Study of oxidative stress cadmium(Cd)-induced in Eisenia fetida based on mathematical modeling
2021-07-16YucuiNINGHaoranZHOUEnzeWANGCongminJINYingYUXuCAOandDongxingZHOU
Yucui NING,Haoran ZHOU,Enze WANG,Congmin JIN,Ying YU,Xu CAO and Dongxing ZHOU,*
1 College of Resources and Environmental Science,Northeast Agricultural University,Harbin 150030(China)
2 College of Hor ticulture Science and Engineering,Shandong Agricultural University,Taian 271018(China)
3 Depar tment of Grain Engineering,Heilongjiang Communications Polytechnic,Qiqihar 161002(China)
4 Institute of Microbiology,Heilongjiang Academy of Sciences,Harbin 150030(China)
(Received December 14,2018;revised May 9,2019)
ABSTRACT As a sensitive biological indicator,earthworms are widely used to monitor various pollutants of soil and provide an early warning for soil pollution.However,because many indices are involved in the exposure-induced oxidative stress response,practical applications of these indices are quite inconvenient.Therefore,it is appropriate to investigate the key monitoring index for use in early warning and pollution monitoring.Using Eisenia fetida as an experimental model in an indoor simulation experiment,the mathematical modelling of the effect on oxidative stress in earthworms under cadmium(Cd)stress was studied.The test lasted 40 d,with the removal of one earthworm every 10 d.The Cd2+concentration gradient was set as 0,1,10,20,100,200,400,and 800 mg kg-1 dry weight.The earthworms were divided into two sections from the clitellum for the determination of total protein(TP)and peroxidase(POD),superoxide dismutase(SOD),glutathione-S-transferase(GST),glutathione peroxidase(GPX),catalase(CAT),malondialdehyde(MDA),and acetylcholinesterase(AChE)activities.Results showed that POD was the key index of oxidative stress in head tissues after 10 d of exposure,TP was the key index at 20 d,and POD became the key index again at 30 and 40 d.By contrast,in tail tissues,MDA and SOD were the key indices at an exposure time of 10 d,GPX at 20 d,CAT and TP at 30 d,and POD and MDA at 40 d.These results contribute to establishing a scientific method for ecotoxicological diagnosis and revealing the mechanism of soil Cd toxicity.
Key Words: biological indicator,biomarker,earthworm,factor analysis,heavy metal,pollution monitoring,natural soil,soil pollutant,TOPSIS
INTRODUCTION
In recent years,international organizations and domestic environmental agencies have recognized that the real influence of cadmium(Cd)on an ecosystem cannot be based solely on monitoring and analysis of the heavy metal content because this does not provide an indication of the harmful effects of heavy metals on the biota(Calisiet al.,2013).Thus,studies on the effects of contamination on the biota and its functions are of great significance and have been highly emphasized by various scholars both at home and abroad.It is an important task to screen sensitive biomarkers,establish a scientific method for ecotoxicological diagnosis,and reveal the mechanism of soil Cd toxicity(Amiardet al.,2006;Zhaoet al.,2015;Akhtaret al.,2016;Wang Y Tet al.,2016).
Earthworms are commonly used as test organisms when testing the toxicity of related pollutants(OECD,1984,2016;WHO,1993;ISO,2008).Numerous scholars have conducted tests of the toxic effects of pollutant stress in earthworms according to such standards.However,most of the tests used the filter paper method(An,2005;Liet al.,2011),solution method(Belfroidet al.,1993;Quet al.,2005)and artificial soil method(Songet al.,2009;Unrineet al.,2012).Few studies have been conducted using the actual environmental substrate or the primary environment of the earthworm:natural soil.
A Cd stress test in artificial soil showed that a factor analysis approach can be used to screen the enzyme indices of oxidative stress in earthworms effectively and directly(Zhouet al.,2016a).Furthermore,the accuracy and scientific value of earthworms as biomarkers for monitoring heavy metals and providing an early warning of contaminated soil were validated(Ninget al.,2018).However,it has rarely been reported or demonstrated that the factor analysis approach could be applied to early warning in an actual polluted environment.
In this study,Eisenia fetidawas used as the test organism,and a Cd stress test was conducted in a natural soil ecological system.In previous studies using artificial soil(Zhouet al.,2016a,b),the key monitoring indices selected at the start and end stages of stress were similar,which led to an inability to accurately define the degree of poisoning or the detoxification mechanism of earthworms under different Cd concentrations.In this study,the model(TOPSIS)Technique for Order Preference by Similarity to an Ideal Solution was used to solve these mechanisms.The goal was to provide a theoretical basis and an accurate,sensitive,and scientific means to achieve early warning of heavy metal pollution in a soil ecosystem.
MATERIALS AND METHODS
Chemicals,animals,and soil tested
The test material used in the study was Cd with CdCl2·2.5H2O as an analytical reagent.It was purchased from Guangfu Science and Technology Development Co.,Ltd.(Tianjin,China).Phosphate buffer saline(PBS)powder,an analytical reagent,was obtained from Coolaber Science Production(Coolaber Science&Technology Co.,Ltd.,China).Each bag was dissolved in deionized water to 1 L,and the pH was 7.3.
Eisenia fetidaworms were obtained from an earthworm farm in Xiangfang District,Harbin,China.Adult earthworms with individual masses of 300 to 500 mg were selected for the toxicity test.
To provide more authentic data on the effects of Cd stress on the protein and enzyme activities of earthworms,the study was conducted in the primordial growth environment of the earthworm,using a mixture of 70%phaeozem and 30%cow dung by weight.The test material was thoroughly mixed,and the moisture was adjusted to 40%of the maximum waterholding capacity with deionized water.The phaeozem was collected from the top 20-cm layer at a nature reserve and was air-dried.Large plant residues and pebbles were removed.The cow dung was semi-decomposed manure collected from the Liangshun Resource Station in Harbin after natural drying.The phaeozem and cow dung were ground and then sifted using a 5-mm sieve,and the agrochemical properties were measured as described by Bao(2000).The results are listed in Table I.

