Review of the potential of mesenchymal stem cells for the treatment of infectious diseases
2021-07-24AmitSharmaAnujaChakrabortyBithiahGraceJaganathan
Amit Sharma, Anuja Chakraborty, Bithiah Grace Jaganathan
Amit Sharma, Anuja Chakraborty, Bithiah Grace Jaganathan, Stem Cell and Cancer Biology Group, Department of Biosciences and Bioengineering, Indian Institute of Technology Guwahati, Guwahati 781039, India
Abstract The therapeutic value of mesenchymal stem cells (MSCs) for the treatment of infectious diseases and the repair of disease-induced tissue damage has been explored extensively.MSCs inhibit inflammation, reduce pathogen load and tissue damage encountered during infectious diseases through the secretion of antimicrobial factors for pathogen clearance and they phagocytose certain bacteria themselves.MSCs dampen tissue damage during infection by downregulating the levels of pro-inflammatory cytokines, and inhibiting the excessive recruitment of neutrophils and proliferation of T cells at the site of injury.MSCs aid in the regeneration of damaged tissue by differentiating into the damaged cell types or by releasing paracrine factors that direct tissue regeneration, differentiation, and wound healing.In this review, we discuss in detail the various mechanisms by which MSCs help combat pathogens, tissue damage associated with infectious diseases, and challenges in utilizing MSCs for therapy.
Key Words: Infectious diseases; Mesenchymal stem cells; Antimicrobial effect; Immunomodulation; Tissue repair; COVID-19
INTRODUCTION
Infectious diseases are a leading cause of morbidity and mortality worldwide; respiratory infections and pneumonia are among the major causes of death globally.Failure of commonly used therapies, drugs and the rising number of new infectious disease outbreaks have increased the necessity to identify novel therapeutic strategies to combat infections, the resulting organ and tissue damage associated with the diseases.Mesenchymal stem cells (MSCs) are non-hematopoietic cells found in the bone marrow and other tissues such as adipose tissue, placenta, dental pulp, synovial membrane, endometrium, umbilical cord blood, Wharton's jelly, and ocular tissues[1-4].Tissues are mechanically or enzymatically dissociated to isolate MSCs, giving rise to plastic adherent cell populations[5].MSCs can also be separated by flow cytometry sorting based on their cell surface marker expression[6,7].MSCs possess extensive selfrenewal, proliferative, and multilineage differentiation potential.They are identified based on the expression of cell surface markers cluster of differentiation 105 (CD105), CD90, CD73, CD 44, CD29 and are negative for markers such as CD45, CD34, CD14, CD11b, CD79α, CD19, and human leukocyte antigen (HLA)-DR[1].However, when stimulated with interferon-gamma (IFN-γ), MSCs express HLA-DR[8-10].
MSCs have multilineage differentiation ability and give rise to adipocytes, osteoblasts, and chondrocytes under standard differentiation conditions.Additionally, MSCs play an important role in tissue repair and homeostasis; thus, they have become an attractive therapeutic option for the treatment of several infectious and degenerative diseases[11-17].In addition, MSCs possess immunomodulatory and immunosuppressive properties, reduce inflammation, and display immune protective functions[1,18,19].Due to the rising number of infectious diseases and associated organ damage, MSCs have been explored as a possible treatment option in recent years.Several pre-clinical and clinical trials with MSCs have yielded encouraging results, improved therapeutic outcomes, and provided the opportunity to utilize MSCs for the treatment of infectious diseases in addition to existing therapeutic options.Further, intravenous administration of MSCs is effective in treating pathogen-induced organ damage in several disease models[20-22].
This review summarizes various studies that tested the therapeutic advantages of MSCs in treating infectious diseases and repairing disease-induced tissue damage.We also discuss the various modes in which MSCs function to clear pathogens and rebuild the damaged tissue, the signaling pathways modulated by MSCs in the host cells during infections, and finally, some of the challenges associated with utilizing MSCs for therapy.
METHODOLOGY
The objective of this review was to analyze various pre-clinical and clinical studies that utilized MSCs for the treatment of infectious diseases and associated tissue damage.PubMed, Scopus, and Web of Science databases were searched without any language restrictions.Studies that utilized MSCs with or without modification in disease models of infection or pathogen-induced tissue damage were selected for inclusion in the review.The research articles were grouped as follows based on their major findings when MSCs were injected: direct anti-pathogen effects, immunomodulatory effects, differentiation into cells of target tissues, and clinical trials.
DIRECT ANTIMICROBIAL EFFECTS OF MSCS
Several studies have reported that administration of MSCs during lung injury and sepsis significantly reduce the bacterial load.MSCs secrete four types of antimicrobial peptides (AMPs): LL-37, hepcidin AMP (HAMP), lipocalin 2 (LCN2), and betadefensin-2 (BD2) (Figure 1).Besides AMPs, several other paracrine factors secreted by MSCs also contribute to the antimicrobial defense.LL-37 is an amphipathic AMP that belongs to the cathelicidin family of AMPs that induces bacterial lysis and enhances antibiotic sensitivity.LL-37 directly binds to and inactivates lipopolysaccharides (LPS), thereby disrupting the bacterial outer membrane.LL-37 can also neutralize the LPS (endotoxin) released by bacteria.LL-37 has chemotactic activity, recruits immune cells to enhance pathogen clearance at the site of infection.However, this recruitment of immune cells such as macrophages does not increase pro-inflammatory cytokines such as tumor necrosis factor α (TNF-α)[23,24].LL-37 also promotes regeneration and angiogenesis by binding to formyl peptide receptor-like 1 expressed on endothelial cells[25].LL-37 secreted by either bone marrow-derived MSCs (BM-MSCs) or adipose tissue-derived MSCs (AD-MSCs) increased the effectiveness of antibiotics, enhanced pathogen killing, and slowed bacterial growth in a pulmonary infection model of cystic fibrosis induced byPseudomonas aeruginosa,Staphylococcus aureus,andStreptococcus pneumonia[26].HAMP, another AMP secreted by MSCs, promotes bacterial clearance by preventing iron uptake by the pathogens.HAMP promotes transport of the cellular iron storage protein, ferritin, into the macrophages and subsequent destruction in lysosomes.This causes iron to be stored inside the macrophages, making it unavailable for bacterial survival.So, by depleting iron, HAMP hampers the growth and survival of bacteria[27].LCN secreted by MSCs also promotes bacterial clearance by blocking iron uptake by the bacterial cells[28,29].BD2 secreted by MSCs reportedly play an important role in pathogen clearance.Sunget al[30] reported that in anEscherichia coli-induced pneumonia model, intratracheal administration of human umbilical cord-derived MSCs (UC-MSCs) resulted in the attenuation of lung injury and led to a significant reduction in inflammation and increase in bacterial clearance from the infected site.Microarray analysis found that toll-like receptor 2 (TLR-2), TLR-4, and BD2 expression levels were significantly upregulated in lung tissue.The TLR-4 signaling pathway is important for BD2 secretion and silencing of TLR-4 but not TLR-2 abolished the anti-bacterial effect of MSCs againstE.coli[30].Depletion of the essential amino acid, tryptophan, by indoleamine 2,3-dioxygenase (IDO) secreted by MSCs also has antimicrobial effects on various pathogens such as toxoplasma, plasmodium, chlamydia, rickettsia, streptococci, staphylococci, and herpes virus[31].In addition, MSCs directly phagocytose bacteria through scavenger receptors (Figure 1).Khanet al[32] found that human MSCs internalizedM.tuberculosisthrough two types of scavenger receptors, namely the macrophage receptor with collagenous structure and scavenger receptor class B member 1.These endocytosed mycobacteria were killed by activation of intrinsic autophagy and nitric oxide secreted by MSCs[32].
