Rice plant growth and yield:foliar application of plasma activated water
2021-07-07RASHIDMamunurRASHIDHASANandTALUKDER
M M RASHID,Mamunur RASHID,2,M M HASAN and M R TALUKDER,∗
1 Plasma Science and Technology Lab,Department of Electrical and Electronic Engineering,University of Rajshahi,Rajshahi 6205,Bangladesh
2 Plant Pathology and Mycology Lab,Department of Botany,University of Rajshahi,Rajshahi 6205,Bangladesh
3 Plant Pathology Lab,Department of Agronomy and Agricultural Extension,University of Rajshahi,Rajshahi 6205,Bangladesh
Abstract Plasma activated water(PAW)was prepared for 10 min to be applied one to five times as a foliar spray to rice plants,to investigate plant growth,yield and the concentrations of total soluble protein and sugar in the rice grains produced.The results reveal that(1)the plant height,stem diameter,dry weight,chlorophyll and total carotene concentrations were improved by ~15%,~25%,~24%,~47%and ~45%,respectively,with respect to control,(2)defense mechanisms of the plants treated with PAW were improved,(3)concentrations of total soluble protein and sugar were enhanced in the rice grains of PAW treated plants and(4)yield was increased by ~14%.
Keywords:plasma agriculture,plasma activated water,rice plant growth,rice yield,total soluble protein
1.Introduction
Demand of food is increasing steadily due to the increased population growth in various countries(i.e.Bangladesh and India)of the world.The government of Bangladesh is emphasizing the importance of enhancing crop yield because of the reduction of cultivatable land area due to rapid industrialization,urbanization,river erosion and climate change.One of the most important requirements for improving crop yield is the development of new technology that can reduce the use of environmentally hazardous chemical fertilizers and pesticides.Rice(Oryza sativa)is the staple food of about 175 million Bangladeshi people.It has been predicted[1]that 44.6 million tons(Mt)of clean rice will be required for the estimated 215.4 million population of Bangladesh in 2050.Increasing climate vulnerability(e.g.drought,floods,salinity)and decreasing total cropped area are the great challenges for increasing food production.Despite the above-mentioned facts,enhanced rice yield could address food security in Bangladesh.Thus,new technological approaches should be adopted in agriculture to enhance rice yield and ensure better ecosystem health through minimal use of chemical fertilizers and pesticides.
Recently,application of plasma technology in agriculture has attracted the attention of researchers due to its potential for enhancing the seed germination rate[2–5],plant growth rate[6–9]and crop yield[10–12].Further,atmospheric pressure cold(APC)plasma treatments of seeds have been found to be effective in improving stress tolerance[10]and detoxification[11]of crops.Further,APC plasma has been found to act as a disinfectant[12,13]against fungi and bacteria as an alternative to conventional environmentally hazardous pesticides.APC plasma can also be utilized for the preparation of plasma activated water(PAW).The properties of water can be modified by plasma treatment,and the treated water can be used as a fungicide,seed treatment and growth enhancer.PAW can also be used[14,15]for seed treatment for the improvement of seed germination rate.Plasma species produced in the medium(gas or liquid)play important roles in seed decontamination[12,13],breaking seed dormancy[2],enhancement of the seed germination rate[2–5],improvement of plant growth[6–9]and crop yields[10–12],and can also act as fungicides[16].
Production of plasma species along with their concentration in the medium depends on several factors,namely the type of power supply used(DC,pulsed DC,low frequency,RF,microwave,etc),the type of discharge(corona,jet,dielectric barrier discharge(DBD),glow,etc),the gas used and so on.Reactive oxygen species(ROS)and reactive nitrogen species(RNS)can act as growth enhancers or stimulators[17]through different signaling pathways,but excess amounts may cause cell death due to membrane damage from oxidative stress[17].On the other hand,PAW not only shows antimicrobial activities but could also be used as fertilizer[18]depending on the types of species produced.Different types of ROS[19]and RNS along with their different concentrations can be produced in water through plasma treatments.The common plasma species of air discharges in water include[20]long-lived species,such as hydrogen peroxidenitritesnitratesand ozone(O3),and short-lived species,such as hydroxyl radicals(·OH),nitric oxide(NO·),superoxideperoxynitrateand peroxynitrite(-OONO).As a consequence,pH,electrical conductivity(EC)and the concentration of dissolved oxygen(DO),ROS and RNS of PAW are likely to be different depending on the type of discharge used for water treatment.