TABLE IAgrochemical properties of test soil and cow dung
Test design
Based on the results of an acute toxicity test and the oxidative stress test in artificial soil(Zhouet al.,2016a,b),the Cd stress concentrations were set at 0(control),1,10,20,100,200,400,and 800 mg kg-1dry weight.The tests were conducted in plastic boxes(19 cm×12.5 cm×4.5 cm),to each of which 200 g of wet-weight test soil was added.The Cd solutions were sprayed into the soil,and the mixtures were thoroughly stirred.The mixtures were allowed to stand for more than 48 h at room temperature to ensure that the soil samples were fully mixed with the Cd solutions.Then,small amounts of deionized water were added to ensure that the moisture content was approximately 40%of the maximum capacity.Five replicates of each concentration were performed.
Earthworm preparation
The earthworms were put on moist filter paper in a dark environment for 24 h to void their gut contents.The surfaces of the earthworms were then cleaned and dried before they were allowed to acclimate to the test soil.At least 2 weeks later,uniformly sized adult worms were selected for toxicity testing after another 24 h of gut cleaning.The earthworm mortality rate was less than 1%during the experiment.
Ten earthworms were placed in each plastic box,which was then covered with perforated film to prevent the soil from drying.The boxes were placed in a climate chamber at 20±2°C,80%humidity,and provided with continuous illumination to prevent earthworm escape.
Sample collection
The experiment lasted 40 d,during which one randomly selected earthworm was removed every 10 d.The surface of the earthworm was washed with deionized water and dried with filter paper.Then,the earthworm was immediately frozen in a-80°C freezer.
Owing to the heterogeneous distribution of various detoxification enzymes(Riberaet al.,2001;Martin-Diazet al.,2009;Zhouet al.,2016a),the earthworm was cut into two sections at the clitellum bottom:the pro-clitellum and clitellum section was designated head tissues(Eh),and the post-clitellum was designated tail tissues(Et).The Ehand Etwere separately placed in glass homogenizers.To each tissue,a volume of PBS buffer solution 9 times the tissue quality(accurate to 0.0001 g)was added.The homogenate was centrifuged for 30 min at 4°C and 3 500 r min-1,and then the supernatant was collected and stored in a-20°C freezer for measurements of the various indices.
Determination of indices
The total protein(TP)content was determined using the Coomassie brilliant blue method(Bradford,1976).The peroxidase(POD)activity was measured using the principle of POD catalysis of hydrogen peroxide(Habiget al.,1974).The superoxide dismutase(SOD)activity was measured as described by Misra and Fridovich in a reaction mixture containing 0.1 mol L-1carbonate buffer,0.2 mmol L-1EDTA,0.34 mmol L-1adrenaline,and 30μL sample(Misra and Fridovich,1972).The rate of adrenaline autoxidation was monitored at 480 nm,and the degree of inhibition was assessed.The glutathione peroxidase(GPX)activity was determined using the 2-thio-2,4-dinitrobenzoic acid colourimetric method(Paglia and Valentine,1967).The glutathione-S-transferase(GST)activity was determined using the 2-chloro-2,4-dinitrobenzene colourimetric method(Songet al.,2009).The catalase(CAT)activity was determined using the method described in Martin-Diazet al.(2009).The acetylcholinesterase(AChE)activity was measured using 1,3,5-trinitrobenzene colourimetry(Ellmanet al.,1961).The malondialdehyde(MDA)content was measured using a reaction with thiobarbituric acid(TBA)(Buege and Aust,1978).
Factor analysis
In this study,factor analysis was used to screen out key monitoring indices after the various exposure times.This mathematical model was established as described in a previous article(Zhouet al.,2016a).The key features of factor analysis are to solve eigenvaluesviathe eigenvalue equation and calculate the contribution rate and cumulative contribution rate of the principal factors using the correction coefficient matrix(Dinget al.,2011;Wang J Jet al.,2016).In this study,principal component analysis was used to extract the principal factors,and the maximum-variance algorithm was used for factor rotation.
After the establishment of the factor model,the factors were expressed as a linear form of the variable,and then the factor scores were calculated and analysed statistically.The entire analysis process was performed by the statistical analysis software SPSS 19.0 and MATLAB 2011.
TOPSIS
The model TOPSIS is a sequential optimization technique of ideal target similarity.It has been recognized as being extremely effective at multi-objective decision analysis,which can significantly improve its scientific accuracy and manoeuvrability(Wang and Elhag,2006;Wang and Lee,2007).In this study,TOPSIS was applied to analyze the degree of toxicity to the earthworms after various stress concentrations and times,and then to explore the detoxification mechanism in earthworms under heavy metal stress.
To eliminate the issues caused by differences between the dimensions of the biochemical indices,the results for the experimental treatments were expressed as the percentage of the control treatment:
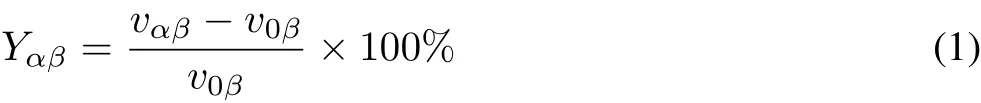
whereYαβis the dimensionless value of the oxidative stress index afterβd ofαmg kg-1Cd stress,vαβis the measured value of the oxidative stress index afterβd ofαmg kg-1Cd stress,andvoβis the measured value of oxidative stress in the blank control group.
There arepCd concentrationsD1,D2,···,Dp,and each concentration hasnoxidative stress indicesX1,X2,···,Xn.The absolute values of the percentage change of indices were used to establish the characteristic matrix:
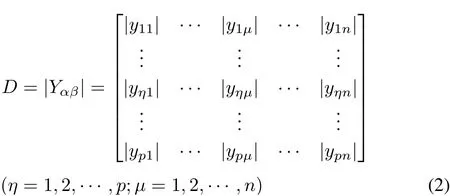
Identifying the optimal solutionand the inferior solution
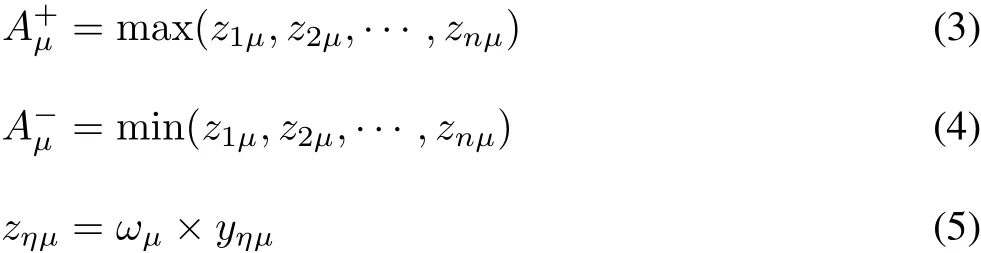
whereωμis the weight of every indicator of oxidative stress.
