玉米芯生物炭对污泥蚯蚓粪中微生物种群及ARGs的影响
2021-07-23关孟欣彭兰生陈景阳
关孟欣,彭兰生,陈景阳,黄 魁,2*,夏 慧
玉米芯生物炭对污泥蚯蚓粪中微生物种群及ARGs的影响
关孟欣1,彭兰生1,陈景阳3,黄 魁1,2*,夏 慧1
(1.兰州交通大学环境与市政工程学院,甘肃 兰州 730070;2.甘肃省黄河水环境重点实验室,甘肃 兰州 730070;3.金风环保有限公司,北京 102600)
较多的抗生素抗性基因(ARGs)积蓄于剩余污泥中降低了污泥蚯蚓粪的农用价值.为削减污泥蚯蚓粪中的ARGs,向污泥中分别添加1.25%和5%(质量比)的玉米芯生物炭(简称玉米芯炭),以无添加为对照组,揭示玉米芯炭对污泥蚯蚓堆肥过程中微生物种群结构及ARGs的影响.结果表明:添加高含量玉米芯炭显著促进污泥有机质的矿化,提升蚯蚓堆肥产物的电导率和pH值(<0.05).同时,添加玉米芯炭能增加污泥蚯蚓粪中细菌16S rDNA和真核生物18S rDNA的丰度,且其丰度均与玉米芯炭添加量呈显著正相关性(<0.05).与对照组相比,高含量玉米芯炭污泥蚯蚓粪中变形菌门、拟杆菌门、放线菌门与浮霉菌门的相对丰度分别降低了11.8%、7.1%、33.3%和20%,但厚壁菌门的丰度显著增加了40%(<0.05).此外,添加玉米芯炭蚯蚓粪中大环内酯类抗性基因(F)和四环素类抗性基因(X)的绝对丰度较对照组分别显著降低了32%~45%和13%~31%(<0.05),但同时整合子基因(I1)和磺胺类抗性基因(2)的丰度分别显著增加了47%~135%和9%~42%(<0.05).研究结果显示,添加玉米芯炭能增加污泥蚯蚓粪中微生物数量和种群多样性,加速有机质矿化,但对ARGs的削减具有选择性.
生物质炭;蚯蚓堆肥;污泥资源化;抗生素抗性基因;蚯蚓粪有机肥;生物污染物
随着我国污水处理能力的不断提升,剩余污泥产量越来越多[1-2],其处理处置已经成为我国生态文明建设过程中急需解决的难题.蚯蚓堆肥是一种蚯蚓协同微生物共同降解有机物的污泥肥料化技术[3],其产物蚯蚓粪富含多种土壤养分及益生菌群[4-5],是一种高质量的生物有机肥.由于我国城市生活污水和工业污水管网的分流,市政污泥中的重金属与有毒有机物含量已不再是污泥蚯蚓堆肥的瓶颈.但新近研究发现剩余污泥中积蓄大量抗生素及其抗性基因(ARGs)[6],亦会造成污泥蚯蚓粪中功能性菌群携带有大量ARGs[7].ARGs作为新型生物污染物,具有很强的遗传性和传播性,难以在生物介质中将其有效去除[8-9].因此,如何有效降低污泥蚯蚓粪中ARGs的环境风险尤为重要[10].
生物质炭具有比表面积大、吸附能力强、稳定性高等特点,能够促进污泥有机物矿化、加速污泥稳定化、吸附污泥中有害物质[11-12].同时,生物质炭为微生物的生长繁殖提供了多尺度的生态位[13].由于大多数ARGs镶嵌在细菌胞内DNA上,其归趋受生境下细菌菌群变化的影响[14-15].因此可推测污泥蚯蚓堆肥体内添加生物质炭,不仅可加速有机物的稳定,而且会改变蚯蚓粪中微生物菌群,进而影响其ARGs的多样性和丰度.已有研究表明生物质炭会影响污泥蚯蚓粪中ARGs[16].但关于不同含量生物质炭对污泥蚯蚓粪中ARGs的影响还鲜有研究.
本实验选取网状多孔结构的玉米芯炭为供试生物质炭,比较不同含量玉米芯炭对污泥蚯蚓堆肥产物中微生物种群结构和ARGs的影响,旨在为减少污泥蚯蚓粪中ARGs的风险提供一种新思路.
1 材料和方法
1.1 供试材料
堆肥蚓种为赤子爱胜蚓(),并经脱水污泥驯化.玉米芯炭购置于大连九成物产公司,表面为100µm的网状多孔结构,理化性质见表1.实验反应器为底部打孔的矩形塑料盒(46cm´17cm´13cm).污泥取自兰州市安宁七里河污水处理厂脱泥车间的新鲜脱水污泥(含水率64%),理化性质见表1.

表1 供试玉米芯炭和供试污泥理化性质
1.2 实验设计
设置3个处理,以无玉米芯炭为对照组(CK组),低含量玉米芯炭(1.25%CL组)和高含量玉米芯炭(5%CH组)为处理组,每组设3个重复.新鲜脱水污泥先用5mm网孔的铁丝网进行造粒,随后将其与玉米芯炭按填加比例分别混匀后,再次造粒作为蚯蚓堆肥基质[16].而后将3kg蚯蚓堆肥基质分别放入对应的反应器中,最后向各基质中接种100 条体重约0.5g的赤子爱胜蚓.蚯蚓投加密度参照之前实验进行[17].为保持水分、湿度和有氧条件,在各反应器上覆盖带孔保鲜膜,每周喷洒一次自来水并进行翻堆.所有反应器均在室温(20~25℃)下进行.蚯蚓堆肥实验共进行60d.污泥蚯蚓粪样品取样时将蚓卵剔除,每个反应器取2个平行样品.所取样品分两份保存,一份存放于无菌塑封袋中,置于-20℃冰箱中冷冻保存,用于DNA相关分析;另一份风干后研磨,过60目筛,然后置于4℃冰箱中保存,用于理化性质分析.
