques,including optical and photoelectronic chemical tests, were used to investigate the structural composition, morphology, and light adsorption ability of these materials. X-ray diffraction, Fourier transform infrared spectroscopy (FT-IR), and X-ray photoelectron spectroscopy results showed that Ni2P/CN nanocomposites with good crystal structure were obtained.Scanning electron microscopy and transmission electron microscopy results revealed that the Ni2P/CN samples had a typical two-dimensional layered structure, and the Ni2P nanoparticles were uniformly loaded on the surface of the CN to form a non-noble metal promoter. UV-Vis diffuse reflectance spectra results demonstrated that the loading of Ni2P nanoparticles effectively enhances the adsorption capacity of CN to visible light. Photoluminescence spectroscopy and photocurrent (PL) results suggested that Ni2P loading to CN is beneficial for promoting the migration and separation efficiency of photogenerated carriers. Photocatalytic H2 production was conducted under visible light irradiation with triethanolamine as a sacrificial agent. The results suggest that the Ni2P/CN composite photocatalysts exhibit excellent photocatalytic reduction performance. In particular, the H2 evolution rate of the optimal Ni2P/CN nanocomposite is 623.77 μmol·h−1·g−1, which is higher than that of CN modified by noble metal Pt, i.e., 524.63 μmol·h−1·g−1. In conclusion, Ni2P nanoparticles are homogeneously attached to the surface of CN, and a strong interfacial effect exists between them,thereby forming an electron transfer tunnel that greatly inhibits the recombination of photoinduced carriers and promotes the migration of electrons from CN to Ni2P. In addition, a possible photocatalytic mechanism is proposed based on the experiments and characterizations. This work has profound significance for developing non-noble metal cocatalysts for the substitution of noble metal cocatalysts for high-efficiency photocatalytic H2 evolution.
Key Words: Cocatalyst; Ni2P; Photocatalytic hydrogen evolution; Interfacial effect, Visible light Received: September 29, 2020; Revised: October 27, 2020; Accepted: October 28, 2020; Published online: November 2, 2020.*Corresponding authors. Emails: 441780131@qq.com (J.W.); gkzhang@whut.edu.cn (G.Z.).
1 Introduction
Owing to the gradually depletion and unsustainable nature of global fossil energy, the development of clean and sustainable energy for the substitution of traditional fossil energy is of great urgency and indispensable for our world. Therefore, looking for an ideal technique for clean energy evolution is of great significance. Among various energy conversion techniques,photocatalytic hydrogen (H2) evolution is one of the most promising technologies to resolve the fossil energy shortage problem and has received more and more attention in the past decade1-4. However, a single photocatalyst is hardly utilized to directly conduct H2evolution experiment owing to its rapid recombination efficiency of photoinduced electron-hole pairs and usually needs to get additive support from the cocatalyst which usually possesses superior electrons trapping ability5-8.To date, many researches about cocatalyst selection in photocatalytic H2evolution are over relied on noble-metal cocatalyst such as platinum (Pt). The expensive cost and easy back reaction with O2and H2to produce H2O of noble-metal cocatalyst largely restrict the practical utilization of H2evolution technique in industry. Therefore, developing non-noble metallic cocatalysts with high efficiency and low price is pivotal for the practical utilization of this technique. So far, several transition metal phosphides, including Co2P, FeP, MoP and Ni2P, have been investigated as promising non-noble metallic cocatalysts to substitute for noble metal cocatalysts9-12. Among them, Ni2P is excellent for its superior electron trapping ability which can effectively inhibit the recombination of photoinduced carriers.Therefore, Ni2P may be a promising non-noble metallic cocatalyst for photocatalytic H2evolution13,14.
Graphite-like carbonitride, a semiconductor driven by visible light, has attracted much attention as a promising photocatalyst mainly owing to its controllable surface, low cost, excellent chemical stability and nontoxicity15-17. Nevertheless, it is usually difficult to achieve a satisfying photocatalytic efficiency by only using graphite-like carbonitride due to the insufficient light adsorption and rapid recombination of photoinduced carriers. Various strategies were therefore investigated to modify graphite-like carbonitride such as metallic/non-metallic heteroatom doping18,19, modifying with defects20, microstructure regulation21and construction of heterojunction22.Especially, cocatalyst modification on the surface of graphitelike carbonitride is considered to be an ideal strategy for boosting photocatalytic H2productionviarapidly transferring interfacial electrons, minimizing the recombination of photoinduced charges, and offering effectively active sites.
