Immunotherapy in the treatment of lymphoma
2021-07-24LazarPopovicGoranaMatovinaBrkoMajaPopovicMilicaPopovicAnaCvetanovicIvanNikolicBiljanaKukicDraganaPetrovic
Lazar S Popovic, Gorana Matovina-Brko, Maja Popovic, Milica Popovic, Ana Cvetanovic, Ivan Nikolic, Biljana Kukic, Dragana Petrovic
Lazar S Popovic, Maja Popovic, Ivan Nikolic, Biljana Kukic, Department for Medical Oncology, Oncology Institute of Vojvodina, University of Novi Sad, Novi Sad 21000, Serbia
Gorana Matovina-Brko, Dragana Petrovic, Department for Medical Oncology, Oncology Institute of Vojvodina, Novi Sad 21000, Serbia
Milica Popovic, Department for Nephrology and Clinical Immunology, Clinical Center of Vojvodina, University of Novi Sad, Novi Sad 21000, Serbia
Ana Cvetanovic, Department for Medical Oncology, Clinical Center of Nis, University of Nis, Nis 18000, Serbia
Abstract Relapsed or refractory non-Hodgkin’s lymphomas, especially diffuse large B-cell lymphoma as well as relapsed or refractory Hodgkin lymphomas are hard-to-treat diseases.Patients who do not respond to initial therapy or experience relapse are treated with salvage regimens, and if eligible for aggressive therapy, treatment is continued with high-dose chemotherapy and autologous stem cell transplantation.Current therapy options can cure substantial numbers of patients, however for some it is still an uncurable disease.Numerous new drugs and cell therapies are being investigated for the treatment of relapsed or refractory lymphomas.Different types of immunotherapy options have shown promising results, and some have already become the standard of care.Here, we review immunotherapy options for the treatment of lymphoma and discuss the results, positions, practical aspects, and future directions of different drugs and cellular therapies for the treatment of this disease.
Key Words: Immunotherapy; Receptors; Chimeric antigen; Antibodies; Monoclonal; Immunoconjugates; Hodgkin disease; Lymphoma; Large B-cell; Diffuse
INTRODUCTION
Diffuse large B-cell lymphoma (DLBCL) is the most common type of non-Hodgkin's lymphoma and accounts for approximately 30%-58% of cases.The combination of rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisolone (R-CHOP) cures approximately 65% of patients[1].Patients who do not respond to R-CHOP therapy or experience relapse are treated with one of the following salvage protocols: rituximab, cisplatin, cytarabine, and dexamethasone (R-DHAP) or rituximab, ifosfamide, carboplatin, and etoposide (R-ICE).If the patient responds and is eligible for aggressive therapy, treatment is continued with high-dose chemotherapy (HDCT) and autologous stem cell transplantation (ASCT)[2,3].Approximately 20% of patients achieve survival with this procedure.Patients who are not eligible for HDCT usually receive second-line therapy with rituximab, gemcitabine and oxaliplatin (R-GEMOX) or rituximab and bendamustine (R-Benda), and a small percentage of these patients survive long-term[4,5].Allogeneic stem cell transplantation (AlloSCT) is usually reserved for patients who have experienced two or more relapses of the disease.Approximately 20%-30% of patients achieve long-term remission but at the cost of high toxicity and treatment-related mortality[6].
The standard chemotherapy regimens doxorubicin, bleomycin, vinblastine and dacarbazine (ABVD) or escalated bleomycin, etoposide, doxorubicin, cyclophosphamide, vincristine, procarbazine, and prednisolone (escBEACOPP) cure more than 80% of patients with classical Hodgkin’s lymphoma (cHL).Salvage chemotherapy followed by HDCT/ASCT in relapsed or refractory patients can cure another 50%.Brentuximab-vedotin and checkpoint inhibitors have already become the standard of care ahead of allogeneic transplantation in patients with R/R cHL because of their high efficacy and significantly lower toxicity than AlloSCT[7-12].
Numerous new drugs and cell therapies are being investigated for the treatment of R/R lymphomas.Different types of immunotherapy options have shown promising results, and some have already become the standard of care.Here, we review immunotherapy options for the treatment of lymphoma and the practical aspects of those therapies.
IMMUNE MECHANISMS OF AVOIDING T-CELL RESPONSES IN LYMPHOMA
The human immune system has two basic tasks.The first is to recognize and neutralize foreign cells, both microorganisms such as bacteria and viruses, and malignant cells.The second is to prevent excessive activation against and destruction of the body’s own cells to avoid the development of autoimmune phenomena and diseases[13].Cellular infiltrates of lymphoma consist of malignant cells and various host immune system cells that significantly affect the onset and progression of malignant disease as well as the immune response against lymphoma[14,15].For example, in cHL, only 1% of tumor infiltrates are malignant Reed-Sternberg cells[16].The T-cell response is triggered by two consecutive signals.The first signal is T-cell activationviathe T-cell receptor (TCR).This process occurs by the TCR binding to lymphoma cells and to the major histocompatibility complex (MHC) receptor, which is most commonly expressed on antigen-presenting cells (APCs).Another important signal is the binding of B7-1 and B7-2 receptors to APCs and the CD28 receptor on T cells.When those two steps are accomplished, effector T cells are activated, differentiated, and expanded[13,17].However, there are several mechanisms by which lymphoma cells avoid effector T-cell recognition and destruction.
One way to avoid the response is to lose the expression of MHC class I and II molecules.Loss of class I MHC molecules occurs in approximately 75% of patients with aggressive lymphomas, while loss of class II MHC molecules is noted in approximately 30%[18].Decreased class I MHC expression is associated with shorter progression-free survival (PFS) in patients with cHL[19].Additionally, loss of class II MHC expression in immune-privileged sites in primary mediastinal lymphoma (PMLCL) and DLBCL leads to a lower objective response rate (ORR) and lower PFS[20,21].
