La-Doped BiOI Microspheres for Efficient Photocatalytic Oxidation of NO under Visible Light Illumination
2021-09-01QianLiJingHuYiZhouHaiqiangWangZhongbiaoWu
Qian Li, Jing Hu, Yi Zhou, Haiqiang Wang , Zhongbiao Wu
Department of Environmental Engineering, Zhejiang University, Hangzhou 310058, China.
Abstract: Photocatalytic oxidation has been widely acknowledged as an economical and effective technology for the treatment of lowconcentration NO. Three-dimensional (3D) BiOI microspheres, which are typical visible-light responsive semiconductor photocatalysts, often suffer from quick recombination of photogenerated carriers and unsatisfactory electrical conductivity when applied in NO photocatalytic oxidation reactions. However, owing to their micro-sized structures, they are usually difficult to couple with other semiconductors and co-catalysts because of their incompact interfaces that provide insufficient contact. In this study, a rare-earth metal (La) doping strategy was first adopted to modify BiOI microspheres via a simple one-step solvothermal method; subsequently,the photocatalytic NO oxidation performance under visible light illumination was systematically investigated. Further, the La precursors and doping contents were optimized. It was found that La(NO3)2 was the best precursor when compared to LaCl3 and La(AC)3. Moreover, 0.3%La/BiOI exhibited the best NO photocatalytic conversion efficiency of up to 74%, which was significantly higher than that of the pure BiOI benchmark (44%). It also exhibited excellent stability during the continuous 5-cycle experiments. Analysis of the physicochemical properties revealed that La doping facilitated the crystallization of BiOI without altering its morphology and structure. La3+ may enter the BiOI lattice by substituting Bi3+ or forming La2O3 nanoclusters that homogeneously scatter in the mesopores of BiOI microspheres. The analysis of the underlying mechanism further revealed that La doping not only enhanced the light harvesting properties by decreasing the bandgaps of BiOI and accelerating the charge separation and transfer dynamics, but also introduced more oxygen vacancies and facilitated the formation of more OH radicals by dissociating the water molecules. All these factors co-contributed to the promotion of NO photocatalytic oxidation activities.Furthermore, NO was mainly oxidized to NO2 over La/BiOI, and the formed NO2 tended to desorb from the catalyst surface, which not only maintained the intactness of active sites and facilitated the sustainable occurrence of NO photocatalytic oxidation reactions, but also prevented the photocatalysts from frequent washing-regeneration;therefore, these factors account for the superior photocatalytic stability of La/BiOI and its long-term operation. The formed NO2 could be easily and totally absorbed by the tail alkaline liquid,thereby effectively avoiding secondary pollution. Therefore, this study elucidates that doping is indeed a feasible and effective approach for the modification of 3D BiOI microspheres, while providing inspiration for the rational design and modification of other 3D semiconductor materials for various photocatalytic applications.
Key Words: La; BiOI microspheres; NO conversion; Photocatalytic oxidation
1 Introduction
Nitric oxide (NO), one of the most harmful gaseous pollutants,is a cause of serious environmental problems such as photochemical smog, haze weather, and acid rain1,2. It can also lead to respiratory diseases, impair the nervous system, and threaten human health3. Thanks to the widespread application of selective catalytic reduction technologies and the three-way catalytic converter (TWC), the high concentrations of NO produced during the combustion of fossil fuels have been effective controlled worldwide and reduced to comparatively lower levels4,5. However, the toxicity and harmfulness of NOxis so strong that even low concentration (in the order of 10−9) can bring severe negative effects. According to the latest air quality standards of China (GB3095-2012), which have taken full effect,the limits for the concentration of NOxare 250 μg·m−3for 1 h averages and only 100 μg·m−3for 24 h averages. Studies on the photocatalytic removal of NO, including photodecomposition,photoreduction, and photooxidation, has once again been raised.Among these techniques, photocatalytic decomposition is hardly achievable under mild solar light, whereas the realization of photocatalytic reduction usually requires the addition of NH3or hydrocarbons, which can hardly operate in practical situations.Hence, photocatalytic oxidation is acknowledged to be the most applicable technology.