Considering the numerous merits of Ni2P, we successfully introduced Ni2P nanoparticles on the surface of graphite-like carbonitride (CN)23. The Ni2P/CN nanocomposites exhibit apparently enhanced photocatalytic H2evolution performance than that of single CN. Especially, the H2evolution performance of the optimal sample is better than that of CN supported by Pt nanoparticles, indicating Ni2P is indeed a promising non-noble metallic cocatalyst. The structures, morphologies, optical and photoelectronic properties of as-prepared nanomaterials were investigated by various characterization techniques. These characterizations demonstrate Ni2P nanoparticles are uniformly attached on the surface of CN and a strong synergistic effect exists between Ni2P and CN, thus forming an electron transfer channel that can greatly improve the charge separation.Moreover, based on the trapping experiments and analyses of characterizations, a possible photocatalytic H2evolution mechanism is proposed. This work offers new insight for synthesizing novel photocatalysts decorated by non-noble metallic cocatalyst such as Ni2P for photocatalytic H2evolution.
2 Experimental and computational section
2.1 Materials preparation
Potassium thiocyanate (KSCN, ≥ 98.5%), potassium persulfate ((NH4)2S2O8, ≥ 99.5%), nickel dichloride hexahydrate(NiCl2·6H2O, ≥ 98.0%) and sodium hypophosphite monohydrate (NaH2PO2·H2O, ≥ 98.0%) were purchased from Sinopharm Chemical Reagent Co., Ltd. All of the reagents were analytical grade without further purified.
2.2 Synthesis of Ni2P/CN samples
Firstly, KSCN and (NH4)2S2O8with a molar ratio of 2 : 1.5 were ground in an agate mortar. After grinding for 1 h in the fume cupboard, the precursor conjugated polymer (SCN)nwas rinsed with deionized water for 4 times and dried to obtain solid powders24. Subsequently, the solid powder was heated to 500 °C for 2 h with a rate of 5 °C·min−1. Ultimately, CN photocatalyst was obtained by cooling naturally to room temperature.
Ni2P/CN composites were prepared by mixing the nickel salt,sodium hypophosphite, pristine CN and heating them in nitrogen(N2) atmosphere. Typically, 10 mg NiCl2·6H2O was dissolved into 5 mL of deionized water. Then, 150 mg as-prepared CN was mixed into the above solution. After that, this mixture was stirred for 1 h, followed by ultrasonication for another 1 h.Subsequently, 50 mg NaH2PO2·H2O was dissolved into the previous mixture. The whole mixed solution was again stirred and sonicated for 1 h each and dried in a vacuum oven. The dried solid was then heated at a rate of 5 °C·min−1up to 200 °C for 1 h under N2atmosphere in a tube furnace and cooled down to ambient temperature. The obtained solid was washed 4 times by ethanol and deionized water, the Ni2P/CN composite with the mass ratio of 2% (w, mass fraction) was fabricated after dried at 60 °C for 8 h. Composites with various loading amounts of Ni2P nanoparticles (1% (w), 3% (w), 5% (w)) were denoted as 1% (w)Ni2P/CN, 3% (w) Ni2P/CN, 5% (w) Ni2P/CN.
2.3 Characterization
The structure and crystallinity of the as-prepared photocatalysts were investigated by X-ray diffraction (XRD,D/MAX-RB, Japan) analysis under the experimental situation of 40 kV and 50 mA. A Fourier transform infrared spectroscopy(FT-IR, Thermo Nicolet, American) was used to study the chemical bonds of these composite samples. X-ray photoelectron spectroscopy (XPS) analyses were performed on a PHI Quantera II system (American) with a monochromatic Mg Ca source to study the chemical states and valence band. All binding energies were referenced to the C 1speak at 284.5 eV.UV-Vis diffuse reflectance spectra (DRS) analyses were performedviaa Shimadzu UV 2550 (Japan) spectrometer with the BaSO4powder as the substrate. Scanning electron microscopy (SEM, JSM-5610LV, American) and transmission electron microscopy (TEM, JEM-2100F, American) were applied to observe the morphology of these samples. And photoluminescence (PL) emission spectra measurements were testedviaa fluorescence spectrophotometer (RF-5301PC,Shimadzu, Japan).
2.4 Photocatalytic measurements
The photocatalytic H2evolution experiments were performed in a 400 mL three-necked Pyrex flask at room temperature. Four 3 W 420 nm LED lamps were employed as the visible light source to trigger the reaction of photocatalytic H2generation.Typically, 20 mg of the as-prepared sample was dispersed in 100 mL aqueous solution containing 10% (volume fraction) of triethanolamine (TEOA) and the dispersion was sonicated for 5 min. Additionally, a control experiment that moderate amount H2PtCl6was added to make sure the pristine CN loaded with 1%(w) Pt by photodeposition route was performed. Before each reaction, the photocatalytic system was bowled with N2for 15 min to pull out the air completely. Finally, photocatalytic H2generation was evaluated by a gas chromatograph (Shimadzu GC-2014C, Japan, with N2as carrier gas), and 200 μL of the gaseous sample was detected at regular intervals (30 min).
