Behavior and respiration responses of the earthworm Eisenia fetida to soil arsenite pollution
2021-07-16YaliWANGYizhaoWUJoCAVANAGHXiuhongWANGJiangpingQIUandYinshengLI
Yali WANG,Yizhao WU,Jo CAVANAGH,Xiuhong WANG,Jiangping QIU and Yinsheng LI,*
1 School of Agriculture and Biology,Shanghai Jiao Tong University,Shanghai 200240(China)
2 School of Environmental Engineering,Henan University of Technology,Zhengzhou 450001(China)
3 Landcare Research,P.O.Box 40,Lincoln 7640(New Zealand)
(Received July 17,2020;revised August 21,2020)
ABSTRACT Soil arsenic(As)pollution from mining and industrial sources is a serious issue in China.Earthworms are considered ecosystem engineers and contribute to soil fertility development and maintenance of soil physico-chemical properties.In this study,earthworms were exposed to soils with different sodium arsenite concentrations(0,5,20,and 80 mg As kg-1)for 60 d to investigate the changes in soil properties and the responses of the earthworms(e.g.,burrowing activity and respiration).Earthworm burrowing activity decreased with increasing arsenite concentrations,and earthworm respiration was significantly lower in soils with 20 and 80 mg As kg-1 compared to 0 mg As kg-1.Changes in soil properties were also observed after incubation of As-amended soil with earthworms.Specifically,soil pH decreased,while soil electrical conductivity and contents of soil NO-3-N,Olsen-P,and available K increased.Our results suggest that arsenite negatively impacts the metabolic activity of earthworms,leading to reduced burrowing activity,which in turn modifies the effects of earthworms on soil fertility and remediation.
Key Words: As contamination,earthworm avoidance,earthworm burrowing activity,maximum burrow length,remediation,soil property
INTRODUCTION
Soil arsenic(As)contamination from mining and industrial activities is a serious issue in China,and soil As concentration can reach as high as 935 mg As kg-1soil(Huanget al.,2006,2013;Liet al.,2010;Wuet al.,2017).The As is a highly toxic metalloid to plants,animals,and humans and contamination of food crops through contaminated groundwater(Hareet al.,2019).The toxicity and bioavailability of As in soil are strongly influenced by its speciation and distribution in different soil fractions(Xuet al.,2016),which in turn influences the success of remediation strategies.
Earthworms support the bioremediation of polluted soils(Singeret al.,2001;Kouet al.,2008),including metal pollution(Sinhaet al.,2010).Metal remediation using earthworms is attributed to changes in metal formation due to earthworm burrowing,thereby increasing metal uptake by plant or leaching by soil solution(Yaoet al.,2012).Earthworms are often referred to as ecosystem engineers,as they can mix and translocate the soil constituents through devouring soil(Wuet al.,2015).In uncontaminated soils,earthworm activity can improve soil physical and chemical properties,such as soil nutrients,soil structure,soil microbial community,and plant biomass(Wanget al.,2008;Wang H Tet al.,2019).Earthworms alter soil physical and chemical properties by consuming organic matter of the soil surface and is often used for testing and assessing the efficiency of soil heavy metal remediation(Udovic and Lestan,2010).
Soil contamination may cause harmful effects on earthworms(Tanget al.,2016).Sub-lethal effects include behavioral responses,such as avoidance of contaminated soil(Yeardleyet al.,1996),changes in burrowing activity(Capowiez,2000),and respiration rate(Dasguptaet al.,2012).Furthermore,earthworm activity may influence the binding of metals to different soil fractions,thus changing their availability(Romero-Freireet al.,2015).Therefore,the sub-lethal effects on earthworms may indirectly affect soil properties.
We used the common earthworm,Eisenia fetida,to assess the effects of soil As amendment on earthworm avoidance,burrowing activity,and respiration.We also assessed changes in soil properties.This study aimed to evaluate the response of earthworms in As-contaminated soils and the corresponding effects on soil.
