Community structure of Lumbricidae in beech woodland of the Bieszczady National Park,Southeast Poland
2021-07-16AnnaMAZURACZKAGrzegorzACZKAJoannaKOSTECKAKevinBUTTMarcinJAROMINMariolaGARCZYSKAandAgnieszkaPODOLAK
Anna MAZUR-P˛ACZKA,Grzegorz P˛ACZKA,Joanna KOSTECKA,Kevin R.BUTT,Marcin JAROMIN,Mariola GARCZYŃSKA and Agnieszka PODOLAK
1 University of Rzeszów,Faculty of Biology and Agriculture,Department of Natural Theories of Agriculture and Environmental Education,1a Cwiklinskiej St.,Rzeszów 35-959(Poland)
2 University of Central Lancashire,Forensic and Applied Sciences,Preston PR1 2HE(UK)
3 Rzeszów University of Technology,Faculty of Chemistr y,Department of Biotechnology and Bioinformatics,6 Powstancow Warszawy Avenue,Rzeszów 35-959(Poland)
(Received May 14,2019;revised August 3,2019)
ABSTRACT As ecosystem engineers,earthworms play a key role in the soil environment.However,due to increasing anthropogenic pressure,soil organisms,including earthworms,are being threatened by habitat loss.In this study,we undertook a qualitative and quantitative investigation of earthworms of the family Lumbricidae in four types of Carpathian beech woodland(Fagetum carpaticum),characterized by their understory vegetation(I,F.c.festucetosum dr ymejae;II,F.c.typicum;III,F.c.lunarietosum;and IV,F.c.allietosum),in the Bieszczady National Park(Eastern Carpathians,Southeast Poland).At each investigated site,soil monoliths(25 cm×25 cm×25 cm)were examined by hand sorting.Earthworms were expelled from deep soil layers using a weak formalin solution(0.4%).Depending on the phytocoenosis,7 species of Lumbricidae were identified at each of the sites I,II,and III and 10 at site IV.Site IV(F.c.allietosum)differed significantly(P<0.05)from the other three sites with respect to earthworm biomass(59.71±39.53 g m-2)and the Shannon-Wiener diversity index(0.52±0.12).Although present three decades ago,the deep-burrowing species Octodrilus transpadanus and the litter-dwelling species Dendrobaena octaedra were not found at site IV in the present study.We suspect that these two species may have been displaced by the invasive Lumbricus terrestris,which was not found at the same site in the 1980s.Such observations warrant further investigation to verify the predicted effects of an invasive earthworm,including the potential effects on soils and other fauna and flora,which have been documented in numerous countries.
Key Words: beech forest,earthworm,invasive species,Shannon-Wiener diversity index,species diversity,species dominance
INTRODUCTION
Earthworms of the family Lumbricidae play a vital role in the soil environment and are considered ecosystem engineers owing to their ability to modify their surroundings(Jouquetet al.,2006;Lavelleet al.,2006).Through their various activities,earthworms make a major contribution to a diverse range of services rendered by ecosystems.Their burrowing activity loosens and aerates the soil,thereby enhancing water circulation(Schonet al.,2017).They also contribute to the circulation of matter by processing dead organic matter for subsequent decomposition by microorganisms(Amosséet al.,2015).Moreover,the presence of earthworms in soils promotes enhanced assimilation of mineral compounds,one of the consequences of which is a positive effect on plant production(Van Groenigenet al.,2014).
Although,to date,approximately 40 species of lumbricid earthworms have been identified in Poland(Plisko,1973;Kasprzak,1986),as a repercussion of mounting anthropogenic pressure,soil organisms,including earthworms,are increasingly being threatened by habitat fragmentation and loss.Currently,the greatest risks posed to these species are changes in soil pH,reductions in soil organic matter content,increased land use,erosion,and soil compaction and contamination(CEC,2006).Furthermore,an additional threat posed to the diversity of local populations of Lumbricidae worms is the colonization of invasive alien species(Pop and Pop,2006;Tiunovet al.,2006;Murchie and Gordon,2013).