3 Results and discussion
3.1 Sample characterizations
The XRD results of pristine CN and Ni2P/CN composites with various Ni2P loading amounts were demonstrated in Fig. 1.The two peaks at 13.0° and 27.8° indicate that both pristine CN and Ni2P/CN composites present similar structural characteristics of graphite-like carbonitride. Meanwhile, peaks of Ni2P/CN composites at 40.8°, 44.6°, 47.3°, 54.2°, 54.9° and 66.2° matched with the characteristic peaks of Ni2P [JCPDS 03-0953]25. The peaks of Ni2P in 1% (w) Ni2P/CN composite are not apparently observed in comparison with other composites,which is attributed to low loading concentration and highly dispersed Ni2P nanoparticles. With further increasing loading concentration, the peaks corresponding to Ni2P can be gradually observed, indicating the successful introduction of Ni2P to CN.Furthermore, no other impurity peaks are found in XRD spectra,confirming Ni2P/CN composites were successfully synthesized.
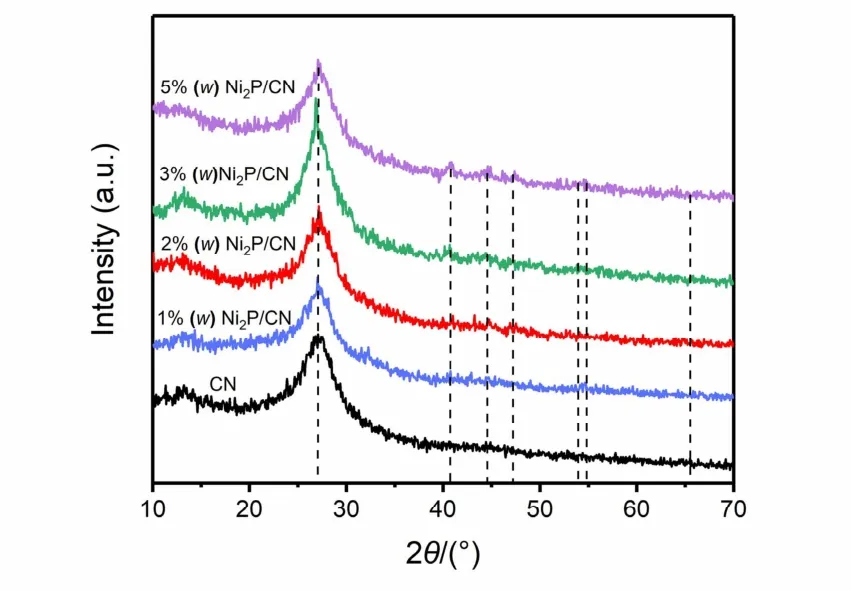
Fig. 1 XRD patterns of pristine CN and Ni2P/CN composites with different Ni2P contents (1% (w), 2% (w), 3% (w), 5% (w)).
The FT-IR analyses (Fig. 2) indicate that the pristine CN backbones are retained after Ni2P nanoparticles hybridization.For the pristine CN and Ni2P/CN composite samples, the characteristic peak at 807 cm−1originates from the unique vibration characteristic of the triazine ring unit. Furthermore,there is a wide absorption band in the region between 1200 cm−1and 1700 cm−1, which can be corresponded to the representative stretching vibration modes of C―N and C=N26. Besides, the absorption band between 2850 cm−1and 3450 cm−1includes residual N―H components and O―H bands, which are related to uncooled amino groups and water molecules adsorbed on the surface. The results confirm that there is no variation in the stretching and bending vibrations of CN on Ni2P nanoparticles loading, which is consistent with XRD analysis27-29.
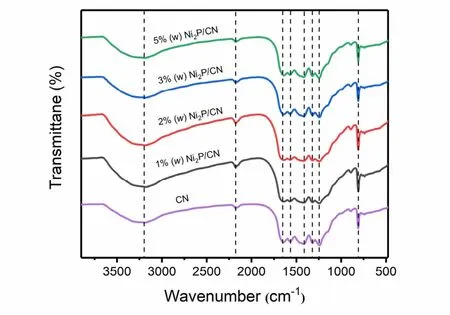
Fig. 2 FT-IR spectra of pristine CN and Ni2P/CN composites with different Ni2P contents (1% (w), 2% (w), 3% (w), 5% (w)).