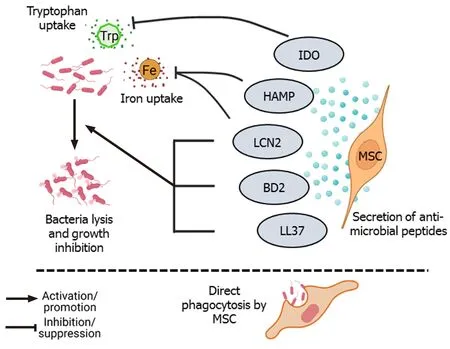
Figure 1 Direct bacterial lysis and phagocytosis.
In addition to the anti-bacterial properties, MSCs also exert anti-viral effects.Rodrigueset al[33] found that MSCs had suppressive effects on human T-lymphotropic virus (HTLV)-infected T cells, similar to that seen with healthy T cells.IDO and prostaglandin E2 (PGE2) secreted by MSCs suppressed the proliferation of infected T cells, and the co-culture of infected T cells with MSCs reduced the expression of HTLV1polgene[33].In a mouse model of lethal herpes simplex virus (HSV-1) infection, MSC administration significantly increased the survival percentage and exerted anti-viral effects by upregulating IFN-γ levels, while decreasing IL-6 and TNFα serum levels[34].
MSCS IN IMMUNOMODULATION
MSCs reduce the infiltration and accumulation of neutrophils and other immune cells at the site of tissue damage and infection.Neutrophils constitute the first line of defense against infections, but their excessive accumulation at the site of infection results in increased secretion of various proteolytic enzymes, matrix metalloproteinases, reactive oxygen species (ROS), and pro-inflammatory cytokines leading to neutrophil extracellular traps (NETosis).Although NETosis helps in pathogen clearance, it also results in tissue damage due to the exaggerated inflammatory response[35].Excessive NET formation and its poor degradation results in tissue damage and has been implicated in sepsis[36,37] and coronavirus disease 2019 (COVID-19)[38].MSCs alleviate the excessive influx of neutrophils through TNF-αstimulated gene-6 (TSG-6) secretion, which inhibits the recruitment of neutrophils by IL-8[39].In addition, MSCs diminish NET formation by delaying the apoptosis of neutrophils and inducing intercellular adhesion molecule 1 expression in neutrophils to facilitate their phagocytosis[40].MSCs also control the tissue damage caused by toxic reactive oxygen and nitrogen species produced by neutrophils through the secretion of antioxidant enzymes such as superoxide dismutase (SOD)[40].
MSCs regulate the function of macrophages during infection.Macrophages play an important role in mediating the inflammatory response and can exist as pro-inflammatory M1-type, which mounts the immune response against pathogens, and antiinflammatory M2-type, which helps in resolving inflammation through secretion of anti-inflammatory cytokines[41].However, during acute respiratory distress syndrome (ARDS), the M1 phenotype is upregulated, disrupting the balance between M1 and M2 macrophages[42].Several studies have reported that MSCs moderate the inflammatory response by promoting the polarization of macrophages towards M2 phenotype through secretion of various factors such as IL-1 receptor antagonist[43], decorin[44], stanniocalcin-2[45], and TSG-6[12].In an LPS-induced acute lung injury (ALI) model, transforming growth factor-β3 (TGF-β3) and thrombospondin 1 (TSP-1) secreted by dental follicle-derived MSCs upregulated M2 phenotype in alveolar macrophages, marked by the increased expression of enzyme arginase 1 and downregulation of M1 macrophage markers such as inducible nitric oxide synthase and CD86[46].Conversely, co-culture of rat BM-MSCs with LPS-treated alveolar macrophages promoted the survival of macrophages through the upregulation of anti-apoptotic Bcell lymphoma 2 (Bcl-2) and inhibition of caspase-3 and Bcl-2-associated X protein expression by modulating the Wnt/β-catenin pathway[47].Furthermore, PGE2secreted by MSCs upregulated the bactericidal activity of M1 macrophages through phosphoinositide 3-kinase and mediated the increase in NADPH oxidase 2 activity and ROS production[41].Interestingly, in a pre-clinical ARDS model, Jacksonet al[48] found that MSCs enhanced pathogen clearance and survival of alveolar macrophages by donating mitochondriaviatunneling microtubules.In addition, intravenous injection of murine BM-MSCs overexpressing hepatocyte growth factor (HGF) attenuated the damage in an LPS-induced ALI model by modulating the function of dendritic cells (DCs).HGF secreted by the MSCs induced mature DCs to differentiate into “tolerogenic” regulatory DCs by activation of the HGF/Akt pathway[49].
Injection of MSCs inhibited the proliferation of septic natural killer (sNK) cells and significantly improved the survival of the experimental animals in a cecal ligation puncture mouse model.Injection of MSCs altered the cytokine profile in the serum and altered the sNK cell function, possibly through modulation of the Janus kinase/signal transducer and activator of transcription (STAT) pathway[50].In preclinical models of acute liver injury and liver necrosis, injection of murine MSCs significantly downregulated the IL-17 level and decreased IL-17-producing NKT cells but enhanced FOXP3+IL10+NKT cells[51].MSCs also suppressed the differentiation of CD4+T cells into IFN-γ-producing T helper type 1 (Th1) cells or IL-17-producing Th17 cells but increased the number of regulatory T cells (Tregs)[52].In a mouse model ofAspergillushyphal extract-induced inflammation, administration of human BM-MSCs decreased IL-4, IL-5, and IL-17 levels and ameliorated inflammation[53].In the presence of IFN-γ and TNF-α, MSCs enhanced the secretion of programmed deathligand 1 (PD-L1) and PD-L2, respectively, which in turn inhibited T-cell proliferation and upregulated FOXO3 expression in these cells[54].CD200, a cell surface protein highly expressed in Wharton’s jelly-derived MSCs (WJ-MSCs), has been implicated in inducing immune tolerance by interacting with CD200R present on CD4+and CD8+T cells[55].
Although MSCs are considered immune privileged, some studies have reported that they are susceptible to NK-mediated killing in an IL-2-dependent manner.Stimulation of TLRs on MSCs leads to shedding of NK cell-interacting ligands such as major histocompatibility complex I (MHC I) and NK group 2 member D, making them less susceptible to killing by activated NK cells[56].TLR4 stimulation increases the survival of MSCs under stress conditions through the upregulation of extracellular signalrelated kinase 1/2 (ERK1/2)[57].Studies that have tracked MSCsin vivofound that MSCs died 24 h post-intravenous injection and accumulated in the lungs and liver[58,59].de Witteet al[58] reported that thein vivo-injected UC-MSCs were rapidly phagocytosed by the monocytes, which then expressed PD-L1 and IL-10 and downregulated TNF-α expression, resulting in acquisition of the regulatory phenotype by these monocytes.Furthermore, phagocytosis of UC-MSCs by lung phagocytes induced the production of (C-X-C motif) ligand (CXCL) 9 and CXCL10 by these cells, which helped to recruit CXCR3+Tregs[60].Keratinocyte growth factor (KGF) secreted by MSCs promotes the survival of monocytes by enhancing Akt phosphorylation, thereby facilitating bacterial clearance[61].In a mouse model of Coxsackie virus infection, secretion of CX3CL1 by the injected human BM-MSCs inhibited the migration of proinflammatory Ly6Chighcells but promoted anti-inflammatory LyC6lowmonocyte migration.By modulating monocyte trafficking to the heart, MSCs reduced inflammation and damage in heart tissue[62].Treatment with BM-MSCs improved lung function and reduced inflammatory cytokines in H9N2-[63] and H5N1-infected mice[64].However, treatment with UC-MSCs was more effective than BM-MSCs in restoring alveolar fluid clearance (AFC) and reducing inflammation in H5N1-infected mice[64].Thus, modulation of immune cells forms the basis for long-term therapeutic effects of MSCs in facilitating pathogen clearance and reducing inflammationmediated tissue damage (Figure 2).