In this study,we sought to undertake a qualitative and quantitative investigation of Lumbricidae in four types of Carpathian beech woodland(Fagetum car paticum)near Ustrzyki Górne in the Bieszczady National Park,Poland.Our objectives were to record the species richness and diversity,population density,and biomass of earthworms at each site,and compare our data with those obtained from the same sites in the 1980s.
MATERIALS AND METHODS
Study site and soil conditions
The Bieszczady Mountains are the most westerly point of the Carpathian range,which runs from northwest to southeast in Southeast Poland and reaches a maximum height of 1 346 m above sea level.In this region,July and February are the warmest and coldest months,respectively(Michna and Paczos,1972).Studies were conducted in the Bieszczady National Park,which is the main zone of the East Carpathian Biosphere Reserve.The area covering the national park and its buffer zone in the upper Vistula basin has a montane climate(Niedźwiedźand Obr˛ebska-Starklowa,1991),where the influence of height is dominant and there is elevational zonation.The region has a high annual rainfall,corresponding on average to the 900 mm isohyet(Nowosad and Wereski,2016).The average number of days with snow cover in the higher parts of the national park is estimated to be 150—155(Michna and Paczos,1972),and the mean annual air temperature is lower than 7°C(Nowosad and Wereski,2016).
Earthworm samples were collected near the town of Ustrzyki Górne,in a region characterized by the dominant association ofDentario glandulosae-FagetumKlika 1927(synonymFagetum carpaticumKlika 1927)beech forest.On the basis of the descriptions by Zarzycki(1963)and Przybylska and Kucharzyk(1999),we distinguished three sub-associations ofF.car paticum,among which four examination sites were selected(I—IV)(Fig.1,Tables I and II).At each of the sampling sites,temperature and soil moisture were measured at depths of 0—10 and 10—25 cm.Soil moisture was determined by oven drying soil samples at 105°C(ISO,1999).Soil samples collected from each examination site
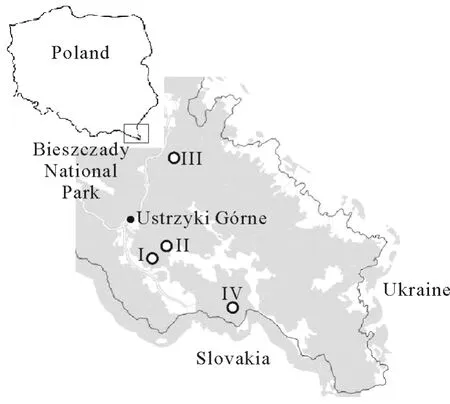
Fig.1 Map showing the four sampling sites(I—IV)within the Fagetum carpaticum beech forest near Ustrzyki Górne,Poland.The sampling sites were selected based on their understory vegetation:F.c.fetucetosum dr ymejae(site I),F.c.typicum(site II),F.c.lunarietosum(site III),and F.c.allietosum(site IV).
were analyzed for:pH in KCl,organic carbon(C)using the Tiurin method modified by Nikitin and Fishman(1969),total nitrogen(N)using a modified Kjeldahl method(ISO,1995),and the contents of the assimilable forms of phosphorus(P),potassium(K),and magnesium(Mg)using the methods described by Mehlich(1984).