Another barrier to the immune response is the expression of immunosuppressive proteins, which are so-called checkpoints, on both tumor cells and other immune cells within the tumor infiltrate.The physiological role of these receptors is to inhibit the autoimmune effects of T cells.The roles of programmed cell death receptor-1 (PD-1) and its ligands programmed cell death ligand 1 and 2 (PD-L1 and PD-L2)[13] have been most extensively studied.Effector T cells in the lymphoma infiltrate frequently express PD-1.The binding of PD-1 to PD-L1/2 receptors on both tumor cells and other cells of the immune system leads to T-cell exhaustion and apoptosis[13,22].In addition to receptors of the PD-1 family, other less investigated checkpoints, such as TIGIT (Tcell immunoreceptor with Ig and ITIM domains), LAG-3 (lymphocyte-activation gene 3), and TIM-3 (T-cell immunoglobulin-3)[23], can modify the immune response and increase the complexity of the inhibition signaling network.
The third way the T-cell response is inhibited in lymphoma is the secretion of inhibitory cytokines by both malignant and immune cells[24-26].Interleukin-10 (IL-10) levels are often elevated in patients with lymphoma and can potentially result in the expansion of myeloid-derived suppressive cells (MDSCs) that negatively affect the immune response[24].IL-12, which initially stimulates the T-cell response but remains present, inhibits the T-cell response and reduces T-cell function[25].Transforming growth factor β (TGFβ) activates a dysfunctional population of effector T cells, which also depletes the immune response[26].
Finally, the fourth mode of inhibition, which is closely related to cytokine stimulation and the immune signaling network, is the increased presence of suppressive and regulatory cells in the tumor infiltrate.MDSCs significantly inhibit Tcell proliferation and activation.Thus, the presence of a large number of these cells results in a reduced immune response against malignant cells[27].In addition, macrophages and monocytes often express PD-L1 and PD-L2, and thus suppress the immune response by binding to the PD-1 receptor of T cells[28].In addition to effector T cells, Fox3+ and CD25+ regulatory T cells are often found in the tumor infiltrate.Regulatory T cells play a role in controlling the immune response and are often responsible for the inadequate or suppressed immune response against malignant cells[29].Lymphoma B-cells alone can induce Fox3 expression in CD4+ lymphocytes, and can thus promote the differentiation of suppressive T-cell clones[30].
STRATEGIES FOR OVERCOMING INHIBITION OF THE IMMUNE RESPONSE IN LYMPHOMA
There are several strategies for overcoming the mechanisms of immune response inhibition.The best known and most commonly used method, first used to treat various solid tumors and then in lymphomas, is the inhibition of PD-1 and PD-L1 binding with immune checkpoint inhibitor drugs.Blocking this checkpoint reduces the exhaustion of effector T cells and restores the ability of T cells to recognize and exterminate malignant cells[13].
Another strategy is the destruction of a malignant clone with an approach that is independent of APC presentation and does not rely on the MHC complex.Chimeric antigen receptor (CAR) T cells are produced by thein vitroengineering of a patient's T cells to render them capable of recognizing a specific antigen, such as malignant CD19-positive clones.CAR T cells act independently of MHC complexes and thus overcome resistance dependent on T-cell signaling pathways[31].
The third method is the direct engagement of CD3/CD16 effector immune cells with bispecific antibodies.One end of these antibodies binds to the CD19 receptor on Blymphoma cells, and the other end binds to the CD3 receptor on T cells or the CD16 receptor on natural killer cells, thus causing a direct stimulation of the immune system and destruction of the malignant clone[32,33].Direct inhibition of MDSCs is being investigated for the treatment of solid tumors and might also be a way to enhance the immune response in lymphomas[34].
MONOCLONAL ANTIBODIES
The anti-CD20 antibody rituximab was the first therapeutic antibody approved in oncology in the late 20thcentury.This drug changed the landscape and prognosis of almost all B-cell lymphoproliferative diseases and became the gold standard of treatment[35-38].Attempts to improve the efficacy of CD20 antibodies have been intensively investigated with two new genetically engineered drugs, atumumab and obinutuzumab (Table 1).Ofatumumab is a humanized type I monoclonal antibody tested in a variety of B-cell lymphomas but to date has only been approved for the treatment of R/R chronic lymphocytic leukemia[39].The type II antibody obinutuzumab has had greater success.The key difference between rituximab and obinutuzumab is the higher affinity of obinutuzumab for FcγRIII receptors on effector immune cells and the decreased FcγRIIb-mediated internalization of the CD20 receptor into lipid rafts, thus increasing antibody-dependent cellular cytotoxicity (ADCC)[40,41].The CLL11 study demonstrated longer PFS when obinutuzumab was combined with chlorambucil (O-Clb)vsrituximab-chlorambucil (R-Clb).At that time, no difference in overall survival (OS) was shown[42].n an updated analysis with a longer follow-up, O-Clb extended OS relative to R-Clb with no new safety issues[43].The GALLIUM study compared obinutuzumab with rituximab in combination with chemotherapy in untreated patients with follicular lymphoma (FL).Obinutuzumab achieved a superior PFS without an OS benefit compared with rituximab[44].In patients refractory to rituximab, obinutuzumab also showed benefit in the GADOLIN study.The combination of O-bendamustin prolonged PFS over bendamustin monotherapy[45].Unlike chronic lymphocytic leukemia (CLL) and FL, obinutuzumab showed no benefit compared with rituximab in combination with CHOP chemotherapy in patients with DLBCL[46].