Several studies have shown that when subjected to an inflammatory environment, MSCs secrete higher levels of anti-inflammatory factors such as TSG-6, IL-10, and PGE2and inhibit nuclear factor kappa B (NF-κB) signaling, which leads to the decreased expression of pro-inflammatory cytokines such as IL-6, TNF-α, and IL-1β[43,51,65-68].In the absence of pro-inflammatory stimulus, MSCs secrete low levels of cyclooxygenase 2 (COX2), PGE2, TGF-β1, HGF, IL-10, PD-1, PD-L1, and PD-L2[69].In contrast, when subjected to an inflammatory environment consisting of TNF-α and IFN-γ, MSCs significantly upregulate the expression of PGE2,COX2, PD1, IDO, HGF, and TGF-β1, which contribute to their immunomodulatory properties[67,69].Furthermore, in the presence of pro-inflammatory cytokines, MSCs also supplement the production of anti-inflammatory lipid mediator lipoxinA4(LXA4) by alveolar type II epithelial (AT-II) cells[70].Secretion of IDO by MSCs, a rate-limiting enzyme involved in the catabolism of tryptophanviathe kynurenine pathway, has been implicated in MSCs-mediated reduction of inflammation[33,71].Inhibition of IDO with 1-methyltryptophan abolished the anti-inflammatory effects of MSCs on a murine hepatitis model[51], and inhibition of kynurenine, a downstream metabolite of IDO, downregulated TSG-6 secretion by MSCs[71].Similarly, in an ALI mouse model, the anti-inflammatory effects of MSCs were abolished when TSG-6 or HGF was silenced[20,72], indicating the role of MSCs-secreted factors in controlling the inflammation.Additionally, netrin-1 expressed by MSCs inhibited neutrophil migration[73].LXA4 and PGE2secreted by MSCs induce heme oxygenase-1 (HO-1) expression in macrophages, resulting in cytoprotection during oxidative stress-mediated inflammation[74,75].HO-1, along with angiopoietin-1 (Ang1), inhibits the TNF-α stimulated migration of leukocytes[76,77].Secretion of antioxidants such as SOD, catalase, glutathione peroxidase, and glutathione reductase by MSCs also reduces oxidative stress[78].
Some studies have also identified a pro-inflammatory role of MSCs, in which MSCs promote the migration of neutrophils, macrophages, and monocytes to the infection site and expedite pathogen clearance[79,80].Petriet al[81] found that secretion of IFNγ by MSCs in the early stages of bacterial infection augmented the function of NK cells but induced the regulatory phenotype in NK cells at the later stages.In aP.aeruginosainduced chronic lung injury model, injection of a high dose of AD-MSCs inhibited bacterial load and downregulated bacteria-induced secretion of PGE2by alveolar cells[82].Downregulation of PGE2levels indirectly enhanced the immune response, leading to higher bacterial clearance[26,83].Further, injection of BM-MSCs inParacoccidioides brasiliensis-infected mice led to increased fungal levels and exaggerated immune responses, with increased accumulation of neutrophils, eosinophils, and M2 macrophages, leading to congestion and edema in lungs[84].Similarly, treatment with BM-MSCs in mice with latentM.bovisinfection resulted in significantly higher mycobacterial number and granuloma formation[85].However, if the MSCs were conditioned with TLR-3 ligand, poly (A:U) prior to the injection, it significantly reduced the pathogen load, suggesting that priming of MSCs was necessary for their anti-mycobacterial effect[85].
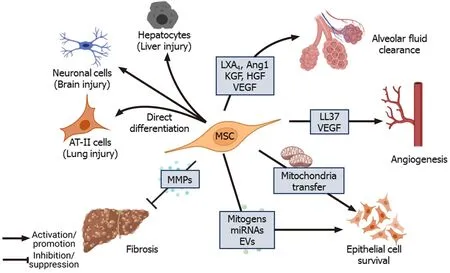
Figure 2 Immunomodulation by mesenchymal stem cells.
MSCS PROMOTE REPAIR OF TISSUE DAMAGE
The regenerative and multipotent differentiation ability of MSCs also aids in the repair of tissue damage caused by infection.Despite employing different therapeutic strategies, the clinical outcome of ALI and acute respiratory distress syndrome is still poor and remains a significant healthcare burden necessitating novel therapeutic interventions[21,86].Apart from controlling infection and inflammation, the paracrine factors secreted by MSCs repair and regenerate the damaged epithelial and endothelial barriers of the alveoli[64].In the alveolar region, AT-I and AT-II epithelial cells (pneumocytes) constitute the continuous alveolar epithelium, separated from the endothelium by a layer of connective tissue.KGF and TSP-1 secreted by MSCs induce the proliferation of epithelial cells and induce the differentiation of AT-II cells into ATI cells, which further promote the regeneration of alveolar epithelium[87,88].Under normal conditions, tight junctions and other cellular junctions maintain the integrity of cellular barriers, allowing the selective flow of fluid.During ALI/ARDS, however, the barrier becomes compromised, and disruption of the ion channel proteins and aquaporins (AQPs) causes fluid leakage into the interstitium and alveolar spaces resulting in edema and compromised gas exchange in the lungs[89,90].Transepithelial ion exchange through Na+ion channels (ENaC), Na+/K+ATPase, and cystic fibrosis transmembrane conductance regulator (CFTR) present on alveolar epithelial cells creates an osmotic gradient that drives the movement of water required for normal AFC.Inflammatory cytokines such as TNF-α, TGF-β1, IFN-γ, IL-4, IL-13, and IL-1β downregulate the expression of ion channel and junction proteins in the alveolar epithelial and endothelial layer leading to the dysregulation of AFC[91].Leeet al[92] found that KGF secreted by MSCs promotes AFC and ameliorates edema during lung injury.KGF secreted by MSCs upregulates the expression of catalytic α1subunit of Na+/K+ATPase and surfactant protein (SP A) in AT-II cells[87].Also, KGF-silenced MSCs failed to dampen pulmonary edema in an LPS-induced ALI mice model[93].
Furthermore, paracrine factors such as LXA4, KGF, Ang1, vascular endothelial growth factor (VEGF), and HGF secreted by MSCs induce the expression of ion channel, cellular junction, and tight junction proteins in epithelial cells, which facilitate repair of alveolar epithelium and restore normal AFC[68,70,94,95].In an ALI mouse model, Fanget al[70] found that intratracheal administration of MSCs significantly increased LXA4 level in bronchoalveolar lavage fluid (BALF) and improved the survival of the experimental animals.LXA4 was found to enhance CFTR expression in AT-II cells damaged by LPS treatment through downregulation of Akt phosphorylation, which led to improved AFC[96].LXA4 also increases the expression of α and γ subunits of ENaC channel[97], and AQP5 in LPS-stimulated epithelial cells[98,99], which contribute to enhanced fluid clearance, improved gas exchange, and pulmonary edema resolution during lung injury.LXA4 improves the integrity of the epithelial barrier by upregulating the expression of junction proteins such as zona occludens 1, claudin 1, and occludin[100].ALI stimulates ROS generation in lung tissue, which induces mitochondria dysfunction, leading to more ROS release, forming a cycle of 'ROS-induced ROS release'[101,102].LXA4activates NF-E2-related factor 2, which is important for maintaining the redox balance in epithelial cells and rescues Ecadherin expression[103].LXA4 increases the proliferation of AT-II cells, reduces caspase-3 levels, and inhibits LPS-induced apoptosis[104].LXA4 secreted by MSCs reduces the permeability of alveolar epithelium by restoring the expression and distribution of tight junctions (Figure 3).MSCs when co-cultured with AT-II cells in the presence of TNF-α, IFN-γ, and IL-1β had significantly high expression of Ang1, which inhibited NF-κB activation and rescued claudin 18 expression in AT-II cells[64,94].Ang1 binding increases active Rac1 levels and subsequently leads to the inactivation of Ras homolog family member A (RhoA) in endothelial cells[105].RhoA activation disrupts actin and myosin contraction and promotes stress fiber formation, thereby increasing the endothelial permeability, whereas Rac1 counteracts RhoA by activating p190GAP and promotes the formation of adherens and tight junctions[106].Ang1 increases Rac1/2/3 activity and downregulates active RhoA levels in AT-II cells[94] (Figure 4).VEGF and HGF secreted by MSCs reduced endothelial permeability by upregulating the expression of junction protein VE-cadherin, and the silencing of VEGF or HGF in MSCs inhibited their ability to reduce paracellular and transcellular endothelial permeability[107,108].Yanget al[95] reported that HGF and VEGF secreted by MSCs acted synergistically to remodel F-actin, and tight junctions in LPSstimulated pulmonary endothelial cells by upregulating Rac1 and downregulating RhoA expression.