MATERIALS AND METHODS
Soils and earthworms
Bulk soil was collected from the suburban district of Shanghai,China,air-dried,and sieved(2 mm)prior to earthworm exposure.A sub-sample of the bulk soil was collected for chemical analysis.
We purchased earthworms(E.fetida)from a commercial breeder and placed them in the collected soils for 3 weeks to acclimate.TheE.fetidais an epigeic species;however,it exhibit a facultative endogeic behavior in homogeneous mineral soil,burrowing to a depth of 20—30 cm,as described in previous studies(Liet al.,2016;Wang Y Let al.,2019).
Arsenite exposure experiment
Before the experiment,we preliminarily assessed the acute toxicity of arsenite onE.fetida,yielding an lethal concentration for 50%(LC50)of 122 mg As kg-1(95%confidence interval:[95.6 mg As kg-1,132 mg As kg-1]).Based on this LC50,we selected 5,20,and 80 mg As kg-1dry soil as the test concentrations.
Sodium arsenite(Merck KGaA,Germany)was added as a solution to bulk soils and well-mixed to yield concentrations of 5,20,and 80 mg As kg-1dry soil and a moisture content of 35%.These treatments were then aged for 1 month,since previous studies have shown that the As bio-accessibility in alkaline soil stabilizes after 2 weeks(Tanget al.,2007).Sub-samples of soil were taken for chemical analyses after aging and prior to the addition of earthworms.
The earthworms(0.3—0.5 g)used in this study had well-developed clitellum.At the end of the experiment,we reweighed the earthworms.Before adding the earthworms to the exposure containers,earthworms were depurated for 24 h.Soils amended with and without(control)As were replicated three times,and each replicate was comprised of 50 earthworms in 3 kg treated soil in a plastic container.The containers were placed in a room at 20±2°C for 60-d incubation as previous studies indicated that the maximum accumulation of As in earthworms occurred at this time(Wang and Cui,2016).Soil moisture was maintained at 35%by adding ultrapure water.After 60 d,all earthworms were removed from the containers for analyses of burrowing activity and respiration.The residual soil was mixed and sub-sampled for chemical analysis.
Avoidance test
Avoidance tests were conducted following ISO 17512-1(ISO,2008).Briefly,one half of a plastic container(17 cm in length,11.5 cm in width,6.5 cm in height)was filled with 300 g artificial soil with arsenite(5,20,and 80 mg As kg-1),and the other half was filled with the 300 g artificial soil without arsenite(OECD,2004).The artificial soil was used as the control substrate to compare our results with other studies.Ten earthworms were added to the space of soils with and without arsenite.After 24 h,we assessed the distribution of the earthworms in the two soils.Earthworm avoidance of the As-amended soil was calculated as follows:

wherencontrol,nAs-soil,andntotalare the number of the earthworms in the control soil without As,the number of earthworms under the side of As-amended soil,and the total number of the earthworms added.There were 3 replicates for each treatment.
Earthworm burrowing activity
We used two dimensional terraria to observe the earthworm burrowing activity(Duet al.,2014;Liet al.,2016;Tanget al.,2016).Farm soil unamended with As was used to fill the terraria,and a single earthworm was added to each terrarium(Fig.1).Four replicate terraria were set up for each exposure concentration.The terraria were kept in a dark room at 20±2°C.We observed the earthworm burrowing behavior every 4 h for 7 dviathe tracing method(Duet al.,2014;Tanget al.,2016).The burrowing tracks were analyzed by the software ArcGIS10.0(ESRI,2011).
3. Great wood: The forest is a recurrent image in German fairy tales, in part because over a quarter of the country is comprised of forest land. In the Grimms tales, the forest is a supernatural world, a place where anything can happen and often does.

Fig.1 Typical earthworm burrowing tracks in a terrarium.Data were collected 6 times a day from March 8,2018 to March 14,2018.Raw data can be seen in File S1(see Supplementary Material for File S1).