Treatments for analysis of earthworm populations
The method used for earthworm sampling in the present study was the mixed method proposed by the International Standards Organization(ISO,2006).Earthworms were sampled by hand from soil blocks of 25 cm×25 cm×25 cm.From deeper layers,earthworms were expelled by flooding the surface of the well remaining after sample collection with 10 L of 0.4%formalin solution.Sampling was performed
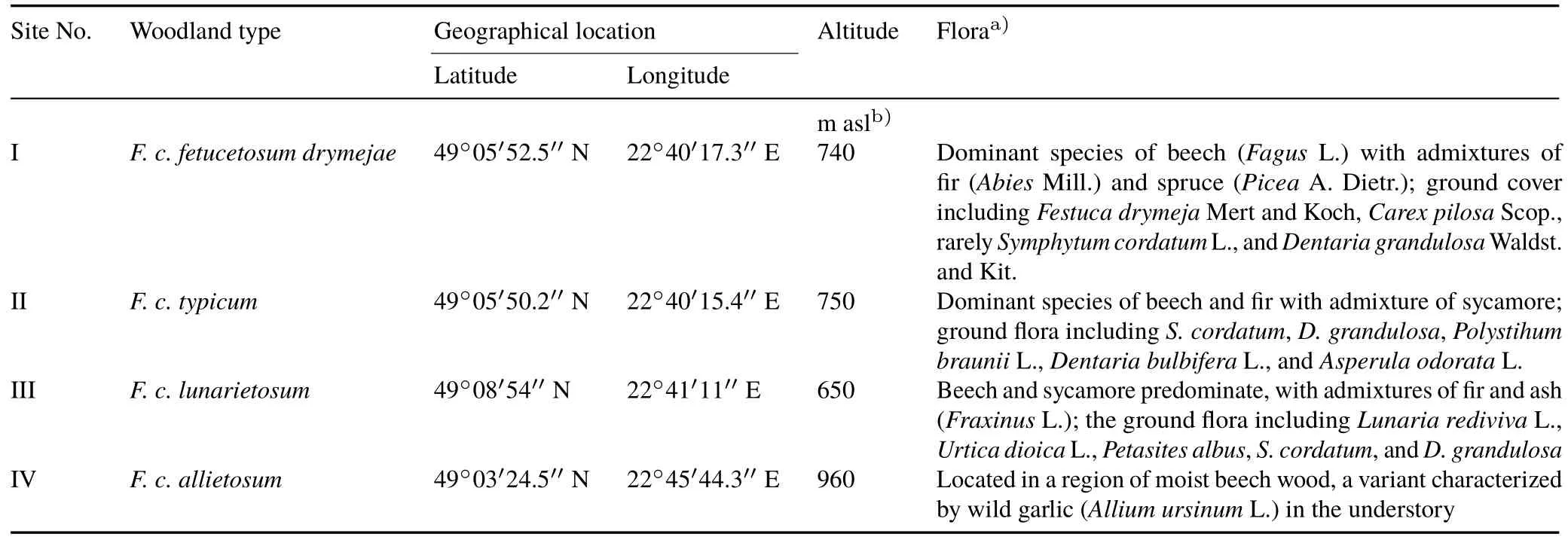
TABLE IGeographical locations,altitudes,and florae of the four Lumbricidae sampling sites in the Fagetum carpaticum beech forest near Ustrzyki Górne,Poland
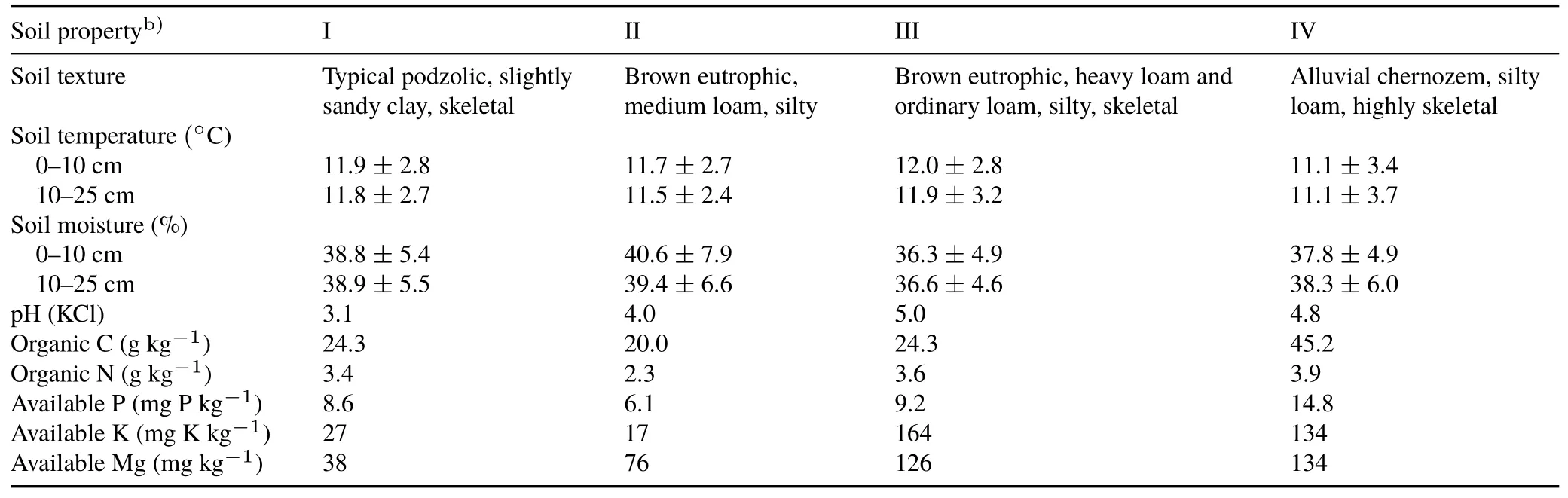
TABLE IISoil properties at the four Lumbricidae sampling sites(I—IV)a)in the Fagetum car paticum beech forest near Ustrzyki Górne,Poland
four times per year(in May,July,September,and October)in two annual cycles(2009 and 2010).According to recommendations of Zisci(1962),eight randomly selected samples were collected each time at each site.Earthworms found were washed in water,then sacrificed in 30%ethyl alcohol,and subsequently transferred into a preservative solution of 4%formalin,which was replenished after 24 h.Thereafter,the collected earthworms were identified,counted,and weighed for their masses including gastrointestinal contents.Earthworm density and biomass in soil monoliths were later recalculated in terms of square meters.The collected specimens were identified to the species level at the University of Rzeszów,using a key to the terrestrial Oligochaeta,family Lumbricidae in Poland(Kasprzak,1986).
Data analysis
To assess the Lumbricidae associations,the Shannon-Wiener species richness index(H′)and dominance index(D)were used:

wherepiis the ratio of the number of theith species to the total number of all the species(Southwood,1978)andnis the number of individuals of the examined group of species in all samples(Kasprzak and Niedbała,1981);

whereNais the number of individuals belonging to the species of interest in all investigated samples.