Recent years have witnessed the development of visible-lightdriven semiconductor photocatalysts, such as graphitic carbon nitride6, bismuth-based materials like Bi2WO67, Bi2MoO68,(BiO)2CO39, and BiOX (X = I, Cl, Br)10-12and so on. Among which, bismuth oxyiodide (BiOI) has sparked remarkable interest because of its excellent optical property. And BiOI microspheres, with a three-dimensional (3D) hierarchical structure, possess a better utilization of light because of the multi-reflection, sufficient surface for the adsorption of reactants and are easy to be recycled, presenting to be a highly efficient photocatalyst for the degradation of organic pollutions in water13and gaseous pollutants10,14. Nevertheless, the photocatalytic activity of BiOI microspheres still suffer from quick recombination rates of photogenerated carriers and unpleasant electrical conductivity15,16. To improve its photocatalytic performance for possible industrial application, various modifications have been adopted, such as co-catalyst decoration11,17, elemental doping18,19, defect engineering13,15,and heterojunction construction20,21. However, 3D BiOI nanospheres, with such a micro size, are difficult to be coupled with other semiconductors or co-catalysts due to the incompact and insufficient contact interfaces17. Excitingly, metal doping,with dopants either intruding the lattice or scattering on the surface with intimate interaction in elementary substance or metal oxides forms, emerges to be an effective modification method for BiOI microspheres18,22. Furthermore, it has been reported that rare earth metal La doping can efficiently suppress the recombination of photogenerated carriers23-25. Therefore, it is expected that La doping may promote the photocatalytic performance of BiOI microspheres. And to the best of our knowledge, the photocatalytic performance of La-doped BiOI microsphere is still a mystery worthy to be systematically studied.
Here, in this work, we synthesized a novel BiOI microspheres material doped with rare-earth metal Laviaa one-pot facile solvothermal method to investigate the NO photocatalytic oxidation performance. Aiming to obtain an optimal activity, the precursor of La as well as its doping contents were also optimized. La(NO3)3was selected to be the optimal La precursor.AllxLa/BiOI (xis the weight ratio between La and BiOI)samples showed superior photocatalytic performance, and 0.3%La/BIOI demonstrated the best conversion efficiency of 74%, far better than that of pure BiOI microspheres (44%). It also exhibited remarkable photocatalytic stability. Various characterizations were conducted to clarify the mechanism behind such an enhanced NO photocatalytic oxidation activity.
2 Experimental
2.1 Sample preparation
Bi(NO3)3·5H2O and KI were purchased from Aladdin Industrial Co. Ltd., Shanghai, China. La(NO3)3·6H2O,LaCl3·7H2O, La(AC)3, ethanol, and ethylene glycol were obtained from Sinopharm Chemical Reagent Co. Ltd., China.All the reagents purchased were analytically pure (AR) and used without further purification.
BiOI microspheres were prepared with Bi(NO3)3·5H2O and KI using the solvothermal method. In a typical process,Bi(NO3)3·5H2O (2.32 g) and KI (0.8 g) were added into ethylene glycol (60 mL). After being thoroughly mixed, the mixtures were transferred into 100 mL Teflon-lined autoclaves and then heated at 160 °C for 12 h. Then the orange precipitates were washed with deionized water and ethanol several times and dried at 60 °C for 12 h. Moreover, La precursors, including La(NO3)3·6H2O, LaCl3·7H2O and La(AC)3, were added in the above process to obtain La-doped BiOI microspheres, includingxLa/BiOI and 0.3La/BiOI-y(wherexstands for the weight ratio between La and BiOI, andyrepresents the La precursors).