Earthworm respiration
We tested earthworm respiration according to Liet al.(2015).Briefly,farm soil unamended with arsenite was sterilized twice according to Zhenget al.(2017)(temperature,121°C;pressure,0.3 Mpa;time,90 min)to avoid CO2production from microorganisms(Wolfet al.,1989).Soil(300 g)was added to a conical filtering flask(Fig.S1,see Supplementary Material for Fig.S1),with 10 pre-exposed earthworms from each bulk treatment,yielding 4 replicates for each of the 0(control),5,20,and 80 mg As kg-1treatments.Respiration was measured as the CO2production over 3 d with results expressed in mL CO2kg-1.
Analysis of soil properties
Individual soil sub-samples(approximately 600 g)were air-dried and passed through a 2-mm sieve prior to analyses.Soil chemical properties were measured following Baoet al.(2000).Soil pH was measured at a soil to water ratio of 1:2.5(weight:volume),and the electrical conductivity(EC)was measured at a soil to water ratio of 1:5(weight:volume).Each soil sample was divided into five As fractions:nonspecifically absorbed As(Av-As),As bound to Al oxides(Al-As),As bound to Fe oxides(Fe-As),As bound to Ca(Ca-As),and the residual As(Re-As)according to Wuet al.(2006).Briefly,soil(1.0 g)was sequentially extracted by:1 mol L-1NH4Cl(20 mL,20°C for 30 min)for Av-As,0.5 mol L-1NH4F(20 mL,20°C for 1 h)for Al-As,0.1 mol L-1NaOH(20 mL,20°C for 17 h)for Fe-As,0.5 mol L-1H2SO4(20 mL,20°C for 1 h)for Ca-As,and HNO3/H2O2(3:1,8 mL,110°C for 5 h)digestion for Re-As.The supernatant was decanted and filtered(0.22μm)prior to As analysis.Soil total K,available K,five As fractions,and total As were analyzed by ICP-OES(Perkin Elmer Optima 8000,Perkin Elmer,USA).
Statistical analyses
All statistical analyses were performed by SPSS 22(SPSS Inc.,Chicago,USA).One-way analysis of variance followed by Duncan multiple comparisons test was used to assess the differences between treatments.Relationships among the earthworm burrowing activity(burrow length),avoidance(avoidance rate),respiration rate,and chemical properties of soils(pH,EC,NH+4-N,NO-3-N,Olsen-P,Available K,Av-As,Al-As,Fe-As,Ca-As,and Re-As)were identified by principal component(PC)analysis.Values were expressed as means±standard errors(SE),andP<0.05 was used as the level of significance.
RESULTS
Earthworm avoidance
Earthworm avoidance increased as the As concentration increased,reaching 82%in the 80 mg As kg-1treatment.It was significantly greater in the 20 and 80 mg As kg-1treatments than in the control(Fig.2).