The Euclidean distance was calculated:
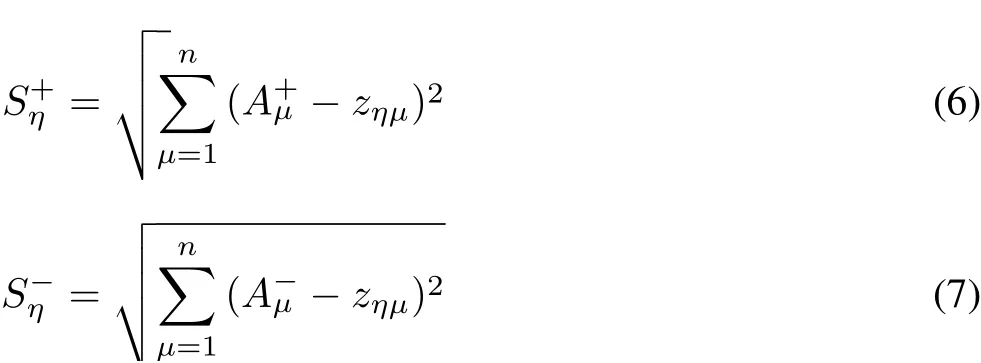
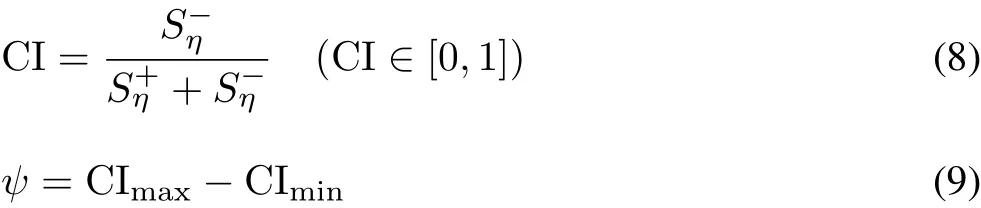
where CImaxand CIminare the maximum and minium values of CI,respectively.Let the weights of the oxidative stress indices be equal and all indices be“expenditure indices”.The entire analysis process is carried out using the statistical analysis software DPS7.05.
RESULTS
Aptness of factor analysis
Some of the oxidative stress indices in different tissues of earthworm were inhibited and some were promoted.Thus,Yαβcan have both positive and negative values and cannot be checked using Kaiser-Meyer-Olkin and Bartlett’s spherical statistics.Based on previous research(Liet al.,2012;Zhouet al.,2016a),this study used the correlation coefficient to check the aptness of the factor analysis.From the correlation coefficient matrix in Fig.1,85.764%of the correlation coefficients were greater than 0.1,indicating that a strong linear dependence existed between the variables.Therefore,it was appropriate to study the relationships among the indices by factor analysis.
As shown in Fig.1,after 10 d of exposure to Cd,there was a significant correlation between GPX and POD in the head tissues,whereas the GPX in the tail tissues showed a significant correlation with MDA.After 20 d of stress,the MDA in the head tissues was significantly correlated with POD,and POD was significantly correlated with TP.When the stress time was extended to 30 d,the GPX was significantly correlated with SOD in the earthworm head tissues,and in the tail tissues,TP was significantly correlated with SOD and CAT.When the stress time reached 40 d,the GPXin the tail tissues was significantly correlated with SOD.
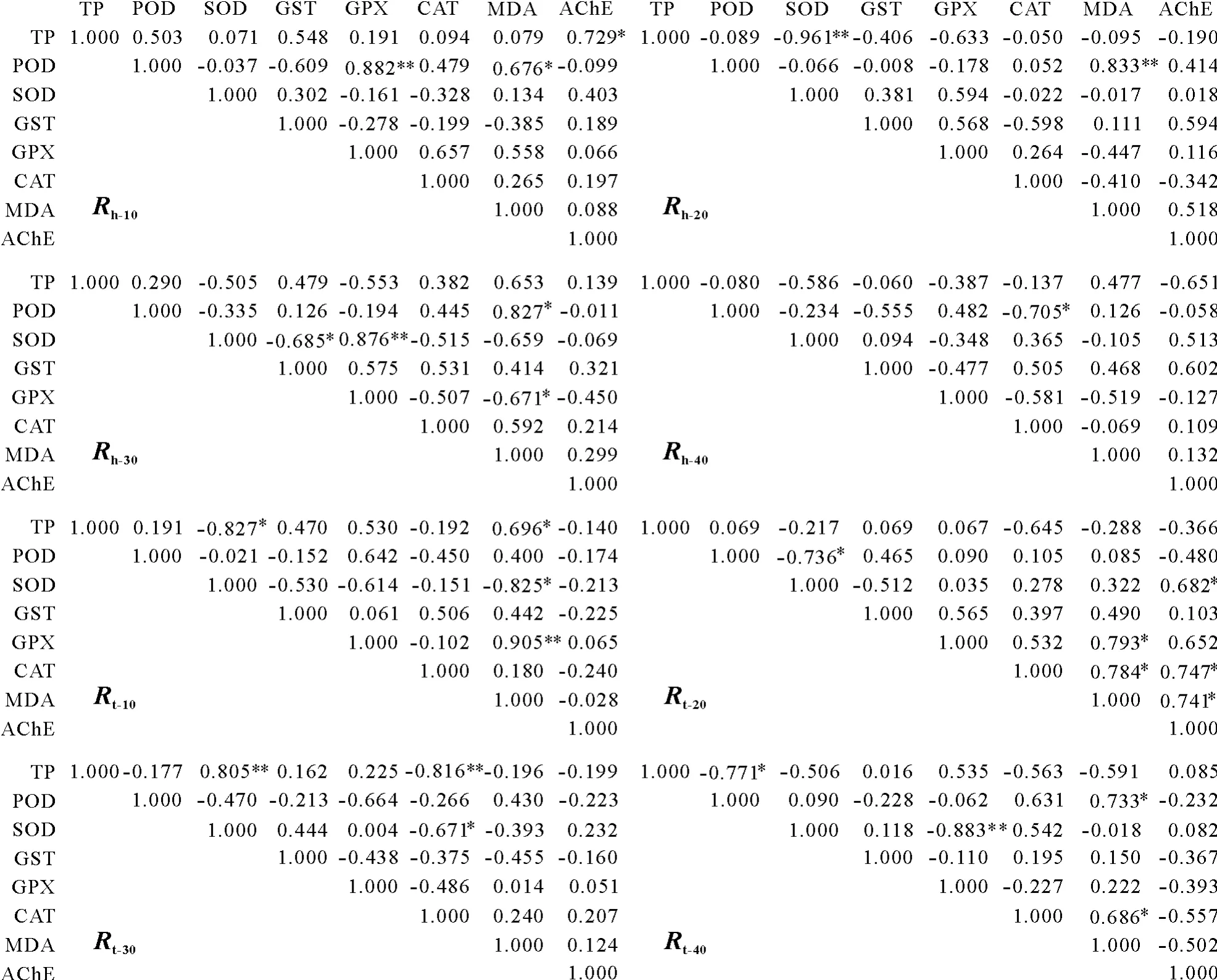
Fig.1 Correlation coefficient matrix of oxidative stress indices of earthworm head and tail tissues under various Cd stress days.*indicates significant correction(P<0.05)and**indicates extremely significant correction(P<0.01).R h-a=the correlation matrix for earthworm head tissues after a d of Cd stress;R t-a=the correlation matrix for earthworm tail tissues after a d of Cd stress;TP=total protein;POD=peroxidase;SOD=superoxide dismutase;GST=glutathione-S-transferase;GPX=glutathione peroxidase;CAT=catalase;MDA=malondialdehyde;AChE=acetylcholinesterase.
Screening of oxidative stress indices
The principal factors were extracted by principal component analysis according to correlation coefficients,and the value of the correlation coefficient was screened by an eigenvalue greater than 1.The variance contribution rate and cumulative variance contribution rate of the principal factors were obtained to check the effects of factor extraction.