MSCs were found to enhance the survival of pulmonary epithelial cells, hepatocytes, and cardiac myocytes by enhancing autophagy in several pre-clinical infection models.Autophagy is also associated with reducing inflammatory signals.Huet al[109] reported that LPS stimulation of mouse lung epithelial cells or human bronchial epithelial cells led to downregulation of autophagy marker MAP1LC3B through activation of mammalian target of rapamycin (mTOR)viaTLR4 signaling.Silencing of mTOR or overexpression of autophagy-related proteins in epithelial cells reduced the production of cytokines IL-6 and IL-8[109].Chenet al[110] found that miR-100 present in human WJ-MSCs-derived exosomes (extracellular vesicles, EVs) downregulated mTOR in rat AT-II cells.Treatment with MSCs-derived EVs activated autophagy but inhibited apoptosis and secretion of pro-inflammatory cytokines in bleomycin-treated rat epithelial cells through mTOR downregulation[110].The protective and repair functions of the MSCs were found to be mediated by p70S6K1[111], an isoform of S6K1, which is the downstream target of mTOR[65].The protective effects of MSCs on injured alveolar epithelial cells are also mediated by the donation of mitochondrial by MSCs to the alveolar epithelial cells.BM-MSCs formed connexin 43 mediated Ca2+transporting gap junctions[112] and transferred mitochondrial to the alveolar epithelial cells with the help of Miro1, a mitochondrial Rho GTPase[113] (Figure 4).In addition to modulating the inflammatory response, treatment with MSCs significantly reduced collagen deposition, fibrosis, and scar formation in injury models involving various organs such as lungs[114,115], liver[116-118], heart[16], bladder[17] and eyes[119].The anti-fibrotic effects of MSCs were mediated by upregulation of matrix metalloproteinases, matrix metalloproteinase 1 (MMP1), MMP13, MMP14, and inhibition of tissue inhibitors of MMP 1[118,120].
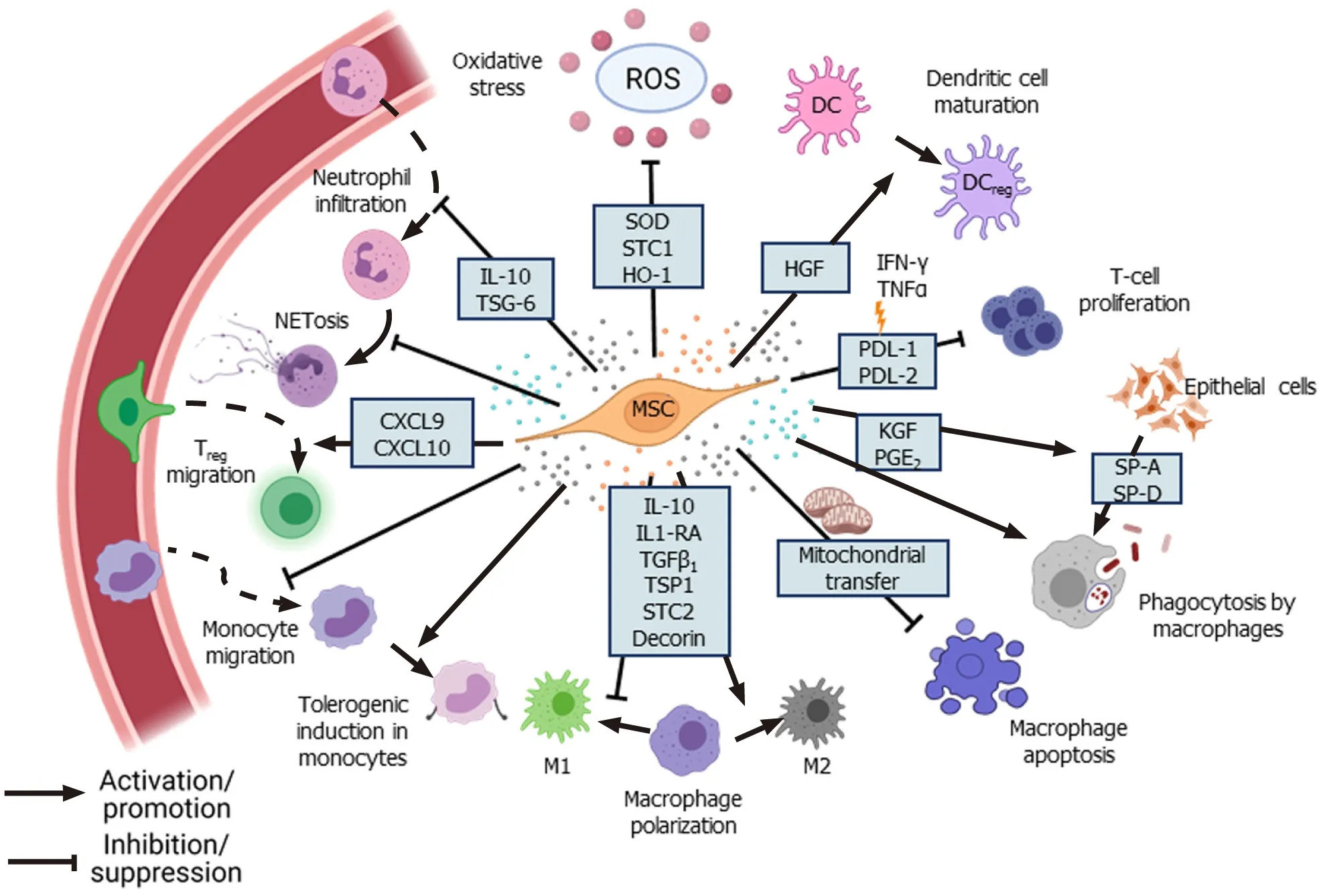
Figure 3 Tissue repair and regeneration.
The ability of MSCs to differentiate into different cell types contributes to the repair of damaged tissue during diseases.In LPS-induced ALI models, activation of canonical Wnt signaling promoted differentiation of mouse BM-MSCs into AT II cells and inhibited lung fibrosis[121].Liuet al[122,123] found significantly high levels of Wnt3a in the lung tissue of ALI mice andin vitroco-culture of mouse BM-MSCs with AT-II cells of ALI or normal mice in the presence of Wnt ligands induced the differentiation of MSCs into AT II cells expressing AQP5, SPB and SPC.Further, intratracheal transplantation of murine MSCs overexpressing receptor tyrosine kinase-like orphan receptor 2 (ROR2), a Wnt5a receptor, into ARDS mice led to differentiation of MSCs into AT II cells, suppressed LPS-induced inflammation, and significantly improved the alveolar epithelial permeability[114].However, under non-inflammatory conditions, inhibition of Wnt signaling promoted epithelial differentiation of murine lung resident MSCs (LR-MSCs)[124].Fanget al[125] found that resident Dermo1+LR-MSCs contributed to various lung epithelial cell types during LPS-induced lung injury.Further, intratracheal administration of fibroblast growth factor 10 (FGF-10) mobilized the LRMSCs, which were more effective in ameliorating the LPS-induced lung injury in rats compared to BM-MSCs[126] and also promoted the differentiation of MSCs into AT II cells[127].Silvaet al[128] showed that LR-MSCs were better than BM-MSCs in reducing neutrophil infiltration, but administration of BM-MSCs or AD-MSCs was more effective at reducing inflammatory cytokines and improving lung function than LR-MSCs.Liet al[115] reported that downregulation of Hippo signaling through silencing of large tumor suppressor kinase 1 in murine BM-MSCs significantly increased their differentiation into AT-II cells and decreased pulmonary edema and inflammation in ARDS lung tissue.
