Monilinia fructicola on loquat:An old pathogen invading a new host
2021-06-02YINLiangfenZHANGShuqinDUJuanWANGXinyuXUWenxingLUOChaoxi
YIN Liang-fen ,ZHANG Shu-qin,DU Juan,WANG Xin-yu,XU Wen-xing,LUO Chao-xi
1 Key Lab of Horticultural Plant Biology,Ministry of Education/Huazhong Agricultural University,Wuhan 430070,P.R.China
2 Experimental Teaching Center of Crop Science,College of Plant Science and Technology,Huazhong Agricultural University,Wuhan 430070,P.R.China
3 Key Laboratory of Crop Disease Monitoring and Safety Control in Hubei Province,College of Plant Science and Technology,Huazhong Agricultural University,Wuhan 430070,P.R.China
4 Key Laboratory of Oasis Agricultural Pest Management and Plant Protection Resources Utilization,College of Agriculture,Shihezi 832003,P.R.China
Abstract Monilinia fructicola has been widely reported as the causal agent of brown rot disease on many Rosaceae family fruits worldwide.It has been reported on stone fruits,e.g.,peach,plum,cherry,apricot and mume;as well as pome fruits,e.g.,apple,pear and hawthorn.Loquat is a member of the Eriobotrya genus in subfamily Maloideae along with apple,pear and hawthorn.So far,loquat has not been reported as the host of any Monilia species.In June 2019,brown rot symptoms were observed on loquat fruits in an orchard in Wuhan,Hubei Province,China.Thirty single spore isolates were obtained and identified as M.fructicola based on morphological characteristics and molecular analysis.This is the first report of loquat brown rot disease caused by Monilia species in the world.Furthermore,upon artificial inoculation,all three Monilia species from peach in China,i.e.,M.fructicola,M.mumecola and M.yunnanensis,could cause typical brown rot disease on loquat fruits.At the same time,M.fructicola isolates from loquat showed virulence similar to those isolates from peach when the pathogenicity test was conducted on peach fruits.These results suggested that loquat could be infected by other Monilia species and that isolates from loquat also have potential to damage other Rosaceae family fruits in practice.
Keywords:Monilia species,brown rot,loquat,Monilinia fructicola
1.Introduction
Loquat,also known asEriobotrya japonicaLindl.,Chinese loquat,and Japanese medlar,is a member of the Rosaceae family.Loquat belongs toEriobotryagenus,along with apple,pear and hawthorn in subfamily Maloideae.It originated in China,and has been cultivated for more than 2 000 years (Morton 1987;Linet al.1999;Lin 2003;Wu 2004;Zhanget al.2006;Zheng 2007,2010;Zhang 2017;Jianget al.2018).As a popular tree in China,loquat is extensively cultivated in most areas of southern China,with more than 80% of the global production (Jianget al.2018).
Loquat trees are susceptible to pests and pathogens,and numerous pathogens have been reported to cause diseases on them.Nevertheless,brown rot disease,the most troublesome disease caused byMoniliaspecies which has been extensively reported on stone fruits peach,plum,apricot,cherry,and mume,and pome fruits apple,hawthorn and pear (Michailideset al.2007;Luo 2017),has not been reported on loquat so far,even though loquat is a near relative to these fruits in the Rosaceae family.
In June 2019,symptoms resembling brown rot disease on loquat fruits were observed in an orchard at Huazhong Agricultural University in Wuhan,China.However,brown rot disease had not been reported on loquat previously in the world.So the objectives of this study were to determine whether this disease was indeed brown rot caused byMoniliaspecies,and,if so,to determine whether the causal agent was different from those from other Rosaceae family fruits reported in China previously.
2.Materials and methods
A total of 15 diseased loquat fruit samples showing brown rot symptoms were collected,two isolates were isolated from each sample by using the single spore isolation method described previously (Yinet al.2015),thus,30 single spore isolates were obtained.In order to identify their taxonomic status,six reference isolates representing threeMoniliaspecies (two isolates from each species) reported previously on peach in China,i.e.,M.fructicolaisolates CEH15-3 and CEH15-4a,M.mumecolaisolates MEH13-2-1b and MEH13-9-1a,andM.yunnanensisisolates YQH12-2-1 and YQH12-3-1a,were selected to compare with three randomly selected representative loquat isolates:CWL19-1a,CWL19-2-7a and CWL19-2-12b.