In this study,factor-loading matrices were obtained by performing an orthogonal rotation using the maximum variance method.At the same time,the information retention of variables was ascertained based on their communalities to verify the scientific accuracy of the screening results.All parameters are listed in Table II.
The cumulative contribution rate of principal factors for the 30-d stress treatment was 69.312%in the head tissues.For all remaining stress time,the cumulative contribution rates were higher than 80%.Specifically,the cumulative contribution rate of three principal factors extracted from the results of the tail tissues after the 20-d stress achieved the maximum value(90.646%)in this study.That is,the three principal factors explained 90.646%of the data information of the oxidative stress indices.Throughout all the treatments,the average cumulative contribution rate was 82.907%,which met the principle of factor analysis.Therefore,the factor extraction method was reasonable.
Factor loadings tended to polarize after the data were rotated,indicating that the principal factors had obvious biological significance.In the head tissues,after 10-d Cd stress,three principal factors were extracted,the first of which explained 37.443%information of the original data.According to the analysis of the factor-loading matrix,POD played a major role in the first principal factor,with a factor loading of 0.928,followed by GPX at 0.876.For the second principal factor,the contribution rate was 26.356%,and the important indices were TP and AChE.The third principal factor explained 17.151%of the information of the original data,and the most important index associated with it was SOD,the factor loading of which was 0.900.
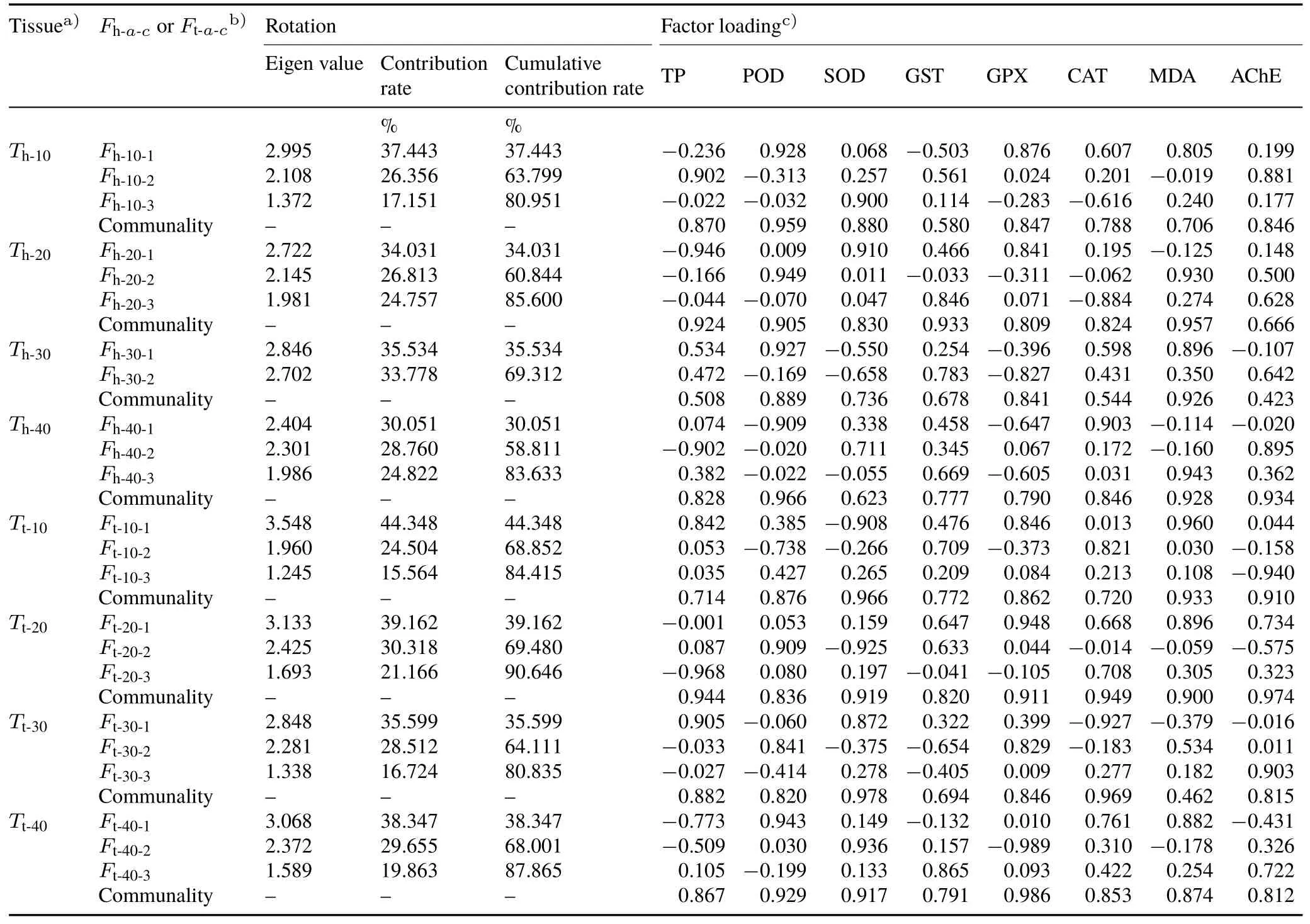
TABLE IISummary of parameters by performing orthogonal rotation on factors and factor loadings of oxidative stress indices under various Cd stress days
Communalities are the degrees by which the principal factors can explain independent variables.Table II showed that,for the tail tissues of earthworms that had been exposed for 20 d,among the three principal factors that were extracted,the six indices,TP,SOD,GPX,CAT,MDA,and AChE,had high communalities those reached more than 0.90,and the variable information retention was perfect;the communalities of POD and GST were 0.836 and 0.820,respectively.Most of the information of the 8 indices can be explained by the extracted principal factors,and the information loss was particularly low.These results showed that