Few studies have also explored the therapeutic role of MSCs during prion infection and found that transplantation of human BM-MSCs intravenously or intrahippocampally improved the survival of prion-infected mice.The transplanted MSCs differentiated into neuronal and glial cells[13].Migration of human MSCs to the site of prion infection was found to be mediated by CCR3, CCR5, CXCR3, and CXCR4, and blocking these receptors in the MSCs inhibited their migration to the infected site[129].Furthermore, treatment with MSCs has been found to promote tissue regeneration and reduction of pathogen load in various parasitic infections such as malaria[14], Chagas disease[130,131], schistosomiasis[116,132,133], and leishmaniasis[134].Thus, MSCs repair pathogen-induced tissue damage by direct differentiation or through the secretion of various mitogens and regulatory factors.
MSCS-DERIVED PARACRINE FACTORS AND EVS
MSCs secrete bilayered lipid microvesicles (100-1000 nm) and exosomes (30-100 nm) that contain cytokines, microRNAs (miRNAs), chemokines, and AMPs[135-138].MiRNAs present in MSCs-derived exosomes play an important role in mediating therapeutic effects.Exosome-derived miR-27a-3p was found to inhibit NF-κB expression and induce M2 polarization in macrophages[139].miRNA-146a found in the exosomes of IL1-β primed MSCs-induced M2 polarization in macrophages by modulating IRAK1, TRAF6, and IRF5 signaling[140].Furthermore, microvesicles from IFN-γ-primed MSCs were more efficient than those of naïve MSCs in inducing M2 phenotype and phagocytosis in macrophages[141].EVs secreted by MSCs contain mRNA of KGF[142] and Ang1[68], which mediate anti-inflammatory effects on LPSinduced ALI mice models.In anE.coli-induced pneumonia mouse model, MSCsderived EVs upregulated the BALF levels of leukotriene B4(LTB4), a lipid mediator that acts as a chemoattractant for T cells, neutrophils, macrophages, and other immune cells, thereby facilitating pathogen elimination[143,144].miRNA-145 present in EVs of MSCs was found to suppress the expression of multidrug resistant protein 1, leading to increased LTB4production, which enhanced microbial clearance[143].miR-100 found in WJ-MSCs-derived EVs enhanced autophagy through mTOR downregulation and improved the survival of alveolar epithelial cells[110] (Figure 4).Treatment with MSCs-derived EVs upregulated KGF, PGE2,IL-10 levels and reduced lung inflammation and endothelial permeability in a pre-clinical model of ischemia reperfusioninduced lung injury[145].Wanget al[146] reported that HGF present in EVs and conditioned media (CM) of BM-MSCs reduced the permeability of endothelial barrier by modulating VE-cadherin and occludin expression.Khatriet al[147] reported that swine BM-MSCs and the EVs derived from them have similar surface marker expression, and treatment with EVs had similar anti-inflammatory effects as that of MSCs themselves in pig ALI.
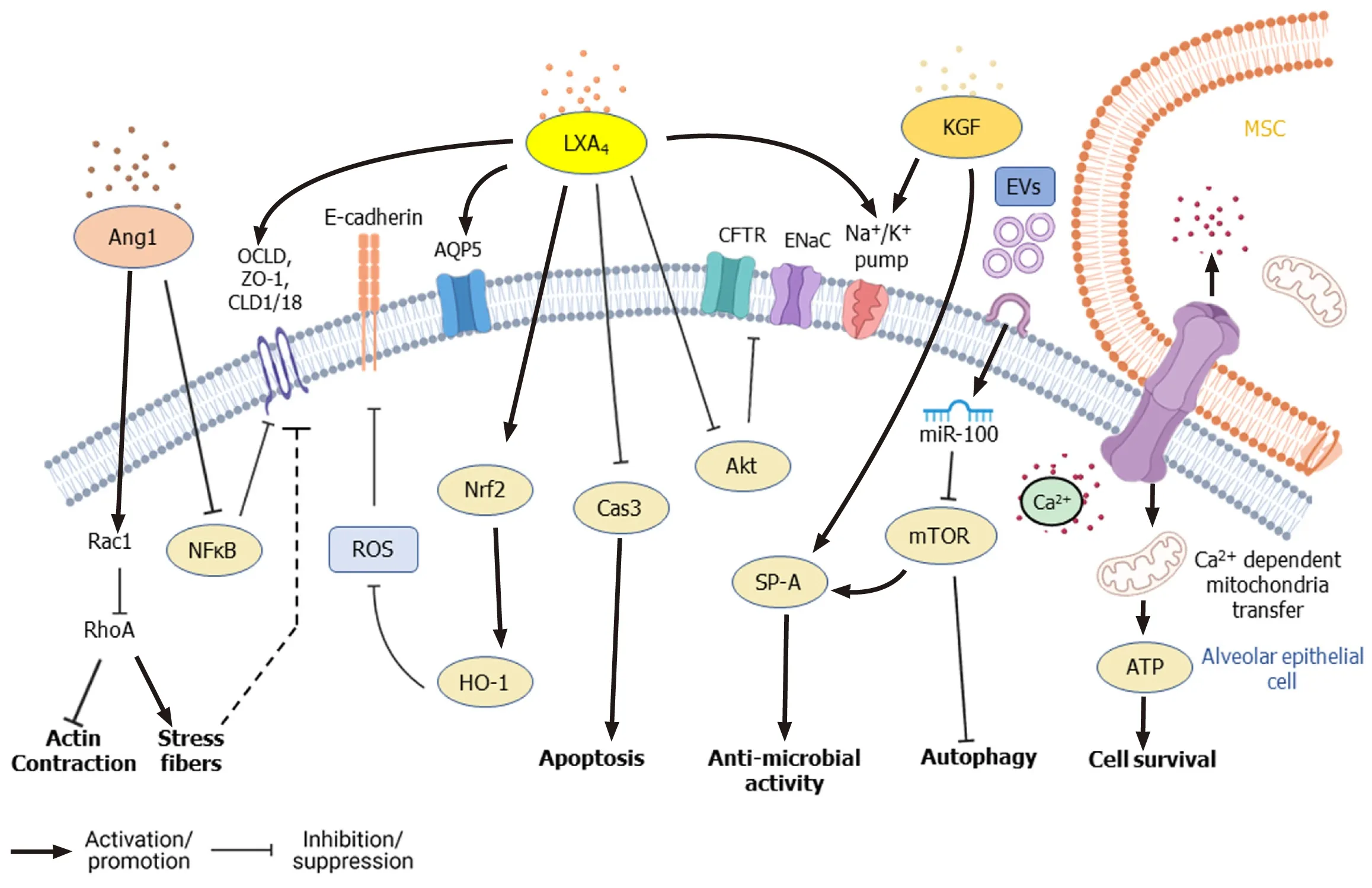
Figure 4 Signaling pathways modified by mesenchymal stem cells in alveolar lung epithelial cells during lung injury.
UC-MSCs-derived EVs inhibited viral replication of hepatitis C virus (HCV) in a pre-clinical disease model, and the anti-viral effect was found to be mediated by miRNAs let-7f, miR-145, miR-199a, and miR-221[148].Human BM-MSCs-derived exosomes were found to induce autophagy but inhibit D-GalN/LP-induced apoptosis of hepatocytes[149] as well as coxsackievirus B3-induced myocarditis[150].Treatment with MSCs-derived exosomes modulated AMPK/mTOR signaling in human cardiomyocytesin vitroand promoted their survival[150].