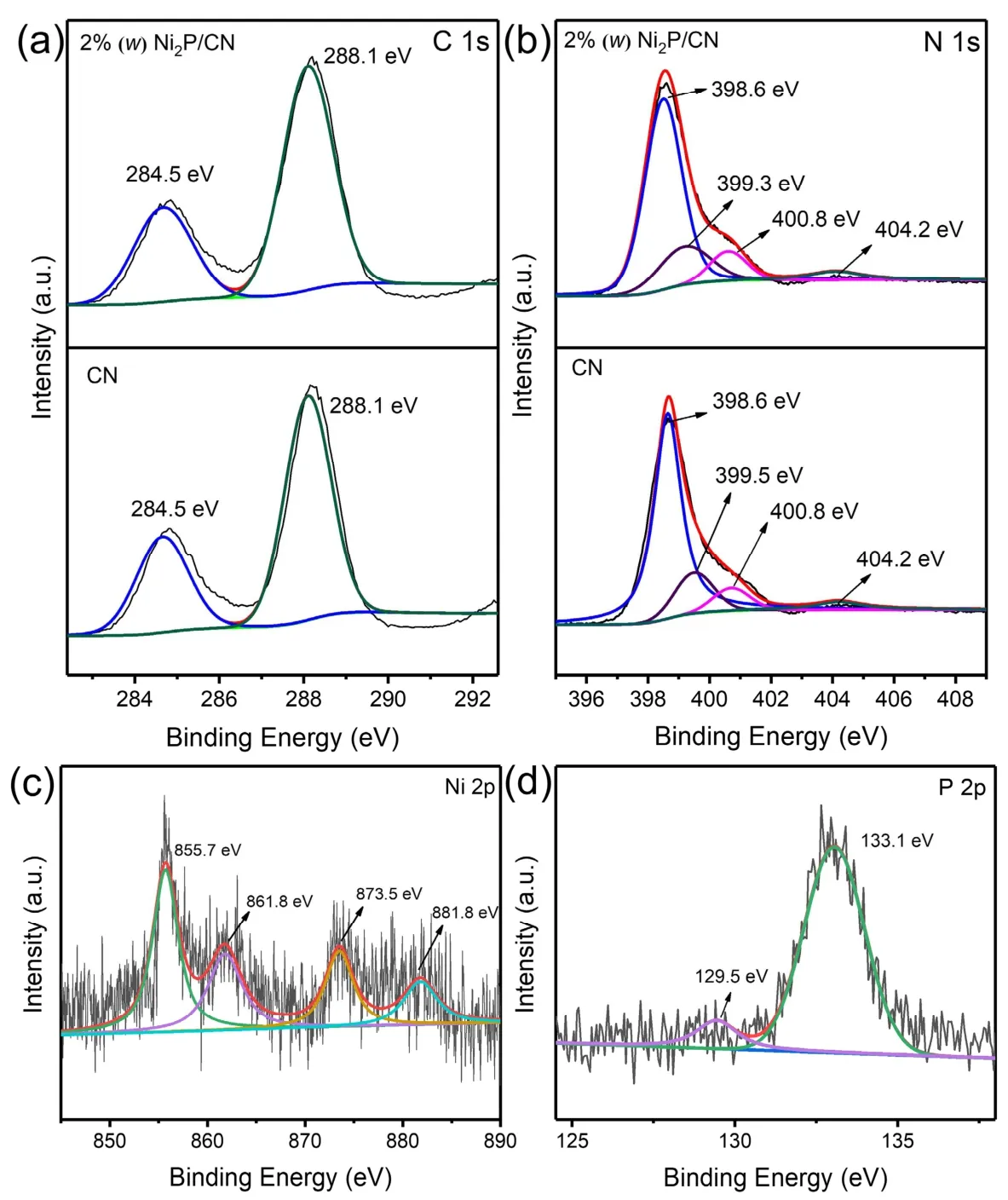
Fig. 3 XPS spectrum of pristine CN and 2% (w) Ni2P/CN: (a) C 1s region, (b) N 1s region, (c) Ni 2p region of 2% (w) Ni2P/CN,(d) P 2p region of 2% (w) Ni2P/CN.