2.2 Characterization
X-ray diffraction (XRD, model D/max RA, Rigaku Co., Japan with CuKαradiation) was conducted to analyze the crystal phase and composition of the samples. The specific surface areas were determined using a static adsorption instrument (JW-BK 132F,Beijing JWGB Sci & Tech Co., China) by the Brunauer-Emmett-Teller (BET) method. The morphology of the samples was obtained by scanning electron microscopy (SEM, SIRION-100,FEI Co., USA) and transmission electron microscopy (TEM,JEM-2010, Japan). The surface properties of samples were analyzed over an X-ray photoelectron spectroscopy instrument(XPS, ESCALAB 250, Thermo, USA) equipped with AlKαXray irradiation (1486.6 eV) at 150 W. The binding energy scale was corrected to the C 1slevel at 284.8 eV. Raman spectra were obtained using a SPEX-1403 laser Raman spectroscope (SPEX CertiPrep Inc., USA) at room temperature. A 514.5 nm Ar-ion laser in a backscattering configuration was used to excite the crystals (Lab RAM-HR, SPEX-1403, France). UV-Vis diffuse reflection spectra (DRS) were measured with an integrating sphere equipped with a UV-Vis spectrophotometer (TU-1901,Beijing Purkinje General Instrument Co., China) using BaSO4as the reference. The photoluminescence spectra (PL) were measured with a steady-state-lifetime fluorescence (Fluorolog-3-TAU, Jobin Yvon, France) using 405 nm lasers as the excitation source. Photocurrent responses and electrochemical impedance spectra (EIS) were performed using an electrochemical system (CHI 660B, Shanghai Chenhua Instrument Co., Ltd., China) under visible light irradiation. A platinum wire was used as the counter electrode, and a saturated calomel electrode was used as the reference electrode. The samples were coated on indium tin oxide (ITO, 20 mm× 50 mm×1 mm, 15 Ω, Meijingyuan Glass Techno. Co. Ltd. China) glass,and the sample film was limited to 1 × 1 cm2. The electrolyte was 0.2 mol·L−1Na2SO4. The spin trapping electron spin resonance (ESR) spectra were collected on a ESR spectrometer(JES-FA200, JEOL Co., Japan) with a Xe lamp to provide visible light; 5,5’-dimenthyl-1-pirroline-N-oxide (DMPO) was employed as a spin-trap reagent to trap the active species of superoxide radicals (·O2−) and hydroxyl (·OH).
2.3 Photocatalytic experiments
Photocatalytic experiments were carried out in a continuous flow reactor, as in our previous study12. A Xenon lamp (CELWLAX500, Beijing Zhongjiao Jinyuan Technology Co. Ltd.,China) was placed above the reactor, and an optical filter was equipped to obtain visible light (420-760 nm). The samples were coated on a glass slide using a dip-coating method and 0.25 g of photocatalyst was used for each test. NO gas (5 × 10−7) was obtained from two air cylinders and a NO cylinder (5 × 10−5,diluted by N2). The relative humidity (RH) was regulated to 55%by passing one air stream through a humidification chamber, and the RH was detected using a relative humidity analyzer (Testo Co., Ltd., Model 605-H1). The flow rate was controlled at 2 L·min−1, and the residence time was about 9 s. After being thoroughly mixed in a gas blender, the streams entered the reactor, followed by the liquid absorption device. When the concentration of NO in the inlet and outlet reached equilibrium,Xenon lamp was turned on, and with the light intensity maintained at at 20 mW·cm−2. The photocatalytic reaction continued for 30 min. The concentrations of NO and NO2were measured using a NOxanalyzer (Thermo Environmental Instruments, Inc., 42i-TL). And the tail gas was absorbed by a solution of sodium hydroxide.
The NO conversion and NO2production were calculated as follows:
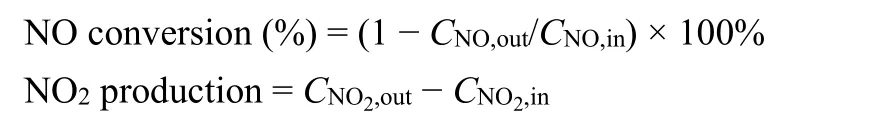
whereCNO,in/outandCNO2,in/outrepresent the NO and NO2concentrations at the inlet and outlet, respectively.
3 Results and discussion
3.1 Physical structure and morphology
The crystal phase of as-synthesized samples were characterized by XRD, with the results displayed in Fig. 1.Tetragonal BiOI was the main crystal phase with characteristic peaks emerging at 29.2°, 31.8°, 45.5°, 55.2°, and 66.4°(PDF#73-2062). Compared with the standard PDF of BiOI, the intensity ratio between (110) facets and (012) facets of BiOI in this work was smaller, which may be caused by the fact that ethylene glycol can preferentially absorb on the (012) facets inhibiting the growth of (012) facets26. After the introduction of La, no characteristic reflections corresponding to La species such as La2O3was detected. What’s more, the peak positions remained almost unchanged while the intensity of characteristic peaks was ever-increasing with the increase of La content, which revealed that the crystal size of BiOI became larger after La doping and La could facilitate the crystallization of BiOI without altering its tetragonal structure27. To further confirm the incorporation of La into BiOI, Raman spectra of as-prepared samples were obtained. As displayed in Fig. 1b, two incisive characteristic vibrational peaks with broad full width at 80 and 100 cm−1corresponded to theA1gstretching modes of Bi―I bonds, and the bands at 150 cm−1could be ascribed to theE1ginternal stretching modes of Bi―I bonds19,28,29. The RamanEgmode in La-doping samples showed slight shift and broadening compared with the prestine BiOI, which were mainly ascribed to the nanosize effect without significant influence of lattice strain or nonstoichiometry. Considering that the ionic radius of La3+was reported to be 0.11 nm, similar to that of Bi3+(0.103 nm), it could be sepculated that La3+may substitute Bi3+into the crystal lattice of BiOI, or forming La2O3nanoclusters homogeneouly scattered in BiOI30,31.

