Visualization of gold nanoparticles formation in DC plasma-liquid systems
2021-07-07ZhaoyuanLIU刘钊源QiangCHEN陈强QinghuoLIU柳清伙andKostyaKenOSTRIKOV欧思聪
Zhaoyuan LIU(刘钊源),Qiang CHEN(陈强),Qinghuo LIU(柳清伙) and Kostya(Ken)OSTRIKOV(欧思聪)
1 Shenzhen Research Institute of Xiamen University,Institute of Electromagnetics and Acoustics,Fujian Provincial Key Laboratory of Plasma and Magnetic Resonance,Key Laboratory of Electromagnetic Wave Science and Detection Technology,Xiamen University,Xiamen 361005,People’s Republic of China
2 Department of Electrical and Computer Engineering,Duke University,Durham,NC 27708,United States of America
3 School of Chemistry and Physics,Queensland University of Technology,Brisbane,Queensland 4000,Australia
Abstract Dual argon plasmas ignited by one direct current power source are used to treat an aqueous solution of hydrogen tetrachloroaurate-(III)trihydrate(HAuCl4·3H2O)which is contained in an H-type electrochemical cell.The solution contained in one cell acts as a cathode,and in the other as an anode.Experiments are carried out to directly visualize the formation process of gold nanoparticles(AuNPs)in separated cells of the H-type electrochemical reactor.The results and analyzes suggest that hydrogen peroxide and hydrated electrons generated from the plasmaliquid interactions play the roles of reductants in the solutions,respectively.Hydrogen peroxide can be generated in the case of the liquid being a cathode or an anode,while most of hydrated electrons are formed in the case of the liquid being an anode.Therefore,the reduction of theions is mostly attributed to the hydrogen peroxide as the liquid acts as a cathode,while to the hydrogen peroxide and hydrated electrons as the liquid acts as an anode.Moreover,the pH value of the solution can be used to tune the formation processes and the final form of the AuNPs due to its mediation of reductants.
Keywords:dual plasma,gold nanoparticles,hydrogen peroxide,hydrated electron,H-type electrochemical cell
1.Introduction
When a discharge plasma is generated with one or both electrodes immersed in a solution,one obtains a plasma electrochemical system because of the electrochemical reactions initiated by the electrons from the gaseous plasma.Besides the usual electrochemical processes which take place at the plasma-liquid interface,a variety of highly reactive species are also produced at the gaseous plasma,the plasmaliquid interface as well as the bulk liquid[1–6].These merits make plasma electrochemical systems widely used in water purification[7–10],nanomaterials fabrication[11–31],plasma medicine[32,33],plasma agriculture[34,35]and some other fields[10].Although the multiplicity of the reactive species in the plasma-liquid systems provides various possibilities for the applications,it is difficult to distinguish what are the actual processes responsible for a specific application.Therefore,to find what processes work for the specific application of the plasma-liquid systems is of great importance.In this paper,we use the formation of gold nanoparticles(AuNPs)as an example to investigate the reactions that are responsible for the AuNPs’ formation in direct current(DC)discharge plasma-liquid systems.
When tetrachloroaurate-(III)is present in the solution,the reduction ofby plasma irradiation can usually lead to the formation of the AuNPs.However,several species produced in the plasma-liquid systems might be responsible for the reduction,including hydrated electron(eaq),atomic hydrogen(H),hydrogen peroxide(H2O2)[36],as well as molecular hydrogen(H2).For a DC discharge plasma-liquid system with the solution as one discharge electrode(a cathode or an anode),the different physical processes at the plasma-liquid interface give rise into a dissolution of different reactive species,and consequently different secondary reactions proceed in the solution[37].
When the solution acts as a cathode,a cathode voltage fall is formed at the interface between gaseous plasma and the solution surface,positive energetic ions are forced to irradiate the solution surface,and in this case much H2O2are formed in the solution[13].The H2O2production rate is dependent on the solution’s properties such as the pH value,conductivity etc.For an aqueous NaCl solution with conductivity of 4800 μS cm−1(pH=5.89),the H2O2production rate for the case with a liquid cathode is 15.35 μmol min−1at a discharge current of 30 mA,while it is almost zero for the case with a liquid anode[13].In addition,the positive space charge at the surface of the liquid cathode will repel the electrons in the plasma to enter the liquid[22],and thereby no or insignificant amount of eaqis formed when the liquid acts as a cathode.However,the electron transfer becomes the major process at the plasma-liquid interface when the solution acts as an anode[38–40].Rumbachet al[39]estimated that the average concentration of the eaqspecies for the liquid as an anode is in the order of 1 mM with an average penetration to the liquid surface of 100 nm.Thus,there is a formation of the eaqand eaq-induced secondary species in the solution,by which the H2O2yield in the solution is suppressed in the case of the solution anode[41].The H2O2production rate is almost zero for an aqueous NaCl solution anode with conductivity of 4800 μS cm−1(pH=5.89)[13],while this production rate can be tuned by the solution’s properties[41,42].