Takahataet al[21]have studied the effects of PAWs on the growth rate of spinach,radish and strawberry.They noted that the application of PAW contributed to faster growth of spinach and radish,while a higher sugar concentration was obtained in strawberry.Sivachandiran and Khacef[21]have investigated the combined effects of plasma seed treatment and application of PAW under laboratory conditions on seed germination and growth of radish,tomato and sweet pepper.They found that stem length increased by ~60% compared with controls when plants were watered with PAW treated for 15 min,while the longer treatment durations showed negative effects.
In this study our aim was to investigate the effects of the application of PAW as a foliar spray on the growth parameters,antioxidant enzymatic activities,total soluble protein(TSP)and sugar(TSS),yield and the concentrations of TSP and TSS in the rice grains produced.To the best of our knowledge,this is the first report to study the effects of application of PAW in field conditions on rice plants.The report is presented as follows:materials and methods are discussed in section 2,including the construction of the PAW reactor in section 2.1,estimation of PAW properties in section 2.2,field experiments in section 2.3,measurements of plant growth and yield parameters in section 2.4,estimation of antioxidant enzymes in plant tissues in section 2.5,estimation of TSP and TSS concentrations in plant tissues and rice grains in section 2.6 and statistical analyses in section 2.7.Results and discussion can be found in section 3 and our conclusions in section 4.
2.Materials and methods
2.1.Reactor for preparing plasma activated water
Figure 1(a)shows the schematic of the atmospheric pressure underwater plasma jet used to prepare PAW;it differs in construction,power supply and water treatment capacity from the reactor in reference[14].The plasma jet was prepared with a Pyrex glass tube(length 170 mm,inner diameter 3 mm).The glass tube was inserted through a Teflon tube(diameter 25 mm,height 45 mm)that was attached with a circular Teflon disk(diameter 90 mm,thickness 10 mm).The Teflon disk was used to enclose the container holding the water to be treated with the plasma jet for the preparation of PAW.A 160 mm tungsten wire(diameter 0.5 mm)was inserted through this tube and used as the power electrode.A 10 mm gap was maintained between the heads of the tungsten wire and glass tube in order to produce a plasma discharge.The water container was a 600 ml Pyrex glass bottle.The lower end of the jet glass tube was dipped into the water and another insulated wire was immersed into the water;this was used as the ground electrode.The upper end of the jet glass tube was used as the gas flow channel.Air was used as the working gas for the production of the underwater air discharge plasma.The flow of air into the jet was controlled with a gas flow controller(KIT 115P).A 1–10 kV,1–10 kHz monopolar pulsed variable power supply was used to operate the plasma jet.A photograph of the underwater air discharge plasma is shown in figure 1(b).
The voltage–current(V-I)characteristics presented in figure 1(c)were measured with a high-voltage probe(HVP-08)and current probe(CP-07C)in combination with a fourchannel digital storage oscilloscope(RIGOL DS1104).The optical emission spectra(OES)from the underwater air discharge plasma were acquired using two spectrophotometers.One(Ocean Optics USB2000+XR1:detector wavelength range180- 1100 nm,slit width25μm,grating 500 lines /mm,optical resolution1.7 nm)was used to identify the plasma species produced in the air discharge.A highresolution spectrometer(Avantes,Avaspec-2018:wavelength range of the detector200- 500 nm,slit width10μm,grating 2400 lines /mm,optical resolution0.07 nm)was used to determine the electronic excitation temperature(Tx),rotational temperature(Tr)and electron density(ne).Note that the electronic excitation temperature and molecular rotational temperature are considered as the electron temperature and gas temperature,respectively[29].It is well known that the electron temperature and density and the gas temperature play key roles in the production kinetics of plasma species in discharges.