1.3 测试方法
1.3.1 理化性质分析 理化测试参照黄魁等[17]方法进行.将研磨后的风干样与去离子水(干样:水=1:50;kg/L)混匀后测定pH值(雷磁PHS-3C,上海)和电导率(雷磁DDS-307,上海).硝酸盐氮采用紫外分光光度法(HJ/T 346-2007),氨氮采用纳氏试剂分光光度法(HJ 535-2009).溶解性有机碳(DOC)为上述混合液稀释10倍后,过0.45μm滤膜,用碳氮分析仪(耶拿MULTI N/C,2100,德国)进行测定.
1.3.2 DNA提取及荧光定量PCR 取约0.25g解冻样品用DNeasy®Power Soil®Kit (Qiagen,德国)试剂盒提取总DNA,并用1%琼脂糖凝胶电泳检测其浓度.本实验选取污泥中常见且含量较高的大环内酯类抗性基因(F)、四环素类抗性基因(MX)和磺胺类抗性基因(1、2)及第一类整合子基因(I1)进行检测.采用荧光定量PCR仪(Takara,TP700,日本)对细菌16S rDNA(V3~V4区),真核18S rDNA(V4区)及上述几种ARGs进行定量.定量反应为25μL体系:TB Green II(Takara,日本)12.5μL, 10μmol/L引物各0.5μL,DNA模板1μL,以及DNA- free水10.5μL.所用引物均购置于生工生物工程(上海)股份有限公司,引物序列和反应条件见表2.标准品为携带目的基因的质粒,制备过程见文献[18].

表2 抗性基因引物及PCR反应条件
1.3.3 PCR和高通量测序 采用带有Barcode碱基的引物515F(5’-GTGCCAGCCGCGGTAA-3’)和806R(5’-GGACTACHVGGGTWTCTAAT-3’)对16S rDNA基因序列V4区进行扩增.使用Phusion High-Fidelity PCR Master Mix with HF Buffer (M0531NEB)高保真酶进行扩增.PCR扩增条件为: 95℃预变性5min;35个循环包括95℃变性30s,58℃退火30s,72℃延伸30s;72℃终延伸10min.所得到的扩增产物使用1%的琼脂糖电泳进行检测,并利用Agencourt AMPure XP 60mL Kit(A63881 Beckman Coulter)对产物进行纯化.使用Qubit dsDNA HS Assay Kit (Q32851Life tech)对文库进行构建,检测合格后使用Illumina HiSeq平台进行上机测序(谷禾信息生物有限公司,杭州).测序结果使用QIIME(1.8.0)软件对序列进行质控过滤,去掉嵌合体,得到有效Clean tags.然后使用MEGA7.0软件(v7.0.1001)将OTUs (Operational Taxonomic Units)进行聚类,相似度达97%的OTU聚为一类.最后与GreenGene(v gg_13_8)和Silva(SILVA128)数据库进行对比并注释,获得各OTU的分类学信息.测序结果已上传至DDBJ数据库.
1.4 统计分析
使用STATISTICA 10.0统计软件进行单因素方差分析(One-way ANOVA)和相关性分析,显著性水平为0.05.使用MEGA7.0软件对DNA测序数据进行聚类,并用HemI1.0软件绘制热图.使用OriginPro 2018(version 9.5)绘图.用CANOCO 4.5软件对环境因素、微生物和ARGs之间的关系进行冗余分析.
2 结果与讨论
2.1 玉米芯炭对堆肥产物理化性质的影响
由表3可知,与CK组相比,CL和CH两组蚯蚓粪中电导率分别提升15%和14%(<0.05),添加玉米芯炭能提升污泥蚯蚓堆肥的矿化水平[13]. Gong等[19]研究发现,高温堆肥中添加竹子生物质炭会提高其产物的电导率.污泥蚯蚓粪中增加的电导率可能与生物质炭中较高的电导率供给有关.与电导率相似,CL和CH两组蚯蚓粪的pH值也显著提升(<0.05).
如表3所示,CH组硝酸盐氮的含量比CK组增加12%(<0.05),但CL与CK组无显著性差异(> 0.05).这可能是因为高含量的玉米芯炭增加了污泥的孔隙率,致使堆体中氧含量增加,进而促进了硝化反应的进行[20].蚯蚓堆肥后各组氨氮含量均有显著性下降(<0.05),但CL和CH氨氮含量比CK组显著提高了1.46倍和1.07倍(< 0.05).这一结果可能与添加玉米芯炭会促进污泥有机物的氨化反应有关[13].玉米芯炭组中同时增多的氨氮和硝氮暗示着生物质炭能提升污泥蚯蚓粪的氮肥肥效.