CD19 is a receptor present in B-cell lymphoma, and is an interesting target for drug development.Tafasitamab is a humanized antibody against the CD19 receptor.Similar to obinutuzumab, tafasitamab has a strong affinity for binding FcγRIII receptors to effector cells of the immune system, and is thus a potent ADCC inducer.In a phase IIa study, single-agent tafasitamab had an ORR of 26% in pretreated patients with DLBCL[47].In a phase II study of L-MIND in R/R DLBCL, tafasitamab in combination with lenalidomide had an ORR of 54%, with 32% of patients achieving a complete response (CR)[48].A phase II/III B-MIND study comparing the combination of tafasitamabbenda with R-Benda in R/R DLBCL and a phase III study comparing R-CHOP with tafasitamab and lenalidomide as first-line treatment of B-cell non-Hodgkin lymphoma(B-NHL)[49,50] are underway.
Magrolimab is a humanized monoclonal antibody that binds to the CD47 receptor that is often expressed in B-cell lymphomas.Unlike CD19 and CD20 antibodies, the magrolimab mechanism of action is not related to the direct killing of malignant cells, ADCC, or cell-dependent cytotoxicity.The SIRPα receptor of macrophages binds to CD47, leading to macrophage invasion and a reduction in the immune response.Magrolimab, by binding to CD47, prevents the binding of SIRPα macrophages to this receptor and thus increases the recognition and destruction of lymphoma cells[51].In phase Ib/II recruiting a heavily pretreated population of DLBCL and FL patients, magrolimab showed an ORR of 50% and a CR of 36% without significant toxicity[52].Mogamulizumab is an antibody against C chemokine receptor 4 (CCR4).In a phase III study in cutaneous T-cell lymphomas (CTCLs), mogamulizumab demonstrated a longer PFS than vorinostat[53].
ANTIBODY-DRUG CONJUGATES
Attempts to increase the effectiveness of antibodies led to the construction of molecules with radioactive elements, such as yttrium-90 (ibrotumab-tiuxetan)[54] and iodine-131 (tositumomab)[55], and more recently, cytotoxic agents (Table 2).
Brentuximab vedotin (BV) is a CD30-targeting antibody-drug conjugate (ADC) that includes the cytotoxic agent monomethylauristatin B on its Fc fragment[56].BV was first approved for the treatment of cHL and systemic anaplastic large-cell lymphoma (sALCL).Tumor reduction was observed in 86% of 46 patients in a phase I study[57].Another cohort of 102 patients with R/R cHL was treated with BV.Those patients had previously received at least two lines of therapy or HDCT/ASCT.The ORR was 75% with a CR of 34%.The median PFS and the duration of response in those who achieved CR were 5.6 and 20.5 mo, respectively[10,58].The AETHERA study evaluated BV maintenance after HDCT/ASCT in patients with a high risk of relapse after ASCT, defined as relapse within 12 mo of initial therapy, primary refractory disease, and/or extranodal presentation.A total of 329 patients were randomized to BV or a placebo.The median PFS was 49.2 mo with BVvs24.1 mo with the placebo (HR 0.57,P= 0.0013)[59].After 5 years of follow-up, the analysis was updated to reveal that relapsefree survival was 59% in patients who received BV and 41% in those given the placebo.The OS difference has not yet been determined, but the need for further treatment in BV-treated patients was reduced[60].Another study compared six cycles of BV in combination with AVD with six cycles of ABVD therapy alone in patients with stage III and IV untreated cHL.After 2 years of follow-up, 82.1% of patients in the BV arm and 77.2% in the control arm were progression free (HR 0.77,P= 0.04)[61].In recent years, BV has also played an important role in the treatment of CD30-positive T-cell lymphomas.The ALCANZA study compared BV with the investigators’ choice of methotrexate or bexarotene in R/R CTCLs.The primary endpoint was the 4-mo objective response (ORR4).The ORR4 was 56.3% in the BV 12.5% in the control arm (P< 0.0001)[62].BV has shown remarkable results for the treatment of R/R ALCL[63].However, probably the greatest progress made using BV has been in the initial treatment of CD30-positive peripheral T-cell lymphomas.The randomized, doubleblind, phase III ECHELON-2 study randomized a total of 601 patients to BV + CHP (ACHP) or CHOP therapy.The median PFS in the A-CHP arm was 48.2 mo compared with 20.8 mo in the CHOP arm (HR 0.71P= 0.011).BV also prolonged OS in comparison to the control (HR 0.66,P= 0.0244)[64].The most common toxicity associated with BV is peripheral sensory neuropathy, which is reversible in most cases[56-64].