Results were analyzed statistically using Statistica software(version 12.5).Given that the biodiversity index(H′)values did not meet the assumptions of normal distribution(Shapiro-Wilk test,P<0.05),the non-parametric Kruskal-Wallis test and a multiple comparison of mean rankspost-hoctest were used to assess the differences between the groups(Siegel and Castellan,1988).As the earthworm density and biomass data met the assumptions of normality and homogeneity of variance,a single-factor analysis of variance(ANOVA)and least significant difference(post-hoc)test were used to assess differences between the groups.
RESULTS
In the forest ecosystem of the Bieszczady National Park,depending on the phytocoenosis of beech sub-associations,there were 7 species of earthworm at each of the sites I—III and 10 species at site IV(Table III).
At all four sampling sites,Dendrobaena alpinawas the dominant species,comprising the highest proportion in the earthworm associations:F.c.festucetosum(83.3%),F.c.typicum(66.1%),F.c.lunarietosum(52.3%),andF.c.allietosum(41.6%)(Table IV).In the soil at theF.c.typicumsite,D.alpinawas accompanied byAllolobophora cernosvitoviana(10.1%),and at theF.c.lunarietosumsite,the eudominants includedAporrectodea caliginosa(18.2%),Aporrectodea rosea(11.1%),andAllolobophora carpathica(11.1%).At theF.c.allietosumsite,A.carpathica(27.3%)andA.caliginosa(10.4%)were recorded as eudominant species.
The lowest proportions of the total number of individuals per association(<1%)at theF.c.festucetosumsite were forA.cernosvitoviana(0.9%)andEisenia lucens(0.6%),whereas at theF.c.typicumsite,E.lucens(0.6%)comprised the lowest proportion,as didDendrodrilus rubidus(0.8%)andOctolasion lacteum(0.8%)at theF.c.lunarietosumsite(Table IV).At the site characterised by the growth of wild garlic(F.c.allietosum),the subrecedents includedE.lucens(0.8%),A.cernosvitoviana(0.5%),D.rubidus(0.4%),O.lacteum(0.4%),andLumbricus terrestris(0.4%).