The XPS measurements of pristine CN and 2% (w) Ni2P/CN were performed to further study the elemental composition and chemical valence of the photocatalysts. As depicted in Fig. 3a,the C 1sorbitals of pristine CN and 2% (w) Ni2P/CN can be fitted to two peaks, and their binding energies can be detected at 284.5 eV and 288.1 eV, which can be matched to the C source adsorbed on the sample surface and the N―C=N bond position in the graphite-like carbon nitride structure30,31. Meanwhile, the N 1sregion of pristine CN and 2% (w) Ni2P/CN involved four contributions in Fig. 3b. The analytical peaks are detected at 398.6 eV, 399.5 eV, 400.8 eV and 404.2 eV, which corresponded to C―N=C, C3―N, C2―N―H, andπ-excitation,respectively32,33. It is noteworthy that the N 1speak of C3―N in 2% (w) Ni2P/CN located at 399.3 eV decreases by 0.2 eV compared to that in CN, implying the combination of chemical bonds between Ni2P nanoparticles and CN layer to form N―Ni bond. Fig. 3c indicates that the Ni 2pregion of the 2% (w)Ni2P/CN can be fitted to four peaks, which can be detected at 855.7, 861.8, 873.5 and 881.8 eV. It shows that there are nickel elements in Ni2P in the valence state of Ni2+34,35. Furthermore,the P 2pregion of 2% (w) Ni2P/CN was shown in Fig. 3d, the peak at 129.5 eV can be attributed to metal―P bonds in Ni2P, the peak at 133.1 eV is ascribed to oxidized and unreduced phosphate in air36,37. These results further affirm the formation of Ni2P in the Ni2P/CN composites.
To investigate the optical properties of pristine CN, as well as Ni2P/CN composites, UV-Vis measurement was carried out and the spectra were displayed in Fig. 4. The pristine CN expresses weak light absorption in the visible light region. Besides,Ni2P/CN composites have better light absorption ability than pristine CN, and the absorption intensity of Ni2P/CN composites in the region of visible light becomes stronger with the increasing amount of Ni2P. Furthermore, the Ni2P/CN composites exhibit a few redshifts of the absorption edge comparing to the pristine CN, indicating the Ni2P/CN composites possess a wider adsorption range of visible light,which is beneficial to H2evolution by generating more photoinduced charge carriers.
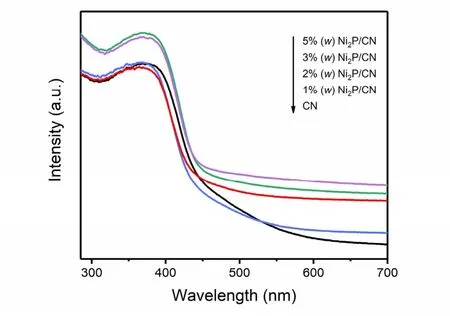
Fig. 4 UV-Vis absorption spectra of pristine CN and Ni2P/CN composites with different Ni2P contents (1% (w), 2% (w),3% (w), 5%(w)).
The morphologies of the synthesized photocatalysts were observed by SEM and TEM techniques. Fig. 5a,b are the SEM images of pristine CN and 2% (w) Ni2P/CN, respectively. It can be observed that the two photocatalysts were composedviathe aggregation of nanoparticles with irregular morphology, and the morphology of the 2% (w) Ni2P/CN composite after secondary calcination did not change significantly. In order to verify the loading status of Ni2P nanoparticles, the morphological characteristics of 2% (w) Ni2P/CN composite was further observed by TEM technology. The TEM images of 2% (w)Ni2P/CN in Fig. 5c-e clearly demonstrated that Ni2P nanoparticles were homogeneous distribution on the surface of CN. Especially, the size of Ni2P nanoparticles ranging from 10 to 50 nm, which is beneficial to enhance the active space area and facilitate electron transport.
3.2 Photocatalytic performance
The photocatalytic H2generation activities of pristine CN,Ni2P/CN and Pt/CN composites were measured with TEOA as a scavenger under visible light illumination. As is revealed in Fig.6a, pristine CN showed little photocatalytic H2evolution (only trace H2can be detected) without noble metal loading.Interestingly, Ni2P/CN composites presented a much superior H2evolution to pristine CN so that loading Ni2P nanoparticles could significantly promote the photocatalytic performance of CN. For 1% (w) Ni2P/CN and Pt/CN, the H2generation rates are 159.66 and 524.63 μmol·h−1·g−1, respectively in Fig. 6b. The highest H2generation rate of 623.77 μmol·h−1·g−1was achieved when using 2% (w) Ni2P/CN composite, despite being more naturally abundant and less price, it is superior to Pt/CN. Nevertheless, as the Ni2P loading content is further increased more than 2% (w),the H2generation gradually decreased but still significantly stronger than the pristine CN, which may be due to excessive Ni2P covers the active sites on the surface of CN and prevents CN from absorbing incident light38.