Selected single spore isolates were inoculated on potato dextrose agar medium (PDA,boiled juice from 200 g potato,20 g dextrose,and 20 g agar L–1),then incubated at 23°C in darkness.The agar plugs (6 mm in diameter) cut from the edge of 4-d-old colonies were placed upside down onto the center of fresh PDA,and incubated at 23°C in darkness.The morphological characteristics of colonies were observed,the diameter of each colony was measured in two perpendicular directions,and mean growth rate of colonies were calculated with three replicates.The experiment was conducted twice.
Pathogenicity tests of representative isolates were executed on detached loquat and peach fruits.Healthy fruits were carefully washed with tap water,then surface disinfected with 75% ethanol and rinsed thoroughly with sterile water.Loquat and peach fruits were punched with a sterile cork borer (6 mm in diameter),then mycelia plugs cut from the edge of a 5-d-old colony with the same cork borer were inserted into the punched wounds.The control was carried out using PDA plugs without pathogen.The inoculated fruits were then placed in a porcelain tray with wet tissue paper at the bottom and covered with cling film to maintain high humidity,and incubated at 23°C with 12 h of dark and 12 h of light.Three fruits of loquat or peach were used for each isolate and the experiments were conducted twice.The developed lesions with conidia were investigated after 2 d (loquat) or 4 d (peach) of incubation,and the lesion diameters were measured by MNT digital caliper (Shanghai Meineite Industry Co.,Ltd.).Statistical analyses for lesion expansion rate were performed by one-way ANOVA with the least-significant difference (LSD) test in SPSS19.0 Software atP=0.05.
Conidia generated on inoculated loquat fruits were collected and observed with a stage micrometer under a Motic BA400 Light Microscope (Motic China Group Co.,Ltd.,Xiamen,China).The size of the conidia was measured by software Digital Camera Viewer 6.0 (Beijing Ruizi Aoheng Vision Technology Co.Ltd.,China).The mean value of each isolate was determined based on the size (width and length) of 50 conidia.The conidial germination pattern was observed by spreading conidia onto PDA medium and incubating at 23°C in darkness.After a 5-h incubation,germ tubes were then observed using a BK5000TR compound light microscope (Chongqing Optical &Electrical Instrument Co.,Ltd.,China).
For molecular identification,DNA of selected isolates was extracted with the Easypure Plant Genomic DNA Extraction Kit (TransGen Biotech,Beijing,China).Isolates were identified to the species level by sequence analysis of the ribosomal internal transcribed spacer (ITS) and multiplex PCR protocol developed previously (Huet al.2011).The PCR reactions were performed in an iCycler Thermal Cycler(Bio-Rad Laboratories Inc.,Hercules,CA,USA) with the following parameters:an initial denaturation at 94°C for 3 min;30 cycles of 30 s at 94°C,30 s at 58°C,and 1 min at 72°C;and a final extension step at 72°C for 5 min.Then PCR products of the ITS region were sent to the Beijing Genomics Institute (Shenzhen,China) for sequencing.Multiplex PCR amplification was performed by using reverse primer Mon-R(5´-ATC TCC AAG ATC CGT GAG GAG-3´) and forward primers Cola-F (5´-CTG TAT GAT GAC CGA GAA GG-3´),Mume-F (5´-AAA GGT AGA AGA CAT CTT AAG G-3´) and Ensis-F (5´-GGA AAC CAA GTG GTT GAG AT-3´).PCR products were tested by 1.2% agarose gels (AGAROS G-10,Gene Company,Hong Kong,China) with 0.5× Tris-Borate-EDTA buffer for 40 min at 110 V.Gels were stained with ethidium bromide and photographed under ultravoilet (UV)light using an Alphalmager EP Image Acquisition System(Alpha Innotec,Santa Clara,CA,USA).
3.Results
3.1.Field and post-inoculation symptoms
Field symptoms of loquat samples resembled brown rot disease,with brown decay and gray pustules of sporulation(Fig.1-A1).In order to determine whether it was brown rot,symptoms were compared with those of peach brown rot caused by threeMoniliaspecies (Fig.1-B1,C1,and D1).Typical field symptoms of brown rot disease on peach caused byM.fructicolawere necrotic areas with numerous incompact brown conidia and conidiophores on the mycelia(Fig.1-B1).Monilinia mumecolacaused similar symptoms,but the covered mycelia and conidiophores/conidia were grayish-green and relatively fewer spores were produced(Fig.1-C1).While obvious pustules were generally produced on the decayed tissues which developed from the infection ofM.yunnanensis,they were different from those caused byM.fructicolaandM.mumecola(Fig.1-D1).In the current study,the loquat fruit and sporulation tufts shrunk due to being out-of-season and drought,and the symptoms were more like those caused byM.yunnanensisthan those caused byM.fructicolaandM.mumecola(Fig.1-A1).