由表3所见,实验后各组污泥蚯蚓粪中溶解性有机碳(DOC)均显著降低(<0.05),这与其他研究相近[17].与CK组相比,CL和CH组DOC含量呈增加趋势(<0.05).研究发现[21-22],生物质炭的添加会增加土壤中的DOC含量.这可能归因于生物质炭芳香结构中的碳被蚯蚓与微生物分解,从而增加了污泥蚯蚓粪中DOC的含量.本研究中DOC含量与玉米芯炭含量有较显著的线性关系(<0.05,=0.96).
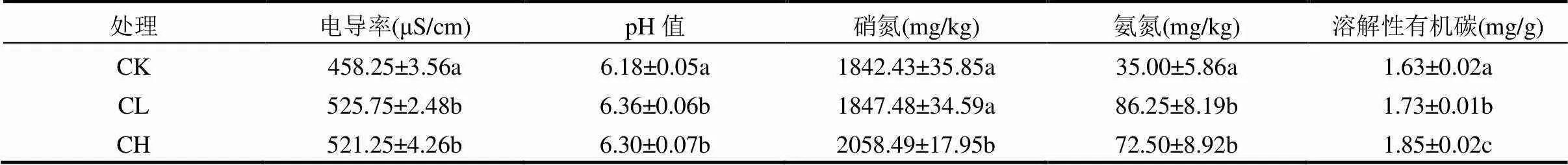
表3 不同处理中污泥蚯蚓粪的理化性质
注:同列指标的两个处理组间存在相同字母表明其两两之间不具有显著性差异(>0.05),同行字母之间没有比较意义,下同.
2.2 玉米芯炭对微生物数量及种群结构的影响
2.2.1 玉米芯炭对微生物数量的影响 如图1所示,与原始污泥相比,蚯蚓堆肥显著增加了各处理组细菌16S rDNA和真核生物18S rDNA微生物的丰度(<0.05).与CK组相比,CL和CH组细菌16S rDNA丰度分别显著增加了0.24倍和1.13倍(<0.05);对于真核生物18S rDNA来说,CL和CH组的数量均显著高于CK组(<0.05).同时,皮尔逊相关性结果显示,细菌与真核微生物的生物量与玉米芯炭含量均有极显著的正相关性(<0.05,=0.96,=0.97).上述结果说明添加玉米芯炭有利于污泥蚯蚓粪中微生物的生长繁殖,这与Xu等[23]在土壤学中的研究结果相似.生物质炭较高的比表面积和孔隙度为微生物的生存提供了多样的环境,其可利用成分可以直接被微生物所利用[24],进而增加了微生物的生物量[25].与细菌相比,CH组中真核生物数量急剧性增长,表明高含量玉米芯炭有利于其生长.这可能是因为高含量玉米芯炭多孔结构为真核微生物提供了好氧环境与庇护所,减少了被蚯蚓捕食的几率[13].
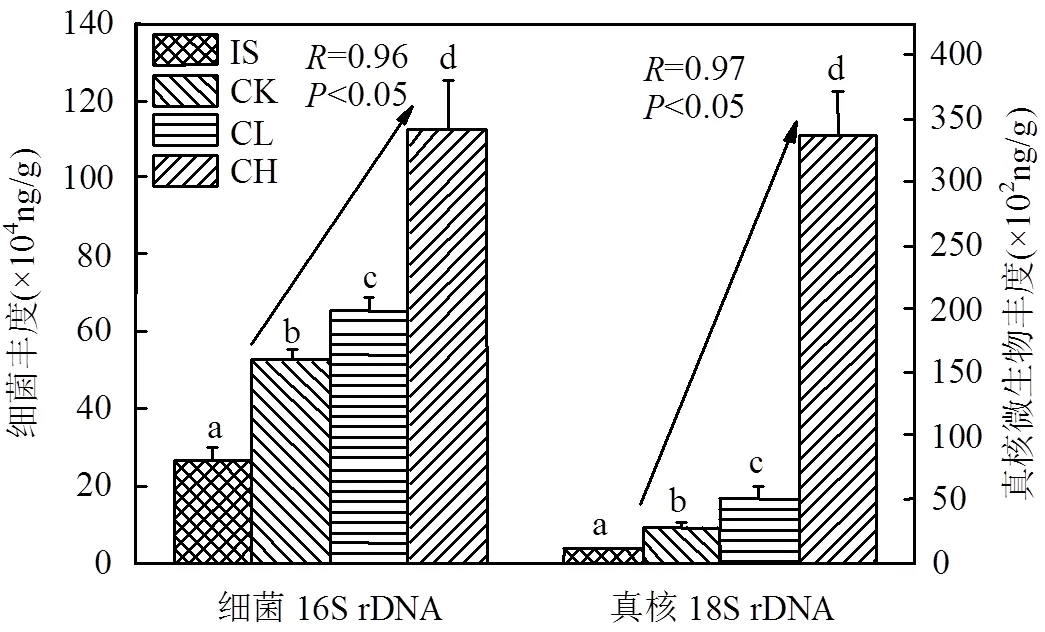
图1 蚯蚓堆肥前后污泥中细菌16S rDNA和真核18S rDNA的绝对含量
2.2.2 玉米芯炭对微生物种群结构的影响 由表4可知,与原始污泥相比,各组Shannon指数和Simpson指数均出现了增加,且最高值都出现在CH组,说明添加高含量玉米芯炭会增加堆肥产物微生物的丰富度和均匀度.这与最近在猪粪高温堆肥的研究[26]结果相似.微生物均匀度的增加,可能是由于生物质炭多孔结构提供了多样的生境,有利于功能性微生物的生长繁殖[25].