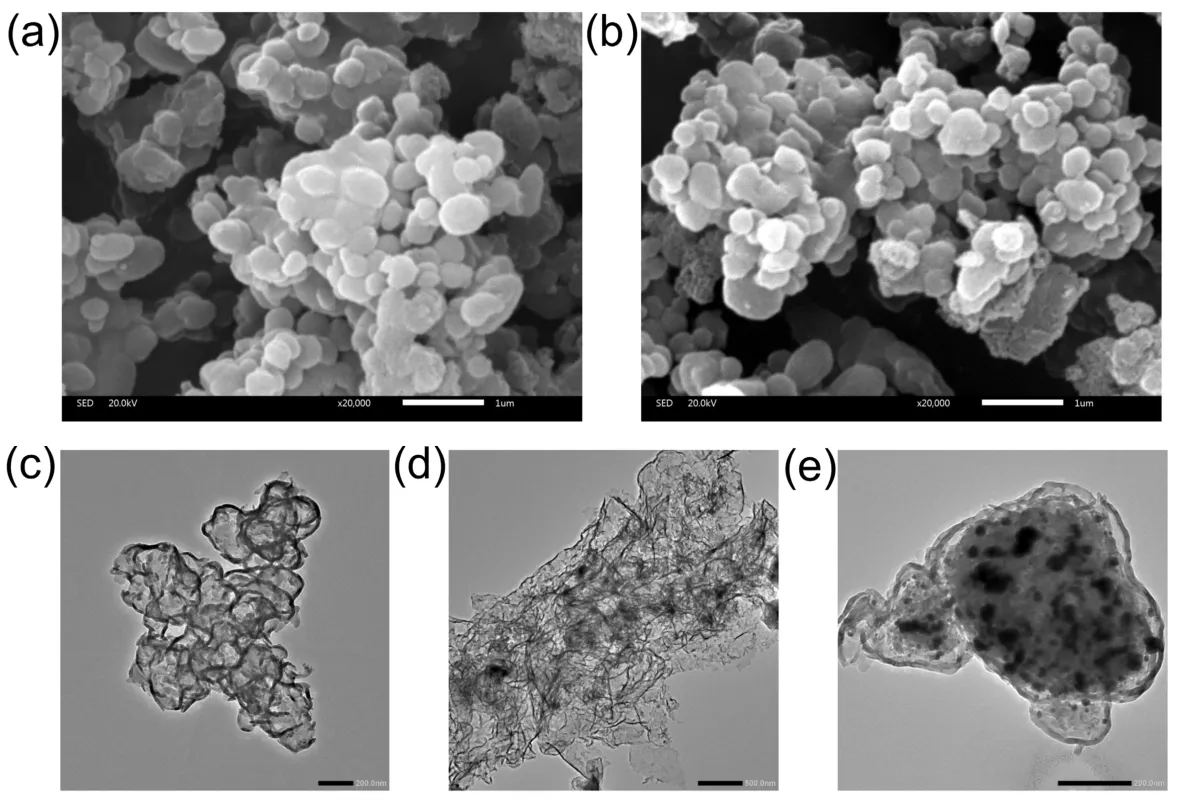
Fig. 5 The SEM images of (a) pristine CN and (b) 2% (w) Ni2P/CN, the TEM images (c–e) of 2% (w) Ni2P/CN.
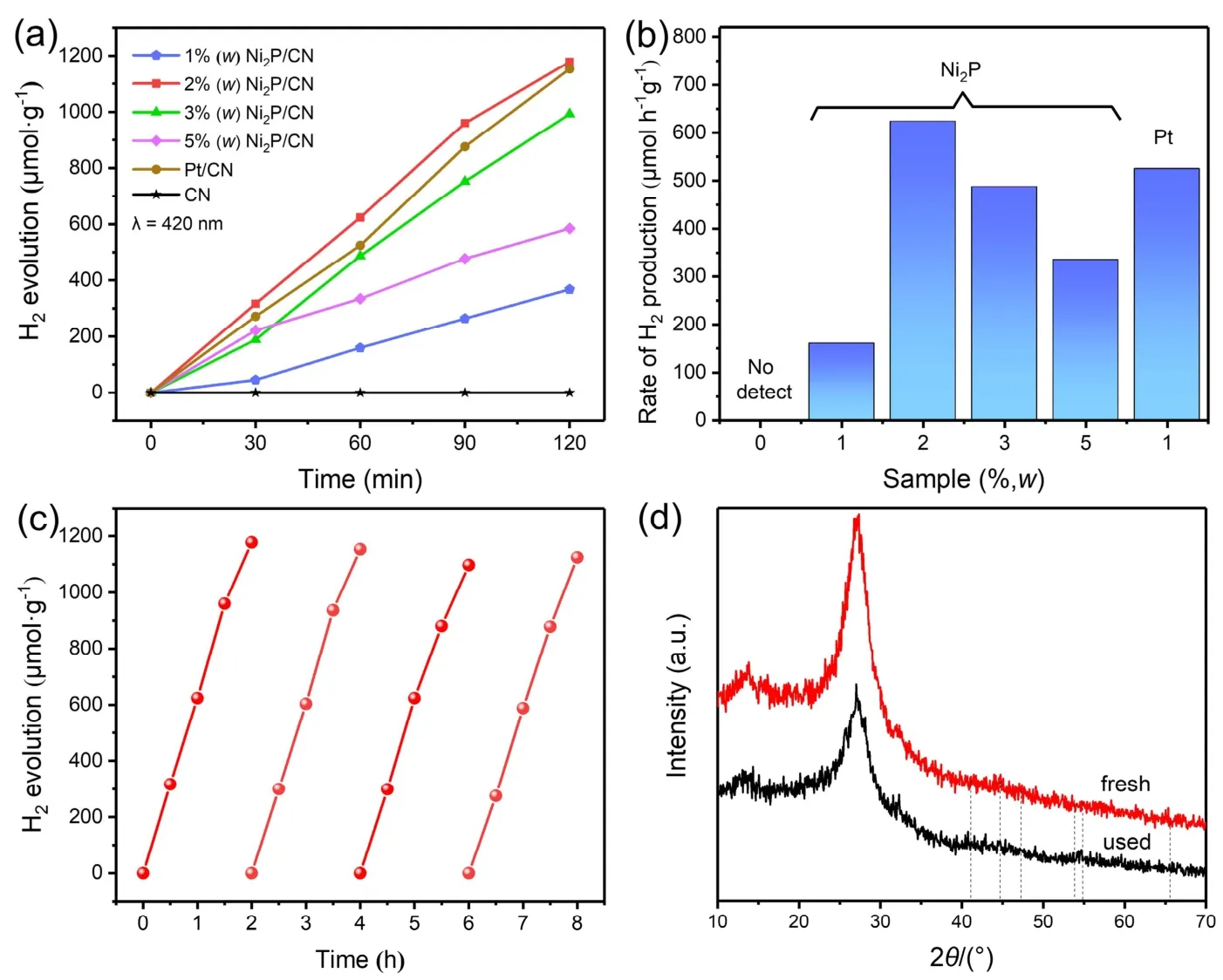
Fig. 6 (a) Time course of H2 generation performance and (b) H2 generation rate of pristine CN, Pt/CN and Ni2P/CN composites with different Ni2P contents (1% (w), 2% (w), 3% (w), 5% (w)). (c) Cyclic H2 evolution curve of 2% (w) Ni2P/CN composite under 420 nm visible light in 10% (volume fraction) TEOA solution and (d) XRD patterns of 2% (w) Ni2P/CN composite before and after the photocatalytic H2 evolution for 4 times.
The stability and durability of photocatalyst are also essential for practical application. Therefore, we used 2% (w) Ni2P/CN as a representative sample to measure the stability of as-prepared composites for photocatalytic H2generation, and the results are depicted in Fig. 6c. It is pretty clear that the 2% (w) Ni2P/CN composite could maintain an efficient and stable photocatalytic property in long-term reaction for four cycles usage. Moreover,XRD analysis confirmed the unchanged basic structure of 2%(w) Ni2P/CN before and after four cycles photocatalytic H2production experiment (Fig. 6d). This further supports the stability and durability of Ni2P as a non-noble metal cocatalyst,which can be used to improve the ability of photocatalytic H2evolution.
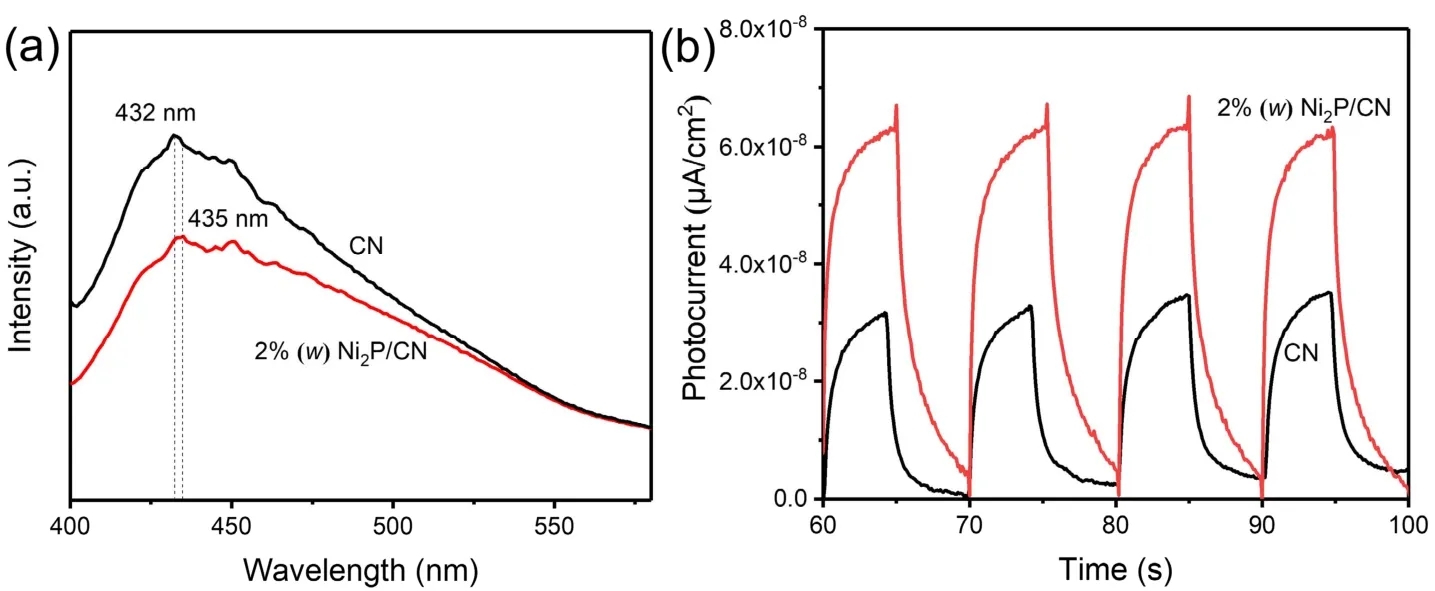
Fig. 7 (a) PL spectra of pristine CN and 2% (w) Ni2P/CN (Ex = 315 nm), (b) the photocurrent response curve of pristine CN and 2% (w) Ni2P/CN.