the principal factor extraction was perfect.In the earthworm head tissues at the same stress time(20 d),among the three principal factors that were extracted,the 7 indices,TP,POD,SOD,GST,GPX,CAT,and MDA,had communalities larger than 0.80 and the variable information retention was perfect,whereas AChE had a smaller communality(0.666).This showed that the factor extraction was more informative.Throughout all the treatments,71.90%of the communalities were greater than 0.80.Only for the 30-d Cd stress,the communalities of AChE in head tissues and MDA in tail tissues were less than 0.50.These results indicated that the overall effects of the extraction factors in this study reached an ideal state.
Taking factor loading>0.900 and communality>0.850 as the standard,the main oxidative stress indices associated with the first principal factor were screened out and identified as the key monitoring indices at each stress time.Asummary is shown in Table III.
Determination of oxidative stress degree(poisoning degree)
The stress time was taken as the main research line to screen out the key monitoring indicators in earthworms after each stress time,which can be used to serve as an early warning system for contaminated soil.TOPSIS was used to calculate the toxicity degree index of earthworms undervarious exposure concentrations and days and to order the indices.The results are listed in Table IV.
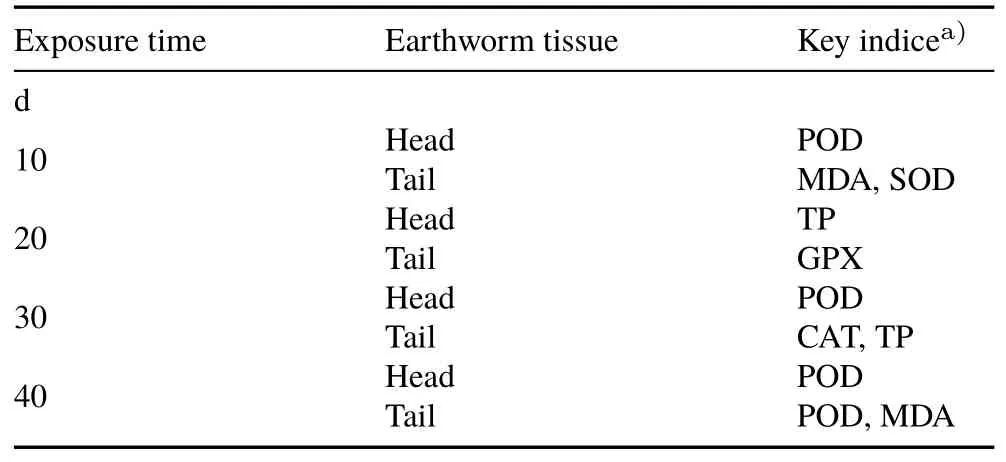
TABLE IIISummary of selected key indices of oxidative stress for head and tail tissues of earthworms under various Cd stress days
After 10 d of stress,for the head tissues of earthworms,a concentration of 200 mg kg-1Cd produced the highest degree of toxicity,followed by 1 mg kg-1,whereas the lowest toxicity occurred at a stress level of 100 mg kg-1;for the tail tissues of earthworms,a concentration of 100 mg kg-1Cd also produced the lowest toxicity,but the concentration that caused the highest poisoning degree was 400 mg kg-1,which was greater than that in the head tissues.The concentration that caused the second greatest toxicity also increased to 10 mg kg-1.In addition,for the 10-d Cd stress,the toxicity in the highest concentration group(800 mg kg-1)was both smaller,ranking only sixth.
As the extension of the stress time to 20 d,the head tissues showed higher toxicity at a low stress concentration of Cd(20 mg kg-1),whereas the tail tissues showed the greatest toxicity at the Cd concentration of 800 mg kg-1.The lowest toxicity of the earthworms(including the head and tail tissues)at the stress time of 20 d still occurred at the Cd concentration of 100 mg kg-1.
