Different physiopsychological changes between AMS- susceptible and AMS-resistant pre-selected Antarctic expeditioners in Tibet
2021-08-18WUXiaopeiLIUShiyingWANGXiWANGJiananQINPengruiXUChengli
WU Xiaopei, LIU Shiying, WANG Xi, WANG Jianan, QIN Pengrui & XU Chengli,2*
Different physiopsychological changes between AMS- susceptible and AMS-resistant pre-selected Antarctic expeditioners in Tibet
WU Xiaopei, LIU Shiying, WANG Xi, WANG Jianan, QIN Pengrui& XU Chengli
Institute of Basic Medical Sciences Chinese Academy of Medical Sciences, School of Basic Medicine Peking Union Medical College, Beijing 100005, China;Center of Environmental and Health Sciences, Chinese Academy of Medical Sciences, Peking Union Medical College, Beijing 100005, China
Throughdynamically monitoring changes of acute mountain sickness (AMS) occurrences, cardiopulmonary function and mood states from Shanghai (4 m) to Lhasa (3650 m) and Yambajan (4300 m), Tibet, we obtained physiopsychological data of the 37th Chinese Antarctic pre-selected expeditioners for Kunlun Station. Through analyzing different physiopsychological changes between AMS-susceptible (AMS-S) and AMS-resistant (AMS-R) expeditioners, we would explore indicators to screen hypoxia-susceptible expeditioners.According to AMS occurrences evaluated by Lake Louise Score (LLS) in Yambajan, we divided the expeditioners (=24, 31.92±5.76 a) into AMS-S and AMS-R groups. Using a series of medical instruments and questionnaires, we monitored their cardiopulmonary function and mood states, and analyzed the differences of physiopsychological parameters between AMS-S and AMS-R groups. Compared with Shanghai, when expeditioners arrived in Yambajan, in both AMS-S and AMS-R groups, oxygen saturation (SpO) significantly decreased, and blood pressure significantly increased (<0.05). As for electrocardiogram (ECG), interval from the beginning to the end of QRS complex wave (QRS), interval from the beginning of QRS complex wave to the end of T wave (QT), interval between 2 adjacent P waves (PP) and interval between 2 adjacent R waves (RR) significantly decreased, heart rate (HR) and HR-corrected QT interval (QTc) significantly increased (<0.05). Cardiac contractility and pumping function significantly decreased, systemic vascular resistance significantly increased (<0.05). Pulmonary airway patency significantly increased (<0.05). Compared with AMS-R group, AMS-S group showed significantly lower SpOand higher stroke volume variation (SVV) in Shanghai, however, significantly lower maximal expiratory flow at 75% of forced vital capacity (MEF), higher levels of anxiety, fatigue and confusion in Yambajan (<0.05). In conclusion, when expeditioners arrived at 4300 m, their cardiopulmonary function and mood states changed significantly. SpO, SVV, MEF, anxiety, fatigue and confusion maybe could be used as clues for screening hypoxia-susceptible individuals.
Antarctic, pre-selected expeditioner, hypoxia, Tibet, acute mountain sickness, cardiovascular function, pulmonary function, mood
1 Introduction
When sea-level residents quickly enter elevations above 2500 m, they are susceptible to acute mountain sickness (AMS). Common symptoms of AMS are insomnia, fatigue, palpitation, headache, dizziness, cyanosis, nausea and loss of appetite (Singh et al., 1969; Hackett and Roach, 2001). But individual resistance to hypoxia is different, and current researches on hypoxia resistance and susceptibility are not completely clear. There are no specific methods for screening people susceptible to hypoxia directly.Therefore, we took the occurrence of AMS as an important indicator to judge the susceptibility to hypoxia. Through exposing people living at low altitude areas acutely to high-altitude environment, we evaluated their AMS occurrences. Furthermore, we compared the physiopsychological differences between the hypoxia- resistant and hypoxia-susceptible people, and tried to find some physiopsychological indicators in those susceptible to hypoxia.
Chinese Antarctic Kunlun Station (80°25'S, 77°6'E, 4087 m) is located in Dome A, the highest point on the Antarctic ice sheet, which is considered one of the most challenging environments on Earth. Kunlun Station has hypobaric hypoxia, extremely cold, strong wind, strong solar radiation in summer and other extreme environmental factors. The most challenging factor for expeditioners is hypoxia. To guarantee the life security of expeditioners of Kunlun Station, under the organization of the administration, we carried out medical screening in Tibet before departure to exclude the hypoxia-susceptible people. When expeditioners took plane from Shanghai (4 m) to Lhasa (3650 m), and then to Yambajan (4300 m) by bus, their physiopsychological changes were dynamically monitored, and AMS incidence was assessed daily. We divided the expeditioners into AMS-susceptible (AMS-S) and AMS-resistant (AMS-R) groups according to AMS occurrences in Yambajan. Through comparing physiopsychological data between AMS-S and AMS-R groups, we tried to find out some physiopsychological indicators which could be used as clues for screening hypoxia-susceptible individuals.
2 Materials and methods
2.1 Participants and procedure
After the clinical physical examination, 27 qualified healthy males from the 37th Chinese Antarctic pre-selected expedition of Kunlun Station participated in this study. Their ages were between 21 and 45 a (31.9±6.1 a). The itinerary and schedule of physiopsychological tests of the pre-selected expeditioners are shown in Figure 1. The pre-selected expedition team flew into Lhasa from Shanghai, took the bus to Yambajan after 3 d of training in Lhasa, and returned to Lhasa by bus after 3 d of training in Yambajan. On the next day, they returned to their living places by plane. Oxygen saturation, pulse rate, blood pressure, heart rate and AMS occurrences were assessed daily. Other data, including electrocardiogram (ECG), cardiovascular function, pulmonary function, and mood states, were collected before departure from Shanghai, on the 2nd day in Lhasa and on the 2nd day in Yambajan.
According to the criteria of AMS evaluation, we eliminated 2 expeditioners with headache in Shanghai and 1 expeditioner who left during the training. The data from 24 participants were analyzed. When expeditioners took physiopsychological examinations on the 2nd day in Yambajan, 7 of them were AMS-S, while 17 of them were AMS-R. Based on the occurrence of AMS in Yambajan where hypoxic conditions were even worse, the team was divided into AMS-S and AMS-R groups.
2.2 Instrumental examinations
The instrumental examinations were carried out during resting state of expeditioners at room temperature (22–25℃), and the results were evaluated by clinical experts.
Blood pressure and heart rate (HR) were measured by an electronic sphygmomanometer (HEM7211, OMRON, Japan). Parameters of blood pressure include diastolic blood pressure (DBP) and systolic blood pressure (SBP). Oxygen saturation (SpO) and pulse rate were measured by a pulse oximeter (DB18, Philips, USA).