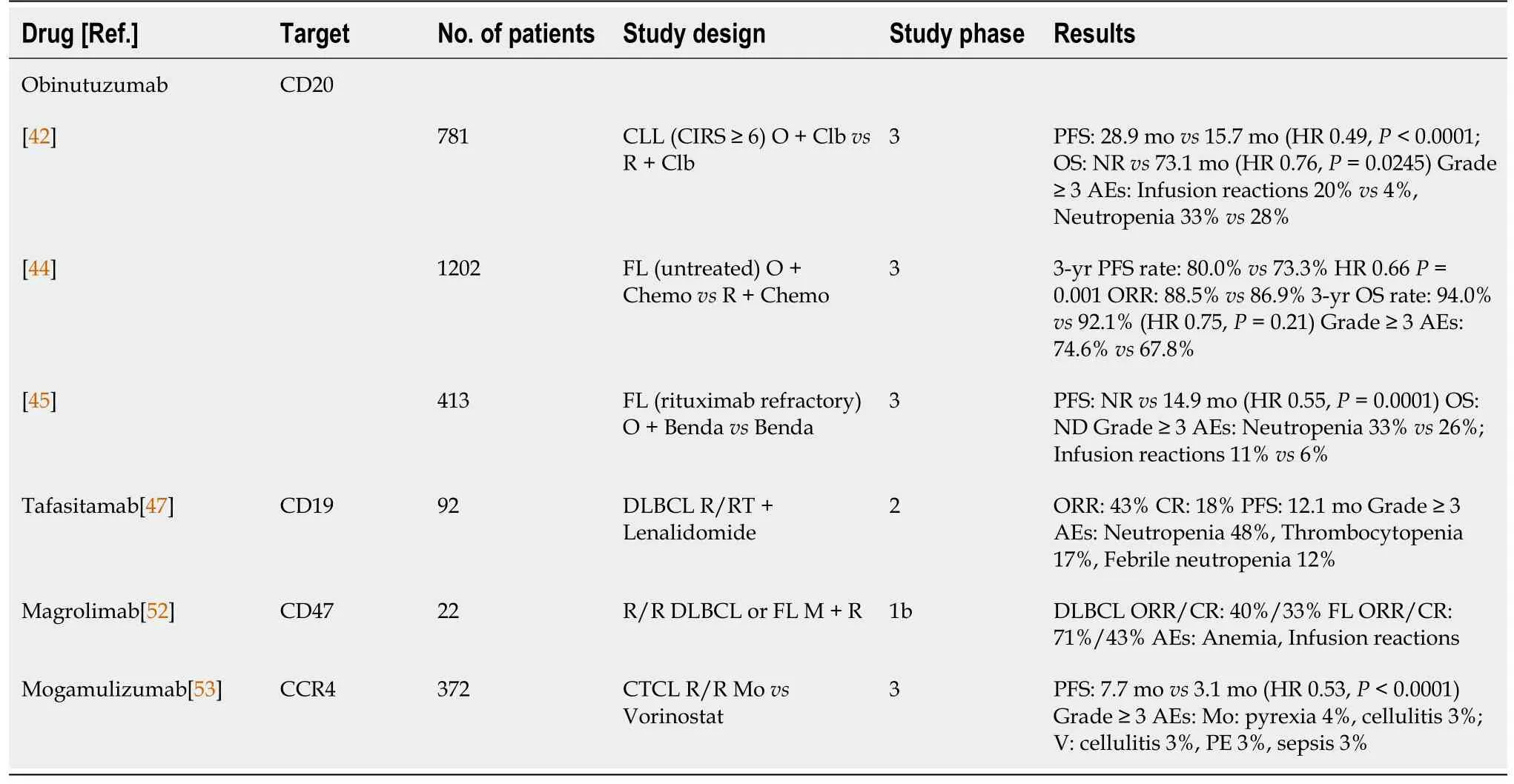
Table 1 Monoclonal antibody studies
Polatuzumab vedotin (PV) is an ADC that targets the CD79b receptor expressed on B-cell lymphomas.PV was approved by the Food and Drug Administration (FDA) based on the results of a phase II study in R/R DLBCL.In that study, the addition of PV was compared with the R-Benda combination.Compared with patients receiving R-Benda, patients receiving PV had a longer median PFS (9.5 movs3.7 mo; HR 0.36,P= 0.001), a longer OS (12.4 movs4.7 mo; HR 0.42,P= 0.002), and a higher CR rate (40%vs17.5%;P= 0.026)[65].Two important clinical trials are underway to further investigate the efficacy of PV for lymphoma, including PV in R/R FL and DLBCL in combination with lenalidomide, and PV as the first-line treatment of the DLBCL with an International Prognostic Index (IPI) score of 2-5 in combination with R-CHOP therapy in the phase III POLARIX study[66,67].Moxetumomab pseudotox was approved for the treatment of R/R hairy cell leukemia (HCL) based on a phase II study in which 30% of patients with HCL who had previously received at least two lines of treatment achieved CR[68].
BISPECIFIC ANTIBODIES/BISPECIFIC T-CELL ENGAGERS
Bispecific antibodies/bispecific T-cell engagers (BITEs) bind two antigens/receptors.One is found on a malignant cell and the other is most often CD3, which is a T-cell surface antigen.In that way, the molecules mediate the direct destruction of lymphoma cells by T cells (Table 3).Blinatumomab is a CD19/CD3 antibody approved for the treatment of R/R B-cell acute lymphoblastic leukemia (B-ALL)[69].Blinatumomab was tested as a therapy for R/R DLBCL in a phase I trial[70].A high rate of neurotoxicity was noted, and the drug did not enter the later clinical trial stages.Long-term follow-up data from those patients have been recently published.The median OS was 7.7 years in patients receiving a dose ≥ 60 μg/m2, indicating that this approach might be an important consideration for future research[71].
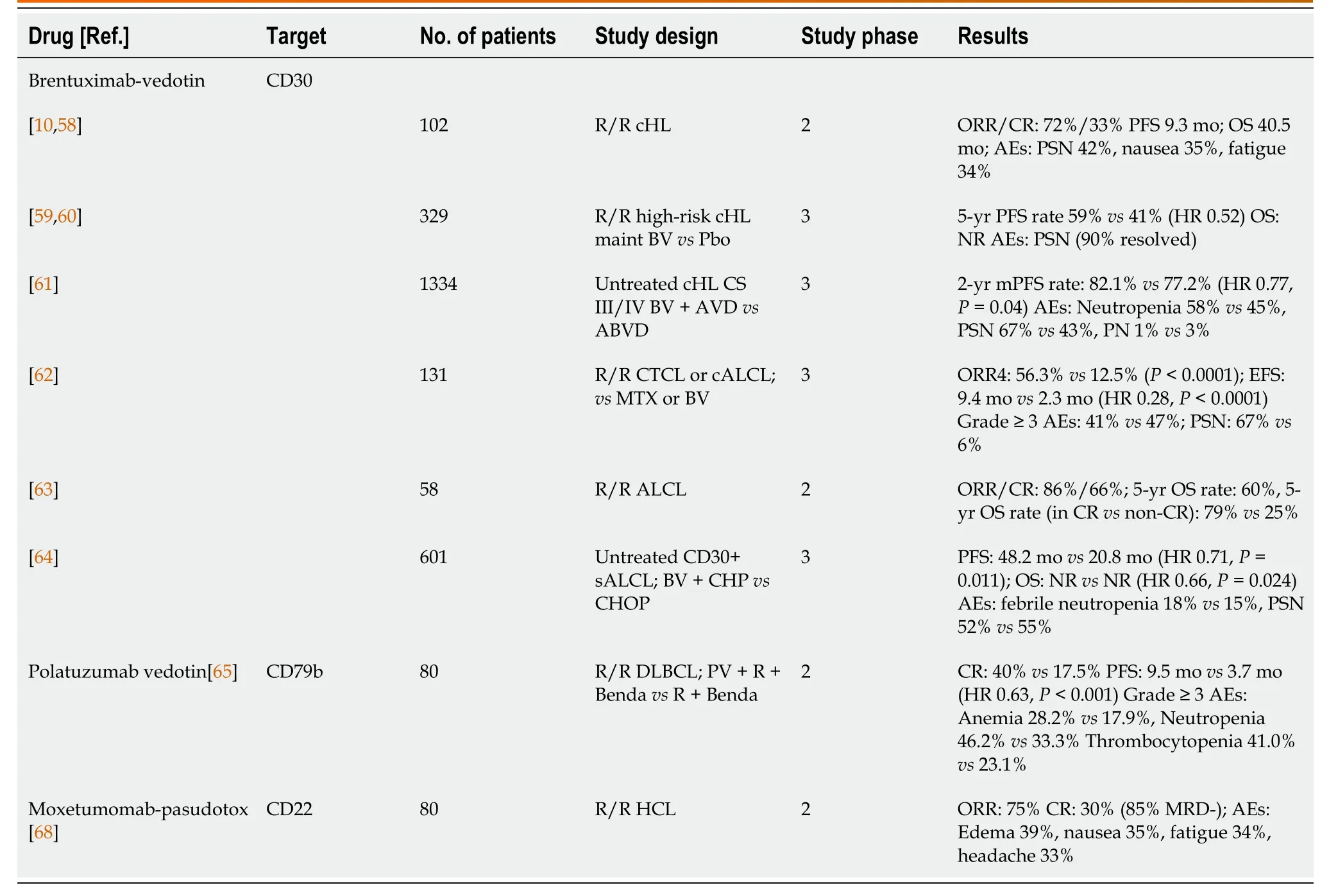
Table 2 Antibody-drug conjugate studies
Mosunetuzumab is a BITE targeting CD20/CD3.It has a longer serum half-life than blinatumomab because of its pharmacological properties and does not have to be administered as a multiday continuous infusion.In a population of heavily pretreated patients with R/R FL and R/R DLBCL, including those who relapsed after CAR T-cell therapy, mosunetuzumab achieved ORRs of 64.1% and 42.2%, respectively, in indolent and aggressive lymphomas.The CR rate was 18.6% for DLBCL and 34.7% for FL.The most limiting blinatumomab and CAR T-cell toxicities were neurotoxicity in 44% and cytokine releasing syndrome (CRS) in 28.4% of patients with mosunetuzumab, mostly grade 1 or 2[72].REG1979 and CD20-TBC are two CD20/CD3 BITEs that have been tested in phase I studies[73-75].CD20-TBC achieved a 47% ORR and 34% CR in heavily pretreated NHL patients.The incidence of CRS was 55% and was mostly grade 1 or 2[75].