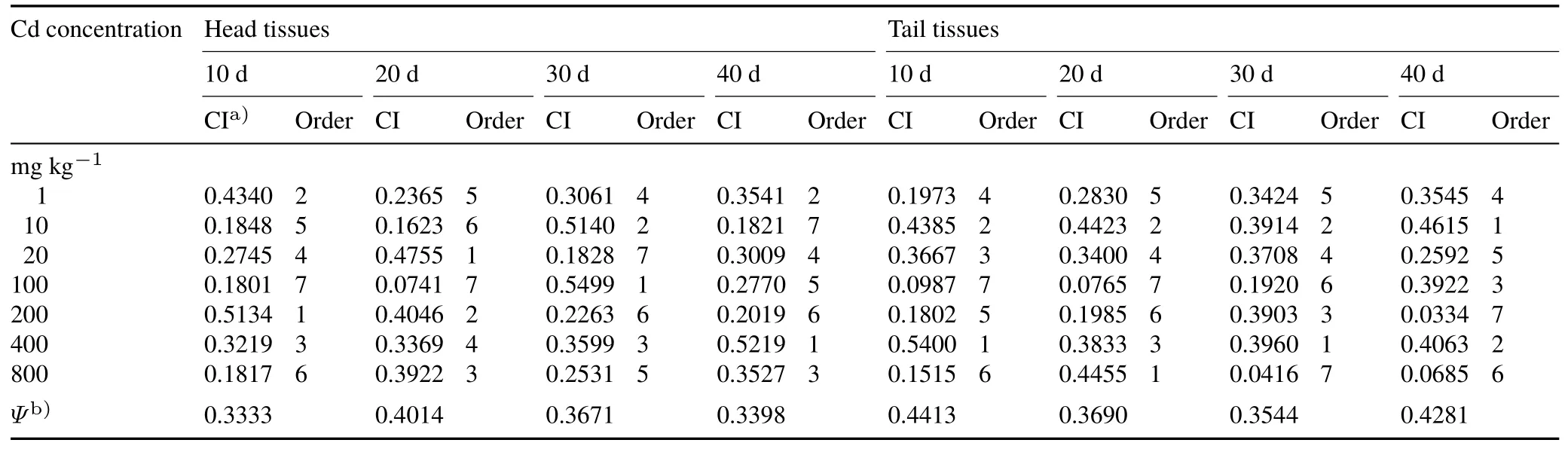
TABLE IVResults of using the model TOPSIS(Technique for Order Preference by Similarity to an Ideal Solution)to calculate toxicity degree of earthworms under various Cd stress concentrations and days
As the stress time was extended to 30 d,the toxicity levels in the head tissues contrasted with those at 20 d:the greatest toxicity was observed at 100 mg kg-1and the lowest at 20 mg kg-1.At the same time,there was a similar toxicity trend in the tail tissues:the toxicity was the lowest at the Cd stress of 800 mg kg-1and the highest at 400 mg kg-1.For the long stress time of the test design(40 d),the Cd concentration causing the lowest toxicity in the head tissues of earthworms decreased to 10 mg kg-1,and the concentration causing the highest toxicity increased to 400 mg kg-1.In the tail tissues,the greatest toxicity level was observed at the low concentration of 20 mg kg-1,but the lowest toxicity level was observed at the high Cd concentration of 200 mg kg-1.Table IV indicated that with an increase of Cd stress concentration,secondary Cd toxicity occurred in the tail tissues at 400 mg kg-1Cd.However,at the high stress concentration of 800 mg kg-1,the level of toxicity was lower.
It can be seen from theψvalues in Table IV that the variation in the toxicity indices of the tail tissues was the greatest after 10 d of stress,followed by that at 40 d of stress.For the head tissues,the variation was the lowest after 10 d of stress,followed by that at 40 d of stress.At the same time,as shown in Table IV,after 10 and 40 d of stress,the levels of toxicity in the tail tissues of earthworms were much higher than those in the head tissues,while after 20 and 30 d of stress,the toxicity levels in the head tissues were slightly higher than those in the tail tissues.
DISCUSSION
An indoor exposure method was used to simulate the earthworm’s natural soil environment,thus reflecting the toxic effects of Cd in the soil ecosystem onEisenia fetidamore authentically.
In traditional biostatistics,the dose-response relationship is an indispensable step of result analysis.However,as a qualitative analysis method,the dose-response relationship cannot clearly and accurately screen out the main oxidative stress indicators of earthworms under certain stress time and concentration.Therefore,we use a mathematical modelling method,factor analysis method,and the TOPSIS method to quantitatively study the oxidative stress effect in earthworms after stress.This approach can be more accurate,scientific,and rapid.The factor analysis method is suitable for the study of the ecotoxicological response mechanism ofEisenia fetidaunder Cd stress in natural soil.Furthermore,the factor analysis can be widely used as an early warning of heavy metal pollution in an actual polluted environment.The core idea of the TOPSIS model is to define the distance between the optimal solution and the worst solution of the decisionmaking problem.Then,the degree of closeness between each scheme and the ideal solution is calculated to order the schemes(Duet al.,2014).This model is widely used in group decision-making such as bridge risk assessment(Wang and Elhag,2006),weapon selection(Dağdevirenet al.,2009),renewable energy planning(Kaya and Kahraman,2010),and supplier evaluation(Wanget al.,2009;Awasthiet al.,2010).However,the TOPSIS model has not been applied in the ecotoxicity research of earthworms.
As shown in Table III the beginning after 10 d of exposure,in the earthworm head tissues,the most important indicator of oxidative stress was POD.This illustrated that the degradation of peroxides mainly occurred in the earthworms.At the same time,Table II showed that the factor loading of GPX on the first principal factor was inferior only to POD,which indicated that the reaction of reduced glutathione with hydrogen peroxide catalysed by POD to form oxidized glutathione had occurred(van der Oostet al.,2003;Chenet al.,2011;Engelmannet al.,2011;Liuet al.,2011).The data also showed that the enzymes that comprise the antioxidant metabolic system in earthworms were mutually promoting and synergistic.Duanet al.(2016)found that POD,GPX,and SOD in the hepatopancreas ofMarsupenaeus japonicushad a synergistic effect in response to the stress owing to resubmersion after desiccation.Studies of Liet al.(2005)and Sunet al.(2018)on thePhellodendron amurenseandArthraxon hispidusalso demonstrated that a certain synergistic effect between the antioxidant enzyme system and ROS(reactive oxygen species)elimination existed when the plant was stressed by drought.