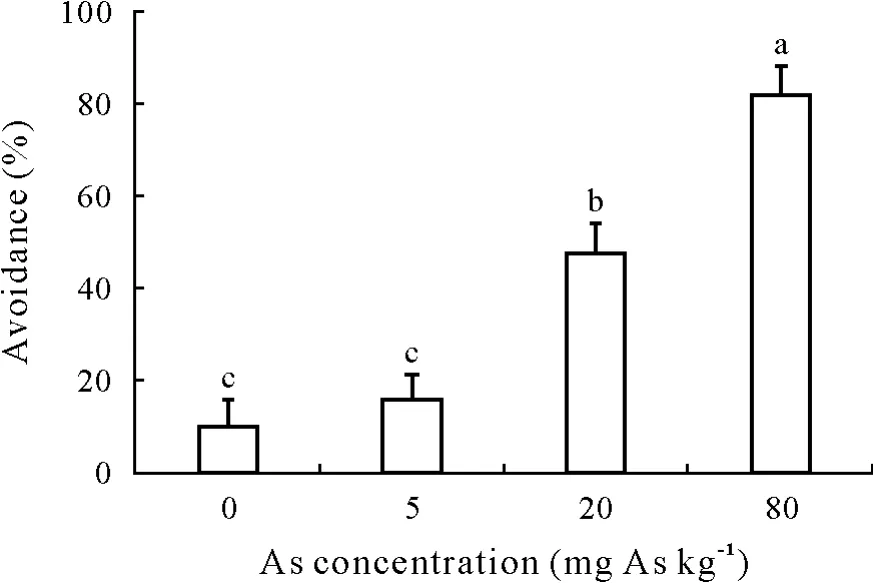
Fig.2 Avoidance of E.fetida exposed to soil amended with sodium arsenite at 0,5,20,or 80 mg As kg-1 for 48 h.Vertical bars indicate standard errors of the means(n=3).Bars with different letters are significantly different at P<0.05.
Earthworm burrowing activity
Earthworm burrow length increased continuously over time for all treatments,but the burrowing activity reduced in all As-amended treatments compared to no As control and almost ceased at the highest As concentration toward the end of the test period(Fig.3).The maximum burrow length(MBL)at day 7 decreased with As concentration,showing a dose-dependent decrease.
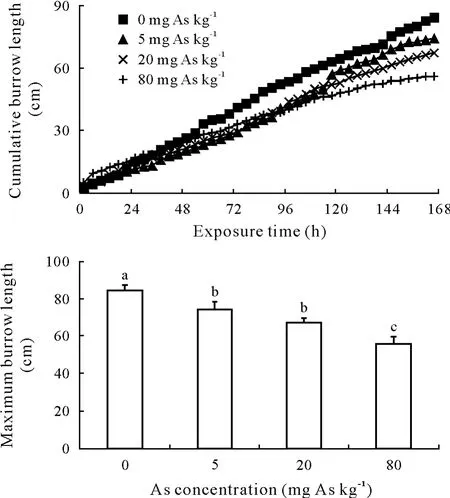
Fig.3 Cumulative earthworm burrow length and the maximum burrow length at day 7 in soil amended with sodium arsenite at 0,5,20,or 80 mg As kg-1.Vertical bars indicate standard errors of the means(n=3).Bars with different letters are significantly different at P<0.05.
Earthworm respiration rate
Earthworm respiration was significantly inhibited in the 20 and 80 mg As kg-1treatments(Fig.4).No changes in CO2production were observed 3 d after earthworms were removed,indicating that the amount of microorganisms introduced by the earthworms was negligible,thus CO2production is solely attributed to earthworm respiration.Earthworm biomass changed significantly after exposure to As-amended soil compared to the earthworm biomass before the experiment(Table SI,see Supplementary Material for Table SI).

Fig.4 Respiration rate of the earthworms(E.fetida)in soil amended with sodium arsenite at 0,5,20,or 80 mg As kg-1.Vertical bars indicate standard errors of the means(n=3).Bars with different letters are significantly different at P<0.05.
Soil chemical properties
The properties of the original soil before arsenite addition are presented in Table I.Soil chemical properties changed over the course of the testing regime.