CHECKPOINT INHIBITORS
Checkpoint inhibitors (CPIs, Table 4) have revolutionized the treatment of solid tumors and have become the absolute standard of care for melanoma, lung cancer, urological tumors, and triple-negative breast cancer[76-83].The rationale for CPI clinical trials for cHLis is high PD-L1/PD-L2 expression as a result of copy-number amplification of the PD-L1 and JAK gene loci on chromosome 9p24[16].Nivolumab and pembrolizumab have shown good results in heavily pretreated patients with R/R cHL.Nivolumab achieved an ORR of 69%, a CR of 40%, and a median DoR of 16.6 mo in patients who relapsed after HDCT/ASCT[84].Similar to nivolumab, pembrolizumab achieved an ORR of 69%, 22% CR, a 6-mo PFS of 72.4%, and an OS of 99.5%[85].The KEYNOTE-204 study compared pembrolizumab with BV in patients who had previously relapsed after HDCT/ASCT or who were not suitable for ASCT.That phase III study included a total of 304 patients.The primary endpoint, median PFS, was longer in the pembrolizumab arm (13.2 movs8.3 mo; HR 0.65,P= 0.00271).Pembrolizumab had demonstrated benefits in all subgroups of patients with primary refractory disease, including those who previously had ASCT or had previously been treated with BV[86].Nivolumab is being studied in previously untreated patients with stage III and IV cHL.The study compares nivolumab and BV in combination with doxorubicin, vinblastine and dacarbazine (AVD) chemotherapy[87].
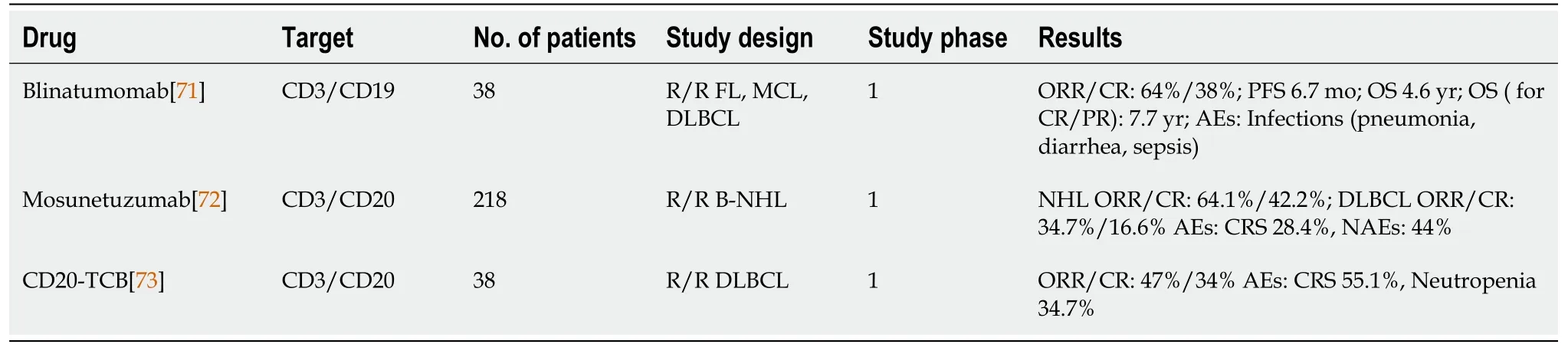
Table 3 Bispecific antibody studies
Nivolumab has also been studied in combination with BV and/or ipilimumab in patients with R/R cHL.The Nivo + BV combination was tested as salvage treatment in a phase I/II trial, after which patients could proceed to ASCT.Therapy was given for up to four cycles.The CR rate was 61% and the ORR 82%.Fewer than 10% of the patients received corticosteroids because of adverse events, and stem cell collection was not compromised[88].The combination of Nivo + BV, ipilimumab + BV, and triple therapy with Nivo + Ipi + BV was investigated in phase I of the study.The ORRs were 89%, 76%, and 82%, respectively, but grade 3 and higher adverse events were observed in 16%, 43%, and 50% of patients, respectively[89].