ECG was recorded by a 12-leadelectrocardiograph (MAC800, GE, USA), and 10 parameters were obtained, including HR, interval from the beginning to the end of QRS complex wave (QRS), interval from the beginning of QRS complex wave to the end of T wave (QT), HR-corrected QT interval (QTc), interval from the beginning of P wave to the beginning of QRS complex wave (PR), interval between 2 adjacent R waves (RR), interval between 2 adjacent P waves (PP), axis of QRS complex wave (QRS axis), axis of T wave (T axis) and axis of P wave (P axis). The P wave represents atrial depolarization, the QRS complex wave represents ventricular depolarization, and the T wave represents ventricular repolarization.
Cardiovascular function was examined by a noninvasive hemodynamic monitor (NiccomoCardioscreen® 2000, Medis, German), and it accessed 36 parameters. Vital signs included SpO, oxygen delivery index (DOI), HR, heart period (HPD), SBP, DBP, mean arterial pressure (MAP) and pulse pressure (PP). Parameters of thoracic fluid content included base impedance (Z), thoracic fluid content (TFC) and thoracic fluid content index (TFCI). Parameters of cardiac pumping included stroke volume (SV), stroke volume variation (SVV), stroke index (SI), cardiac output (CO) and cardiac index (CI). Parameters of cardiac contractility included velocity index(VI), acceleration index (ACI), Heather index (HI),pre-ejection period (PEP), left ventricular ejection time (LVET), systolic time ratio (STR), systolic time ratio index (STRI), ejection time index (ETI), ejection time ratio (ETR) and flow time corrected (FTc). Parameters of cardiac work included cardiac power (CPO), cardiac power index (CPI), left cardiac work (LCW), left cardiac work index (LCWI) and left stroke work index (LSWI). Parameters of systemic vascular resistance included total arterial compliance (TAC), total arterial compliance index (TACI), systemic vascular resistance (SVR), systemic vascular resistance index (SVRI) and stroke systemic vascular resistance index (SSVRI).
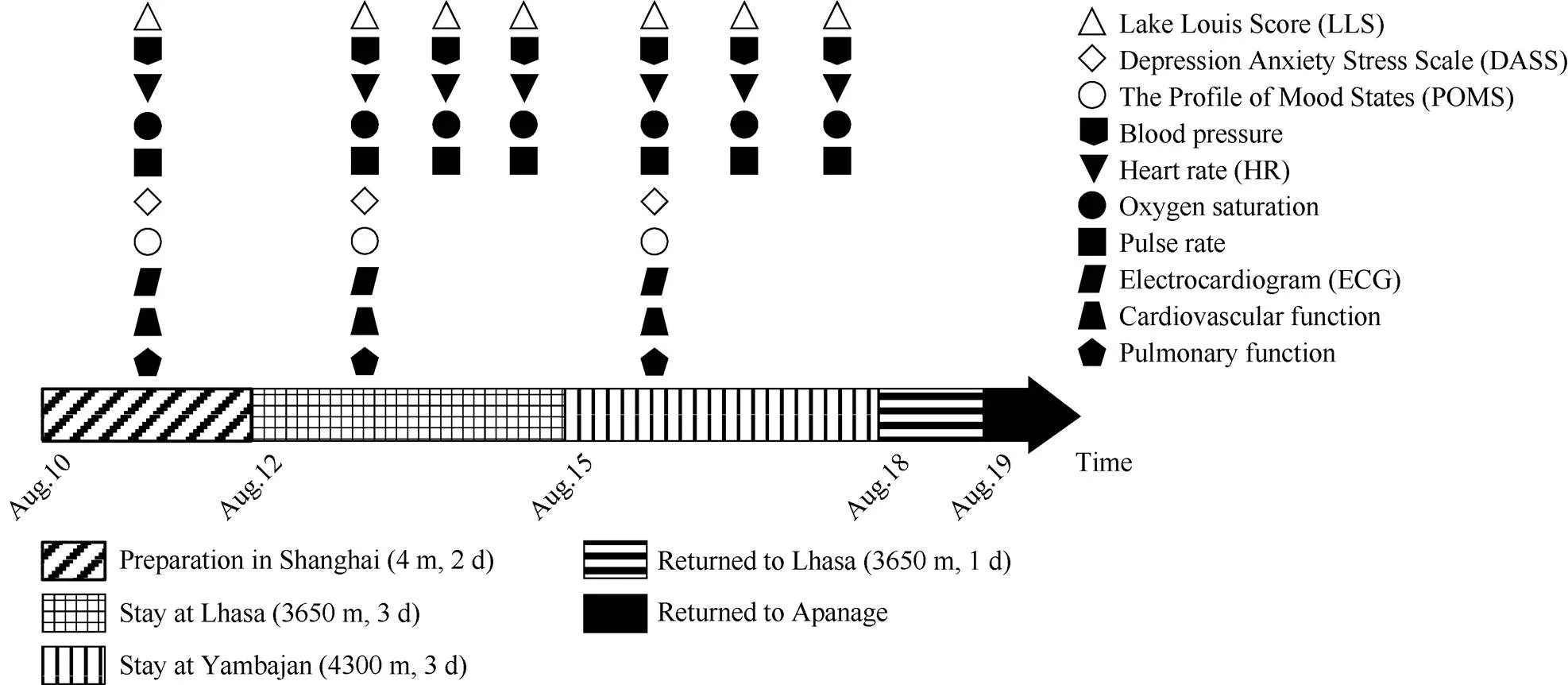
Figure 1The itinerary and schedule of physiopsychological tests of the pre-selected expeditioners of Kunlun Station (=27).
Pulmonary function was examined by a digital spirometer (MasterScope, Jaeger, German), 12 parameters were recorded. Vital capacity maximum (VC) and forced vital capacity (FVC) reflected vital capacity.Forced expiratory volume in 1s (FEV) and FEV/FVC reflected pulmonary ventilatory function. Peak inspiratory flow (PIF), peak expiratory flow (PEF) and maximal expiratory flow at 75% of FVC (MEF) reflected large airways ventilatory function. Maximal inspiratory flow at 50% of FVC (MIF), maximal expiratory flow at 25% of FVC (MEF), maximal expiratory flow at 50% of FVC (MEF), and maximal mid-expiratory flow (MMEF) reflected small airways ventilatory function. MEF/MIFreflected the site of airway obstruction.
2.3 Questionnaires
Lake Louise Score (LLS) was adopted to evaluate the incidence of AMS (Roach et al., 2018). The 5 main symptoms were evaluated, including headache, gastrointestinal symptoms, fatigue and/or weakness, dizziness/light-headedness, and sleep difficulty (Hackett and Oelz, 1992). Expeditioners whose total scores of LLS ≤2 were determined as AMS-R, while those whose total scores ≥3 along with headache were determined as AMS-S (Julian et al., 2011). 3–5 points are mild AMS, 6–9 points are moderate AMS, and 10–12 points are severe AMS (Roach et al., 2018).