CM derived from MSCs cultured in xenofree conditions has been hypothesized as a reasonable approach to cell-free therapy.The CM was found to be rich in exosomes, EVs, and several paracrine factors[151].In an LPS-induced ALI mouse model, Suet al[152] reported that mice injected intravenously with the CM of MSCs showed reduced neutrophil infiltration and accumulation.MSCs-CM was also shown to induce apoptosis in neutrophils bothin vitroandin vivoby inhibiting NF-κB signaling.BALF of MSCs-CM-treated mice had reduced levels of anti-apoptotic proteins such as Bcl-xL and Mcl-1[152].Treatment with MSCs-derived CM reduced TNF-α, IL-6 levels and increased IL-10 secretion by macrophages stimulated with TLR ligands or liveS.pneumoniae[153].However, Hayeset al[154] showed that administration of MSCs was significantly more effective in improving ventilation-induced lung injury than treatment with MSCs-CM alone.In anex-vivoperfusion lung injury model of pneumonia, Parket al[155] found that EVs derived from human BM-MSCs treated with TLR3 ligand, poly (I:C) significantly reduced the bacterial load, inflammation, and protein permeability compared to EVs derived from naïve MSCs.CM of murine BM-MSCs was found to exhibit pathogen-related differences in their therapeutic effect, where treatment with MSCs-CM inhibited herpes virus replication but not dengue or enterovirus[148].
GENETIC MODIFICATIONS AND PRIMING OF MSCS
Several studies have reported that genetic modifications of MSCs improved their efficacy and therapeutic potential (Table 1).Martínez-Gonzálezet al[156] found that intravenous injection of MSCs overexpressing sST2, a soluble decoy receptor for IL-33, was highly effective at reducing inflammation and preserving the lung architecture compared to naïve MSCs in a murine ALI model.IL-33/IL-1 receptor-like (ST2) signaling 'alarms' and activates the immune cells upon damage of epithelial or endothelial cells[157].Similarly, administration of MSCs overexpressing Ang1 or HO-1 reduced vascular endothelial permeability and inflammatory cells in the lungs of LPSinduced ALI animal models[78,158,159].Intratracheal administration of TGF-β1 overexpressing MSCs increased Treg cells but decreased Th17 cells in the lungs of LPSinduced ARDS mice.MSCs expressing TGF-β1 induced occludin protein expression and improved vascular permeability[160].In anE.coli-induced pneumosepsis experimental model, injection of human UC-MSCs that overexpressed IL-10 were highly effective in reducing the percentage of alveolar neutrophils and macrophages and also increased the phagocytic function of macrophages compared to naïve MSCs, leading to significantly reduced bacterial counts[75].Murine BM-MSCs overexpressing either developmental endothelial locus-1 or FGF2 were found to attenuate lung injury and infiltration of immune cells and reduced TNF-α levels compared to control MSCs in an LPS-induced ALI mouse model[161,162].HGF overexpressing MSCs reduced apoptosis and cell permeability in LPS-treated pulmonary endothelial cells by upregulating occludinviathe mTOR/STAT3 signaling pathway[163].BM-MSCs overexpressing β-catenin, Wnt5a receptor, ROR2, or p130/E2F4 showed higher retention in the lungs and differentiation into AT II cells compared to control MSCs, leading to significant improvement in lung tissue structure in LPS-induced ARDS mouse models[114,121,164].Angiotensin-converting enzyme (ACE) and its homolog ACE2 are cell membrane-linked enzymes that have important catalytic functions in the renninangiotensin system.Although ACE, angiotensin II type 1a receptor, and angiotensin II are involved in the progression of ARDS by increasing edema and disturbing lung function, its homolog ACE2 and angiotensin II type 2 receptor play a protective role during sepsis-induced lung injury[165].Heet al[166] reported that treatment with MSCs expressing ACE2 led to a significant reduction in neutrophil counts and inflammatory cytokines, IL-6, and TNF-α levels in the lungs of LPS-induced ARDS mice compared to the control BM-MSCs-treated group.However, ACE2 was found to be the functional receptor for coronaviruses including severe acute respiratory syndrome (SARS)-associated coronavirus (SARS-CoV-1), and SARS-CoV-2 and has been implicated in the progression of SARS-induced ARDS[167,168].Thus, ACE2 overexpressing MSCs might not be a suitable option for the treatment of SARS-induced ARDS.
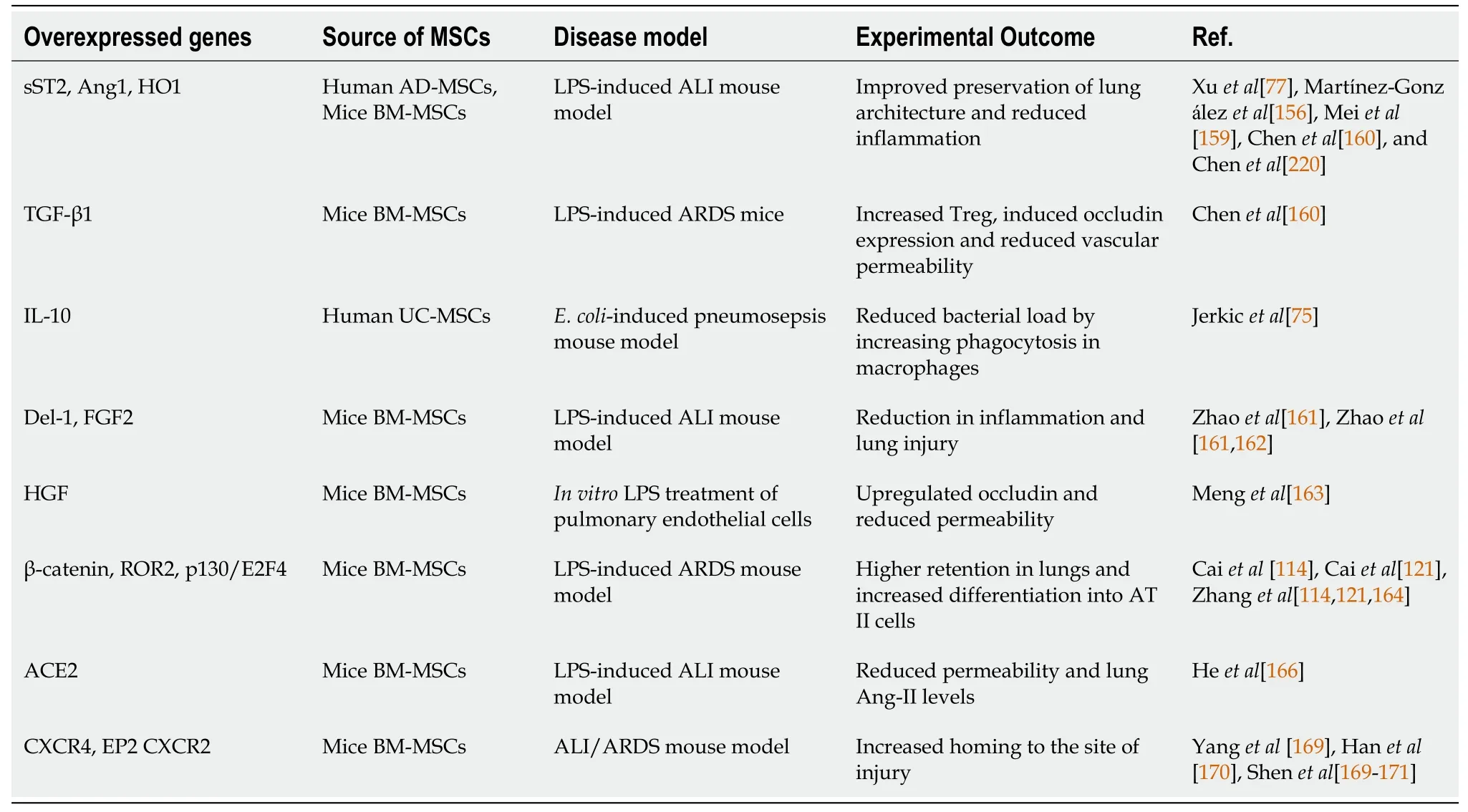
Table 1 Genetic modifications to enhance mesenchymal stem cells potential for treatment of infectious diseases and associated tissue injury
Migration of MSCs to the site of infection and injury is required for MSCs to exhibit their therapeutic effects.Overexpression of CXCR4, a receptor for stromal cell-derived factor-1α (SDF-1) in MSCs, improved their migration potential to the injured lungs, which in turn contributed significantly to controlling the tissue damage compared to control MSCs[169].Because PGE2levels increase significantly during lung injury, Hanet al[170] overexpressed the E-prostanoid receptor (EP-2), a receptor for PGE2,in MSCs to improve their homing to the injured lung.EP-2 expressing MSCs showed significantly high migration to the injured lung and repaired the damaged tissue in the ARDS model[171].In an oral mucositis rat model, Shenet al[172] found that overexpression of CXCR2 facilitated the migration of MSCs to the infected site.CXCR2 is a receptor for NAP2, secreted by NK cells at the injury site, and can act as a chemoattractant for MSCs expressing CXCR2[171].