In NHL, unlike in cHL, CPIs have not shown a high level of response.Only in PMBCL, a subtype of DLBCL, does response to CPI occur, because PMBCL shares some features with cHL and expresses PD-L1[91].Pembrolizumab achieved an ORR of 45%, with a median DoR not reached after 1 year of follow-up in the KEYNOTE-170 study.The CR rate was 13%[91].Pembrolizumab also achieved a response rate of 38% in patients with R/R CTCL[92].
CHIMERIC ANTIGEN RECEPTOR T-CELL THERAPY
Chimeric antigen receptor T cells are therapies involving thein vitrogenetic engineering of patient T cells capable of recognizing and killing CD19-positive Blymphoma cells.CAR T cells consist of an extracellular variable fragment targeted to tumors, a hinge antigen-transmembrane region, and an intracellular domain.The development of CAR T cells began with a first-generation receptor that consisted of the CD3ζ signaling domain and had limited persistence and efficacy.The addition of a costimulatory domain to second-generation CAR T-cell receptors led to greater efficacy.The most important feature of CAR T-cell action is that it does not depend on MHC costimulation and acts by activating T-cell signals and costimulatory pathways (Table 5)[31,93,94].
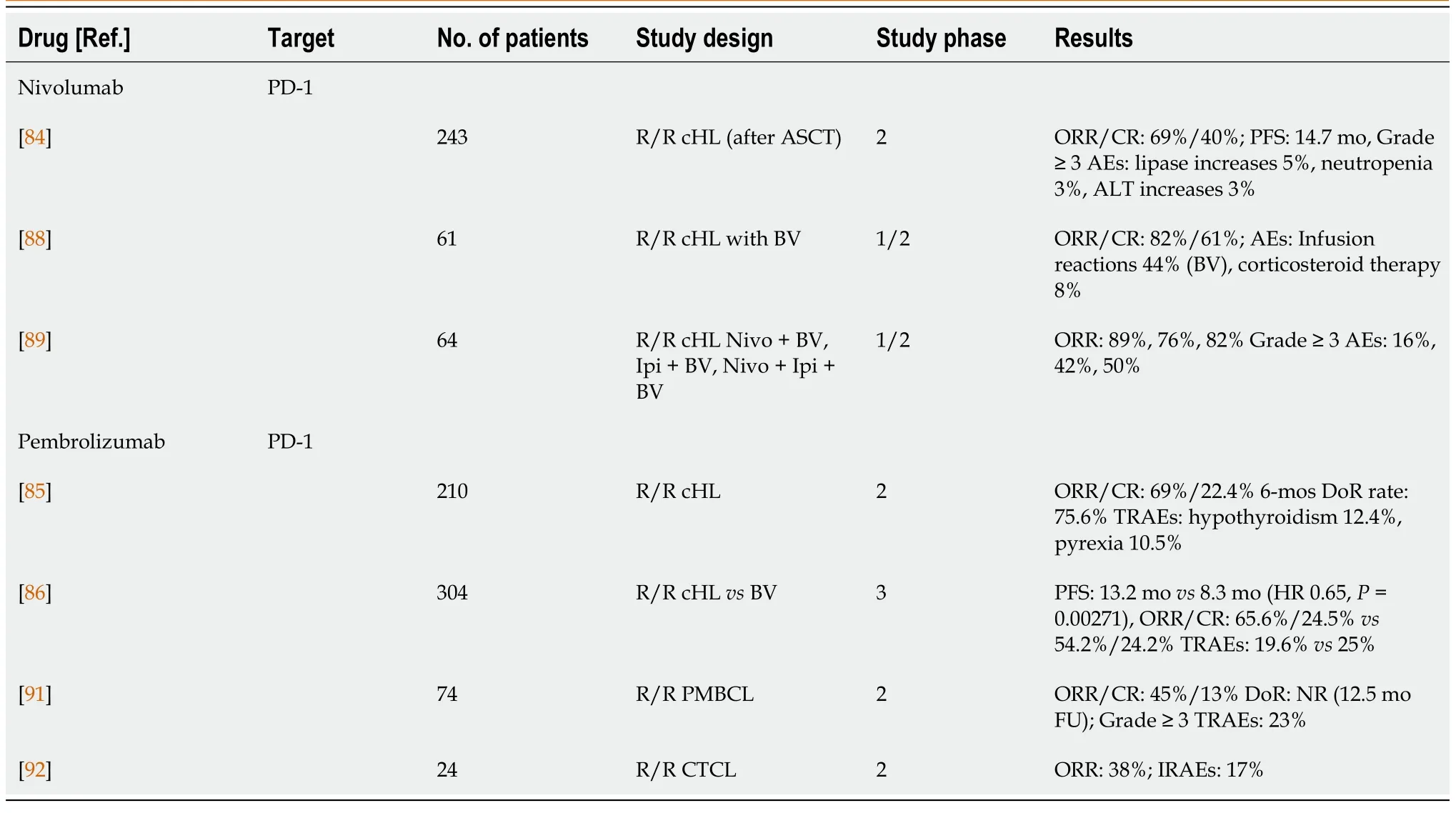
Table 4 Checkpoint inhibitor studies
Two CAR T-cell products have been approved by the FDA in the absence of randomized trials, based on comparison with the historical data on R/R DLBCL treatment[95].The axicabtagene-lisoleucel (axi-cel) CAR T-cell receptor expresses the CD28 costimulatory domain and was examined in a phase 2 trial in patients with R/R DLBCL.One hundred-eleven patients were included in the study.The production of CAR T cells was successful in 110 patients, and the therapy was applied in 101 patients.The ORR was 82%, with a CR rate of 54%.After a median follow-up of 15.4 mo, 42% of the patients were still without progression, and 40% were still in complete remission.The total 18-mo survival was 