Herein,an H-type electrochemical cell is used to study the influence of the above mentioned processes and reactive species on the AuNPs synthesis in a DC plasma-liquid system.A similar experimental setup has been utilized to study the chemical reactions[43],and to synthesize AuNPs,AgNPs as well as Ag–Au alloy[44],while our focus here is mainly on the discussion of specific species for the reduction of AuCl4−ions.
2.Experimental setup
Figure 1 presents the illustration of the H-type plasma electrochemical reactor.The height,outside and inner diameters of a single cell are 102.1 mm,26.9 mm and 22.6 mm respectively.The length and the inner diameter of the connecting part for the two cells are 45.6 mm and 16.1 mm,respectively.60 ml of aqueous solution of hydrogen tetrachloroaurate-(III)trihydrate(99.99%,HAuCl4·3H2O,Alfa Aesar Co.,Ltd)and polyvinylpyrrolidone(PVP,GR,Sinopharm Chemical Reagent Co.,Ltd)is contained in the plasma electrochemical cell for the plasma treatment.The concentration for both HAuCl4·3H2O and PVP is 0.1 mM,and PVP acts as a surfactant for the AuNPs’ formation.Two identical slightly-tapered-hollow tungsten steel electrodes are placed just above the solution surface with a distance of 3 mm.The hollow tungsten steel electrode is 1.02 mm/6.35 mm in inner/outer diameter.Argon gas is supplied through two hollow electrodes(total flow rate of 40 sccm).The discharge current is 20 mA for all cases.In some cases,a single plasma is used to treat the solution,where the Ar flow rate is 20 sccm.The electrode at the left cell is connected to the high voltage point of a DC power source(BOHER HV,LAS-20 kV–50 mA,positive polarity),and the ground point of the power source is connected to the right electrode.As a result,the solution in the left cell acts as a cathode and in the right cell as an anode.When the applied voltage is greater than a certain value,dual atmospheric pressure Ar discharge plasmas are ignited in both separated cells between the electrode tip and the solution surface.Chemical reactions in the solution are induced by the plasma-liquid interactions under continuous plasma treatment.
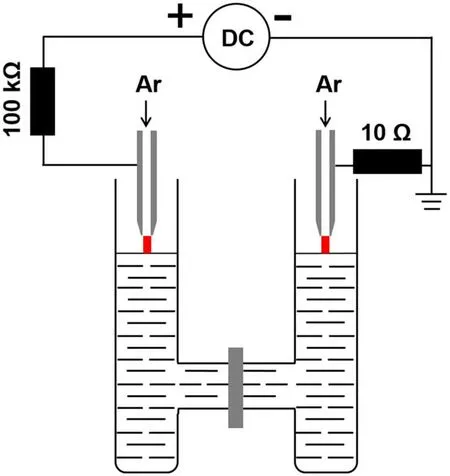
Figure 1.Schematic diagram of the H-type plasma electrochemical reactor.Dual Ar discharge plasmas are simultaneously operated in the separated cells for treating the solution.The solution in the left cell acts as a cathode and in the right cell acts as an anode.
The appearance of H2O2is observed by a colorimetric method which is based on the formation of a yellow-colored complex(H2TiO4)when H2O2is mixed with titanium sulfate[Ti(SO4)2]inastronglyacidicenvironmentTheH2O2quantification is based on the feature of the proportionality between the H2TiO4absorption intensity at 407 nm and the H2O2concentration.Relevant details can be found in[13].The formaldehyde(HCHO)produced by plasma treatment of dimethyl sulfoxide(DMSO)is also quantified by a colorimetric method which is based on the Hantzsch reaction[45].100 ml of test aqueous solution of ammonium acetate(50 g),acetic acid(6 ml),and acetylacetone(0.5 ml)is prepared for the HCHO quantification.Equal volume of test solution and the plasma treated DMSO solution is mixed,and then bathed in heated water(60°C)for 15 min.After that,the mixed solution is cooled to room temperature.The absorption intensity of the mixed solution at 410 nm is proportional to the HCHO concentration[45–47],based on which the HCHO concentration is measured.Experiments are performed thrice to obtain a statistic result.