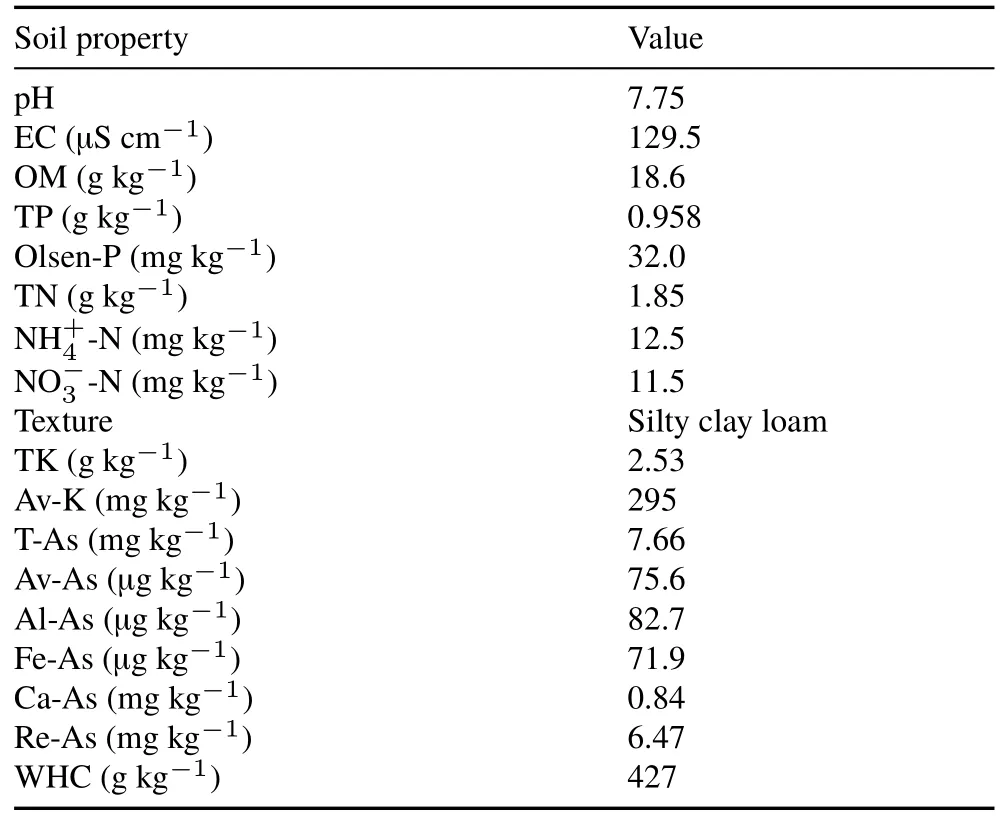
TABLE IProperties a)of the soil collected from the suburban district of Shanghai,China
Soil As fractions
Total As content(7.7,12.1,26.4,and 86.4 mg As kg-1for soils amended with 0,5,20,and 80 mg As kg-1,respectively)in all soils did not change significantly after 1 month of aging,although they were lower after removal of earthworms after 60 d exposure.However,significant differences were only observed in the soil amended with 80 mg As kg-1(Fig.5).Contents of As in the five As fractions decreased in the order of Re-As>Ca-As>Fe-As>Al-As>Av-As,with Re-As comprising 74.5%of the total As in the control and Ca-As comprising 11.1%of the total As in the soil amended with 80 mg As kg-1.Total As content only significantly changed in the 80 mg As kg-1treatment after incubation with earthworms,but the distribution of As fractions changed in all As-amended treatments,with a decrease of Re-As fraction,and increases of the Fe-As,Al-As and Av-As fractions.