52%.The most common side effects were cytopenias, while CRS and immune effector cell-associated neurotoxicity syndrome (ICANS) of grade 3 and higher occurred in 13% and 28% of patients, respectively[96].Tisagenlecleucel (tisa-cel) is a CAR T-cell product, similar to axi-cel, directed against the CD19 antigen of B-cell lymphomas but with a different 4-1BB costimulatory domain.Tisa-cel showed an ORR of 52%, of which 40% achieved CR in a study of 93 patients with R/R DLBCL.After 12 mo of follow-up, 65% of patients were progression free, 79% of whom achieved CR[97].Lisocabtagen-maraleucel (liso-cel) is another anti-CD19 4-1BB CAR T-cell product that has shown significant results in R/R DLBCL.The study included 344 patients who underwent leukapheresis, of whom 269 received at least one dose of liso-cel.Almost all patients (97%) had previously received at least two lines of therapy, 42% were older than 65 years of age, 67% had chemotherapyrefractory disease, and seven had secondary CNS involvement.The overall response was 73%, with 53% of patients achieving CR.Grade 3 and higher chronic inflam-matory response syndrome (CIRS) and ICANS occurred in 2% and 10% of patients, respectively[98].
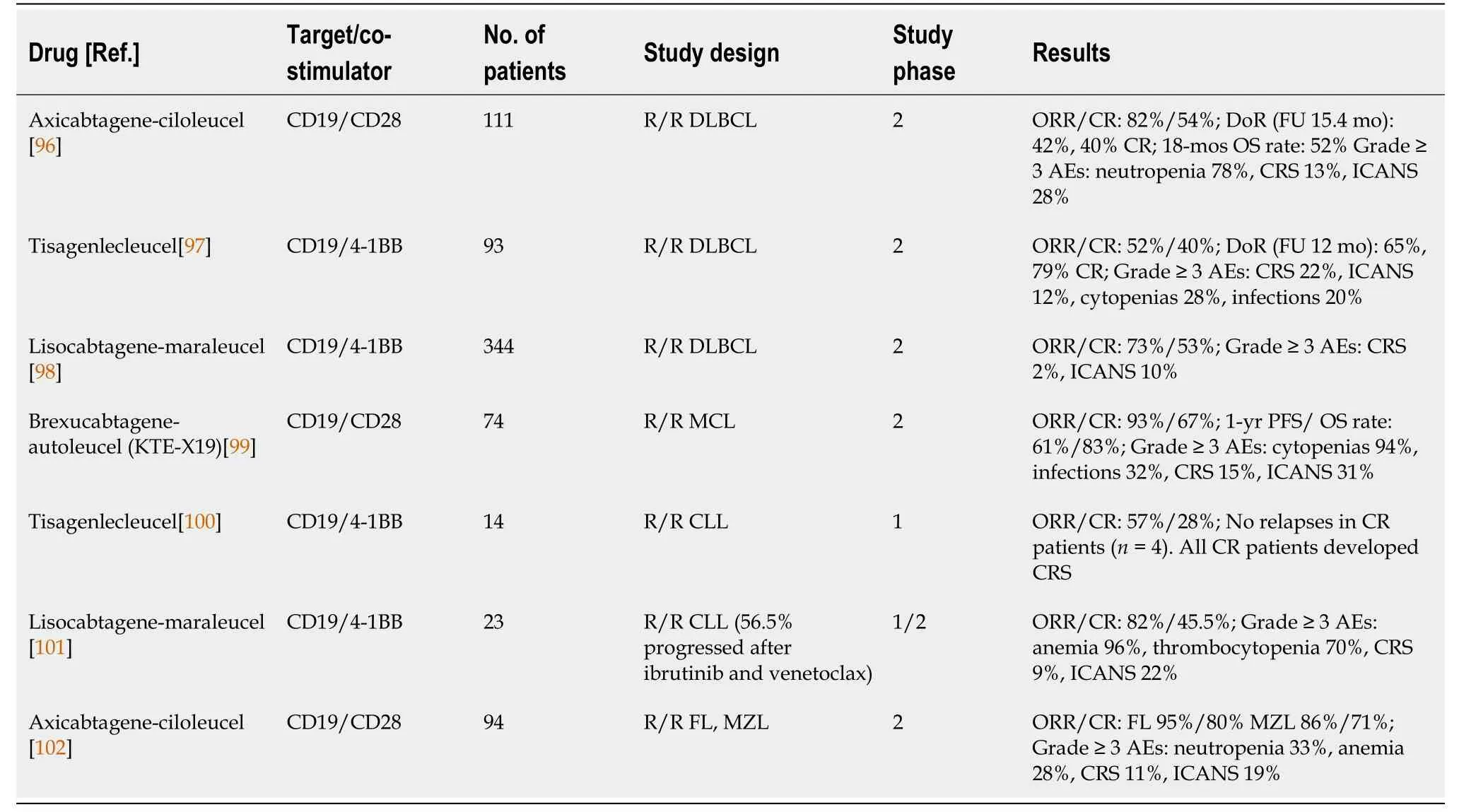
Table 5 Chimeric antigen receptor T cell studies
CAR T-cell therapies have also been shown to be effective for mantle cell lymphoma (MCL) and CLL in addition to DLBCL.Brexucabtagene-autoleucel was investigated in pretreated patients with MCL.The ORR was 93%, and the CR rate was 67%.The 1-year PFS rate was 61%.The toxicity profile was similar to that of other CAR T-cell products tested for DLBCL[99].CLL is one of the first diseases in which CAR T-cell therapy was tested.Tisa-cel and liso-cel had substantial and long-lasting responses in R/R CLL[100,101].Similar studies have shown a high ORR and long remission after CAR T-cell therapy in patients with FL, marginal zone lymphoma (MZL), and Epstein-Bar virus (EBV)-associated lymphoma[102-104].