To study the influence of pH value on the AuNPs’ formation,solutions with pH values from acidic to basic range are prepared.The pH value of pre-prepared HAuCl4·3H2O/PVP solution is 3.34.The solution with pH value of 11.22 is obtained by mixing some sodium hydroxide(NaOH)solution with the HAuCl4·3H2O/PVP solution.Solutions with pH values between 3.34 and 11.22 are prepared by firstly adjusting the pH value of the HAuCl4·3H2O/PVP solution to neutral(by adding some NaOH solution),and then mixing these neutral solutions with a phosphate buffer(10 mM in buffer strength).The pH values of the solutions are measured by a pH detector(Yesmylab SX620).
Videos of the electrochemical cell are taken during the plasma treatment in order to directly visualize the change of the treated solution.All the videos are available in the supplementary information(SI)(available online at stacks.iop.org/PST/23/075504/mmedia).Screenshot photographs(every two min)are presented in the paper to give a direct visualization of the solution change at different experimental conditions.
In some cases,a OH radical scavenger,DMSO(≥99.0%Sinopharm Chemical Reagent Co.,Ltd),and an electron scavenger,5-Bromouracil(5-BrU,99%,Shanghai Macklin Biochemical Co.,Ltd),are also added in the solution to control the processes in solutions.
To investigate the formed AuNPs,the plasma treated solutions taken from the electrochemical cell are centrifuged once,and then the collected products are centrifuged twice in ethanol.The final products are dispersed in ethanol for further characterization.The morphology of the synthesized AuNPs is investigated by a scanning electron microscope(FE-SEM,Sigma HD,Carl Zeiss).
The absorbance of the solution is measured in a quartz cell(10 mm in optical path length)using Ocean Optics spectrometer(USB2000+)affiliated with a light source(DH-2000,200–2500 nm).
3.Results and discussion
Figure 2(a)presents the temporal photographs of plasma treated solutions of HAuCl4(0.1 mM)/PVP(0.1 mM)(SI video 1).In both cells,there is a transparent-to-red color change during the plasma treatment,which is a typical characteristic of the AuNPs formation.Although some reactants generated through the plasma-liquid interactions enter the solution from the solution surface,the color change in both cells does not appear immediately at the solution surface after the plasma ignition,but suddenly appears at a certain range of the solution after the plasma treatment of about 1 min(SI video 1).It was reported that tetrachloroaurate-(III)dominantly takes the form ofanddepending on the pH values[48].When the solution’s pH value is less than 4,is the main form of tetrachloroaurate-(III).Then,in the solution will be reduced to atomic gold by reductant X produced from the plasma-liquid interactions(equation(1)).It is well known that the concentration of atomic gold in the solution is necessary to reach a critical value for starting the nucleation of AuNPs[49].This explains the above observed phenomena.
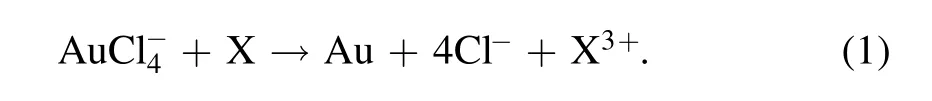

Figure 2.Temporal photographs of the plasma treated aqueous solutions of(a)HAuCl4(0.1 mM)/PVP(0.1 mM)(SI video 1),and(b)acidic Ti(SO4)2.The pH value is 3.34 for the solution of HAuCl4/PVP,and 0.81 for the solution of acidic Ti(SO4)2(SI video 3).
In addition,we observe that the color at the right cell shifts to the bottom with increasing treatment time and finally migrates into the left cell.However,the color at the left cell does not change obviously.This finding can be explained as follows.The movement of theion in the solution is driven by the electric force,and it will move towards the left discharge electrode based on the applied electrical polarity.Consequently,the concentration ofions at the right cell will present an increasing gradient from the solution surface to the bottom.The reduction rate ofions is proportional to theconcentration,and therefore the reductions ofions will demonstrate an increasing gradient from the solution surface.This phenomenon will lead to an increasing distribution of the AuNPs’concentration from the solution surface to the bottom at the right cell,which causes the color distribution at the right cell since the solution color intensity of AuNPs is positively related to the AuNPs’concentration.This movement ofions might be also one of the reasons for the much more intense color appearing at the left cell.