Depression Anxiety Stress Scale (DASS) was used to assess some core symptoms of depression, anxiety and stress (Norton, 2007). The Profile of Mood States (POMS) was used to assess 6 dimensions of mood, i.e. vigor-activity, tension-anxiety, depression-dejection, anger-hostility, fatigue-inertia and confusion-bewilderment (McNair et al., 1971).
2.4 Statistical analysis
Continuous data were expressed as mean±SD, discontinuous data were expressed as median (interquartile range). All data were analyzed using IBM SPSS Statistics 25 software (SPSS Inc, Chicago, IL). Normal distribution was tested by the Shapiro-Wilks test. Data with normal distribution were analyzed by T test. Abnormally distributed data were analyzed by the Friedman test. When<0.05, data were considered to be significantly different.
3 Results
3.1 Demographic and physiological characteristics
When expeditioners took physiopsychological examinations on the 2nd day in Yambajan, 7 of them were AMS-S, while 17 of them were AMS-R. Based on the occurrence of AMS in Yambajan where hypoxic conditions were even worse, the team was divided into AMS-S and AMS-R groups. In the AMS-S group, all were mildly AMS.
There were no significant differences in demographic and physiological characteristics between the AMS-S and AMS-R groups before departure from Shanghai (Table 1).
3.2 Changes of SpO2, HR, blood pressure and pulse rate
Compared with before departure from Shanghai, in Lhasa and Yambajan, DBP, HR and pulse rate of expeditioners significantly increased, SpOsignificantly decreased, and SBP significantly increased only in Lhasa (<0.05)(Figure 2). There were no significant differences in SpO, HR, blood pressure and pulse rate between AMS-S and AMS-R groups.
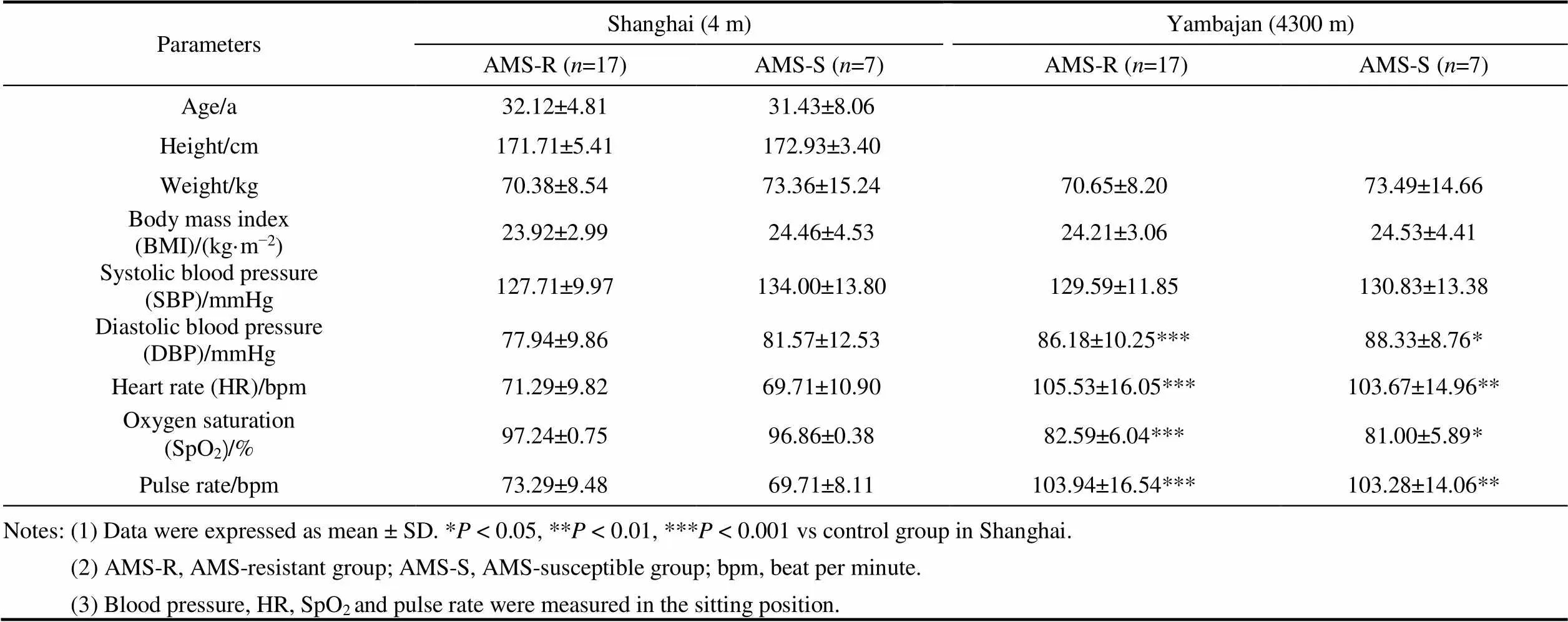
Table 1 Demographic and physiological characteristics
3.3 Changes of ECG
Compared with before departure from Shanghai, ECG conduction of expeditioners changed in Yambajan (Table 2). HR and QTc of both AMS-S and AMS-R groups significantly increased (<0.01). However, QT, QRS, PP and RR significantly shortened (<0.05). There were no significant differences of ECG conduction between AMS-S and AMS-R groups.