When isolates of these species were inoculated on loquat fruits,typical brown rot symptoms developed on the inoculated loquat fruits.Isolates from loquat (Fig.1-A2)produced a lesion appearance similar toM.fructicolafrom peach,which showed brown rot with light tan mycelia tufts and areas of sporulation (Fig.1-B2).Monilinia mumecolafrom peach produced brown rot with little or no mycelia tufts (Fig.1-C2).Monilinia yunnanensisfrom peach also produced brown rot with mycelia tufts and areas of sporulation,however,it produced fewer but larger sporodochia which were off-white in color (Fig.1-D2).
3.2.Colony morphology,growth rate,conidia morphology and germination pattern of loquat isolates compared to other Monilia species
The colony morphology and growth rate of loquat isolates were compared with threeMoniliaspecies from peach on PDA.Loquat isolates showed similar colony morphology withM.fructicolaisolates from peach,which were gray to brown and had many tufts of sporulation (Fig.2-A1 and-B1).The growth rate of isolates from loquat was 15 mm while that ofM.fructicolaisolates from peach was 14 mm d–1on average at 23°C in darkness.Colonies ofM.mumecolaandM.yunnanensisfrom peach were whitish to grayish,without obvious areas of sporulation,and had the same growth rate of 11.8 mm d–1on PDA (Fig.2-C1 and-D1).However,the growth pattern ofM.mumecolaisolates is distinct from other species due to its mycelia with lobbed margins.
Isolates from loquat had lemon-shaped conidia similar toM.fructicolafrom peach (Fig.2-A2 and B2),the mean conidia size of loquat isolates was 13.63 (8.34 to 17.28) μm×10.43 (7.35 to 14.45) μm,andM.fructicolafrom peach was 13.47 (9.39 to 16.77) μm×10.46 (7.58 to 13.34) μm.Furthermore,they had similar germination patterns that produced only one germ tube per conidium,and most of the germ tubes were initiated from the middle of the conidia(Fig.2-A3 and B3).Monilinia mumecolaproduced lemonshaped but larger conidia of 22.57 (13.35 to 32.46) μm×16.3(11.67 to 22.84) μm (Fig.2-C2).MostM.mumecolaconidia produced more than two germ tubes (Fig.2-C3).Conidia produced byM.yunnanensiswere long and thin,measured 16.65 (9.16 to 21.39) μm×10.42 (7.21 to 13.14) μm(Fig.2-D2),produced one or two germ tubes,and differed from those ofM.fructicolaandM.mumecolaas their germ tubes generally developed from the pointy sides of the conidia (Fig.2-D3).
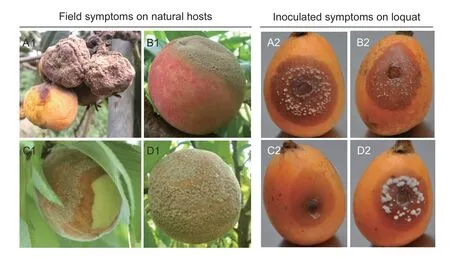
Fig.1 Field symptoms on natural hosts and inoculated symptoms on loquat fruits of Monilinia fructicola from loquat (A1 and A2)and M.fructicola (B1 and B2),Monilia mumecola (C1 and C2) and Monilia yunnanensis (D1 and D2) from peach.Inoculated loquat fruits were incubated at 23°C for 2 d under a 12 h light/12 h dark regime.