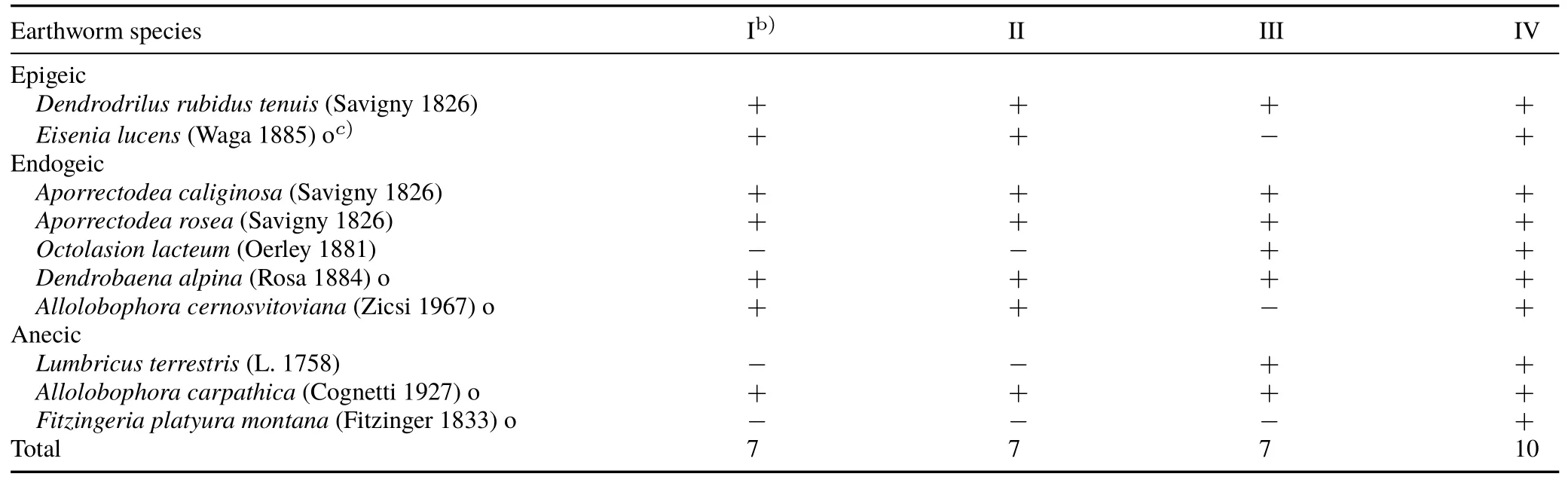
TABLE IIIEarthworm species identified at the four sampling sites(I—IV)a)in the Fagetum carpaticum beech forest near Ustrzyki Górne,Poland
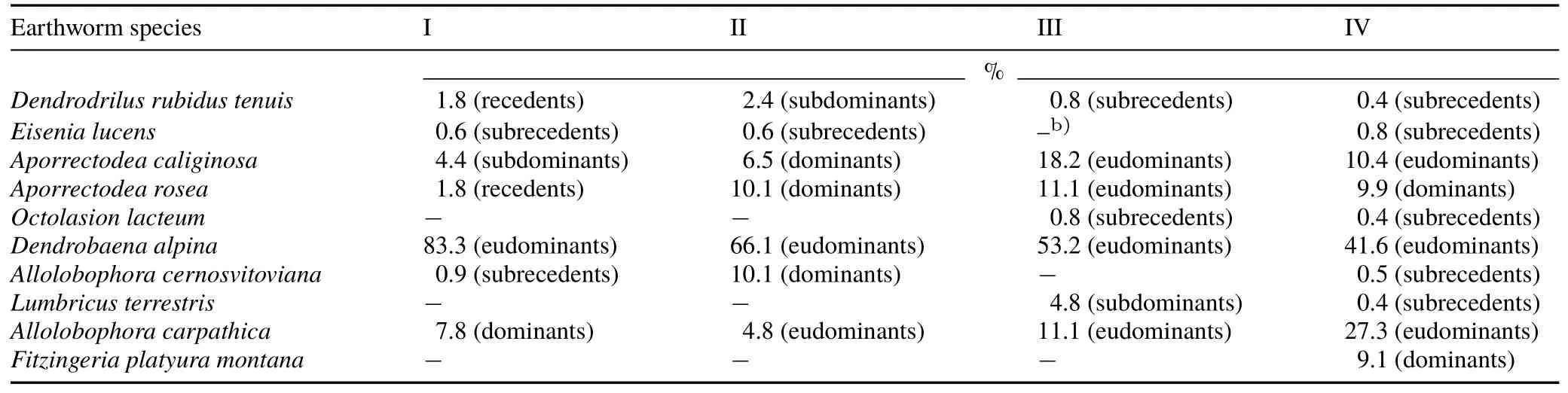
TABLE IVDominance of earthworm species,expressed as a percentage of the lowest proportion of the total number of individuals per association,at the four sampling sites(I—IV)a)in the Fagetum car paticum beech forest near Ustrzyki Górne,Poland
TheH′values for theF.c.fetucetosum dr ymejae,F.c.typicum,F.c.lunarietosum,andF.c.allietosum.sites were 0.23±0.16,0.40±0.20,0.43±0.23,and 0.52±0.12,respectively,and theF.c.allietosumsite was found to be significantly different(P<0.05)from the other three sites with regards toH′(Fig.2).
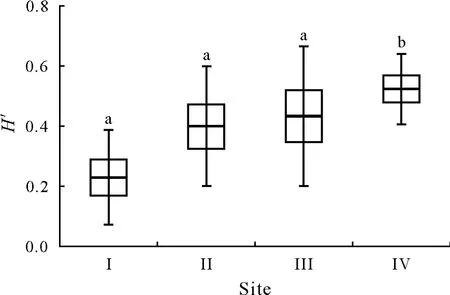
Fig.2 Box-plot of Shannon-Wiener species richness index(H′)for the four earthworm sampling sites(I—IV)in the Fagetum car paticum beech forest near Ustrzyki Górne,Poland.Horizontal lines within boxes are means(n=7),boxes are means±standard errors,and vertical lines are means±standard deviations.Different letters above the bars indicate significant differences(P<0.05)between the means.See Table I for detailed descriptions of the four sampling sites.