As mentioned in the introduction,eaq,H,H2O2and H2are potential reductants in the plasma-liquid systems.Thereductions involving these species are expressed by equations(2)–(6)
The standard Gibbs free energy change(ΔGo)[50]can be expressed as ΔGo=−nFEofor a chemical reaction,wherenis the number of moles of electrons transferred in the reaction,Fis Faraday constant(9.65×104C mol−1),andEois the standard reduction potential of the chemical reaction.A simple estimate provides us the ΔGoof equations(2)–(6)based on values ofEotaken from[50–54].These AuCl4−reduction reactions can spontaneously proceed due to the negative values of ΔGo.The potentiality of the reactions is dependent on the values of ΔGo.The more negative ΔGois,the more thermodynamically possibility is for the reactions to occur.However,the occurrence of the reactions also depends on other variables such as the reactant concentration and the existence of competing reactions,the actual reactions do not firmly follow the thermodynamically possibility.
As described above,H2O2,H2,H and eaqare the possible candidates for thereduction in the plasma-liquid interactions.Hence,these species might contribute to the formation of AuNPs to some extent,respectively.For further understanding and control of the AuNPs’ formation,one would like to know what the dominant contributors are in specific conditions.In the introduction,we mentioned the different processes at the plasma-liquid surface,energetic ion irradiation for the solution cathode and electron shower for the solution anode.OH and H produced by the dissociation of water in plasma are present in both cells,while eaqonly exists at the solution anode.As a result,the typical reactions in both cells can be summarized in table 1.
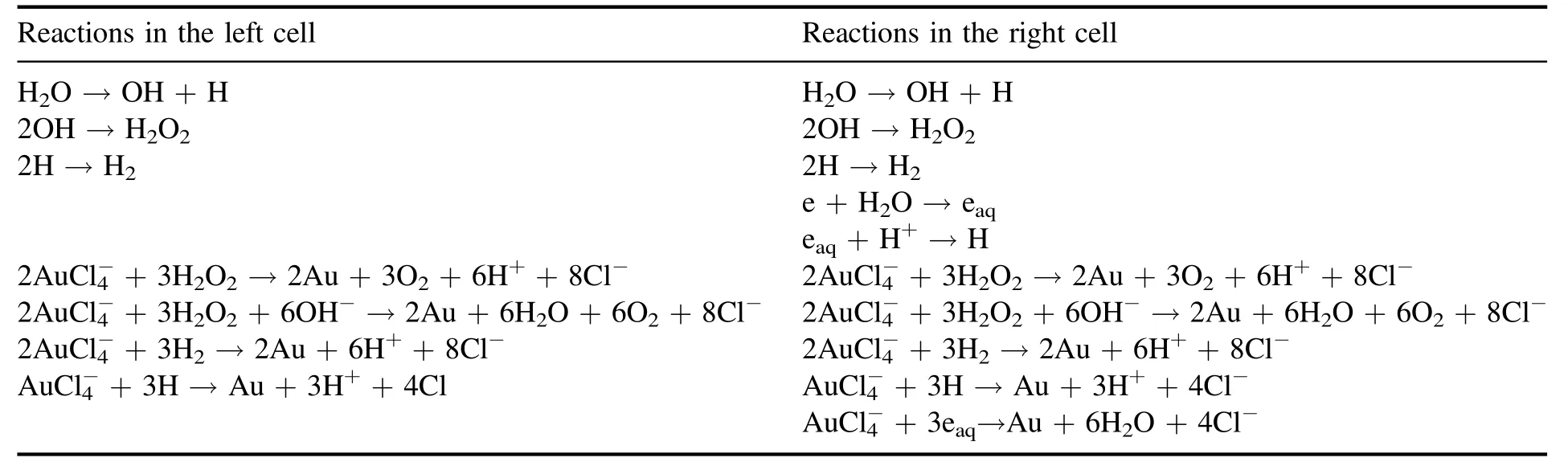
Table 1.Possible typical reactions in both cells according to the different physical processes in the plasma-liquid system with the solution as a cathode or as an anode.
By replacing the plasma in the right cell with an immersed helical gold wire,the solution cathode is treated by a single plasma(SI video 2).Many bubbles appear at the gold wire during the treatment,and those bubbles should be H2due to the electrolysis of water at the right cell.However,no observable AuNPs are found at the right cell,which excludes H2from the possible reductants of.