MSCs modify their paracrine secretome depending on the environmental cues.Preconditioning MSCs with different environmental cues was found to alter their immunomodulation and differentiation abilities.MSCs pre-conditioned with the serum of ARDS mice or inflammatory cytokines had high expression of IL-10, IL-6, and IL1RA but significantly lower expression of inflammatory cytokines[140,173,174].Similarly, stimulation of MSCs with pro-inflammatory cytokines TNF-α and IL1-β induced the expression of HGF, FGF2, heparin-binding EGF-like growth factor that contributed to the healing of airway epithelial cellsin vitroby modulating ERK1/2 phosphorylationviaEGFR activation[66].Similarly, MSCs primed with IL1-β were more effective at reducing TNF-α and IL-6 levels and increasing IL-10 levels in serum of ARDS mice[140].During co-culture of human BM-MSCs and macrophages, TNF-α secreted by activated M1 macrophages induced MSCs into the immunosuppressive phenotype.This effect was amplified by IL-10 produced by M2 macrophages, which further increased PGE2secretion by MSCs[175].Treatment of MSCs with either IFN-γ or TNF-α increased PGE2expression, but IDO and PD-L1 levels increased only in IFNγ-treated MSCs[69], which suggests that the composition of the inflammatory milieu alters the function of MSCs and their anti-inflammatory potential.Pre-treatment of UC-MSCs with TGF-β1 prior to transplantation improved their long-term survival in the lungs[176].Long-chain fatty acids such as eicosapentaenoic acid treatment improved the therapeutic effects of MSCs, which led to a reduction in lung injury and increased secretion of inflammation resolving factors such as resolvin D1, IL-10, TGFβ, and PGE2in a CLP-induced sepsis model[177].Exposure to IFN-γ or TLR3 ligand poly (I:C) increased HLA-I expression in MSCs, which protected the cells from killing by NK cells[56,178].Furthermore, murine BM-MSCs pre-conditioned with TLR3 ligand poly (A:U) were more efficient than naïve MSCs in eliminatingM.bovisin an experimental model[85].TLR4 activation in LPS-stimulated MSCs expedited wound healing by promoting neutrophil migration and NETosis at the site of infection[79].Extracellular vesicles from IFN-γ-primed MSCs significantly increased the phagocytic ability of THP1 monocytic cellsin vitroand improved the lung histopathology and survival ofE.coli-induced ARDS mice[141].
In a pre-clinical model, Liuet al[117] reported that transplantation of MSCs expressing short hairpin RNA against hepatitis viral proteins (HBV S and HBV X) significantly reduced HBV antigens in the liver and serum.Masalovaet al[179] tested the efficacy of utilizing MSCs as an immunization agent against HCV and found that murine BM-MSCs expressing five non-structural HCV proteins induced significantly higher proliferation of lymphocytes, IFN-γ secretion, and IgG2a levels compared to naked DNA immunizations suggesting the feasibility of utilizing modified MSCs as vaccine agents.Hypoxic pre-conditioning also improves the migration, survival, and anti-inflammatory properties of MSCs[174,180,181].MSCs cultured under hypoxic conditions (1% O2) had high expression of SDF-1α receptors CXCR4 and CXCR7, which promoted their migration to the site of infection[180].Although both short- and long-term hypoxia increased metabolic activity of BM-MSCs compared to normoxic conditions, short-term hypoxia was superior to long-term hypoxia in augmenting the therapeutic characteristic of MSCs.Hypoxic treatment altered the secretome of porcine BM-MSCs and human BM-MSCs differently, indicating species-specific variations in MSCs characteristics[181].Pre-conditioning of MSCs from human bone marrow and adipose tissue with hypoxia (2% O2) significantly inhibited the differentiation potential but increased the metabolic activity of MSCs.Treatment with cytokine mix consisting of IL-1β, TNF-α, and IFN-γ increased the secretion of anti-inflammatory cytokines such as IL1RA and IL-10 as well as thrombogenic tissue factor in both AD-MSCs and BM-MSCs[174].Similarly, subpopulations of MSCs selected based on the expression of specific cell surface markers showed a higher therapeutic effect compared to the bulk population.Mastersonet al[7] found that intravenous administration of a homogenous population of syndecan2 (CD362)-positive BM-MSCs significantly improved the lung function and reduced inflammatory response during lung injury induced byE.colicompared to CD362-negative BM-MSCs.Similarly,PDGFR+Sca1+TER119-(PαS) BMMSCs with high CFU-F ability were reported to reduce bacterial load, ameliorate inflammation, and increase survival in mice model of ALI induced byKlebsiella pneumonia[6]; albeit, the results were not compared with the effects seen with bulk MSCs population.
The route of administration of MSCs can also modify the therapeutic outcome.Danchuket al[20] found that, whereas administration of human BM-MSCs through intravenous, oropharyngeal, or intraperitoneal routes reduced the pulmonary inflammation to a similar extent in an ALI mouse model, MSCs were not detected in the lung after intraperitoneal injection during their analysis period.Interestingly, intravenous administration of murine BM-MSCs was found to be beneficial in ameliorating ALI caused by intratracheal rather than intravenous injection of LPS.Extrapulmonary organ damage induced by intravenous LPS injection reduced the migration and retention of MSCs in the lungs and accounted for the difference in therapeutic effects observed between these two modes of injury[182].In a mouse model of prion infection, intravenous or intrahippocampal administration of human BM-MSCs enhanced the survival of infected mice; however, the survival rate was higher in the experimental group where MSCs were transplanted intrahippocampally[13].
CLINICAL TRIALS WITH MSCS
Liver injury
In a clinical trial involving 56 patients with hepatitis B infection, intra-hepatic administration of autologous BM-MSCs along with anti-viral drug Entecavir resulted in a significant reduction of inflammation and improvement in liver function[183].Similar therapeutic benefits were observed in HBV-induced decompensated liver cirrhosis patients when UC-MSCs were intravenously administrated along with standard therapy[184]; however, UC-MSCs administration did not alter the prognosis in HBV infection-related acute-on-chronic liver failure patients[185].Conversely, prolonged treatment with UC-MSCs for more than 4 wk was found to be effective at improving some but not all liver injury markers in HBV-related liver failure and liver cirrhosis patients[186].In a clinical trial involving hepatitis C patients with end-stage liver disease, intravenous administration of autologous BM-MSCs was found to be well tolerated and effective at reducing liver injury markers and fibrosis[187].