The application of CAR T cells is complex and requires careful patient selection and a series of procedures from leukapheresis, bridging therapy, laboratory treatment, lymphodepletion, and infusion of the CAR T-cell product to the management of acute and chronic toxicities[93].Clinical studies with axi-cel and tisa-cel included fit patients (ECOG 0-1)[96,97].Analysis of real-world data showed that patients with poorer performance status could also be treated successfully[105].Some patients are not eligible for this type of therapy and are excluded from clinical trials, including those with renal impairment (creatinine clearance < 40), liver or lung damage, or a left ventricular ejection fraction < 40%)[96-98,105].Patients with neurological diseases or cognitive impairment are also not eligible for this type of therapy[105].The DLBCL subtype did not affect the ORR[96,97].Although CAR T cells have not been registered for the treatment of DLBCL with CNS involvement, several studies that included such patients have shown CAR T-cell efficacy in this group.On the other hand, patients with high disease volume and high lactate dehydrogenase (LDH) have shorter PFS times[105].Some patients need bridging therapy after leukapheresis because of the aggressiveness of the disease and the 4-6 wk it takes to make a CAR T-cell product.This therapy may include the R-ICE or R-DHAP protocols[2] as well as the combination of polatuzumab with rituximab and bendamustine[65].
CAR T cells have two specific toxicities in addition to pancytopenia, febrile neutropenia, and infections, which are CRS and ICANS.CRS is characterized by high fever, but as the clinical condition progresses, capillary leak syndrome, hypotension, and hypoxia of varying degrees occur.Any organ in the body can be affected[106,107].On the other hand, ICANS is characterized by various neurological symptoms, including decreased level of consciousness, motor dysfunction, and speaking difficulties.The progression of symptoms can lead to convulsions and cerebral edema[106].Tocilizumab, an anti-IL-6 antibody, is used to treat those symptoms.In addition to tocilizumab, corticosteroid therapy has shown results in ICANS therapy[106,107].Clinical trials are underway with various drugs, including tocilizumab, corticosteroids, and anakinra, in an attempt to provide prophylaxis for CRS and ICANS after CAR Tcell therapy[108,109].
CONCLUSION
Are we going to replace stem cell transplantation with immunotherapy?
Relapsed or refractory DLBCL has a poor prognosis.The SCHOLAR-1 study showed that the median survival time of refractory disease was 6.3 mo[95].The median survival time was approximately 13 mo in our patients, and a large number of patients were not eligible for HDCT/ASCT because of refractory disease or poor performance status[110].HDCT/ASCT for fit patients remains the standard of care, but many patients are not eligible for that procedure, and some relapse after ASCT.Tafasitamab and selinexor have shown promising results, but PV in combination with rituximab and bendamustine has been largely adopted as the standard of care.We are waiting for the results of clinical trials investigating polatuzumab and tafasitamab in the initial treatment of DLBCL[50,67].Despite the complexity of the CAR T-cell procedure itself, the results obtained with CAR T-cell therapy have pushed AlloSCT to later lines of therapy, and three studies comparing different CD19 CAR T cells with HDCT/ASCT are underway[111-113].Mosunetuzumab and other BITEs have shown promising results in the early stages of research and are a potential therapeutic option after relapse following CAR T-cell therapy or in countries where CAR T cells are not available.CRS and ICANS are potentially fatal complications of both CAR T-cell therapy and BITE, and significant education is needed in terms of recognizing and treating these complications.
Hodgkin's lymphoma is a curable disease in over 80% of cases.HDCT/ASCT is an important part of relapse treatment.Most patients are eligible for that type of treatment, and the procedure can cure approximately 50% of patients[7-9].BV improved treatment outcomes in patients with stage III and IV cHL and prolonged PFS when administered after HDCT/ASCT was given as maintenance therapy in patients at a high risk of relapse[59,61].AlloSCT has also been significantly suppressed by the CPI of pembrolizumab and nivolumab, which are now standard fourth-line treatments.Pembrolizumab has achieved even longer PFS and OS than brentuximabvedotin[86] and is slowly becoming the first treatment option after disease progression following ASCT.Promising results achieved by the combination of BV and nivolumab provide optimism for the results achieved with this type of treatment.The results of first-line studies comparing nivolumab with chemotherapy and BV with chemotherapy[87,88].
杂志排行
World Journal of Stem Cells的其它文章
- Genome engineering and disease modeling via programmable nucleases for insulin gene therapy; promises of CRISPR/Cas9 technology
- Recent trends in stem cell-based therapies and applications of artificial intelligence in regenerative medicine
- Epigenetic regulation of autophagy: A key modification in cancer cells and cancer stem cells
- Review of the potential of mesenchymal stem cells for the treatment of infectious diseases
- Growing and aging of hematopoietic stem cells
- Therapeutic potential of periodontal ligament stem cells