The previous studies demonstrated that H2O2and eaqare the main products among the above mentioned species in solutions when the solutions are the cathode and the anode,respectively[13,38,39].Therefore,we firstly explored the roles of H2O2and eaqin the AuNPs’ formation.Figure 2(b)presents a visual evidence of the H2O2production in both cells with acidic Ti(SO4)2solution(SI video 3).The solution color in the left cell is more intense than that in the right cell,indicating a higher H2O2yield in the solution cathode.Although the H2O2yield in the solution cathode is much greater than that in the solution anode[41],the existence of H2O2in both cells indicates that they should be responsible or partially responsible to the AuNPs’formation,since the H2O2has already been confirmed to be an effective reducing species of the[36].
There are several sources for the formation of the liquidphase H2O2in a plasma-liquid system,including the dissolution of gaseous H2O2,the dimerization of the dissolved OH radicals.The dissolved OH radicals might form the dissolution of the gaseous OH radicals or form the generationin situplasma-induced photolysis of water.For a RF or low frequency plasma jet where liquid is not an discharge electrode,the contribution of the gaseous H2O2dissolution to the liquid-phase H2O2is not negligible.However,it is found that the H2O2in the solution is predominantly formed from the recombination of dissolved gaseous OH radicals in DC plasma-liquid systems[41,55,56].Thus,it is possible to control the H2O2production by adding OH scavenger into the solution[46].The OH and electron scavengers(DMSO and 5-BrU)are used to study the effects of H2O2and eaqon thereduction,and the results are presented in figure 3(SI videos 4 and 5).

Figure 3.Temporal photographs of the plasma treated aqueous solutions of(a)HAuCl4(0.1 mM)/PVP(0.1 mM)/DMSO(100 mM)(SI video 4),and(b)HAuCl4(0.1 mM)/PVP(0.1 mM)/5-BrU(5.24 mM).The pH value is 3.34 for the solution of HAuCl4/PVP/DMSO,and 3.45 for the solution of HAuCl4/PVP/5-BrU(SI video 5).
For a plasma treated flowing solution,we have found that 100 mM of DMSO is enough to suppress the formation of H2O2[55].There is no formation of AuNPs in the right cell,implying that either the yield of H and H2is very low or they have no contributions to the AuNPs’ formation in our experimental conditions.However,the AuNPs’ formation is observed in the left cell confirmed by the purple color and the AuNPs-related localized surface plasmon resonance(figure 5),although the H2O2yield is limited by the 100 mM of DMSO.This is probably related to the products of the reaction between DMSO and OH radicals as shown in equations(7)–(9).OH radicals can be scavenged rapidly by DMSO[(CH3)2SO]via equation(7)to form CH3(k=6.6×109M−1s−1)[57,58]which in turn reacts with molecular oxygen to produce HCHO.Molecular oxygen is available in the left cell due to the electrolysis of water at the plasma-liquid interface.(See a single plasma treatment of the solution anode in SI video 6.)Formaldehyde has been reported as a strong reducing species which can reduce theions to form the AuNPs[59]

Figure 5.UV–vis absorption of the plasma treated aqueous solutions of HAuCl4(0.1 mM)/PVP(0.1 mM)/DMSO(100 mM)(pH=3.34),and HAuCl4(0.1 mM)/PVP(0.1 mM)/5-BrU(5.24 mM)(pH=3.45)at the left cells.

The information of the H2O2and HCHO is achieved from the plasma treated aqueous solution of PVP(0.1 mM)/DMSO(100 mM).The solution pH value is 3.34.2 ml of the treated solutions is taken near the solution surface every two minutes during the plasma exposure.The H2O2and HCHO concentrations are estimated separately using the above mentioned colorimetric methods.Figure 4 provides the evolutions of the H2O2and HCHO concentrations.The H2O2and HCHO exist in both cells,although both concentrations are higher in the left cell than those in the right cell.Because the solution is not circulated in our experiments,the DMSO at the solution surface might be quickly consumed by equation(7),which leads to that DMSO concentration at the solution surface is not enough to quench the dissolved OH radicals.Therefore,the H2O2can still be formed when DMSO is present.The molecular oxygen for the formation of the HCHO in the right cell might be from the dissolved oxygen in the solution.

Figure 4.Temporal evolutions of(a)the H2O2 and(b)the HCHO concentrations of the plasma treated aqueous solution of PVP(0.1 mM)/DMSO(100 mM)in the left cell(liquid as cathode),and in the right cell(liquid as anode).The pH value of the solution is 3.34(adjusted by H2SO4).