Fig. 8 Mechanism for photocatalytic H2 evolution from water over Ni2P/CN composite.
3.3 Mechanism for enhanced photocatalytic performance
The PL measurements of pristine CN and 2% (w) Ni2P/CN were carried out to investigate the separation efficiency and recombination process of photoinduced carriers39. As revealed in Fig. 7a, the peak value of the fluorescence spectrum of 2% (w)Ni2P/CN composite is lower than that of pristine CN, illustrating that the recombination rate of photoinduced carriers is hindered impressively with Ni2P nanoparticles loading. Moreover, the absorption peak of 2% (w) Ni2P/CN composite shifted from 432 nm to 435 nm compared with pristine CN because of the addition of Ni2P nanoparticles, which was more conducive to the transfer of photoinduced carriers from CN to Ni2P. Additionally, the photocurrent response was measured as well in Fig. 7b. It is clearly observed that pristine CN showed pretty low photocurrent response and photocurrent density, but 2% (w)Ni2P/CN composite presented much higher photocurrent density than pristine CN. The increased photocurrent intensity indicates the enhanced migration efficiency of photoinduced charge carriers, thus finally promoting the photocatalytic efficiency of H2production40.
On the basis of the results above, the enhanced photocatalytic H2generation performance may be attributed to the effective interfacial charge transfer from CN to the Ni2P (Fig. 8)41,42. As a cocatalyst, Ni2P mainly plays two roles: (1) reducing the charge recombination effect after charge separation, and (2) promoting the surface chemical reaction of pristine CN. The facilitated electrons in Ni2P cocatalyst would reduce H+into H2, while holes on CN can oxidize the sacrificial electron donor TEOA.