Lung injury
Respiratory tract infections claim more than 1.5 million lives annually, and with epidemic and pandemic outbreaks, the number of deaths and disabilities can be devastatingly high (SARS outbreak in 2002, H1N1 flu in 2009, Middle East respiratory syndrome coronavirus outbreak in 2012, and COVID-19 outbreak in 2020)[188].Based on the successful outcomes observed in pre-clinical models of bacterial pneumonia and respiratory infection, the potential benefits of MSCs administration were explored for treating infection-associated lung injury.Avian influenza viruses associated with high mortalities in poultry pose a risk to cross the interspecies barrier to give rise to influenza strains that can cause pandemics[189,190].In a clinical trial involving 17 patients infected with H7N9, treatment with allogeneic menstrual blood-derived MSCs significantly reduced the death rate (54.5% in controlvs17.6% in MSCs treated) and improved lung function without any adverse side effects over a follow-up period of 5 years[191].In a case study, intra-atrial injection of allogeneic BM-MSCs facilitated resolution of ARDS in a deteriorating critically ill 58-year-old patient with H1N1 infection[192].
COVID-19
MSCs are immune to infection by SARS-CoV-2 as they lack the expression of ACE2 and serine protease TMRSS2, which are essential for SARS-CoV-2 infection[193].In a clinical trial of a critically ill 54-year-old man with COVID-19 pneumonia, administration of allogeneic WJ-MSCs showed no side effects, improved lung function, and diminished the infection by the 6thd of transplantation[194].In another clinical trial involving seven COVID-19 patients with severe pneumonia, MSCs treatment was found to be safe and effective at reducing inflammation[193].MSCs transplantation was reported to act synergistically with convalescent plasma therapy and improve lung injury in another critically ill 66-year-old COVID-19 patient[195].Severely ill COVID-19 patients are at a risk of thromboembolism that can lead to multiorgan failure.The rationale for using MSCs in treating COVID-19 was discussed in several reports[196-198]; however, treatment of COVID-19 patients with MSCs requires further analysis considering certain aspects of COVID-19-related pathology.Since MSCs often have a high expression of procoagulant tissue factor CD142, intravenous administration of MSCs can be detrimental in patients at risk of systemic coagulation[199], and intratracheal or intramuscular administration can obviate this risk.In another clinical trial of 25 COVID-19 patients receiving MSCs transplantation once, twice, or thrice at intervals of 5 d, three patients developed complications such as liver failure, heart failure, and allergic reactions[200].
CHALLENGES IN UTILIZING MSCS
Although MSCs have potent anti-inflammatory and multipotent differentiation properties, some studies have reported that MSCs can act as “safe harbors” for some bacterial and viral pathogens and help them evade the immune response and therapeutic drugs.Anin vitrostudy by Naiket al[201] reported that BM-MCs could be infected by both virulent (M.tuberculosis) and avirulent (M.bovis, M.smegmatis) mycobacteria.However, MSCs effectively eliminated the intracellular avirulent species but not the virulent mycobacteria.M.boviselimination was mediated by activation of TLR2/4 pathway.In contrast, intracellular survival ofM.tuberculosiswas facilitated by bacteria-induced downregulation of CRAMP, an AMP expressed in BM-MSCs[201].IntracellularM.tuberculosisin MSCs were drug-resistant, attributed to the expression of drug-efflux pumps ABCC1 and ABCG2, and immune protected, due to PGE2secretion by MSCs[202].Lopeset al[203] found that CD271+Sca1+BM-MSCs served as a niche forLeishmania infantum in vivo, which protected the parasite from anti-parasite drugs, possibly through active drug pump ABCG2 expressed by MSCs.
Qiaoet al[204]and Solandet al[205] reported that human MSCs were fully permissive to human cytomegalovirus infection, and the highest infection rate was observed in lung perivascular MSCs, suggesting that MSCs in different organs might act as a viral reservoir in humans.Further, Meiselet al[206] found that MSCs infected with CMV lose their immunosuppressive and antimicrobial properties, and Sundinet al[207] found that parvovirus B19 persisted in BM-MSCs even after several years of infection.Human placenta-derived MSCs were found to be permissible to infection with HSV such as HSV1 and HSV2, and fetal membrane-derived MSCs are susceptible to infection with Varicella Zoster Virus[208].Human BM-MSCs were found to be susceptible to HBV infection[209], and Wanget al[210] reported that while BM-MSCs from patients with chronic HBV infection had defective differentiation potential, ADMSCs were not permissible to HBV infection and differentiated effectively into functional hepatocyte-like cells.Therefore, AD-MSCs might be a better therapeutic option than BM-MSCs in patients with HBV infection.Similarly, avian influenza virus H5N1 was also reported to infect and induce cell death in human BM-MSCs and cord blood-derived MSCs[211].MSCs are also susceptible to HIV infection since they express receptors and co-receptors for HIV-1.Cotteret al[212] reported that HIV-1 infection alters the differentiation potential of MSCs, and MSCs exposed to sera from patients with high viral load showed proadipogenic phenotype.BM-MSCs from HIV transgenic mice showed a reduction in proliferation and therapeutic effects on an acute kidney injury model compared to normal BM-MSCs[213].Cervenakovaet al[214] reported that BM-MSCs from mice infected with prions were able to propagate TSE agents or prions when transplanted into healthy animals.Thus, due considerations on the susceptibility of MSCs to various infectious agents should be given while utilizing MSCs for therapy.
Although several studies have reported that MSCs are non-immunogenic due to lack of MHC II and the co-stimulatory molecules CD40, CD80, or CD86 and that the allogenic MSCs are well tolerated[191,192], some studies have found that allogenic MSCs elicit an immune response in the recipients leading to transplantation failure[215-217].Furthermore, MSCs from different tissue sources have varied differentiation ability and secrete a unique set of immunomodulatory factors which might influence the clinical outcome, and these source-specific differences are reviewed in detail elsewhere[198,218].Further studies are required to understand the immune response elicited by allogeneic MSCs transplantation as well as the diverse effects of utilizing MSCs isolated from different tissue sources.An important point to consider is that several pre-clinical studies were performed in animal models with non-human MSCs or human MSCs from various tissue sources.The non-human inflammatory milieu might not exactly resemble the disease conditions seen in humans, and thus additional precautions should be taken while interpreting the potential benefits of utilizing MSCs for the treatment of infectious diseases.
CONCLUSION
Exaggerated immune response and inflammation during infections cause tissue damage, which is one of the major reasons for infectious disease-induced mortality.However, treatment with MSCs was reported to provide therapeutic benefits by reducing inflammation, pathogen load, and tissue damage in several disease models.By expediting pathogen clearance through secretion of AMPs and direct phagocytosis and by reducing inflammation through secretion of several anti-inflammatory cytokines, MSCs combat tissue damage at the site of infection.MSCs play an important role in tissue regeneration by secreting various mitogens as well as differentiating into cells of the target tissue.During ARDS, secretion of LXA4, Ang1, HGF, and VEGF by MSCs upregulate the expression of ion channel and tight junction proteins and thus restore AFC and reduce endothelial permeability.MSCs-derived EVs contain several therapeutically beneficial cytokines, miRNAs, and treatment with MSCs-EVs showed promising results in clinical trials involving patients with liver injuries and severe COVID-19 pneumonia.However, caution should be exercised in utilizing MSCs for treatment as they can harbor harmful pathogens and might cause unfavorable outcomes in patients with pre-existing conditions.
杂志排行
World Journal of Stem Cells的其它文章
- Genome engineering and disease modeling via programmable nucleases for insulin gene therapy; promises of CRISPR/Cas9 technology
- Immunotherapy in the treatment of lymphoma
- Recent trends in stem cell-based therapies and applications of artificial intelligence in regenerative medicine
- Epigenetic regulation of autophagy: A key modification in cancer cells and cancer stem cells
- Growing and aging of hematopoietic stem cells
- Therapeutic potential of periodontal ligament stem cells