Interestingly,the appearance of AuNPs in the left cell is initially found at a location around 10 mm below the solution surface,and this is also observed when the solution cathode is treated by a single plasma(SI video 7).The reason for this phenomenon still remains unclear at present.In the right cell,the H2O2formation is suppressed.H2is produced from the electrolysis of water instead of O2,while some formaldehyde is also formed in the right cell probably due to the dissolved O2.The limited H2O2,the formed formaldehyde,and the eaqformed by the dissolution of gaseous plasma electrons should have caused the formation of AuNPs.However,no obvious AuNPs are observed in the right cell.For the eaq,because of the high H+concentration(10−3.34M,higher than theconcentration,10−4M)and the high rate constant of eaqand H+(k=2.3×1010M−1s−1)[57],eaqin the right cell is consumed by H+etc(equations(10),(11))
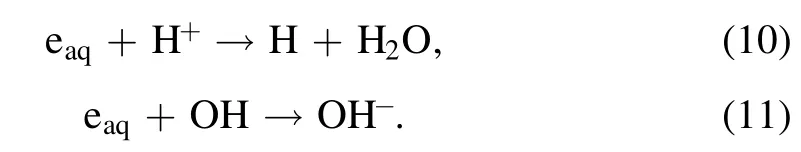
Although some H2O2and formaldehyde are existent in the right cell when DMSO is used,theions are not obviously reduced.At present,the reason for this phenomenon is still not clear.
The electron scavenger,5-BrU,is used to investigate the effect of eaq,and the result is presented in figure 3(b).In the right cell,there are no observable AuNPs due to the consumption of eaqby 5-BrU as well as the above mentioned pathways.A yellow color appears at the upper solution in the left cell during the plasma treatment,but an absorption measurement of the yellow solution shows that the products are not AuNPs due to the lack of AuNPs-related localized surface plasmon resonance(figure 5).This result indicates that OH is also eliminated by 5-BrU,and the yellow material is the product.Thereby,the H2O2is also limited in the left cell,resulting in the suppression of thereduction.
In figure 3,the solutions have low pH values,which might affect the DMSO and 5-BrU effects because of the competing reactions of 5-BrU and H+with eaq.Therefore,we carried out the same experiments in a neutral environment.The results shown in figure 6(SI videos 8 and 9)are similar to those in figure 3,except the AuNPs in the left cell do not initially appear at a location below the solution surface.In this case,the H+concentration is 10−7M which is significantly less than theconcentration(10−4M),eaqshould reduce thein the right cell when DMSO is present.Consequently,we consider that eaqmight be eliminated by other competing reactions.Equations(6),(10)and(11)are competing reactions involving eaqin the right cell.As a result,thereduction by eaqwill be tuned by the existence of those substances included in equations(6),(10)and(11).