由图2可见,原始污泥中优势菌门为变形菌门(25%)、拟杆菌门(13%)、厚壁菌门(13%).蚯蚓堆肥后各处理组中变形菌门、放线菌门、浮霉菌门均显著增加(<0.05),而厚壁菌门显著减少(<0.05).与CK组相比,CL组细菌菌群结构无显著性变化.但CH组中厚壁菌门较CK组显著增长了40%,这与黄家庆等[26]所得到的结果一致.厚壁菌门的主要功能为降解大分子有机物如蛋白质、脂肪类、纤维素等[17],因此可推断高含量玉米芯炭会促进有机物质的转化.而与CK组相比,CH组放线菌门和变形菌门分别减少了33.3%和11.8%.Cui等[27]也发现在鸡粪高温堆肥中添加5%的稻草生物质炭会降低变形菌门的比例.这说明高含量玉米芯炭会抑制堆肥体中变形菌门和放线菌门的生长.CH组中浮霉菌门和拟杆菌门与CK组相比也有明显减少.以上结果表明,添加高含量玉米芯炭显著影响了堆肥产物中门水平的细菌群落组成.
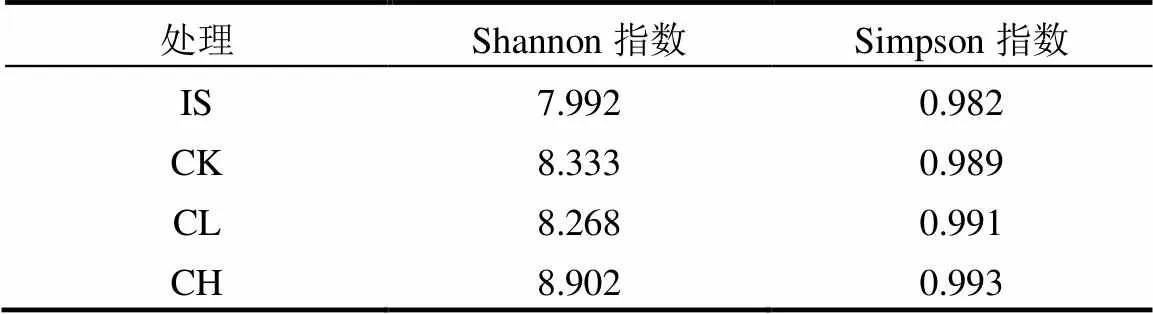
表4 不同处理中微生物Shannon与Simpson指数
注:IS为原始污泥.
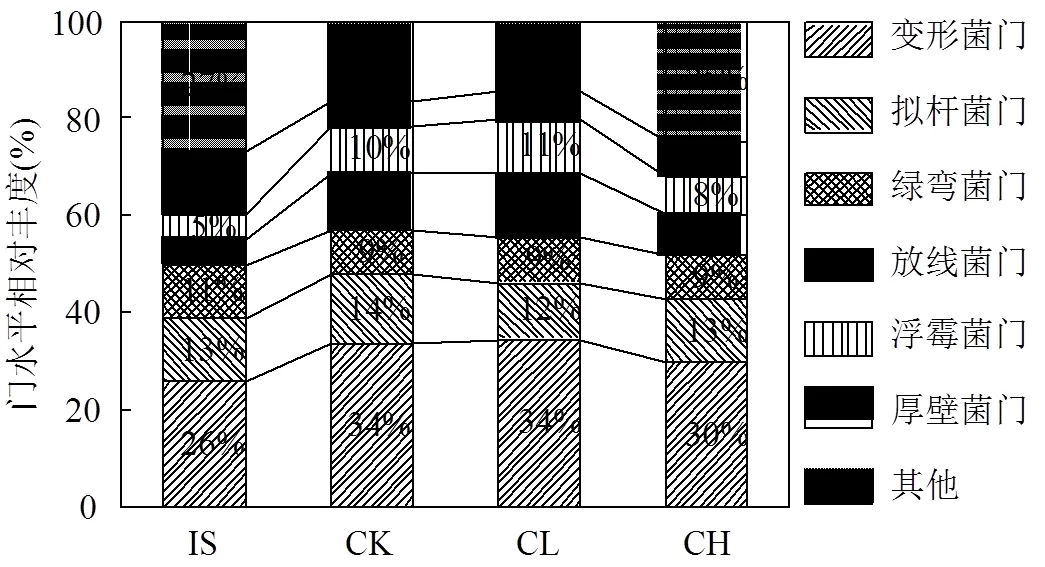
图2 堆肥前后污泥中细菌门水平结构变化

图3 堆肥前后污泥中细菌属水平相对丰度
如图3所示,原始污泥中优势菌属为、、Blvii28.而堆肥后3个处理组中Unclassified-1、、、、、SWB02、、、、、、和均有不同程度的增加.其中,、、、和从无到有,这说明蚯蚓堆肥可以显著增加细菌种群多样性[17].与CK组相比,CL组Unclassified-1和Nocardioides增加了28%和14%,而CH组中Nocardioides降低了36%,SWB02降低了34%,、、和降低了40%.结果显示玉米芯炭的含量对堆肥产物细菌群落组成有较大的影响.聚类分析可见,CK与CL组种群结构差异性较小,而CH组与其余两组种群结构有较大差异性,这说明高含量玉米芯炭对细菌属水平结构影响较大.