In terms of earthworm density,comparable values were recorded between theF.c.festucetosum(32.57±20.45 individuals m-2),F.c.typicum(48.00±17.96 individuals m-2),andF.c.lunarietosum(36.00±19.46 individuals m-2)sites,as well as between theF.c.typicum(48.00±17.96 individuals m-2)andF.c.allietosum(66.01±32.52 individuals m-2)sites(P>0.05)(Fig.3a).Similarly,the biomass of Lumbricidae was not significantly different between theF.c.fetucetosum dr ymejae,F.c.typicum,andF.c.lunarietosumsites(10.16±5.03,14.40±5.81,and 19.22±12.31 g m-2,respectively),although it was significantly higher(P<0.05)at theF.c.allietosumsite(59.71±39.53 g m-2)than at the other three sites(Fig.3b).
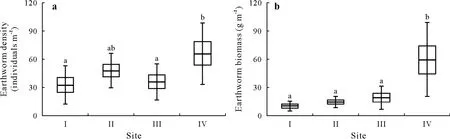
Fig.3 Box-plots of earthworm density(a)and biomass(b)for the four sampling sites(I—IV)in the Fagetum carpaticum beech forest near Ustrzyki Górne,Poland.Horizontal lines within boxes are means(n=7),boxes are means±standard errors,and vertical lines are means±standard deviations.Different letters above the bars indicate significant differences(P<0.05)between the means.See Table I for detailed descriptions of the four sampling sites.