3.4 Changes of cardiovascular function
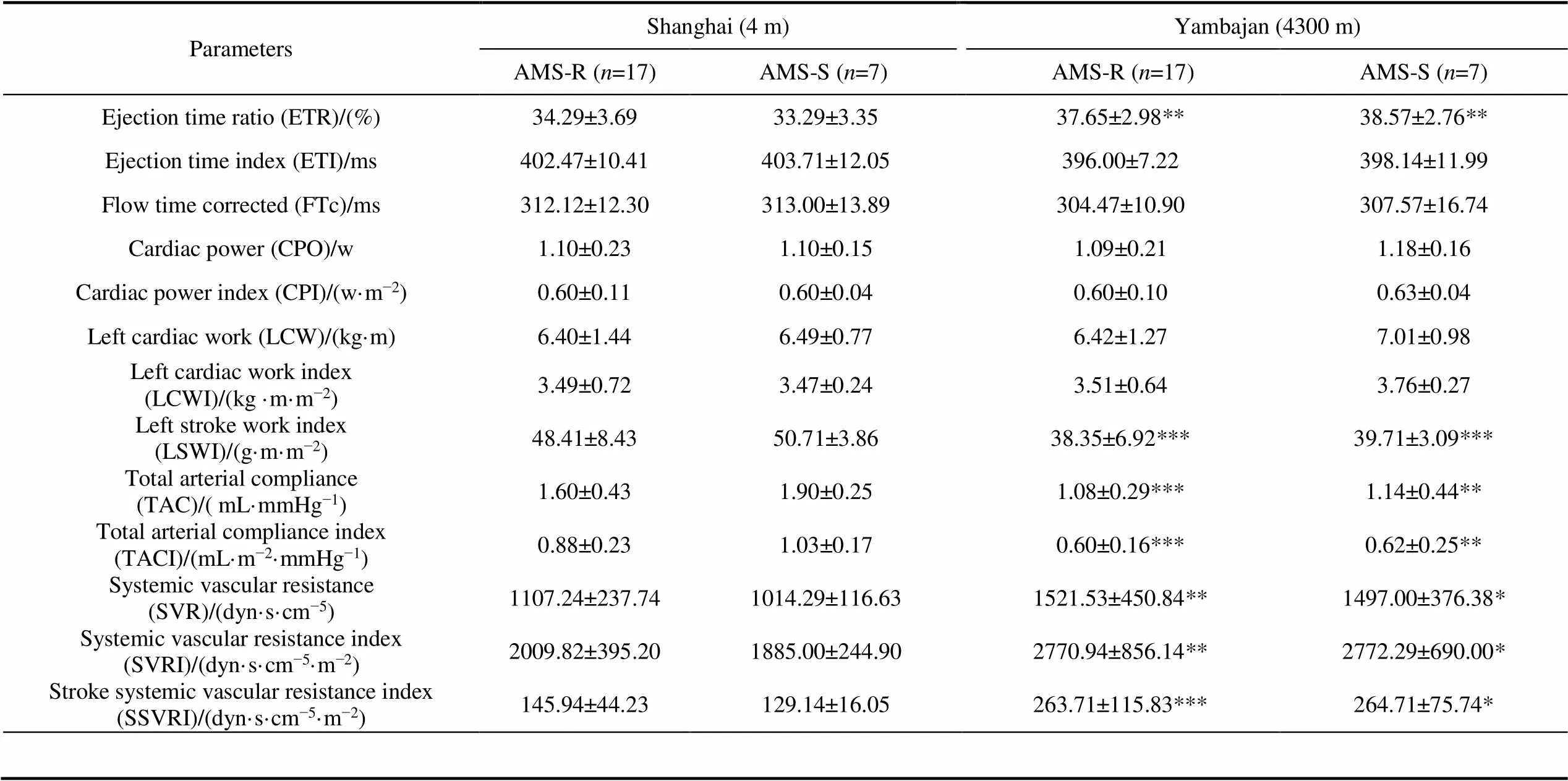
When expeditioners arrived in Yambajan, compared with before departure from Shanghai, in both AMS-S and AMS-R groups, SpO, DOI and HPD significantly decreased, HR, SBP, DBP and MAP significantly increased (<0.01). For cardiac pumping function, SV, SI, CO and CI significantly decreased (<0.01). For cardiac contractility, VI, ACI, HI and LVET significantly decreased, STR, STRI and ETR significantly increased (<0.01). For systemic vascular resistance, TAC and TACI significantly decreased, but SVR, SVRI and SSVRI significantly increased (<0.01, Table 3).
Compared with AMS-R group, AMS-S group showed significantly lower SpOand higher SVV before departure from Shanghai (<0.05).
3.5 Changes of pulmonary function
Compared with before departure from Shanghai, only FEV/FVC significantly increased in both AMS-S and AMS-R groups in Yambajan (<0.01). VC, FEV, PIF, PEF, MEFMEF, and MMEF only significantly increased in AMS-R group in Yambajan (<0.05, Table 4).
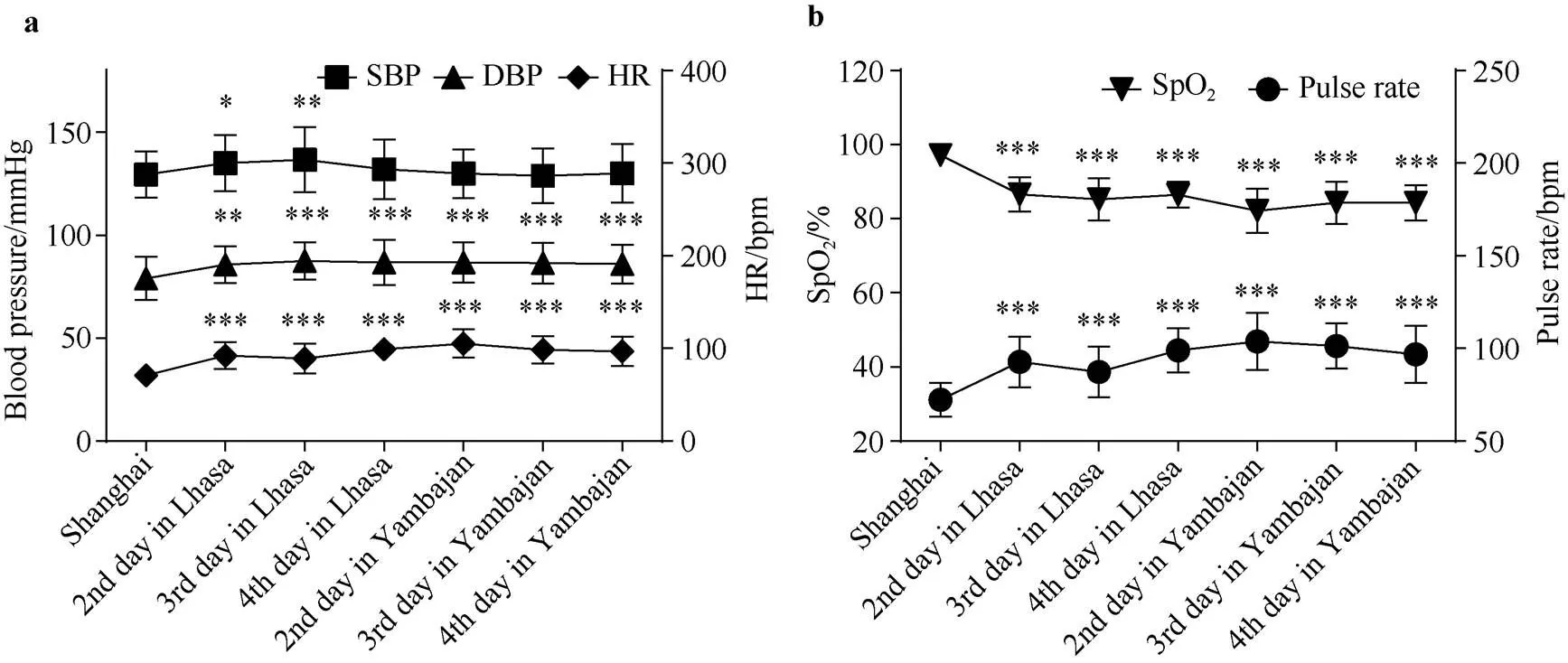
Figure 2 Changes of blood pressure and SpOof the pre-selected expeditioners at different altitudes (=24). Notes: (1) Data were expressed as mean ± SD. *< 0.05, **< 0.01, ***< 0.001, vs control group in Shanghai. (2) SBP, systolic blood pressure; DBP, diastolic blood pressure; HR, heart rate; SpO, oxygen saturation. (3) All parameters were measured in the sitting position.