Figure 1.(a)Schematic of the atmospheric pressure underwater air discharge plasma jet for the preparation of PAW.(b)Image of the underwater air discharge plasma jet.(c)Voltage–current characteristics measured for the underwater air discharge plasma conditions.(d)Optical emission spectra(OES)acquired from the underwater air discharge plasma jet.
2.2.Estimation of PAW properties(pH,O3,H2O2, and )
We treated 250 ml of distilled water(pH=7.58)for 10 min at a time with an atmospheric pressure underwater air discharge plasma.The pH and the concentrations ofand in the PAW treated for 10 min were measured.The pHwas measured instrumentally with a pH meter(Hanna Instrument,USA,model HI 2002-02:pH range-2.00 to 16.00,resolution 0.01,accuracy±0.01).Concentration of O3(Hanna Instruments,USA,test kit model HI93757-0),(Hanna Instruments,test kit model HI3844-0),(Hanna Instruments,test kit model HI3873-0)and(Hanna Instruments,test kit model HI3874-0)were determined by color development.The absorbances ofandwere measured(following the manufacturer’s instructions)at352 nm,390 nm,548 nm and527.2 nm,respectively using a UV-VIS spectrophotometer(Shimadzu Corporation,Japan,model UV-1900i).After treatment of distilled water with an atmospheric pressure air discharge plasma jet for 10 min,the following PAW parameters were obtained:pH=6.50 ±0.07,O3=0.42±0.01 mg l-1,H2O2=9.15 ±0.01 mg l-1,0.06 mg l-1and.
2.3.Field experiments
The field experiments were carried out from mid February to May 2020 in a plot at the agricultural project area of the University of Rajshahi,Bangladesh.Rice seeds(Oryza sativaL.,cv.variety BRRI dhan 28),were collected from the Regional Rice Research Institute,Shampur,Rajshahi.The rice seeds were immersed in tap water for24 h.The seeds were then taken out of the water and kept covered with gunny bags.The seeds started to germinate after 48 h and were sown after72 h in a previously prepared wet nursery bed.The muddled plot was prepared by applying a standard amount of chemical fertilizer(as per the instructions of the Bangladesh Rice Research Institute,BRRI),namely urea(150 kg ha-1),triple super phosphate(60 kg ha-1)and murate of potash(45 kg ha-1).The plot was then divided into 18 subplots(three control plots,15 plots for application of PAW)with an area of 10 m2each.Subplots were selected following the randomized complete block(RCB)method in order to carry out the described experiments.After 35 days after sowing the seedlings were uprooted and transplanted into the well-muddled subplots with three replications.The subplots were designated asWx(where PAW was appliedxtimes as a foliar spray).Urea was applied three times:50% during the preparation of the muddled field,25% at 20 days after transplant(DAT)and the remaining 25%at 50 DAT.PAW was sprayed onto the rice plants one to five times(at 22,29,36,43 and 50 DAT)within the vegetative growth stage(20–50 DAT).Note that PAW was sprayed onto the paddy plants 30 min after its preparation.
2.4.Measurement of plant growth and yield parameters
The roots and shoots of the plants were collected from the study field at 70 DAT and washed with tap water.Their lengths were measured with a millimeter scale and digital slide caliper and the data were recorded.Dry weights of roots and shoots were measured with an analytical balance(Shinko Denshi Co.Ltd,Japan)after being dried for72 h at 70° C.For the determination of chlorophyll concentration,fresh leaves were collected(at 70 DAT)randomly,weighed and ground with a pestle and mortar in 90% methanol;the homogenized mixture was centrifuged at 5000 rpm for 5 min.The absorbances of the collected supernatants were measured at 666 nm and 653 nm(for chlorophyllaandb,respectively)using a spectrophotometer(Shimadzu Corporation,UV-1900i)and the chlorophyll concentrations were estimated from the absorbance data using standard methods[30].Total carotene concentration was estimated by measuring the absorbances at 666 nm,653 nm and 470 nm using the method described in[31,32].Yield contributing parameters,i.e.length of panicle and number of grains per panicle,were measured from randomly collected panicles and the yield was estimated for each subplot.
2.5.Estimation of antioxidant enzymes in plant tissues
Concentrations of catalase(CAT),superoxide dismutase(SOD)and ascorbate peroxidase(APX)were estimated in roots and leaves of rice plants.Methods related to the determination of their concentrations will be described briefly.One hundred milligrams of seedling tissue(collected at 70 DAT)[33,34]was ground using a pestle and mortar with5 ml of phosphate buffer(100 mM,pH 7.0).The mixture was homogenized and centrifuged at 8000 rpm for 10 min.The supernatants were collected in several Eppendorf tubes.For the estimation of CAT activity,1.5 ml of reaction mixture was prepared by mixing100μl tissue extract,400μlH2O2(200 mM)and 1 ml of 100 mM potassium phosphate buffer(pH 7.0).The same spectrophotometer was used to measure the optical density of the reaction mixture at240 nm from 30 -90 s by the decrease in absorbance.The concentration of CAT was estimated from the recorded data(extinction coefficient0.03 mM-1cm-1).