DISCUSSION
Studies on earthworms in the Carpathian basin date back to the beginning of the 19th century,and consequently the earthworm fauna in this region is probably the best characterized in Europe(Pop,1997;Csuzdi and Pop,2008;Popet al.,2012).To date,approximately 97 earthworm species have been identified in this region,and these are notably distinguished by a high degree of endemism(40.21%)(Csuzdiet al.,2011).Among these,21 species in the family Lumbricidae have been found in the Carpathian region(Kostecka,1988;Dumnicka and Kostecka,2004),and it is estimated that the number of lumbricid earthworm species inhabiting Bieszczady National Park constitute almost 57%of the total Polish fauna(Plisko,1973;Kasprzak,1986).The abundance of Lumbricidae species in the Western Bieszczady Mountains can,to a large extent,be attributed to the abundance of natural food provided by the annual leaf fall,and the wide availability of plant litter and accumulated humus.Zhanget al.(2008)showed that the quality of plant litter as a substrate for Lumbricidae increases with the contents of N,P,K,and calcium(Ca).A further important contributory factor is the limited anthropogenic pressure in this region,which can be ascribed to the introduction of bans that directly or indirectly restrict human activity in the area of the national park(Seym of the Republic of Poland,2004).The landscape of Bieszczady comprises parallel mountain ranges,running from the northwest to southeast,and the middle reaches of rivers cut through the mountain ranges perpendicularly,thereby allowing numerous animal and plant species to penetrate into remote mountainous areas(Zarzycki,1963).These conditions create specific microclimates and facilitate the development of unique habitats for numerous species of earthworms,which include a number of endemic species(Dumnicka and Kostecka,2004).Half(five species)of the earthworms identified in the present study,namely,E.lucens,D.alpina,A.cernosvitoviana,A.car pathica,andFitzingeria platyura montana,are rare and have a limited range of distribution(Table II).Among these species,D.alpinawas characterized by its highest proportion in the association at all sites examined in Ustrzyki Górne(category of eudominant)(Table III).This is a montane species,which in Europe may be encountered in the Alps,Southern Carpathians,and Balkans(Csuzdiet al.,2005;Popet al.,2007).In Poland,it has been recorded in the Bieszczady Mountains and Eastern Beskids,where it inhabits woodland soils(Kasprzak,1986).According to Popet al.(2007),this species plays a dominant role in producing a thick(10—12 cm)black organic layer in the soil horizons and is present in soils of different types containing raw humus or mor.In the 1980s,D.alpinawas also a dominant species in Carpathian beech forests of the Bieszczady region(minimum of 11.4%to maximum of 56.0%,depending on site)(Kostecka and Butt,2015).
In the present study,the highest richness of earthworms species(10)was recorded in soils associated with the wild garlic(F.c.allietosum)variant of Carpathian beech forest(Table III),which was also characterized by the highestH′value(Fig.2).It has been shown that with an increase in the complexity of the phytocoenoses of Carpathian beech woodlands,there is an increase in several earthworm species within the three different ecological groups(Pop,1997).The high species diversity at this site could be attributable to the soil’s optimal organic carbon content(45.2 g kg-1)(Table II).
On the basis of Lumbricidae densities at the selected sampling sites(Fig.3a),it is difficult to clearly identify the factors that may contribute to the observed differences and similarities in numbers,although the underlying causes are probably related to differences in ecological niche.Among the four sites we examined,site IV(F.c.allietosum)was characterized by the highest biomass(Fig.3b),which can be explained in part by the presence of three large species,i.e.,
L.terrestris,A.carpathica,andF.platyura montana.The higher Lumbricidae biomass in soils underF.c.allietosumvegetation can also probably be ascribed to the greater availability and quality of food at this site.Each spring,site IV is flooded with water derived from the melting of snow on a polonina lying at a distance of some 300 m,which deposits large amounts of organic matter.In the present study,we failed to detect the large deep-burrowing speciesOctodrilus transpadanus(Csuzdiet al.,2015)at theF.c.allietosumsite.This species was recorded at the same site in 1986 and 1987,accounting for 10%(dominant)of the total number of species in the association(Kostecka and Skoczeń,1993).The speciesO.transpadanusoccurs in fertile mountain soils,fertilized by nearby watercourses(Kasprzak,1986),and we suspect that