2.3 玉米芯炭对ARGs的影响
如图4(a)所示,与原始污泥相比,CK组中I1的数量显著降低了0.48倍(<0.05),显示出蚯蚓堆肥可以降低污泥中I1的丰度[17,28].但与CK组比较,添加玉米芯炭使I1的数量呈现出增长趋势,且其丰度与玉米芯炭含量有显著正相关性(<0.05,= 0.88).这表明添加玉米芯炭对I1丰度的增加有促进作用.相关研究证实,环境中较高的细菌数量和多样性增多了ARGs的潜在宿主及细菌胞外接触的可能性,进而增大了I1在生物介质中水平传播的可能性[29-30].本研究中,增加的I1丰度与CL和CH组中较高的细菌数量和多样性密切相关,因为污泥蚯蚓粪中的细菌数量和I1呈正相关关系(<0.05,=0.88).这一结果暗示着添加生物质炭将会增加污泥蚯蚓粪中ARGs的传播风险.Duan[31]在研究中也发现生物质炭的添加并不会降低土壤中I1丰度.
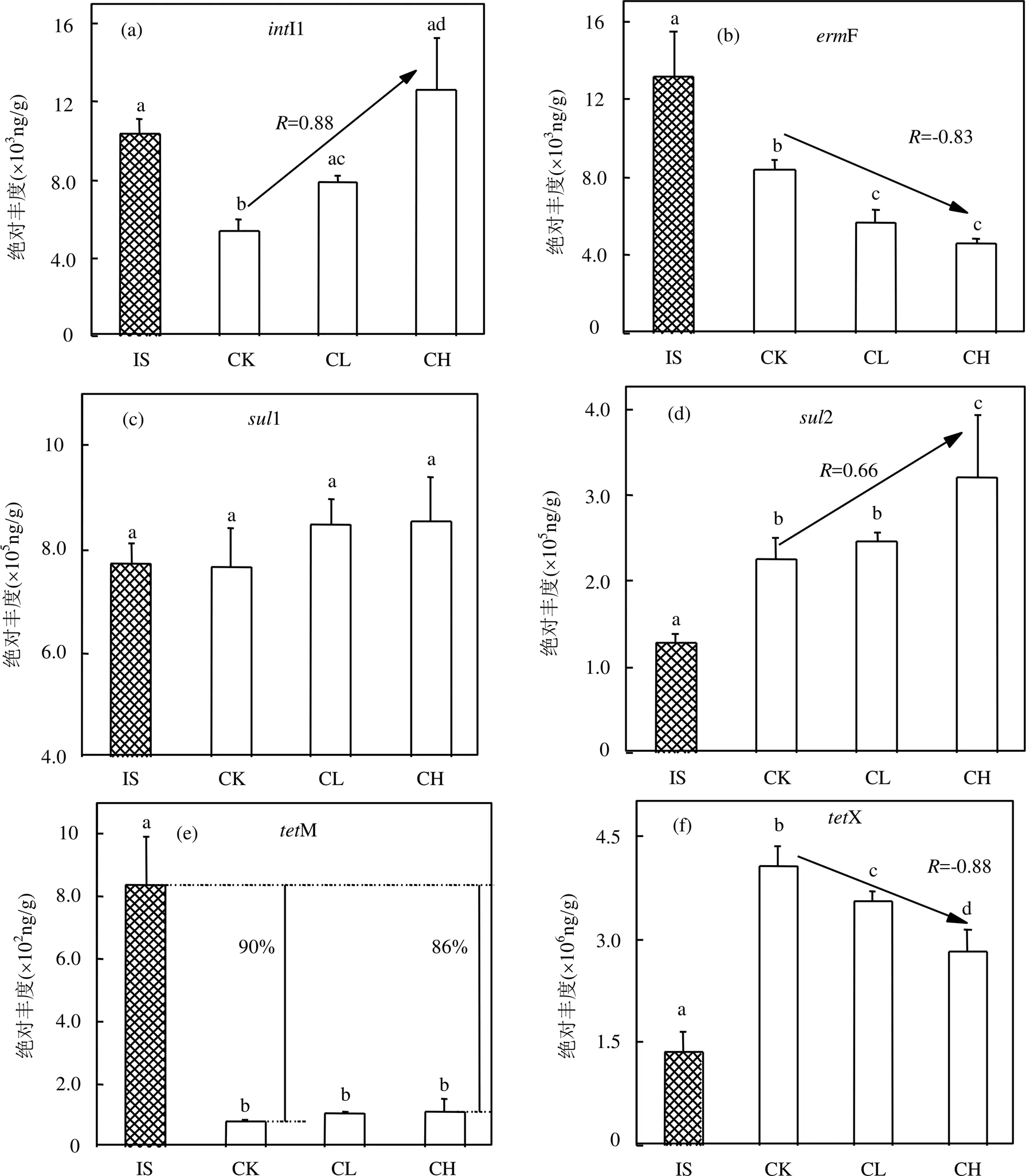
图4 蚯蚓堆肥前后ARGs的绝对丰度
对ARGs而言(图4b~f),各组中F(大环内酯类抗性基因)和M(四环素类抗性基因)的丰度在堆肥处理后均出现显著减少(<0.05),其中M与原始污泥相比减少了86%~90%.并且M各组间并无显著性差异(>0.05),说明添加玉米芯炭对污泥蚯蚓粪中M的丰度无显著影响.但对F来说,随着玉米芯炭含量的增加,污泥蚯蚓粪中F的丰度逐渐减少,且与玉米芯炭含量有较强的负相关性(<0.05,=-0.83).这一现象表明,玉米芯炭可以减少蚯蚓堆肥中F的丰度.Li等[32]也发现向鸡粪高温堆肥中添加竹子生物质炭,F的丰度会减少.此外,与原始污泥相比,X(四环素类抗性基因)的丰度在蚯蚓堆肥后会出现显著增长(<0.05),见图4(f).但与CK组相比,CL和CH组X的丰度显著下降了13%和31%,且与玉米芯炭含量存在显著负相关(<0.05,=-0.88),说明添加玉米芯炭会降低污泥蚯蚓粪中X的丰度.与之相反,在添加玉米芯炭后,1和2(磺胺类抗性基因)的丰度均出现增加.磺胺类抗性基因的增多可能与污泥蚯蚓粪中I1丰度有关[31].Chen[33]在I1的保守区发现了磺胺类抗性基因,因此I1的增加会导致1和2的增加.相比而言,CH组中2丰度与CK组相比显著增加了42%,且其丰度与玉米芯炭含量存在显著正相关(<0.05,0.66).Wang等人[34]也发现高含量玉米秸秆生物质炭能增加动物粪便高温堆肥中1和2的丰度.另外,最高的1和2丰度均出现在CH组,暗示着高含量玉米芯炭会提升蚯蚓堆肥产物中磺胺类抗性基因的丰度.综上结果显示玉米芯炭对蚯蚓污泥堆肥体系中ARGs的去除具有选择性,且去除效率与玉米芯炭的含量密切相关.