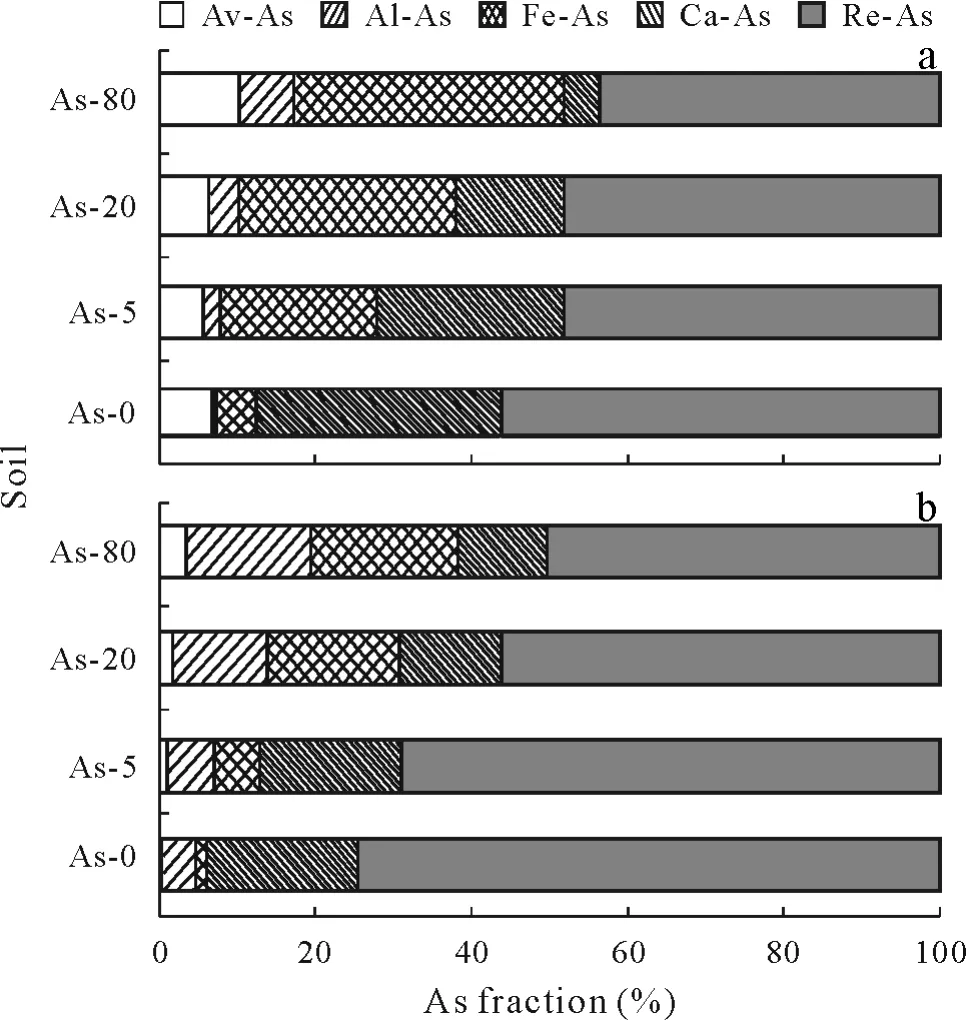
Fig.5 Arsenic fractions in soils amended with sodium arsenite at 0(As-0),5(As-5),20(As-20),or 80(As-80)mg As kg-1 after 30-d aging(a)and 60-d incubation with earthworms following 30-d aging(b).Av-As=non-specifically absorbed As;Al-As=As bound to Al oxides;Fe-As=As bound to Fe oxides;Ca-As=As bound to Ca;Re-As=residual As.
Soil pH and EC
After 1 month of aging,pH in As-amended soils was significantly lower than the control soil without As,but there were no significant differences among As-amended soils(Fig.6).Addition of earthworms decreased soil pH after 60-d incubation,with the lowest pH in the 20 mg As kg-1treatment.Soil EC increased slightly with increasing As concentrations in the aged soils amended with As,and this effect was more pronounced in soils after 60-d incubation with earthworms.

Fig.6 pH and electrical conductivity in soils amended with sodium arsenite at 0,5,20,or 80 mg As kg-1 after 30-d aging(Soil 1),followed by 60-d incubation with earthworms(Soil 2).Vertical bars indicate standard errors of the means(n=3).Bars with different letters are significantly different at P<0.05.