Fig. 1 XRD patterns (a) and Raman spectra (b) of asprepared samples.
SEM and TEM images of BiOI and 0.3%La/BiOI were investigated to analyze the morphology of the samples; with results shown in Fig. 2a,b. The synthesized BiOI microspheres were well-shaped with diameters ranging from 1 μm to 2 μm,assembled hierarchically by plenty of nanosheets. Differences in the SEM images between BiOI and 0.3%La/BiOI could hardly be observed, indicating that La doping had no influence on the morphologies of BiOI. The TEM images of 0.3%La/BiOI in Fig.2c further confirmed the size of the microspheres, in agreement with the SEM results. In the enlarged view (Fig. 2d), distinct lattice fringes with a spacing of 0.282 nm could be clearly observed, corresponding to the (110) facet of BiOI. The EDS mapping of 0.3%La/BiOI were also recorded, the results (Fig.2e-h) vividly showed that La species was indeed present and uniformely dispersed in 0.3%La/BiOI.
The surface areas, pore volumes and diameters of BiOI andxLa/BiOI were obtained from the N2adsorption-desorption isotherms and pore distribution curves. As displayed in Table 1,the doping of La somewhat decreased the surface area, pore size and pore volume of BiOI. For BiOI, the pore volume originates from the intra-aggregation and intercrossed of bismuth oxyiodide crystallites and nanosheets32, consistent with the selfassembled hierarchical morphology displayed in SEM.Moreover, all samples presented characteristic type IV isotherms with an H3 hysteresis loop from 0.4 to 1.0P/P0(see Fig. 3a),which was a typical evidence for a mesopores-predominant structure33. Fig. 3b showed that the diameters of all samples were mainly centered at 2-8 nm and shared similar pore size distribution. It should be noted that, both the pore volume and pore size slightly decreased upon the addition of La, which may be caused by the aggregation of smaller crystallite or the embedding of La2O3nanoclusters into the pore canal of BiOI34,35.
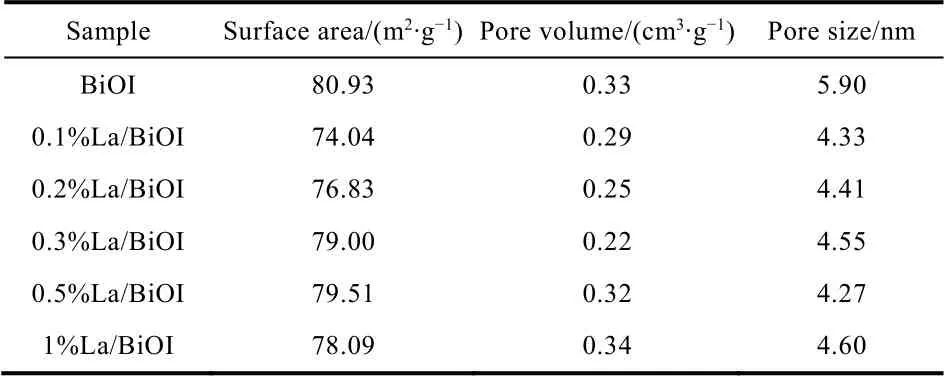
Table 1 Physical characteristics of the as-prepared samples.

Table 2 Bandgaps and band edge positions of as-synthesized samples.

Fig. 2 SEM images of BiOI (a) and 0.3%La/BiOI (b); TEM image (c), HRTEM image (d) and EDS mapping (e–h) of 0.3%La/BiOI.