To estimate[35]the concentration of SOD,100μl of plant tissue extract was mixed with0.1 mM ethylene diamine tetra acetic acid(EDTA),50 mM sodium carbonate/bicarbonate buffer(pH 7.0)and0.6 mM epinephrine enzyme.Data regarding the formation of adrenochrome for4 min were registered with the spectrophotometer at475 nm and the concentration of SOD was thereby estimated from the amount of enzyme required for the inhibition of 50% epinephrine oxidation.
To estimate APX activity a reaction mixture was prepared[36]by taking0.10 mM EDTA,0.50 mM ascorbic acid,50 mM potassium phosphate buffer(pH 7.0),0.10 mM H2O2and0.10 ml tissue extract.The optical absorbance of the reaction mixture was measured at290 nm using the spectrophotometer.The APX activity was calculated from the dependence of the extinction coefficient(2.80 mM-1cm-1)on absorbance.
2.6.Estimation of TSP and TSS concentrations
Concentrations of TSP and TSS in roots,leaves and rice grains were determined[37]employing spectroscopic methods.In brief,the collected(at 70 DAT)fresh leaves and roots of rice plants were washed,weighed and ground in an icecold pestle and mortar together with an assay buffer containing50 mM Tris-HCl(pH 7.5),2 mM EDTA and 0.04%(v/v)2-mercaptoethanol for protein isolation.The homogenized mixture was centrifuged for 10 min at 7000 rpm at a temperature of25° C.The extracted supernatant was mixed with 1 ml Coomassie brilliant blue in a glass cuvette.The optical absorbance of the prepared solution was measured with a spectrophotometer at595 nm and the data were recorded.The concentration of TSP was estimated by comparing the recorded data with the standard curve of bovine serum albumin(BSA).
The TSS concentrations in leaves,roots and rice grains were estimated[38]by a spectroscopic method.Homogenized solutions were prepared from roots,leaves and rice grains using hot aqueous ethanol(80% v/v)centrifuged at 5000 rpm for 5 min and then collected in glass test tube.A hot water bath was used for incubation for 8 min and the samples were then kept on ice.Optical absorbance at 620 nm was recorded from the ice-cold samples and compared with the standard curve of BSA in order to estimate the concentration of TSS.
2.7.Statistical analyses
Three independent replicates were used for all investigations.Each group of data was analyzed statistically for the level of significance atp≤ 0.05 by one-way analysis of variance.The analyses were carried out under Duncan’s multiple test range(DMRT)using SPSS statistics 20 software.The figures presented in this article were prepared using Microcal Origin 6.0 software.
3.Results and discussion
TheV-Icharacteristics,shown in figure 1(c),were used to estimate the amount of power absorbed by the atmospheric pressure underwater air discharge plasma.The absorbed power is calculated by the relationwherev(t),i t()andTare the voltage,current and period,respectively,of the corresponding waveforms.The estimated electrical power absorbed by the plasma was ~16 W.
The major electronic,atomic and molecular transitions obtained from the OES spectrum are shown in figure 1(d):the nitrogen second positive system(SPS)in the wavelength range280- 390 nm,the band transitionin the wavelength range 306 -312 nm,the frist negative system(FNS)band in the wavelength range391- 405 nm,thetransition in the range620- 750 nm;a small amount ofin the range 306 -312 nm,theline at486.1 nm andat777 nm were also found in the underwater air discharges.
The fundamental properties of plasma discharges are electron temperature and density and gas temperature.The electronic excitation temperature(Tx)(also called electron temperature),rotational temperature(Tr)and electron density(ne)play active roles in the species production mechanisms.The band of the FNS,was used[39]for the determination ofTrby fitting the measured emission spectrum with the LIFBASE simulation software[40]and the simulated result gaveThetransition was used following the method employed by Royet al[29]for the determination ofneand a density of1014cm-3was obtained.On the other hand,was determined utilizing the method described in[19]and it was found that.
As per the present experimental conditions,the plasma discharge tube was submerged in water and air was used as the working gas.Therefore,the production ofand O in the gas phase,shown in figure 2(d),can be obtained through different collision mechanisms in both gas and liquid phases depending on the plasma properties mainly electron temperature and density,and the gas temperature.Thereby,the production ofandin water as found in PAW will be described briefly.
Royet al[39]studiedpost-discharge plasma species production and their interactions with water using Fourier transform infrared spectroscopy(FTIR)in the midinfrared wavelength range of4545- 14286 nm for DBD discharge in water.The post-discharge species,as identified from the FTIR spectra,areand O3as well as a band ofH2O in the gas phase.On the other hand,OES data(shown in figure 2(d))reveal the production of OH,and O in the wavelength range of200- 1100 nm in an underwater air discharge.Depending on the OES data,the mechanism of production of the gas phase species will be described below.
Strong photoemissions were produced[13,41–45]by deexcitation or recombination of the excited or ionized atoms or molecules.Therefore,N andspecies can mostly be excited or ionized by the following collision processes of nitrogen molecules.Excited N2can be produced by direct electron impact excitations,wherekrepresent their respective collision or reaction rate coefficients,