Table 2 Differences in ECG between AMS-R group and AMS-S group
Notes: (1) Data were expressed as mean ± SD. *< 0.05, **< 0.01, ***< 0.001 vs control group in Shanghai.
(2) AMS-R, AMS-resistant group; AMS-S, AMS-susceptible group; º, degree.
(3) QRS, interval from the beginning to the end of QRS complex wave; QT, interval from the beginning of QRS complex wave to the end of T wave; QTc, HR-corrected QT interval; PR, interval from the beginning of P wave to the beginning of QRS complex wave; PP, interval between 2 adjacent P waves; RR, interval between 2 adjacent R waves; P axis, axis of P wave; QRS axis, axis of QRS complex wave; T axis, axis of T wave.
(4) All parameters were measured in the lying position.
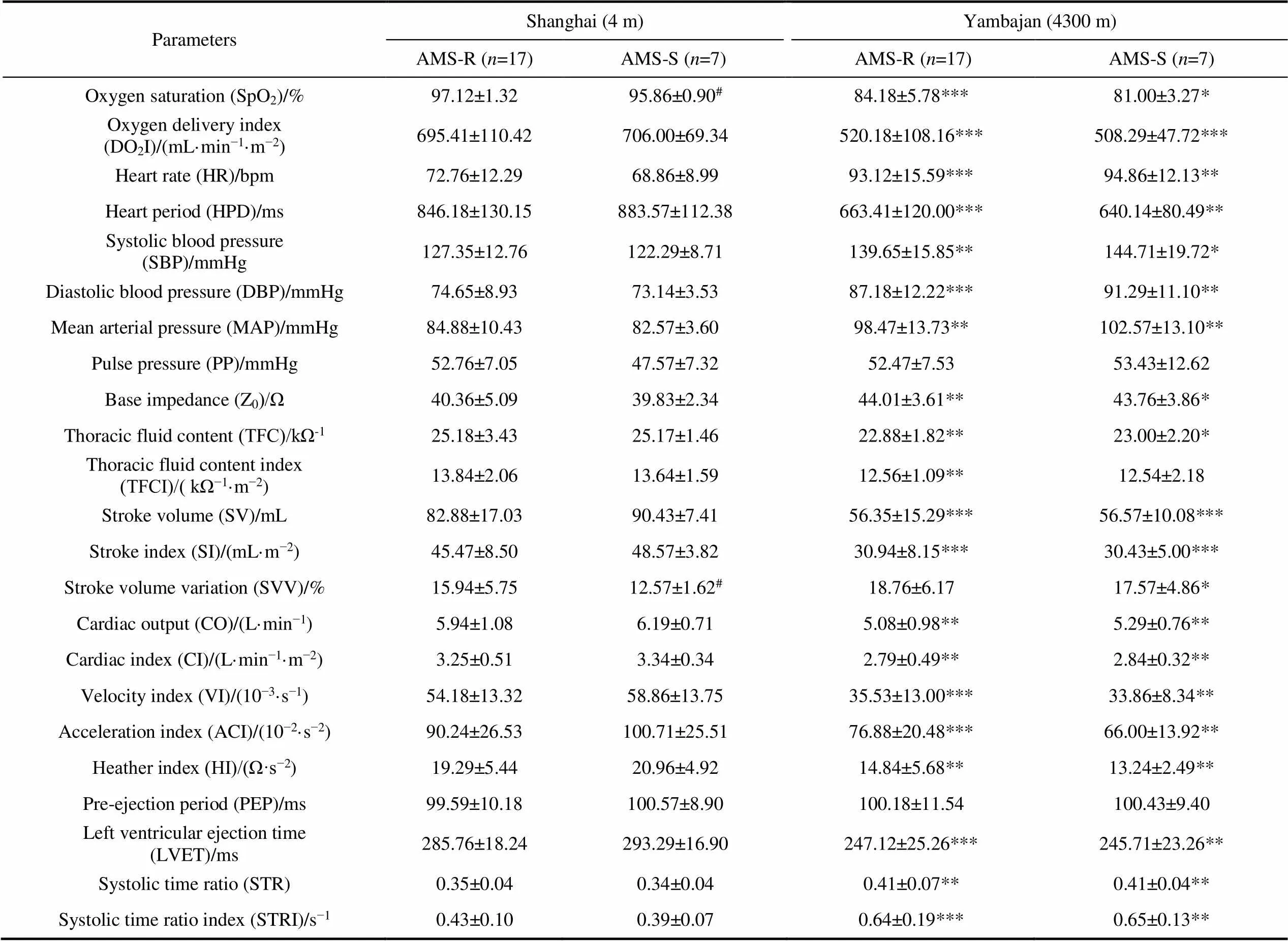
Table 3 Differences in cardiovascular function between AMS-R and AMS-S expeditioners
Continued
Notes: (1) Data were expressed as mean ± SD. *< 0.05, **< 0.01, ***< 0.001 vs control group in Shanghai.< 0.05, vs AMS-R group.
(2) AMS-R, AMS-resistant group; AMS-S, AMS-susceptible group; w, watt; dyn, dyne; Ω, ohm.
(3) All parameters were measured in the lying position.
(4) 1 mmHg = 133.322 Pa.