3.2 Chemical composition
To investigate the chemical states and detailed elemental compositions of BiOI andxLa/BiOI, XPS spectra were obtained and carefully analyzed. No remarkable differences appeared for the spectra of Bi 4forbits for BiOI andxLa/BiOI (Fig. 4a); the peaks centered at 159.4 eV and 164.7 eV were respectively attributed to Bi 4f7/2orbits and Bi 4f5/2orbits of Bi3+. As for the spectra of I 3d, peaks at 630.7 and 619.2 eV for BiOI were corresponded to I 3d3/2and I 3d5/2orbits6, with the increase of La doping amount, the shifts towards higher binding energy of both peaks became more and more distinct, and nearly 0.4-0.5 eV shift emerged in 1%La/BiOI (Fig. 4b). The increased binding energy reflected that the decrease of the electron cloud density around I decreased after La doping36,37. Surprisingly, La doping led to more conspicuous changes in spectra of O 1s(Fig. 4c).The O 1sspectra of BiOI was deconvoluted into only two overlapping peaks. Peaks centered at 530.2 eV was assigned to Bi―O bonds in BiOI20, while the peak at 531.6 eV was attributed to structural H2O or hydroxyl groups38. Both two species also presented in the XPS spectra ofxLa/BiOI with a slight shift. Besides, a third peak located at 533.5 and 532.7 eV emerged in 0.3%La/BiOI and 1%La/BiOI. These species were supposed to be associated with oxygen vacancies having been replenished by oxygen and/or water, and were reported to be related with a cationic migration39. Fig. 4d exhibited spectra of La 3dforxLa/BiOI. Unfortunately, characteristic peaks of La for 0.3%La/BiOI and 1%La/BiOI were too weak to analyze due to the low doping content. Then 5%La/BiOI was further prepared to testify the existence of La and determine the chemical state of La. Excitingly, two peaks centered at 838.0 eV and 854.8 eV could be observed, which were respectively represented the La 3d5/2and La 3d3/2orbits of La3+40.
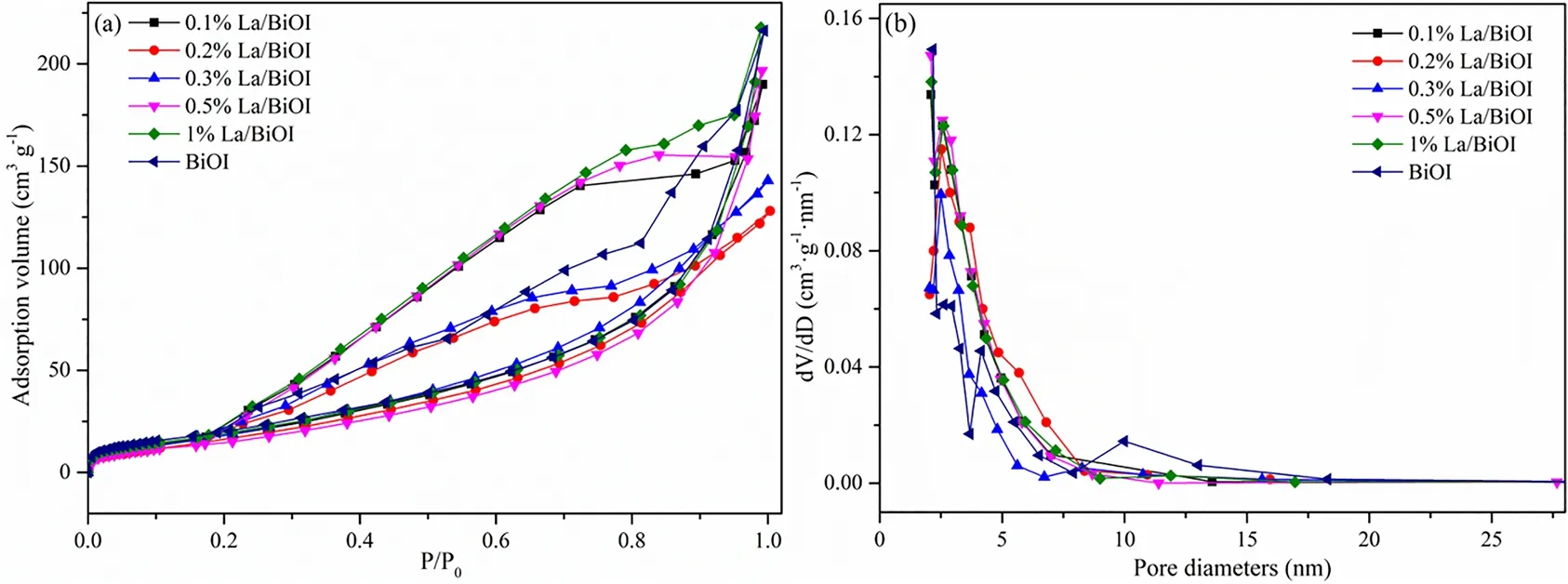
Fig. 3 Nitrogen adsorption-desorption curves (a) and pore size distribution (b) of as-prepared samples.