In the tissues of the earthworm tails,after 10 d of Cd stress,MDA and SOD showed an“antagonistic”phenomenon.The toxicity of Cd led to“overloading”of the oxidation-antioxidant system,and slight membrane lipid peroxidation occurred in the body.Both Liuet al.(2014)and Sunet al.(2016)observed similar antagonism.However,Liuet al.’s study on the protection of lycopene against ethanolinduced toxicity in mice showed a decline in the MDA content and an increase in the SOD activity.By contrast,Sunet al.found that the SOD activity in the myocardium of mice decreased while the MDA content increased in a study that explored the cardiac toxicity of apigenin in doxorubicintreated mice.The reason for such differences may be that the composition of antioxidant enzymes differs among species.It is also possible that the distribution of antioxidant enzymes is different in various body tissues.Moreover,the key ecological indices of oxidative stress in earthworm head tissues and tail tissues were diverse.Therefore,when earthworms are used as biomarkers to monitor soil heavy metal pollution and provide early warning,a sectional study is necessary.
After 20 d of stress,TP was the most important indicator of stress in the head tissues,and its factor loading reached the maximum(-0.946)in the negative direction of the first principal factor Ft-20-1(Table II).This effect may be owing to an awareness in the earthworms of“danger”in the environment after Cd exposure.Earthworms instinctively reduced ingestion to reduce the intake of toxic substances.At the same time,their physical activities increased to find a way to escape from the dangerous environment.This could result in a steep drop in TP content at this stress stage.A study by Tianet al.(2007)showed that after earthworms were treated with flumorph at 700 mg kg-1,they appeared to tumble,escape,and accelerate their activity on the surface of the soil and refused to enter the toxic soil.In a previous experiment on the growth inhibition rate of earthworms after Cd stress in artificial soil,we found that the earthworm activities increased and their weight decreased at the early stage of Cd stress(Zhouet al.,2016).
Furthermore,research by Luet al.(2017)confirmed that food shortage and increased physical activity does lead to a significant reduction in the TP content of the organism.Superoxide dismutase is a type of metalloenzyme that is mainly distributed in the cytoplasm and mitochondria.Superoxide dismutase catalyses the disproportionation reaction of superoxide anion radicals and is one of the key enzymes in the system that defends against oxidative damage.In the present study,the activity of SOD in the earthworm head tissues was induced by an increase in free radicals.The expression of the SOD protection system,which stimulates the synthesis of SOD,is an adaptive response to pollutant stress(Fanget al.,2004).
However,in the tail tissues of earthworms exposed to Cd stress for 20 d,GPX was the most important indicator of the response to oxidative stress.Malondialdehyde was the secondary indicator,illustrating that with the extension of stress time,the Cd toxicity in earthworms was enhanced,the oxidation-antioxidant system collapsed,and membrane lipid peroxidation was aggravated.This is similar to the results of the authors’study on artificial soil(Zhouet al.,2016a),except that the time of collapse lagged behind that of the artificial soil test.Davieset al.(2003)and Lock and Janssen(2003)found that the toxicity of pollutants in artificial soil is higher than that in natural soil.The reason for this disparity may be that the effectiveness of heavy metals differs in different types of soil(Zhouet al.,2008).
At the same time,as shown in Table II,the factor loading of SOD on the second principal factor Ft-20-2(Table II)reached-0.925,which was the maximum in the negativeaxis direction.This result indicated that the earthworm tails were subjected to moderate environmental stress and accumulated high levels of reactive oxygen species in the cells,thus causing damage to the body.Therefore,it is speculated that under the same Cd concentration gradient for 20 d of exposure,the toxicity in the tail tissues of the earthworms was greater than that in the head tissues.Moreover,the SOD in the earthworm head tissues had a positive correlation with the first principal factor,while in the tail tissues,SOD was negatively correlated with the second principal factor.The reason for this difference may be that the head tissues contain the brain,heart,pharynx,gizzard,and other organs.Therefore,the response to exogenous pollutants is partly caused by direct contact damage of the pollutant,and the remaining response comes from feedback after the tail tissues are injured.This study demonstrated that the feedback was the main source of the difference.
With the extension of the stress time to 30 d,the toxicity of the head tissues was greater than that at 10 d,and the membrane lipid peroxidation had begun.Glutathione peroxidase is a peroxidase that exists widely in the organism and has the dual functions of removing peroxides and detoxification.In the head tissues under stress for 10 and 20 d,the factor loadings of GPX at the positive axis of the first principal factor were both larger(0.876 at 10 d of stress,and 0.841 at 20 d of stress).Under 30-d exposure to stress,the factor loading was-0.827.These results indicate that Cd can induce GPX at a lower stress intensity.This may be because the detoxification enzymes have produced a large amount of oxygen free radicals,which can induce the generation of GPX to remove the lipid peroxide and hydrogen peroxide to protect the cell membrane from oxidative damage(Mohammadifard and Mottaghipisheh,2017;Ninget al.,2018).With a prolonged period of stress,this induction ability first increased and then decreased until the GPX activity was inhibited.These results correspond with previous work onSinopotamon yangtsekiense(Jinet al.,2011)andParalichthys olivaceus(Wanget al.,2007),but contrast with the observation of Bai and Zeng(2013).The differences may be owing to the differences in both the pollutants used and the test method.These authors studied a pesticide using the filter paper method,while the present study examined the toxicity of a heavy metal in natural soil.
Several previous studies proved that exogenous chemicals cause changes in the metabolic characteristics and toxicity in earthworms(Sukumwang and Umezawa,2013;Wuet al.,2013;Huet al.,2016).The content became the most important indicator in the tail tissues when the earthwormwas stressed with Cd for 30 d.This was observed to be associated with sluggish movements and reduced activity under this stress condition(Zhouet al.,2016b).It is speculated that the absorption and accumulation of heavy metal by the earthworms interferes with the biosynthesis of protein,which leads to a loss in necessary proteins or a decrease in activity(Gaoet al.,2015;Hogenhout and Bos,2011).Goswamiet al.(2014,2016)and Paulet al.(2018)found that metallothionein in earthworms(Eisenia fetidaandLampito mauritii)was changed in the process of vermicomposting with heavy metal contaminated materials.