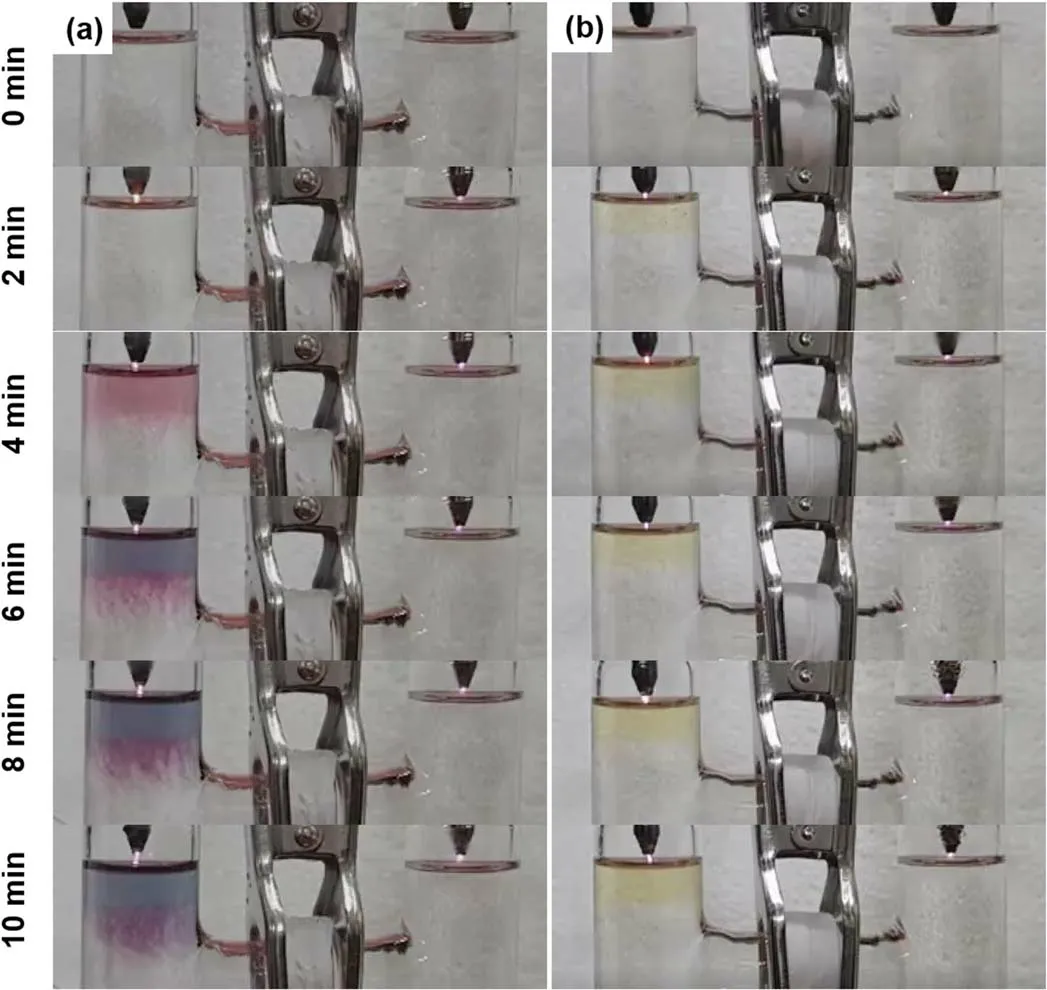
Figure 6.Temporal photographs of plasma treated aqueous solutions of(a)HAuCl4(0.1 mM)/PVP(0.1 mM)/DMSO(0.1 M)(SI video 8),and(b)HAuCl4(0.1 mM)/PVP(0.1 mM)/5-BrU(5.24 mM)(SI video 9).The pH value is 7.00 for the solution of HAuCl4/PVP/DMSO,and 6.96 for the solution of HAuCl4/PVP/5-BrU.
The concentration of H+ion is an important parameter for eaqinvolved reactions.Thence,we further carried out dual plasma treatment of HAuCl4(0.1 mM)/PVP(0.1 mM)solutions with different pH values for studying the influence of pH values on the formation of AuNPs.The results are shown in figure 7(SI videos 1,10,11 and 12).Evidently,the color of plasma treated solution is affected by the pH values,which is correspondent to the shape,size and aggregation of the formed AuNPs[60].Compared to the results at pH=3.34,the colors in the right cell are more located near the solution surface after 10 min plasma treatment at the pH values of 7.20,8.81 and 11.22.As the pH value increases,the H+concentration decreases,while the OH−concentration increases.H+is a scavenger of eaq[26],while OH−is a scavenger of OH radicals[41].Therefore,at higher pH values,eaqspecies work as thereductant in the right cell,meanwhile the reduction reaction will be suppressed at the left cell due to the low H2O2yield,which is demonstrated in figure 7.
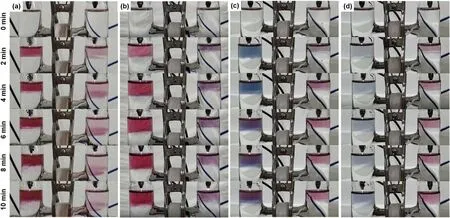
Figure 7.Temporal photographs of the plasma treated aqueous solutions of HAuCl4(0.1 mM)/PVP(0.1 mM)at pH values of(a)3.34(SI video 1),(b)7.20(SI video 10),(c)8.81(SI video 11),and(d)11.22(SI video 12).The pre-prepared HAuCl4/PVP solution(pH=3.34)is adjusted to neutral by adding NaOH solution,and then a phosphate buffer(10 mM in buffer strength)is used to sustain the pH values of 7.20 and 8.81 during the plasma treatment,while the pH values of 11.22 is obtained by only mixing some NaOH solution with the HAuCl4/PVP solution.
The samples produced from experiments in figure 6 are collected and morphologies of the AuNPs formed at different pH values are imaged by SEM(figure 8).For the solution acting as the cathode,the size of particles decreases with increasing pH values,and some rod-like particles are observed when the pH values are 3.34,7.20 and 8.81.When the pH value approaches 11.22,the products are aggregated AuNPs with small-sized particles.For the solution as the anode,some rod-like and triangle-shaped particles exist when the pH value is 3.34.The particles obtained at the other pH values are aggregations of small-sized AuNPs more or less.