by electronic stepwise excitations

and by direct electron impact ionization of excited nitrogen species

A small number of atomic oxygen radicals were generated in the air discharge.They can be produced[42,44]through direct electron impact dissociation and dissociative ionization of oxygen molecules by the following collision processes:

Ozone was also produced in the non-equilibrium atmospheric pressure air discharge plasmas.O3can be generated through
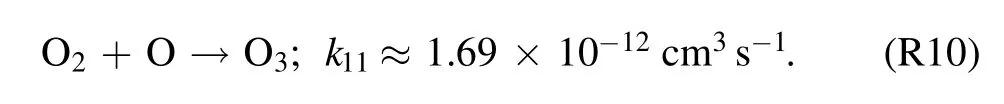
Oxygen and nitrogen species as produced[41,42]in the air discharge can interact and subsequently create oxy-nitrogen species:


A small number of OH radicals are present in the OES spectrum,as can be evidenced from figure 1(d).The following are the most probable OH production channels in both in gas and liquid because of its high production rate coefficient.One of them is the direct electron impact dissociation of water molecules[29,45]

A significant number ofN2(A)excited molecules are available in the gas phase and thus interact with water molecules;OH radicals are and subsequently produced through the following dissociative collision process[45]:

Another probable electronic collision process for the production of OH with water ion is dissociative electron attachment[45]

Further,OH can be generated through the following threebody dissociation interaction[29,45]:

H2O2can be produced[46]through the following reaction process:

The gas phase plasma species,as mentioned above,will be transported into the water due to flowing air as well as by diffusion,and consequently they will interact with water molecules.Production mechanisms ofandin water due to interactions of gaseous species with water molecules will now be described briefly.
OH(aq)can be produced through conversion ofH2O2by plasmaUV radiation[45]

The most significant interaction processes[47,48]that may occur at the plasma–liquid interface and in the water bulk for the production of ROSare considered below:

On the other hand,the following interactions are likely to be most relevant for the production([20]and references therein)of RNS:

Figures 2(a)–(d)show the effects of PAW as a foliar spray on plant height(PH),stem diameter(SD),dry weight(DW),chlorophyll and total carotene(TC)concentrations,respectively.It is observed from figure 2(a)that theW5treatment produced the highest PH of ~36,~54 and ~92 cm measured at 30,50 and 70 DAT with respect to control,i.e.an increase of~15%in PH is found at 70 DAT.Figures 2(b)and(c)reveal that the highest SD and DW,~13.23 mm and~22.23 g,respectively,are found in theW5treated plants,i.e.they are enhanced by ~25% and ~24%,respectively compared with control.Further,it is observed from figure 2(d)that the highest concentrations of chlorophyll and TC are 4.85 mg ·g-1and74.44 mg ·g-1,respectively,which are produced byW5treated plants measured at 70 DAT.Chlorophyll and TC are increased by ~47% and ~45%,respectively,with respect to control.