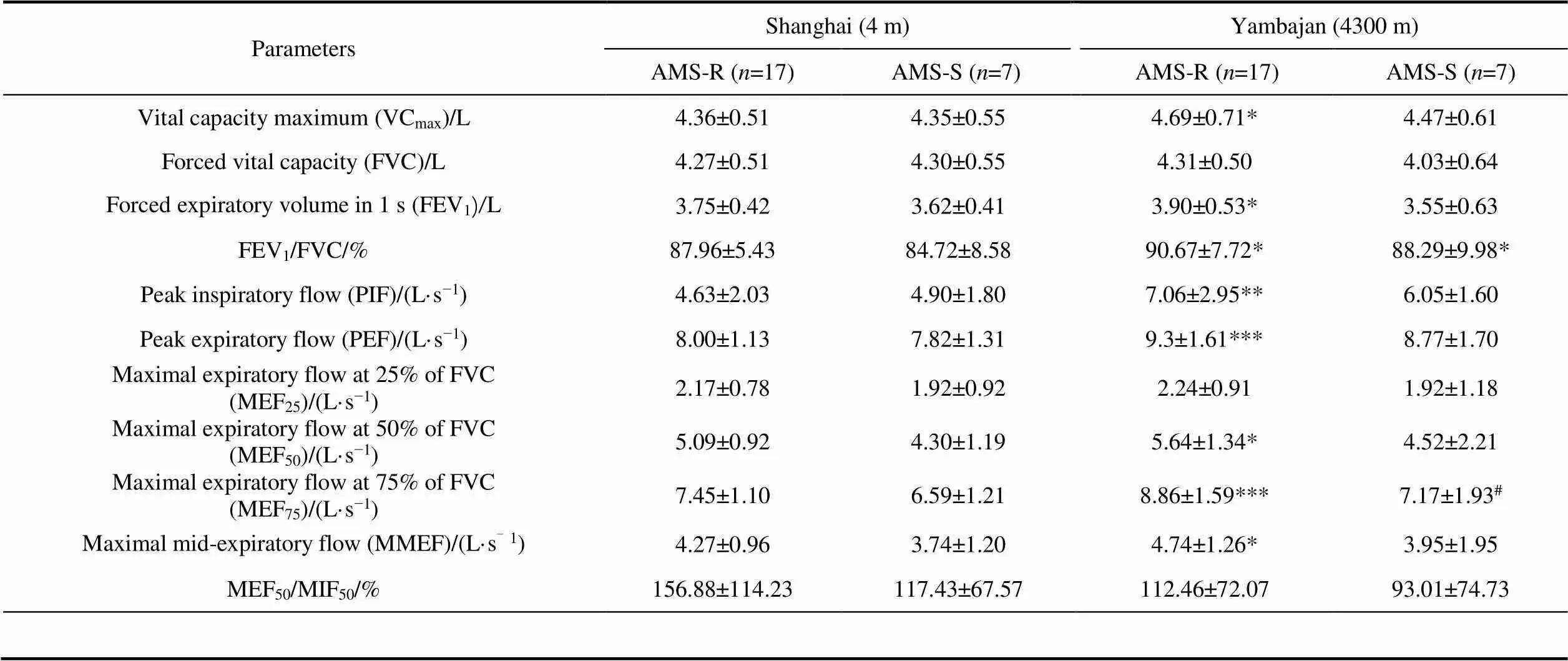
Table 4 Differences in pulmonary function between AMS-R and AMS-S expeditioners
Notes: (1) Data were expressed as mean ± SD. *< 0.05, **< 0.01, ***< 0.001 vs control group in Shanghai.< 0.05, vs AMS-R group.
(2) AMS-R, AMS-resistant group; AMS-S, AMS-susceptible group.
(3) All parameters were measured in the sitting position.
Compared with AMS-R group, AMS-S group in Yambajan showed significantly lower MEF(<0.05).
3.6 Changes of mood states
Compared with before departure from Shanghai, after arriving in Yambajan, the level of anxiety significantly increased only in AMS-S group, the levels of depression and anger significantly decreased only in AMS-R group (<0.05, Table 5). According to the diagnostic criteria of DASS, the anxiety level of AMS-S group was normal in Yambajan.
Compared with AMS-R group, the levels of anxiety, confusion and fatigue significantly increased in AMS-S group in Yambajan (<0.05).
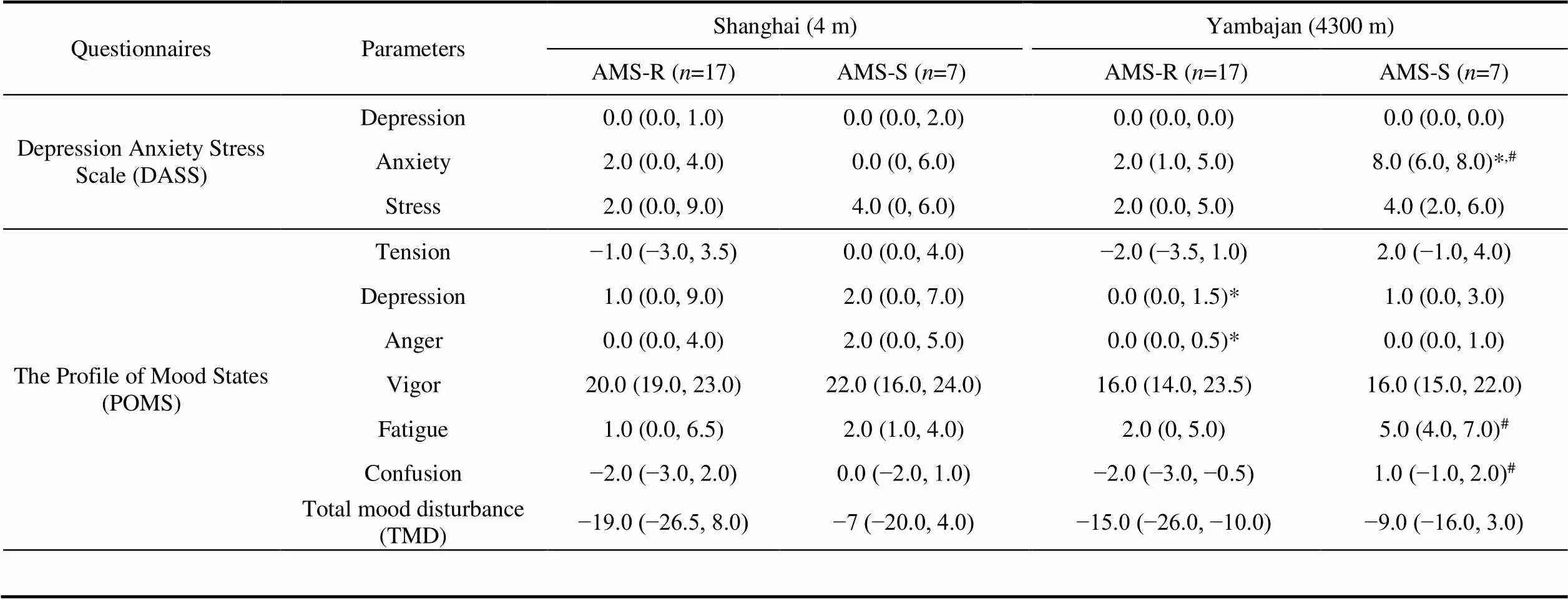
Table 5 Differences in mood state between AMS-R and AMS-S expeditioners in Tibet
>Notes: (1) Data were expressed as median (interquartile range). *< 0.05, vs control group in Shanghai.< 0.05, vs AMS-R group.
(2) AMS-R, AMS-resistant group; AMS-S, AMS-susceptible group.
4 Discussion
Hypoxia is the greatest challenge for expeditioners at Kunlun Station (4087 m). Therefore, through dynamically monitoring changes of AMS incidence, cardiopulmonary function and mood states of the 37th Chinese Antarctic pre-selected expeditioners of Kunlun Station from Shanghai (4 m) to Lhasa (3650 m) and Yambajan (4300 m), we obtained physiopsychological data of AMS-S and AMS-R groups. By comparing different changes of AMS-S and AMS-R groups, our study found that compared with AMS-R group, AMS-S group had lower SpOand higher SVV before departure from Shanghai, lower MEFand higher levels of negative moods, including anxiety, confusion, fatigue, in Yambajan.