Soil nutrient properties

TABLE IINutrient properties of soils amended with sodium arsenite at 0(As-0),5(As-5),20(As-20),or 80(As-80)mg As kg-1 before(Soil 0)and after(Soil 1)30-d aging,followed by 60-d incubation with earthworms(Soil 2)
Relationships between earthworm behavior and soil properties
Pearson’s correlation analysis results are shown in Table SI.These results can be considered in 6 blocks:i)correlations between earthworm behavior data(respiration was strongly negatively correlated with earthworm avoidance,while MBL was positively correlated with respiration;ii)correlations between total As and As fractions(all parameters were significantly strongly positively correlated with each other,except for Ca-As);iii)correlations between total As and As fractions and earthworm behavior(total As and As fractions were positively correlated with avoidance,but negatively correlated with MBL and respiration rate);iv)correlations between general soil properties(only EC was positively correlated withv)correlations between As fractions and general soil properties(generally As fractions were positively correlated with EC,and Av-K,but negatively correlated with pH,,and Olsen-P);and vi)correlations between earthworm behavior data and general soil properties(EC was positively correlated with avoidance,whilewas negatively correlated with avoidance,was negatively correlated with MBL,and EC andwere negatively correlated with respiration).
Principal component analysis of the aged soils showed that the first two PCs(PC 1 and PC 2)accounted for 89.2%of the overall variability(Fig.7a,Fig.S2a,see Supplementary Material for Fig.S2a).The PC 1,comprising soil total As,five As fractions,andaccounted for 78.2%of the total variability.After incubation with earthworms for 60 d,EC andconcentration were more tightly clustered within the two clusters,while Av-K was associated with a completely different cluster(Fig.7b,Fig.S2b,see Supplementary Material for Fig.S2b).Distribution of the aged soils with different concentrations of As changed after incubation with earthworms(Fig.S2).Soil without As amendment was initially located on the far left,but soils with 0 and 20 mg As kg-1moved to the right after incubation with earthworm.The first two PCs accounted for 90.1%of the total variability,with PC 1,including earthworm avoidance and soil EC,Av-K,total As,and five As fractions accounting for 78.8%.

Fig.7 Principal component(PC)analysis of soil properties and earthworm behavior after 30-d aging(a),followed by 60-d incubation with earthworms(b).EC=electrical conductivity;Av-K=available K;T-As=total As;Av-As=non-specifically absorbed As;Al-As=As bound to Al oxides;Fe-As=As bound to Fe oxides;Ca-As=As bound to Ca;Re-As=residual As;MBL=maximum burrow length.
DISCUSSION
Earthworms were sensitive to As amendment,with a significant increase in avoidance behavior at≥20 mg As kg-1(Fig.2).Other studies have also demonstrated earthworm avoidance of chemical contamination,including Cu(Xinget al.,2017),ether(Duet al.,2014),enrofloxacin(Liet al.,2015),and Hg(Tanget al.,2016).Reduced burrowing activity has been observed in soils contaminated with a range of chemicals,including pesticides(Capowiez and Bérard,2006;Dittbrenneret al.,2011),ethers(Duet al.,2014),and heavy metals(Tanget al.,2016,Wang Y Let al.,2019).We did the post-exposure behavioral test in this study,with similar results of earthworms toAporrectodea caliginosaandLumbricus terrestrisunder imidacloprid exposure(Dittbrenneret al.,2011).The total burrow length decreased with increasing pollutant concentrations.Our results suggest that the effect of As amendment on burrowing behavior may be caused by physiological damages in the earthworms,which was consistent with the results of Dittbrenneret al.(2011).This effect persisted even after the earthworms were removed from the As-amended soil,suggesting permanent injury to the earthworm.Similarly,we observed that respiration was reduced in earthworms previously exposed to As,suggesting decreased metabolic activity.Other studies also reported reduced respiration after exposure to chemicals(Tanget al.,2016).Reduced metabolic activity of earthworms may provide an explanation for the reduced MBL,i.e.,earthworms could not sustain their burrowing activity.