4 Conclusions
In conclusion, Ni2P/CN photocatalysts were successfully synthesized by solid-phase grinding, ultrasonic mixing and calcination method. Ni2P nanoparticles can be served as an active and highly stable free-noble metal cocatalyst under visible light irradiation to enhance the photocatalytic H2evolution efficiency of pristine CN. The optimized H2production rate is 623.77 μmol·h−1·g−1for 2% (w) Ni2P/CN composite, which is pretty higher than that of Pt/CN composite. The improvement of photocatalytic performance is mainly ascribed to the effective transfer of charge from CN to Ni2P, which inhibits the recombination of photoinduced electron-hole pairs in CN. Most significantly, the long-term stability and excellent performance of continuous H2evolution under visible light irradiation promote the practical application of the system made of freenoble metal elements as cocatalyst.
杂志排行
物理化学学报的其它文章
- Defect Engineering in Two-Dimensional Graphitic Carbon Nitride and Application to Photocatalytic Air Purification
- 理论计算评价光催化剂VOCs 降解性能:g-C3N4 量子点/石墨烯
- 富含缺陷的2D/2D 异质结促进光催化清洁能源转化
- Development of Iron-Based Heterogeneous Cocatalysts for Photoelectrochemical Water Oxidation
- 二维光催化材料电子结构和性能调控策略研究进展
- Selective Exposure of BiOI Oxygen-Rich {110} Facet Induced by BN Nanosheets for Enhanced Photocatalytic Oxidation Performance