Our study found SpOdecreased, while blood pressure and HR increased when expeditioners arrived in Tibet. In hypoxic environments, the oxygen content of the breath decreased, SpOdecreased, and would stimulate the peripheral chemoreceptors, leading to increase of sympathetic activity (Bartsch and Gibbs, 2007). As a result, HR, SBP, DBP and pulse rate increased. Previous studies reported that SpOcan be used to predict the development of AMS (Liu et al., 2014; Burtscher et al., 2019). Our results also showed that expeditioners with lower SpObefore departure were at increased risk for AMS when arrived in Yambajan.
We didn’t find differences in ECG between AMS-R and AMS-S groups. But after reaching 4300 m, our results showed that QTc lengthened, while QT, QRS, RR and PP shortened in both AMS-S and AMS-R groups.ECG changes under hypoxia condition are related to energy metabolism. Our previous research (Guo et al., 2012) conducted in the 25th and 26th Chinese Antarctic pre-selected expeditioners of Kunlun Station found that after entering the plateau by train, at 4300 m, their QTc lengthened, but QRS shortened. This was consistent with our result.
Hypoxia at high altitude could reduce cardiovascular function. Stembridge et al. (2019) found that 12 healthy adult men showed reduced stroke volume (SV) and left ventricular diastolic volume at 3800 m. Sareban et al. (2019) found that after rapid ascent to 4559 m, left ventricular passive filling volume and SV of 50 healthy subjects decreased. Our group (Guo et al., 2012) found that at 3650 m and 4300 m, systemic vascular resistance (SVR) of the 25th and 26th Chinese Antarctic pre-selected expeditioners of Kunlun Station increased, while left ventricular pumping and contractile function weakened. This study is consistent with previous reports. Under hypoxic environments at 4300 m, left ventricular systolic and pumping function of both AMS-S and AMS-R groups decreased. Meanwhile, systemic vascular resistance increased. Acute hypoxia can cause transient pulmonary hypertension, and then increase pulmonary vascular resistance, leading to right ventricular dilatation. As a result, left ventricular diastolic volume decreased correspondingly (Mahmud et al., 2002). Therefore, SV and SI reduced, while SVR increased. Due to reduced oxygen supply, energy metabolism is impaired and myocardial energy supply is inadequate, resulting in decreased myocardial contractility (Chacaroun et al., 2016). Our study also found decreases in velocity index (VI), acceleration index (ACI) and left ventricular ejection time (LVET), increases in systolic time ratio (STR) and ejection time ratio (ETR). Under hypoxia conditions, increased sympathetic activity could lead to persistent vascular tension (Duplain et al., 1999), coupled with reduced reflex sensitivity of vascular baroreflex (Cooper et al., 2005). These changes lead to reduced vasodilation response, thus, resulting in increased SVR. Our results also showed expeditioners with higher SVV were susceptible to AMS when arrived in Yambajan. The relationship between SVV and AMS hadn’t been reported, which needs further research in the following pre-selected expeditions.
Previous studies have reported pulmonary ventilation function changed in hypoxia environments. Cremona et al. (2002) found FEV/FVC increased when 262 climbers arrived at 4559 m. Dehnert et al. (2010) found FEV/FVC and MMEF of 34 subjects significantly increased at 4559 m. Our study found VC, FEV/FVC, PEF, PIF, MEF, MEFincreased after reaching 4300 m. Under hypoxic conditions, decreased POin blood caused hyperventilation (Burgess and Ainslie, 2016). Pulmonary function can be used to monitor the occurrence of AMS (Roach et al., 2000; Beidleman et al., 2004). Yang et al. (2020) took pulmonary function tests on 121 healthy men at 4100 m, they suggested that subjects with low MEF(FEF) values at low altitude are susceptible to high levels of pulmonary artery pressure at high altitude but not the incidence of AMS following short-term high-altitude exposure. But our results also found expeditioners who were susceptible to AMS had lower MEFin Yambajan, indicating that expeditioners with good big airway ventilation at high altitude may be tolerant to hypoxia.
Hypoxia at high altitude can cause mood disorders. In hypoxic environments, low oxygen led to increase of sympathetic activity (Bärtsch and Gibbs, 2007), which may impair mood states. Our study found anxiety, fatigue, and confusion of AMS-S group increased in Yambajan compared to AMS-R group. These were coincidence with several studies. Boos et al. (2018) found anxiety at high altitude was independently associated with AMS and its severity. Fatigue was one of the main symptoms of AMS. de Aquino Lemos et al. (2012) also found fatigue increased and vigor reduced in 10 healthy men after arriving at 4500 m. Bian et al. (2015) analyzed risk factors of AMS in 163 participants who were acute exposure 3700 m, they found the AMS population exhibited more negative mood states, including anxiety, depression, hostility, fatigue and confusion.
Limited by one-week adaptive training in Tibet, our study only monitored physiopsychological changes of pre-selected expeditioners of Kunlun Station during the acute period of hypoxia. Although we have suggested some physiopsychological indicators which may be used as clues for screening hypoxia-susceptible individuals, however, because of the small sample size in this study, we still need to accumulate more data from multiple teams to verify our findings.
5 Conclusion
Our study found that cardiopulmonary function and mood states could be affected when expeditioners were acutely exposed to 4300 m. After comparing the differences of physiopsychological parameters between AMS-S and AMS-R groups at Shanghai and Yambajan, we found that compared with AMS-R group, AMS-S group showed significantly lower SpOand higher SVV before departure in Shanghai, however, significantly lower MEF, higher levels of anxiety, fatigue and confusion in Yambajan. Maybe these changed physiopsychological parameters could be used as clues for screening hypoxia-susceptible individuals.
Acknowledgement We would like to thank the Chinese Arctic and Antarctic Administration (CAA) of State Oceanic Administration (SOA), and the Polar Research Institute of China for the support and organization of the fieldwork. We also appreciate the participation and cooperation of the 37th Chinese Antarctic pre-selected expeditioners of Kunlun Station. This study was funded by the CAA (Grant no. JDXT2019-03), the National Antarctic Expedition Training Base of China (Medical Monitoring and Screening of the 37th Chinese Antarctic Pre-selected Expeditioners of Kunlun Station During Plateau Adaptive Training in Tibet), and the CAA (Formulation of Technical Specifications for Physiological Health Monitoring of Antarctic Expeditioners). We would like to thank the reviewer Dr. Mauro Marzorati, another anonymous reviewer and Associate Editor, Dr. Ian Allison for their valuable suggestions and comments regarding further improvement of this article.
Bärtsch P, Gibbs J S R. 2007. Effect of altitude on the heart and the lungs. Circulation, 116(19): 2191-2202, doi:10.1161/CIRCULATIONAHA. 106.650796.
Beidleman B A, Muza S R, Fulco C S, et al. 2004. Intermittent altitude exposures reduce acute mountain sickness at 4300 m. Clin Sci, 106(3): 321-328, doi:10.1042/CS20030161.