Many factors may influence the availability and binding nature of As in soil,including concentrations of phosphate,Al,Fe,and organic matter and pH(Bhumbla and Keefer,1994;Huang,1994;Langdonet al.,2003).Sequential extraction techniques provide a method to assess how As is bound to different soil components.Using this approach,the non-specifically absorbed As(Av-As)is the most weakly bound and is considered the most bioavailable form(Yu and Liao,2016),whereas the residual component is the most strongly bound and unavailable.We found that incubation with earthworms increased the potential bioavailability of As as reflected by a reduction in Re-As and an increase in the more available Fe-As.This is consistent with Sizmuret al.(2011),who observed soil residual As fraction decreased after incubation with earthworm.The mechanism underlying this change is unclear.
Earthworms play an important role in agro-ecosystems in maintaining soil fertility through improving soil nutrient properties by earthworm movementviaingestion of soil particles and modification of soil microflora(Choosaiet al.,2010;Pérèset al.,2011).Soil pH was lower after incubation with earthworms(Fig.6a).Increased soil microbial activities following earthworm inoculation and secretions of earthworms may change the degradation or metabolism of organic matter in soils,thereby changing soil pH(Wanget al.,2013;Xuet al.,2019).However,the mechanism of pH decrease in this study requires further exploration.Soil EC increased with increasing As concentrations(Fig.6b),perhaps due to the addition of metal salt and increased Av-K in soil.Elevated Av-K induced by earthworm has been reported in other studies(Choosaiet al.,2010),and it is attributed to K-solubilizing bacteria(Wuet al.,2012).
Soil incubation with earthworms also greatly influencedconcentrations,which in turn suggests an influence on N cycling in soil.Specially,the increasedmay arise from the stimulation of microorganisms that mineralize organic N(Liet al.,2002).Soil nitrification process can increase the transformation of(Wanget al.,2008).In this study,soilwas negatively correlated with(correlation coefficient=-0.884),confirming the transformation of(Table SII,see Supplementary Material for Table SII).
Incubation of soil with earthworms markedly increased soil Olsen-P in this study,similar to previous study(Singhet al.,2016).Earthworm activity influences soil Olsen-Pviatheir escape and avoidance behavior(Givaudanet al.,2014).Moreover,increased earthworm activity may cause soil particles in the gut to be more finely ground,thus enhancing the release of nutrients or minerals from organically bound N,P,and K(Singhet al.,2017).This is similar to the result of Suárezet al.(2004)that earthworm activity influenced soil total P and different P fractions.
CONCLUSIONS
Arsenite addition reduced the respiration rate and burrowing activity of earthworms.Inoculation of earthworms markedly changed soil properties and As fractionation.Earthworms made As more active in soils,which may induce more toxic effects to soil organisms and enhance the bioaccumulation of hyperaccumulators during soil remediation.Physiological responses of earthworms to As amendment helped to develop the biomarker of soil As contamination.Understanding the interaction of soil contaminants and earthworms and the subsequent changes in soil properties caused by earthworms increased our knowledge of As environmental toxicity and provided valuable information for future environmental monitoring,assessment,and remediation of soil As contamination.
ACKNOWLEDGEMENT
This study was financed by the National Key R&D Program of China(No.2017YFD0501405),the National Natural Science Foundation of China(No.41471203),the Natural Science Projects of Henan University of Technology,China(No.2019BS037),and the Key Projects of Shanghai Municipal Agricultural Commission,China(No.2016 6-3-2).
SUPPLEMENTARY MATERIAL
Supplementary material for this article can be found in the online version.
杂志排行
Pedosphere的其它文章
- Preface Earthworms in soil ecology and organic waste management
- Letter to the Editor Field and laboratory investigations of Lumbricus badensis ecology and behaviour
- Strategies to mitigate the adverse effect of drought stress on crop plants—influences of soil bacteria:A review
- Advances in fungal-assisted phytoremediation of heavy metals:A review
- Study of oxidative stress cadmium(Cd)-induced in Eisenia fetida based on mathematical modeling
- Biological weathering of phlogopite during enriched vermicomposting