this species may have been displaced at theF.c.allietosumsite by the invasiveL.terrestris,which was not found at this site in studies conducted in the 1980s.The earthworm speciesL.terrestrisis typically found in soils under city lawns and in minor agricultural areas associated with the cultivation of vegetables(Kasprzak,1986).It is conceivable that this alien species has been incidentally introduced into this forest siteviatourist traffic and work associated with the securing of hiking trails.Field experiments conducted in Germany have shown thatL.terrestrismay well colonize areas previously devoid of earthworms,such as the acid soils of spruce forests,which indicates the broad adaptability of the species(Judaset al.,1997).As indicated by the findings of previous studies,the invasiveL.terrestrishas a significant influence on soil profiles,as well as the dynamics of nutrients and organic matter(Hendrix and Bohlen,2002).From a long-term perspective,invasion of alien species,such asL.terrestris,at a given site may promote pronounced changes in the local fauna of various taxonomic groups(McLeanet al.,2006;Migge-Kleianet al.,2006;McCay and Scull,2019).Displacement of native earthworms,including rare and endemic species,byL.terrestrishas been observed in the Romanian Carpathians(Pop and Pop,2006)and in the Russian Federation(Tiunovet al.,2006).The expansion ofL.terrestristypically leads to taxon disappearance,resulting in the simplification of Lumbricidae associations and contributing to reduced species diversity(Pop and Pop,2006).
In the present study,the leaf litter speciesDendrobaena octaedra(Savigny 1826)was not found at theF.c.allietosumsite,whereas it comprised 1.6%(recedents)of the total Lumbricidae number atF.c.allietosumin the 1980s(Kostecka and Skoczeń,1993).On the basis of the known ecology of this species,its current absence is not readily explicable,although in common withO.transpadanus,it is conceivable that this species has also been displaced by the invasiveL.terrestris,which has been reported to out-compete species from various ecological groups(Pop and Pop,2006).
According to Hendrixet al.(2006),the invasive potential of an alien species stems not only from its inherent properties,but also from abiotic and biotic properties of the habitat,including climatic conditions,soil-related factors,and the native fauna and flora.Therefore,F.car paticumsub-associations should be monitored for transitions,and particularly for the disappearance of dominant plants within the sub-associations,namely,Festuca dr ymejae,D.grandulosa,Lunaria rediviva,andAllium ursinumat sitesF.c.fetucetosum dr ymejae,F.c.typicum,F.c.lunarietosum,andF.c.allietosum,respectively.It would also be important to monitor soil erosion in areas with open trekking trails.From a long-term perspective,the active protection of earthworms living in Bieszczady could be considered as a worthwhile conservation measure,in conjunction with population restorations based on the introduction of Lumbricidae species into areas where they have undergone recent population declines.
CONCLUSIONS
A comparison of the findings of the present study conducted in theF.car paticumwoodlands of the Ustrzyki Górne region with those conducted in the same region in the 1980s may indicate a decrease in earthworm species diversity,in addition to a structural simplification of earthworm associations.These changes may have been caused by natural and/or human-induced succession resulting from the spread of the invasive speciesL.terrestris,despite the imposition of statutory protective measures during recent decades.To observe the long-term effect of alien species on the local Lumbricidae populations,further earthworm monitoring is required and should be coupled with the monitoring of plant communities and soil conditions in the Bieszczady National Park and its surroundings.Strategies designed to conserve the biodiversity of soil fauna and flora,including the possibility of active protection of earthworm species inhabiting Bieszczady,should be considered.
ACKNOWLEDGEMENT
We would like to thank the management of the Bieszczady National Park,Southeast Poland for making the research possible.
杂志排行
Pedosphere的其它文章
- Preface Earthworms in soil ecology and organic waste management
- Letter to the Editor Field and laboratory investigations of Lumbricus badensis ecology and behaviour
- Strategies to mitigate the adverse effect of drought stress on crop plants—influences of soil bacteria:A review
- Advances in fungal-assisted phytoremediation of heavy metals:A review
- Study of oxidative stress cadmium(Cd)-induced in Eisenia fetida based on mathematical modeling
- Behavior and respiration responses of the earthworm Eisenia fetida to soil arsenite pollution