Figure 2.Effects of PAW as a foliar spray on plant(a)height,(b)stem diameter,(c)dry weight(measured at 30,50 and 70 days after transplant(DAT))and(d)chlorophyll and carotene concentrations(measured at 70 DAT).Error bars indicate standard errors of three replicates.Letters represent statistically significant differences(p<0.05).
The first three plant growth parameters(PH,SD and DW)reveal the significance of the application of PAW.PH,SD and DW increase almost linearly with increasing number of applications of PAW and were the highest with respect to control in plants that had five applications of PAW.Similar results are found in case of chlorophyll and TC concentrations,which increased with increasing number of applications of PAW.Plants use nitrogen in the forms ofandNitrogen is the most important element for the biochemical and physiological processes in growth and development of plants.Therefore,proper use of nitrogen is indispensable and unavoidable for the enhancement of crop growth and yield.On the contrary,excess or improper use of nitrogen can cause reduced yield.Foliar spraying of PAW,due to the presence of a significant amount ofandin PAW,can provide more nitrogen to plants and consequently contribute to greater plant growth and development.
Figures 3(a)–(c)display the effects of the applications of PAW on the antioxidant enzymes APX,CAT and SOD,respectively,extracted at 70 DAT from plants grown under the treatment conditions considered herein.It is seen from figure 3(a)that the maximum APX concentrations of21.78 and18.31 nmol min-1(mg protein)-1are found in the roots and leaves of the plants grown with treatmentW.5Whereas,the highest CAT concentrations(figure 3(b))of21.39 and 5.27 nmol min-1(mg protein)-1are found in roots and leaves,respectively,when treatmentW5was applied.On the other hand,the concentrations of SOD(figure 3(c))are found to be24.60(minimum)and24.75 nmol min-1(mg protein)-1(maximum)in the roots and leaves,respectively,with treatmentW5.Note that SOD concentrations decrease in the roots but increase in the leaves with increasing number of PAW applications.Concentrations of APX and CAT increased by~17% and ~28%,respectively,but SOD was reduced by~31% in roots with respect to control;concentrations of all three enzymes increased by ~10%,~90% and ~53%,respectively,in the leaves.

Figure 3.Effects of the application of PAW as a foliar spray on the concentrations of antioxidant enzymes(a)ascorbate peroxidase(APX),(b)catalase(CAT)and(c)superoxide dismutase(SOD)in roots and leaves of the rice plants(measured at 70 DAT).Bars indicate standard errors of three replicates.Letters represent statistically significant differences(p<0.05).
Antioxidant enzymes(figure 3)provide plants with defense against environmental stresses.Production of ROS(mainly H2O2)can be increased in plant cells during both biotic and abiotic stress and in excess they can cause oxidative damage to cells.The enzyme APX plays a key role as a catalyst in the detoxification of ROS through conversion of H2O2into H2O using ascorbate as an electron donor[50].Concentrations of APX are higher in the roots with respect to leaves,but APX concentration is slightly increased both in roots and leaves with increasing number of PAW applications.PAW was applied in the vegetative stages(from 20 to 50 DAT)of the rice plant growth cycle[51]because plants need more nitrogen during this stage.The greater nitrogen requirement in this vegetative stage of plants is likely to be fulfilled up PAW application,because PAW contains a significant concentration of RNS in the forms ofand.As a consequence,it is reasonable to consider that the concentration of photosynthetic pigments(chlorophyll and TC,figure 2(d))and APX(figure 3(a))are increased in the leaves through enhanced application of PAW to plants.
CAT,one of the most important relevant enzymes,is responsible for seed germination in the early stage[50]but later on peroxidase plays an even more significant role.The activities of CAT are increased in both roots and leaves with increasing number of PAW applications.This probably occurs because of the increased presence ofH2O2due to increasing application of PAW(H2O2is mitigated by CAT through production of water and oxygen)[50].There are several mechanisms[52]of conversion of H2O2within the plant cells.APX isoforms prevail in chloroplasts,mitochondria,peroxisomes and the cytosol.H2O2is converted into H2O using ascorbate as an electron donor.However,CAT,which occurs in peroxisomes[53],catalyzes the dismutation reaction without using any reductant.
SOD acts as the main agent providing defense against oxidative stress produced by ROS in plants.SOD is a metalloenzyme that dismutases superoxide radicals through the production of H2O2and oxygen.The activities of SOD were found to be higher in roots than in leaves.This result is consistent with the findings of Kucerovaet al[51].The concentrations of SOD decrease in roots but increase in leaves with increasing number of PAW applications.Application of PAW results in higher levels of SOD in the leaves,indicating that the higher SOD concentration provides better defense to plants against oxidative stresses with respect to control plants.Alternatively,this result could indicate that the higher concentrations of RNS provide better defense to the plants.
Figures 4(a)and(b)show the effects of the application of PAW on TSP and TSS in roots,leaves(collected at 70 DAT)and grains,respectively.The maximum concentrations of TSP,225,367 and492 mg g-1FW,are found in roots,leaves and grains,respectively,for treatmentW5,as can be seen in figure 4(a).Note that the concentrations of TSP and TSS are decreased by ~26% and ~30% in roots but are increased by~78% and ~23%,respectively,in leaves compared with control.On the other hand,the concentrations of TSP and TSS are increased by ~69% and ~13%,respectively,in grains with increased application of PAW.
The concentration of TSP is decreased in the roots while it is increased both in leaves and grains with increasing number of PAW applications(figure 4(a)).Comparison between control and PAW treated plants shows that the application of PAW provides tends to generate a higher TSP concentration.Most probably,TSP is transconducted from roots to leaves and thereafter from leaves to grains during the grain filling stage.TSS may produce[54]an adaptive response against stresses.These stresses include drought,pathogen attack,low temperature,anoxic injury and surplus excitation energy.The level of exogenous sugar concentration(figure 4(b))is decreased in the roots but is increased by PAW treatment with respect to control.Leaves have a higher TSS concentration than roots.It is interesting to note that the highest TSS concentration is found in rice grains from the PAW treated plants.TSS is likely to be transferred from roots to leaves and then from leaves to grains,and subsequently the concentration of TSS is also increased in the grains.Further experiment should be carried before definitive conclusions can be made.