2.4 环境因子、微生物和ARGs之间的关系
图5冗余分析可知,CK组中总ARGs的丰度最高,EC、pH值以及NH4+对堆肥体内ARGs的总丰度有一定的抑制作用.CH组高含量玉米芯炭及DOC、NO3-的含量与2和M的丰度有显著正相关性(<0.05),表明高含量玉米芯炭对2和M的丰度有积极的影响,这与CH组中高含量2结果一致.此外,Firmicutes(厚壁菌门)与2和M之间较强的相关性表明其可能为2和M的潜在宿主细菌.Sun等[35]在牛粪厌氧消化中添加生物质炭也发现类似结果.RDA结果还显示CL组Planctomycetes (浮霉菌门),Actinobacteria(放线菌门)及Proteobacteria(变形菌门)的丰度较高,表明了在蚯蚓堆肥过程中添加低含量玉米芯炭会促进上述菌门细菌的生长,细菌门水平的变化对堆肥过程中ARGs的变化有一定的影响.因此,环境变量对ARGs丰度的影响主要取决于它们对其潜在宿主细菌的影响[10,36-37].本研究显示污泥中环境因子与微生物种群的变化受生物质炭含量的影响较大,进而对蚯蚓粪中ARGs分布和丰度产生影响[7,28,38].但由于污泥蚯蚓堆肥体系的复杂性,玉米芯炭对污泥蚯蚓堆肥中微生物和ARGs的影响机制仍须进一步研究.

图5 ARGs、细菌菌群和环境因子的冗余分析
3 结论
3.1 高含量玉米芯炭污泥蚯蚓粪中硝氮含量和电导率分别增加了12%和14%,促进了污泥蚯蚓堆肥中有机物的矿化,提升其产物的稳定性.
3.2 高含量玉米芯炭组提升了污泥堆肥产物中微生物种群多样性,、、Blvii28、、Unclassified-1、、、、、和为生物炭污泥蚯蚓粪的优势菌属.
3.3 玉米芯炭降低了污泥中占主导地位的F和X的丰度,但I1和2的丰度在添加玉米芯炭组中分别增加47%~135%和9%~42%,玉米芯炭蚯蚓粪的生态风险需进一步研究.
[1] 薛重华,孔祥娟,王 胜,等.我国城镇污泥处理处置产业化现状、发展及激励政策需求[J]. 净水技术, 2018,37(12):41-47.
Xue C H, Kong X J, Wang S, et al. Industrialization status, development analysis and incentive policy demands of municipal sludge treatment and disposal industry in china [J]. Water Purification Technology, 2018,37(12):41-47.
[2] Qu J, Wang H, Wang K, et al. Municipal wastewater treatment in China: Development history and future perspectives [J]. Frontiers of Environmental Science and Engineering, 2019,13(6):88.
[3] Domínguez J, Aira M, Gómez-Brandón M. Vermicomposting: earthworms enhance the work of microbes [M]. Berlin, Heidelberg: Springer, 2010:93-114.
[4] Huang K, Xia H, Li F, et al. Optimal growth condition of earthworms and their vermicompost features during recycling of five different fresh fruit and vegetable wastes [J]. Environmental Science and Pollution Research, 2016,23(13):13569-13575.
[5] Sharma K, Garg V K, Vermicomposting: A green technology for organic waste management [M]. Singapore: Springer, 2018:199-235.
[6] 安新丽,苏建强.活性污泥抗生素抗性基因研究进展[J]. 微生物学通报, 2019,46(8):2069-2079.
An X L, Su J Q. Resistome in activated sludge: current knowledge and future directions [J]. Microbiology China, 2019,46(8):2069-2079.
[7] Huang K, Xia H, Wu Y, et al. Effects of earthworms on the fate of tetracycline and fluoroquinolone resistance genes of sewage sludge during vermicomposting [J]. Bioresource Technology, 2018,259: 32-39.