On the other hand,it is possible that the presence of heavy metals severely damages the body tissues.This causes a disorder of the protein metabolism and the production of some pathological proteins and resistance proteins,which result in the changes in TP.In a study of the chemical defence system ofPinus tabuliformis,Wang Y Cet al.(2016)found upregulated protein spots,downregulated protein spots,specific protein expression,and the loss of some protein spots.Ling(2004)used the electrophoresis method in an analysis of water-soluble proteins and confirmed that changes of protein bands in theMisgurnus anguillicaudatusmuscle after dichlorvos stress were significant and new bands appeared.
As the stress time was extended to 40 d,POD was still the most important indicator in earthworm head tissues,closely followed by CAT.The correlation coefficient matrix(Fig.1)showed that POD was significantly correlated with CAT.Thus,it is conjectured that the poisonous effects of heavy metal on the earthworm head tissues were relatively weak after the self-detoxification and repair reaction(including the antioxidant system,lipid peroxidation,and protein metabolism)during the previous stress stage.However,in the earthworm tail tissues,PODshowed an extremely significant correlation with MDA.The factor loading of POD reached the maximum in the positive-axis direction of the principal factor Ft-40-1(Table II),followed by MDA.It can be inferred that even at a prolonged stress time,the toxic effects were still very strong,and the lipid peroxidation of the earthworms was not complete.
At the same time,CAT was also shown to have an extremely significant correlation with MDA.Liuet al.(2008)confirmed that the role of CAT in earthworms was not only to remove excessive hydrogen peroxides but also to mitigate or even block the initiation of lipid peroxidation.Therefore,with a further extension of the stress time,the stress intensity increases.In addition,there are serious risks:that the lipid peroxidation reaction will be complete,the disorder of the protein expression will be aggravated,and the individual will exhibit epidermal folding,body breaking,and even death and dissolution(Skalickaet al.,2012;Namet al.,2015).This accounts for the phenomenon that the body breaks in earthworms often appear in the tail after being poisoned by heavy metals(Zhenget al.,2013;Zhouet al.,2016a).
As shown in Table IV,for the treatments of 10—20 d of stress,the Cd concentration that caused the lowest toxicity was the same for the head and tail.However,the concentration that caused the greatest toxicity in the head was lower than that in the tail.This phenomenon was aggravated with the increase in the stress time and illustrated that the earthworm head tissues were more sensitive to the stress of Cd than the tail tissues.In addition,at 20 d,Cd concentration of 20 mg kg-1caused the highest level of toxicity in the head tissues,whereas the lowest toxicity appeared at the 100-mg kg-1stress level.This illustrated that the resistance of the head tissues to heavy metal toxicity was weaker than that of the tail tissues.This may be because the poison is ingested by earthworms through the pharynx and oesophagus,then passes into the gizzard,and after physical grinding,enters the intestine.
The stress time of the head tissues to these toxins was earlier than that of the tail,so the head tissues were more sensitive.On the other hand,the detoxification enzymes mainly exist in the tails of earthworms.Sanchez-Hernandezet al.(2009)reported that the intestinal epithelial cells of earthworms are the main source of carboxylesterases(CBE),which are the key enzymes for the detoxification of pesticides.Furthermore,because the distribution of the CBE in earthworms was not uniform,Sanchez-Hernandez and Wheelock(2009)called for selective evaluations when the earthworm was used as a biomarker to monitor pesticide contamination.It is also possible that these variations may be owing to microorganisms in the earthworm.
Zhang(1997)found that the digestive enzymes inPontoscolex corethruruswere mainly synthesized by the microorganisms of the digestive tract.Therefore,it was speculated that these microorganisms synthesized some enzymes with detoxification functions,thereby enhancing the resistance of the earthworm to pollutants.At the same time,the variation in the levels of toxicity(ψ)in the head tissues was lower than that of the tail tissues(Table IV).This may be caused by a homeostatic mechanism that is more effective in the earthworm head tissues.A study by Panget al.(2016)on mosquitos confirmed that a homeostatic mechanism can produce an immune defence against pathogenic bacteria.At the same time,with the recent studies on the effects of accompanying anions on plant toxicity(Xie and Han,2011;Tanet al.,2013)and heavy metal morphology(Yanget al.,2015;Wanget al.,2017),the effects of Cl-on the oxidative stress inEisenia fetidahave yet to be determined.
CONCLUSIONS
Mathematical modeling based on the combination of factor analysis and TOPSIS can quickly,efficiently,and intuitively screen the relevant enzyme indices of oxidative stress in earthworms exposed to heavy metals.This can improve the accuracy and scientific value of earthworms as biomarkers for monitoring heavy metal pollution of the soil and provide an early warning of soil pollution.When earthworms were exposed to Cd in natural soil for 10 d,POD was the key index of oxidative stress in the head tissues,and TP was the key factor at 20 d.However,POD once again became the key index at 30 and 40 d.In the tail tissues,MDA and SOD were the key indices at an exposure time of 10 d,GPXat 20 d,CATand TP at 30 d,and PODand MDAat 40 d.These results contribute to establishing a scientific method for ecotoxicological diagnosis and revealing the mechanism of soil Cd toxicity.
ACKNOWLEDGEMENT
This research was supported by the“Youth Talents”Project of Northeast Agricultural University,China(No.19QC12)and the National Key Research and Development Program of China(No.2018YFD0300106).
杂志排行
Pedosphere的其它文章
- Preface Earthworms in soil ecology and organic waste management
- Letter to the Editor Field and laboratory investigations of Lumbricus badensis ecology and behaviour
- Strategies to mitigate the adverse effect of drought stress on crop plants—influences of soil bacteria:A review
- Advances in fungal-assisted phytoremediation of heavy metals:A review
- Behavior and respiration responses of the earthworm Eisenia fetida to soil arsenite pollution
- Biological weathering of phlogopite during enriched vermicomposting