Figure 4.Effects of the applications of PAW as a foliar spray on the concentrations of(a)total soluble protein(TSP)and(b)total soluble sugar(TSS)in roots and leaves of rice plants measured at 70 DAT and in grains.Bars indicate standard errors of three replicates.Letters represent statistically significant differences(p<0.05).
Figures 5(a)and(b)represent the effects of PAW application on yield-related characters and yield,respectively.Figure 5(a)shows the panicle length and the number of grains per panicle.This figure reveals that the panicle length and grains per panicle are enhanced by ~13% and ~21%,respectively,forW5treated plants.Figure 5(b)shows the 1000-grain weight and yield of rice.The 1000-grain weight and yield are also found to increase by ~9% and ~14%,respectively,after treatmentW5.

Figure 5.Effects of the applications of PAW as a foliar spray on(a)panicle length and grains per panicle and(b)1000-grain weight and rice yield.Bars indicate standard errors of three replicates.Letters represent statistically significant differences(p<0.05).
The increased panicle length and grains per panicle were probably due to the appropriate supply of nitrogen to the plants through application of PAW.It is to be noted that there is a great scarcity of data regarding the yield of rice with plasma technology.However,our previous results[8,9]regarding the yield of wheat using plasma technology can be considered here.In those wheat experiments,an 18%–20%increased yield was obtained.But in the case of rice,the yield is increased by~14%with respect to control when treatmentW5was applied.
4.Conclusion
Plant height,stem diameter,dry weight,and chlorophyll and carotene concentrations were increased due to application of PAW to rice plants.The antioxidant enzymatic activities reveal that the plant defense mechanisms improved with the application of PAW.A better adaptive response against stresses is produced in the plants because of enhanced TSS concentrations produced by PAW treatment.The highest TSS and TSP concentrations were produced in rice grains from the plants grown with PAW treatment.This indicates that the parameters concerning the food value of the grains are likely to improve as a consequence of PAW application.Finally,the yield of rice was increased by ~14% with respect to control after PAW treatment.
Acknowledgments
MRT would like to acknowledge the Ministry of Education(no.LS2017544),Government of the People’s Republic of Bangladesh,and the University of Rajshahi(No.62/5/52/RU/Engg-05/2020-2021)for their partial financial support to carry out this work.The authors also would like to thank Mizanur Rahman,Lab Technician,Plasma Science and Technology Lab,Department of Electrical and Electronic Engineering,University of Rajshahi for providing time in the lab and research field.
杂志排行
Plasma Science and Technology的其它文章
- Numerical simulation of carbon arc discharge for graphene synthesis without catalyst
- Enhanced removal of ultrafine particles from kerosene combustion using a dielectric barrier discharge reactor packed with porous alumina balls
- Visualization of gold nanoparticles formation in DC plasma-liquid systems
- An active tunable Fano switch in a plasmafilled superlattice array
- Comparison of sample temperature effect on femtosecond and nanosecond laser-induced breakdown spectroscopy
- Magnetic field induction and magnetic force distribution profiles in plasma focus discharge device