Bian S Z, Jin J, Zhang J H, et al. 2015. Principal component analysis and risk factors for acute mountain sickness upon acute exposure at 3700 m. PLoS One, 10(11): e0142375, doi:10.1371/journal.pone.0142375.
Boos C J, Bass M, O'Hara J P, et al. 2018. The relationship between anxiety and acute mountain sickness. PLoS One, 13(6): e0197147, doi:10.1371/journal.pone.0197147.
Burgess K R, Ainslie P N. Chapter 19: Central sleep apnea at high altitude//Kikis E A, Gidalevitz T , Morimoto R I. Advances in experimental medicine and biology. Boston, MA: Springer US, 2016: 275-283, doi:10.1007/978-1-4899-7678-9_19.
Burtscher M, Philadelphy M, Gatterer H, et al. 2019. Physiological responses in humans acutely exposed to high altitude (3480 m): Minute ventilation and oxygenation are predictive for the development of acute mountain sickness. High Alt Med Biol, 20(2): 192-197, doi:10.1089/ham.2018.0143.
Chacaroun S, Borowik A, Morrison S A, et al. 2016. Physiological responses to two hypoxic conditioning strategies in healthy subjects. Front Physiol, 7: 675, doi:10.3389/fphys.2016.00675.
Cooper V L, Pearson S B, Bowker C M, et al. 2005. Interaction of chemoreceptor and baroreceptor reflexes by hypoxia and hypercapnia – a mechanism for promoting hypertension in obstructive sleep apnoea. J Physiol, 568(2): 677-687, doi:10.1113/jphysiol.2005.094151.
Cremona G, Asnaghi R, Baderna P, et al. 2002. Pulmonary extravascular fluid accumulation in recreational climbers: a prospective study. Lancet, 359(9303): 303-309, doi:10.1016/S0140-6736(02)07496-2.
de Aquino Lemos V, Antunes H K M, dos Santos R V T, et al. 2012. High altitude exposure impairs sleep patterns, mood, and cognitive functions. Psychophysiology, 49(9): 1298-1306, doi:10.1111/j.1469- 8986.2012.01411.x.
Dehnert C, Luks A M, Schendler G, et al. 2010. No evidence for interstitial lung edema by extensive pulmonary function testing at 4559 m. Eur Respir J, 35(4): 812-820, doi:10.1183/09031936.00185808.
Duplain H, Vollenweider L, Delabays A, et al. 1999. Augmented sympathetic activation during short-term hypoxia and high-altitude exposure in subjects susceptible to high-altitude pulmonary edema. Circulation, 99(13): 1713-1718, doi:10.1161/01.cir.99.13.1713.
Guo Z M, Huang F M, Lu H, et al. 2012. Effects of different altitudes on cardiac hemodynamics and electrocardiogram of healthy male adults. Chin J Appl Physiol, 28(1): 1-4.
Hackett P H, Oelz O. 1992. The Lake Louise Consensus on the definition and quantification of altitude illness. Burlington: Queen City Press, 327-330.
Hackett P H, Roach R C. 2001. High-altitude illness. N Engl J Med, 345(2): 107-114, doi:10.1056/nejm200107123450206.
Julian C G, Subudhi A W, Wilson M J, et al. 2011. Acute mountain sickness, inflammation, and permeability: new insights from a blood biomarker study. J Appl Physiol (1985), 111(2): 392-399, doi:10.1152/japplphysiol.00391.2011.
Liu Y, Zhang J H, Gao X B, et al. 2014. Correlation between blood pressure changes and AMS, sleeping quality and exercise upon high-altitude exposure in young Chinese men. Mil Med Res, 1: 19, doi:10.1186/2054-9369-1-19.
Mahmud E, Raisinghani A, Hassankhani A, et al. 2002. Correlation of left ventricular diastolic filling characteristics with right ventricular overload and pulmonary artery pressure in chronic thromboembolic pulmonary hypertension. J Am Coll Cardiol, 40(2): 318-324, doi:10.1016/S0735-1097(02)01959-9.
McNair D M, Lorr M, Droppleman L F. 1971. Manual for the profile of emotion states. San Diego, Educational and Industrial Testing Services.
Norton P J. 2007. Depression Anxiety and Stress Scales (DASS-21): psychometric analysis across four racial groups. Anxiety Stress Coping, 20(3): 253-265, doi:10.1080/10615800701309279.
Roach R C, Hackett P H, Oelz O, et al. 2018. The 2018 lake louise acute mountain sickness score. High Alt Med Biol, 19(1): 4-6, doi:10.1089/ham.2017.0164.
Roach R C, Maes D, Sandoval D, et al. 2000. Exercise exacerbates acute mountain sickness at simulated high altitude. J Appl Physiol, 88(2): 581-585, doi:10.1152/jappl.2000.88.2.581.
Sareban M, Perz T, Macholz F, et al. 2019. Impairment of left atrial mechanics does not contribute to the reduction in stroke volume after active ascent to 4559 m. Scand J Med Sci Sports, 29(2): 223-231, doi:10.1111/sms.13325.
Singh I, Khanna P K, Srivastava M C, et al. 1969. Acute mountain sickness. N Engl J Med, 280(4): 175-184, doi:10.1056/nejm1969 01232800402.
Stembridge M, Ainslie P N, Boulet L M, et al. 2019. The independent effects of hypovolaemia and pulmonary vasoconstriction on ventricular function and exercise capacity during acclimatisation to 3800 m. J Physiol, 597(4): 1059-1072, doi:10.1113/JP275278.
Yang Y Q, Liu C, Yu S Y, et al. 2020. Pulmonary function tests at low altitude predict pulmonary pressure response to short-term high altitude exposure. Respir Physiol Neurobiol, 282: 103534, doi:10.1016/j.resp.2020.103534.
: Wu X P, Liu S Y, Wang X, et al.Different physiopsychological changes between AMS-susceptible and AMS-resistant pre-selected Antarctic expeditioners in Tibet. Adv Polar Sci, 2021, 32(3):239-247,doi:10.13679/j.advps.2021.0010
11 April 2021;
21 July 2021;
30 August 2021
10.13679/j.advps.2021.0010
Corresponding author, E-mail:xuchengli@pumc.edu.cn
杂志排行
Advances in Polar Science的其它文章
- The marine environmental evolution in the northern Norwegian Sea revealed by foraminifera during the last 60 ka
- Application of unmanned underwater vehicles in polar research
- An introduction to the riometer system deployed at China-Iceland joint Arctic observatory and its beam- forming correction method based on the preliminary data
- Characteristics of hydrogen/oxygen isotopes in water masses and implications for spatial distribution of freshwater in the Amundsen Sea, Southern Ocean
- Marine biogenic aerosols and their effects on aerosol- cloud interactions over the Southern Ocean: a review
- Utilization of clean energy and future trend of Antarctic research stations