Fig. 4 XPS spectra of BiOI and xLa/BiOI: (a) Bi 4f, (b) I 3d, (c) O 1s, and (d) La 3d.
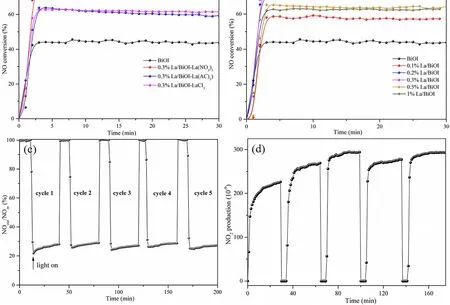
Fig. 5 Photocatalytic oxidation of NO over (a) xLa/BiOI, (b) 0.3%La/BiOI with different La sources; (c) NO conversion efficiency and(d) NO2 production in the cycling tests over 0.3%La/BiOI under visible light illumination.
3.3 Photocatalytic activity and stability
Different La precursors were first utilized to synthesize 0.3%La/BiOI-ysamples, and their photocatalytic oxidation performance was evaluated and compared. As shown in Fig. 5a,NO conversion equilibrium could be established within 5 min for all the samples. Additionally, 0.3%La/BiOI-La(NO3)3exhibited better photocatalytic activity than 0.3%La/BiOI-LaCl3and 0.3%La/BiOI-La(AC)3. Furthermore, the effect of La doping on the photocatalytic conversion efficiency of NO was also investigated on the basis of the La(NO3)3precursor. As shown in Fig. 5b, 44% of NO was photocatalytically oxidized over pure BiOI microspheres after 30 min of visible-light irradiation. AllxLa/BiOI samples exhibited better photocatalytic performance than that of the pure BiOI, and the NO photocatalytic conversion showed a volcano-like relationship with the La doping amount. Specifically, the NO conversion efficiency first increased with an increase in the La content, then decreased. 0.3%La/BiOI stood out to be the optimal sample,exhibiting a very high NO photocatalytic conversion of 74%. Its stability was also evaluated. As shown in Fig. 5c, the NO conversion efficiency was very stable during five continuous 5 cycles. This was because NO was mainly oxidized to NO2over La/BiOI (see Fig. 5d) and the formed NO2was not easily adsorbed on the surface; thus, the active sites could be greatly exposed instead of being occupied.
3.4 Light absorption, charge separation, and carrier lifetime
UV-Vis DRS spectra were employed to investigate the lightharvesting properties of the as-prepared samples. As depicted in Fig. 6a, all samples exhibited intensive light absorbance ranging from UV light to visible light. After La doping, the absorption edge of BiOI was slightly red-shifted, which revealed that the bandgap of BiOI decreased after La doping and more photoexcited carriers would be generated under visible light irradiation. Since charge transfer and separation is a crucial process for photocatalysts in photocatalytic reactions, steadystate photoluminescence (PL) spectra, photocurrent responses and AC impedance spectra were employed to gain more insights into the influence of La doping on the electron transfer dynamics. Fig. 6b displayed the PL result excited at 405 nm.xLa/BiOI showed lower emission peak in comparison with the pure BiOI, indicating that charge recombination was efficiently suppressed after La doping. This meant that more photoinduced species could participate in the photocatalytic reactions. The smaller radius of 0.3%La/BiOI in the arc part further proved that photogenerated electrons in 0.3%La/BiOI transferred faster than that of BiOI (Fig. 6c). Besides, 0.3%La/BiOI exhibited higher transient photocurrent intensity than BiOI (Fig. 6d),which could be attributed to the improved light harvesting, quicker charge transfer and inhibited recombination of photogenerated charges.Besides, it should be noted that a cathodic spike was observed over BiOI the moment turning on the light. anodic overshoot appeared simultaneously when switching off the light.According to the basic principles of photoelectrochemistry41,this could be assigned to the rapid capture of photogenerated electrons by surface vacancies and the faster transfer dynamics of photogenerated holes. Similar phenomena were also reported in previous literatures42,43.