[8] Aminov R I, Mackie R I. Evolution and ecology of antibiotic resistance genes [J]. FEMS Microbiology Letters, 2007,271(2): 147-161.
[9] 张 宁,李 淼,刘 翔.土壤中抗生素抗性基因的分布及迁移转化 [J]. 中国环境科学, 2018,38(7):2609-2617.
Zhang N, Li M, Liu X. Distribution and transformation of antibiotic resistance genes in Soil [J]. China Environmental Science, 2018, 38(7):2609-2617.
[10] Huang K, Xia H, Zhang Y, et al. Elimination of antibiotic resistance genes and human pathogenic bacteria by earthworms during vermicomposting of dewatered sludge by metagenomic analysis [J]. Bioresource Technology, 2020,297:122451.
[11] Zhang J N, Lü F, Shao L M, et al. The use of biochar-amended composting to improve the humification and degradation of sewage sludge [J]. Bioresource Technology, 2014,168:252-258.
[12] 周 楫,余亚伟,蒋 越,等.生物炭对污泥堆肥及其利用过程重金属有效态的影响[J]. 环境科学, 2019,40(2):987-993.
Zhou J, Yu Y W, Jiang Y, et al. Effect of biochar on available heavy metals during sewage sludge composting and land application of compost [J]. Environmental Science, 2019,40(2):987-993.
[13] Lehmann J, Rillig M C, Thies J, et al. Biochar effects on soil biota–a review [J]. Soil Biology and Biochemistry, 2011,43(9):1812-1836.
[14] Miller J H, Novak J T, Knocke W R, et al. Survival of antibiotic resistant bacteria and horizontal gene transfer control antibiotic resistance gene content in anaerobic digesters [J]. Frontiers in Microbiology, 2016,7:263.
[15] 黄 薇,刘兰英,刘 洋,等.鳗鲡养殖废弃物抗性基因赋存特征及其与抗生素和微生物群落的相关性 [J]. 应用与环境生物学报, 2020,26(5):1275-1281.
Huang W, Liu L Y, Liu Y, et al. Analysis of occurrence of antibiotic resistance genes in eel culture waste and its correlations with antibiotics and microbial community [J]. Chinese Journal of Applied and Environmental Biology, 2020,26(5):1275-1281.
[16] Huang K, Chen J Y, Guan M X, et al. Effects of biochars on the fate of antibiotics and their resistance genes during vermicomposting of dewatered sludge [J]. Journal of Hazardous Materials, 2020,379(5): 122767.
[17] 黄 魁,夏 慧,陈景阳,等.蚯蚓对城市污泥蚯蚓堆肥过程中微生物特征变化的影响[J]. 环境科学学报, 2018,38(8):3146-3152.
Huang K, Xia H, Chen J Y, et al. Effects of earthworms on changes of microbial feature during vermicomposting of municipal sludge [J]. Acta Scientiae Circumstantiae, 2018,38(8):3146-3152.
[18] Cui G, Bhat S A, Li W, et al. Gut digestion of earthworms significantly attenuates cell-free and-associated antibiotic resistance genes in excess activated sludge by affecting bacterial profiles [J]. Science of the Total Environment, 2019,691:644-653.
[19] Gong X, Cai L, Li S, et al. Bamboo biochar amendment improves the growth and reproduction of Eisenia fetida and the quality of green waste vermicompost [J]. Ecotoxicology and Environmental Safety, 2018,156:197-204.
[20] 李 明,李忠佩,刘 明,等.不同秸秆生物炭对红壤性水稻土养分及微生物群落结构的影响[J]. 中国农业科学, 2015,48(7):1361-1369.
Li M, Li Z P, Liu M, et al. Effects of different straw biochar on nutrient and microbial community structure of a red paddy soil [J]. Scientia Agricultura Sinica, 2015,48(7):1361-1369.
[21] El-Naggar A, El-Naggar A H, Shaheen S M, et al. Biochar composition-dependent impacts on soil nutrient release, carbon mineralization, and potential environmental risk: a review [J]. Journal of Environmental Management, 2019,241:458-467.
[22] Agegnehu G, Srivastava A K, Bird M I. The role of biochar and biochar-compost in improving soil quality and crop performance: A review [J]. Applied Soil Ecology, 2017,119:156-170.
[23] Xu N, Tan G, Wang H, et al. Effect of biochar additions to soil on nitrogen leaching, microbial biomass and bacterial community structure [J]. European Journal of Soil Biology, 2016,74:1-8.
[24] Farrell M, Kuhn T K, Macdonald L M, et al. Microbial utilization of biochar-derived carbon [J]. Science of the Total Environment, 2013,465:288-297.
[25] Zhu X, Chen B, Zhu L, et al. Effects and mechanisms of biochar- microbe interactions in soil improvement and pollution remediation: a review [J]. Environmental Pollution, 2017,227:98-115.
[26] 黄家庆,叶 菁,李艳春,等.生物炭对猪粪堆肥过程中细菌群落结构的影响[J]. 微生物学通报, 2020,47(5):1477-1491.
Huang J Q, Ye J, Li Y C, et al. Effect of biochar on bacteria community structure of pig manure composting [J]. Microbiology China, 2020,47(5):1477-1491.
[27] Cui E, Wu Y, Zuo Y, et al. Effect of different biochars on antibiotic resistance genes and bacterial community during chicken manure composting [J]. Bioresource Technology, 2016,203:11-17.