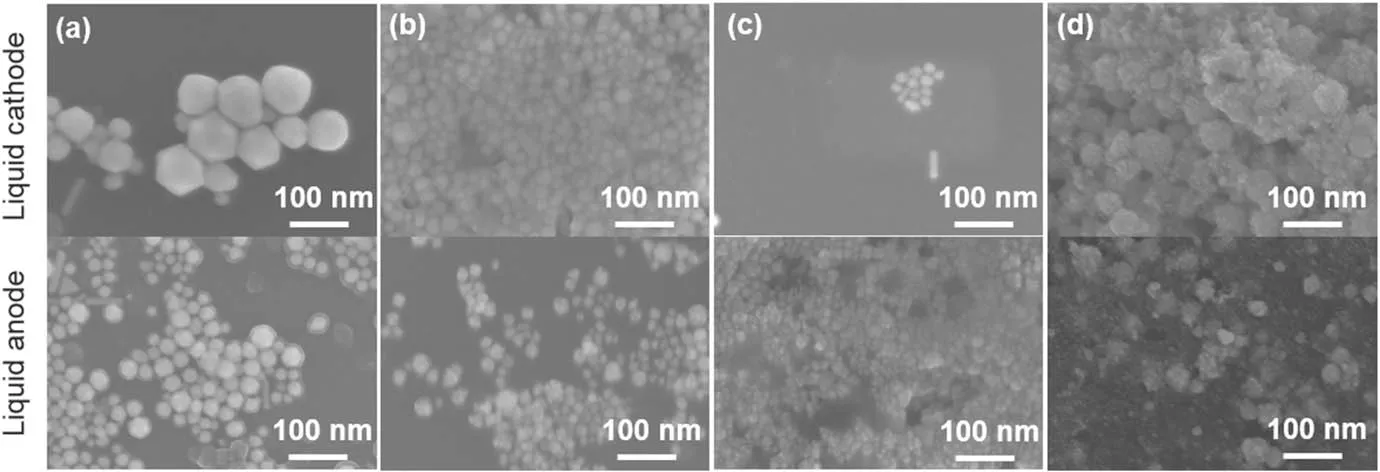
Figure 8.SEM images of plasma treated aqueous solutions of HAuCl4(0.1 mM)/PVP(0.1 mM)at pH values of(a)3.34,(b)7.20,(c)8.81,and(d)11.22.The pre-prepared HAuCl4/PVP solution(pH=3.34)is adjusted to neutral by adding NaOH solution,and then a phosphate buffer(10 mM in buffer strength)is used to sustain the pH values of 7.20 and 8.81 during the plasma treatment,while the pH value of 11.22 is obtained by only adding some NaOH solution into the pre-prepared solution.
4.Conclusion
AuNPs are synthesized in the H-type electrochemical reactor using atmospheric pressure DC dual plasmas in argon.The formation processes of AuNPs for the cases when the solution acts as a cathode or an anode are visualized.A rigorous analysis of the results indicates that the reduction ofis dependent on the experimental conditions.Although H2O2,hydrated electrons,atomic hydrogen,and molecular hydrogen produced in the plasma-liquid system own reducing ability,they contribute to thereduction differently.The H2O2works as the reducing species in both cells.However,the reducing role for theof the hydrated electrons existing in the solution is highly dependent on the pH value of the solution.In addition,the experiment results confirm that the reducing roles of atomic hydrogen and molecular hydrogen are minimal.These results are generic and are relevant for the development of diverse applications based on plasma-aided nanofabrication utilizing plasma-liquid systems.
Acknowledgments
Q Chen thanks the Basic Research Program of Science and TechnologyofShenzhen,China(No.JCYJ20190809162617137)and National Natural Science Foundation of China(No.52077185)for partial financial support.K Ostrikov thanks the Australian Research Council(ARC)and QUT Center for Materials Science for partial support.
猜你喜欢
杂志排行
Plasma Science and Technology的其它文章
- Numerical simulation of carbon arc discharge for graphene synthesis without catalyst
- Enhanced removal of ultrafine particles from kerosene combustion using a dielectric barrier discharge reactor packed with porous alumina balls
- Rice plant growth and yield:foliar application of plasma activated water
- An active tunable Fano switch in a plasmafilled superlattice array
- Comparison of sample temperature effect on femtosecond and nanosecond laser-induced breakdown spectroscopy
- Magnetic field induction and magnetic force distribution profiles in plasma focus discharge device