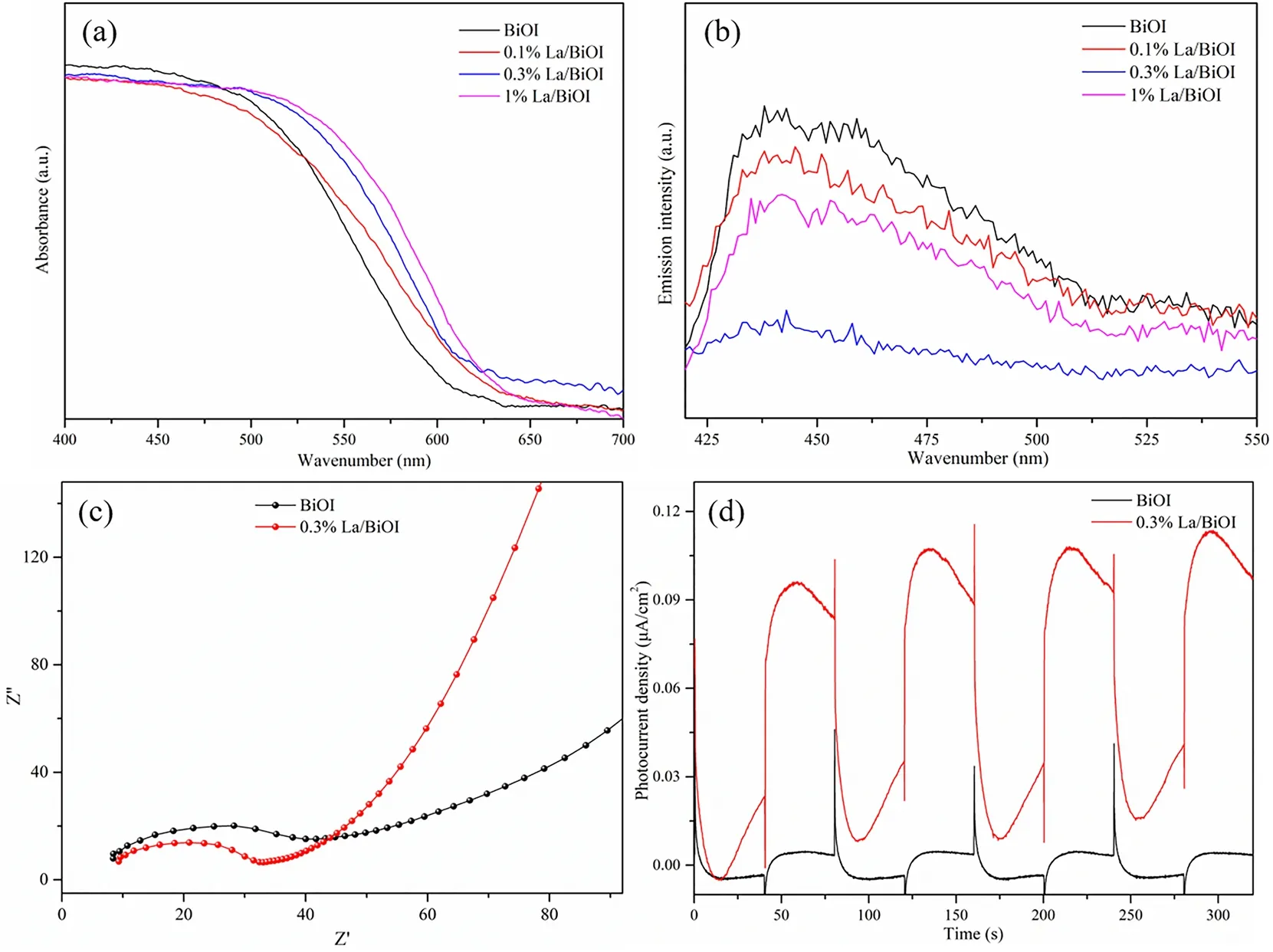
Fig. 6 Photocatalytic oxidation of NO over (a) xLa/BiOI, (b) 0.3%La/BiOI with different La sources; (c) NO conversion efficiency and(d) NO2 production in the cycling tests over 0.3%La/BiOI under visible light illumination.

Fig. 7 (a) XPS VB spectra and band gaps calculated from the Tauc plots, (b) flat-band potentials, DMPO-ESR spin-trapping for (c) ·O2− and(d) ·OH in darkness and under visible light illumination of the as-synthesized samples.