[28] 陈景阳,夏 慧,黄 魁,等.四环素对污泥蚯蚓粪中微生物种群和抗性基因的影响[J]. 环境科学, 2019,40(7):3263-3269.
Chen J Y, Xia H, Huang K, et al. Effects of tetracycline on microbial communities and antibiotic resistance genes of vermicompost from dewatered sludge [J]. Environmental Science, 2019,40(7):3263-3269.
[29] Cui G, Lü F, Zhang H, et al. Critical insight into the fate of antibiotic resistance genes during biological treatment of typical biowastes [J]. Bioresource Technology, 2020,317:123974.
[30] Zhou G, Qiu X, Wu X, et al. Horizontal gene transfer is a key determinant of antibiotic resistance genes profiles during chicken manure composting with the addition of biochar and zeolite [J]. Journal of Hazardous Materials, 2020,408:124883.
[31] Duan M, Li H, Gu J, et al. Effects of biochar on reducing the abundance of oxytetracycline, antibiotic resistance genes, and human pathogenic bacteria in soil and lettuce [J]. Environmental Pollution, 2017,224:787-795.
[32] Li H, Duan M, Gu J, et al. Effects of bamboo charcoal on antibiotic resistance genes during chicken manure composting [J]. Ecotoxicology and Environmental Safety, 2017,140:1-6.
[33] Chen B, Liang X, Nie X, et al. The role of class I integrons in the dissemination of sulfonamide resistance genes in the Pearl River and Pearl River Estuary, South China [J]. Journal of Hazardous materials, 2015,282:61-67.
[34] Wang J, Sui B, Shen Y, et al. Effects of different biochars on antibiotic resistance genes during swine manure thermophilic composting [J]. International Journal of Agricultural and Biological Engineering, 2018,11(6):166-171.
[35] Sun W, Gu J, Wang X, et al. Impacts of biochar on the environmental risk of antibiotic resistance genes and mobile genetic elements during anaerobic digestion of cattle farm wastewater [J]. Bioresource Technology, 2018,256:342-349.
[36] Ma Y, Wilson C A, Novak J T, et al. Effect of various sludge digestion conditions on sulfonamide, macrolide, and tetracycline resistance genes and class I integrons [J]. Environmental Science and Technology, 2011,45(18):7855-7861.
[37] Zhang R, Gu J, Wang X, et al. Contributions of the microbial community and environmental variables to antibiotic resistance genes during co-composting with swine manure and cotton stalks [J]. Journal of hazardous materials, 2018,358:82-91.
[38] Sun W, Qian X, Gu J, et al. Mechanisms and effects of arsanilic acid on antibiotic resistance genes and microbial communities during pig manure digestion [J]. Bioresource Technology, 2017,234:217-223
Effects of corncob biochar on the microbial communities and ARGs during vermicomposting of dewatered sludge.
GUAN Meng-xin1, PENG Lan-sheng1, CHEN Jing-yang3, HUANG Kui1,2*, XIA Hui1
(1.School of Environmental and Municipal Engineering, Lanzhou Jiaotong University, Lanzhou 730070, China;2.Key laboratory of Yellow River Water Environment in Gansu Province, Lanzhou 730070, China;3.Goldwind Environmental Protection Co., LTD, Beijing 102600, China)., 2021,41(6):2744~2751
The higher content of antibiotics resistance genes (ARGs) accumulated in excess sludge lowers its agricultural value of vermicompost. To eliminate the content of ARGs in sludge vermicompost, this study aimed to reveal the effects of corncob biochars added in sludge on microbial communities and ARGs of vermicomposting. For this, 1.25% and 5% of corncob biochars, were separately added to dewatered sludge, comparing with the counterpart without addition of biochars. The addition high content of corncob biochars significantly (< 0.05) promoted the mineralization of organic matter, thus increasing the conductivity and pH of sludge vermicompost. In addition, the corncob biochars (< 0.05) enhanced the gene abundances of bacterial 16S rDNA and eukaryotic 18S rDNA in the vermicompost, resulting in a significantly (< 0.05) positive correlation between the microbial abundance and biochar concentration. Compared to control, the relative abundance of Proteobacteria, Bacteroidetes, Actinomycetes, and Planctomycetes in sludge vermicompost with high content of corncob biochar decreased by 11.8%, 7.1%, 33.3% and 20%, respectively. However, its abundance of Firmicutes significantly increased by 40% (< 0.05). Besides, the absolute abundance of macrolide resistance genes (F) and tetracycline resistance genes (X) in vermicompost with corncob biochars significantly decreased by 32%~45% and 13%~31% (< 0.05), respectively. But, the abundance of class 1integron (I1) and sulfonamides resistance genes (2) significantly increased by 47%~135% and 9%~42% (< 0.05) in the final vermciompost, respectively. The addition of corncob biochar in sludge can promote the mineralizaiton of sludge by enhancing microbial number and diversity of microbial, but this addition only selectively reduce the ARGs in vermicompost.
biochar;vermicomposting;sludge recycling;antibiotics resistance gene;vermicompost fertilizer;biological pollutants
X171.5
A
1000-6923(2021)06-2744-08
关孟欣(1996-),男,山西运城人,兰州交通大学硕士研究生,主要研究污泥资源化技术.发表论文2篇.
2020-11-13
国家自然科学基金资助项目(51868036;52000095);甘肃省高等教师创新能力提升项目(2019A-040);兰州交通大学百人计划项目
* 责任作者, 副教授, huangk1199@hotmail.com