3.5 Band structure, active species analysis, and possible mechanism
The bandgaps of as-prepared samples were calculated from the Tauc plots (Fig. 7a). To be noted, the bandgap of BiOI nanospheres was much smaller than BiOI nanosheets in our previous work12and other reported studies44,45. This was because bandgaps of semiconductors could be influenced by the morphologic structure. The valence band positions (EVB) of BiOI andxLa/BiOI, obtained by the XPS VB spectra, was almost the same at 1.30 eV (Fig. 7b), which indicated that La doping didn’t affect the valance bands. Then the conduction band positions(ECB) could be calculated through the equationECB=Eg−EVB,with results shown in Table 2. Further, ·O2−radicals and ·OH radicals were detected by DMPO-ESR spin-trapping under visible light illumination to determine the reactive radicals involved in the photocatalytic reaction process. As displayed in Fig. 7c, almost no ·O2−radicals could be found both in darkness and under visible light irradiation. This was quite understandable, as the conduction band positions of as-prepared samples shown in Table 2 were all more positive than the standard potentials of ·O2−/O2(−0.32 eV,vs.NHE).Nevertheless, four characteristic peaks with an intensity ratio of 1 : 2 : 2 : 1, corresponding to the formation of ·OH radicals, were surprisingly observed for both pure BiOI and La doped BiOI samples (Fig. 7d). In fact, theEVBwas less positive than the standard potential of ·OH/H2O (2.27 eVvs.NHE) and ·OH/OH−(1.99 eVvs.NHE), ·OH radicals should not be detected theoretically. This may be attributed to the existence of oxygen vacancies (Fig. 8a), evidenced by the characteristic ESR signals atg= 2.046,47. It was reported that oxygen vacancies could facilitate the dissociation of H2O molecules, and then decrease the reaction potentials of ·OH/OH−, thus promoting the generation of ·OH radicals12. It is worth to be mentioned that more oxygen vacancies were formed after La doping (Fig. 8a),which may be caused by the electronic structure change or even lattice distortion of BiOI after La doping. This may also account for the much stronger intensity of ·OH radicals overxLa/BiOI(Fig. 7d). And due to the abundant ·OH radicals formed over La/BiOI, NO could be efficiently converted to NO2. Based on the above discussion, the mechanism of the enhanced NO photocatalytic oxidation performance over La/BiOI was graphically illustrated in Fig. 8b.
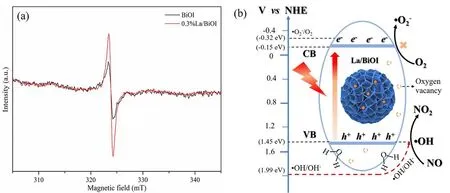
Fig. 8 (a) EPR spectra of BiOI and 0.3%La/BiOI, (b) schematic diagram of the possible NO photocatalytic oxidation mechanism over La/BiOI.
4 Conclusion
In this work, the rare-earth metal La doping strategy was adopted to modify BiOI microspheresviaa facile one-step solvothermal method, which were first applied to NO photocatalytic oxidation. Experimental results showed that La(NO3)2was the best La precursor compared with LaCl3and La(AC)3. AllxLa/BiOI samples showed higher NO photocatalytic conversion efficiency than BiOI. 0.3%La/BiOI exhibited the highest NO conversion (74%) and remarkable stability. After being subjected to various characterizations, it was found that La doping facilitated the crystallization of BiOI without altering its tetragonal structure. Besides, La doping not only improved the light harvesting by decreasing the bandgap of BiOI, but also accelerated the charge separation and transfer. In addition, abundant oxygen vacancies were introduced,facilitating the dissociation of H2O molecules and promoting the formation of ·OH radicals. In addition, NO was mainly oxidized to NO2over La/BiOI, and the formed NO2tended to desorb from the catalyst surface, which not only maintained the intactness of active sites, kept the sustainable occurring of NO photocatalytic oxidation reactions, but also avoided frequent washingregeneration. It is expected that our work could provide some perspective on the rational modification of 3D semiconductor materials and inspire the design of more efficient photocatalysts for NO conversion and other environmental problems.
杂志排行
物理化学学报的其它文章
- Defect Engineering in Two-Dimensional Graphitic Carbon Nitride and Application to Photocatalytic Air Purification
- 理论计算评价光催化剂VOCs 降解性能:g-C3N4 量子点/石墨烯
- 富含缺陷的2D/2D 异质结促进光催化清洁能源转化
- Development of Iron-Based Heterogeneous Cocatalysts for Photoelectrochemical Water Oxidation
- 二维光催化材料电子结构和性能调控策略研究进展
- Selective Exposure of BiOI Oxygen-Rich {110} Facet Induced by BN Nanosheets for Enhanced Photocatalytic Oxidation Performance