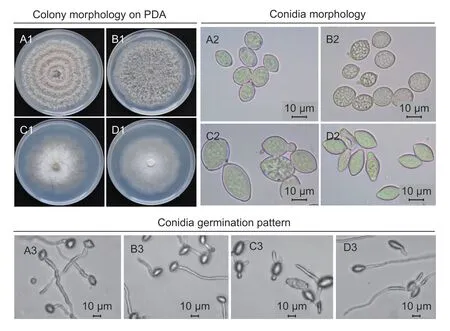
Fig.2 Colony morphology,conidia morphology and germination patterns of Monilinia fructicola from loquat (A1–A3) and M.fructicola(B1–B3),Monilia mumecola (C1–C3) and Monilia yunnanensis (D1–D3) from peach.The isolates were grown on PDA medium for 5 d at 23°C in darkness.Conidia were obtained from inoculated loquat fruits.Photos were taken under ×400 microscope;bars represent 10 μm (A2–D2).For investigation of germination patterns,conidia were spread on PDA medium and incubated at 23°C for 5 h in darkness.The photos were taken under ×200 microscope;bars represent 10 μm (A3–D3).
Based on the colony and conidia morphology and germination pattern,the causal agent of loquat brown rot was preliminarily identified asM.fructicola.However,the morphological observation based identification needed to be confirmed by ITS sequence analysis and species-specific PCR identification.
3.3.ldentification by lTS sequencing and speciesspecific PCR
The ITS regions of three selected isolates from loquat,CWL19-1a,CWL19-2-7a and CWL19-2-12b were amplified and sequenced.The BLASTn comparison showed that their ITS sequences were identical to theM.fructicolasequences of isolates from Bulgaria (GenBank accession no.MN453261),Mexico (no.MN179292),China (no.MK834757) and USA (no.KY038837).
In species-specific PCR identification,all three loquat isolates amplified a 534-bp fragment,which was the same as twoM.fructicolaisolates from peach,but different from twoM.mumecolaand twoM.yunnanensisisolates from peach,which amplified 712-and 237-bp fragments,respectively (Fig.3).
Therefore,the molecular analysis further confirmed that the causal agent of brown rot disease on loquat wasM.fructicola.
3.4.Pathogenicity evaluation
In order to test whether isolates from loquat could infect other Rosaceae family fruits,a pathogenicity test was performed on detached peaches.The results showed that three isolates from loquat (Fig.4-B,C and D) were as virulent on peach asM.fructicolaisolate from peach (Fig.4-A).This result suggested that the brown rot isolates from loquat have potential risk to infect other hosts in Rosaceae family.
On detached loquat fruits (Fig.1-A2,B2,C2 and D2),the lesion expansion rates of loquat isolates andM.fructicolafrom peach were 17.4 and 15.9 mm d–1,which are higher than those ofM.mumecolaandM.yunnanensisisolates from peach,which were 10.5 and 10.8 mm d–1,respectively(P<0.05).The lesion expansion rate on detached loquat fruits was in accordance with the colony growth rate on PDA.
4.Discussion
Loquat is widely cultivated in more than 30 countries and areas around the world,mainly in Asia,Europe,South Africa,America and Australia (Linet al.1999;Hu and Lin 2002;Wu 2004;Lin 2007).China stands out as the leading producer with a planting area of more than 130 000 ha and annual production of 650 000 t,which is more than 80% of the world production (Jianget al.2018).Loquat grows in 20 out of 32 regions in China,and Fujian has been replaced by Sichuan as the leading province in recent years (Hu and Lin 2002;Lin 2007;Jianget al.2018).In addition to China,Spain,Japan and Brazil are the other main loquat producers in the world (Hu and Lin 2002;Wu 2004).
Corresponding to its wide distribution in the world,studies on loquat diseases have also been carried out extensively.However,despite being the most disastrous disease on stone fruits and an important disease on pome fruits,brown rot disease caused byMoniliaspecies had not been reported on loquat previously,even though loquat is a member of Rosaceae family and a near relative of stone and pome fruits.
Thus far,sixMoniliaspecies,M.fructicola,M.fructigena,M.laxa,M.mumecola,M.yunnanensis,andM.polystroma,have been reported to cause brown rot disease on stone and pome fruits (Luo 2017).However,even in countries where both loquat diseases and brown rot disease on other Rosaceae family fruits have been reported,there have been no reports of loquat brown rot disease.For instance,China is the leading producer in the world not only of loquat,but also some stone and pome fruits,e.g.,peach and apple.Loquat diseases such as stem blight (Fenget al.2016)and leaf spot (Zhang 2017) have been reported.Brown rot diseases of stone and pome fruits caused byMoniliaspecies have also been frequently reported (Huet al.2011;Yinet al.2013,2014a,b,2015,2017;Zhaoet al.2013;Chenet al.2016;Zhuet al.2016;Luo 2017),but loquat brown rot caused byMoniliaspecies has not been reported so far.
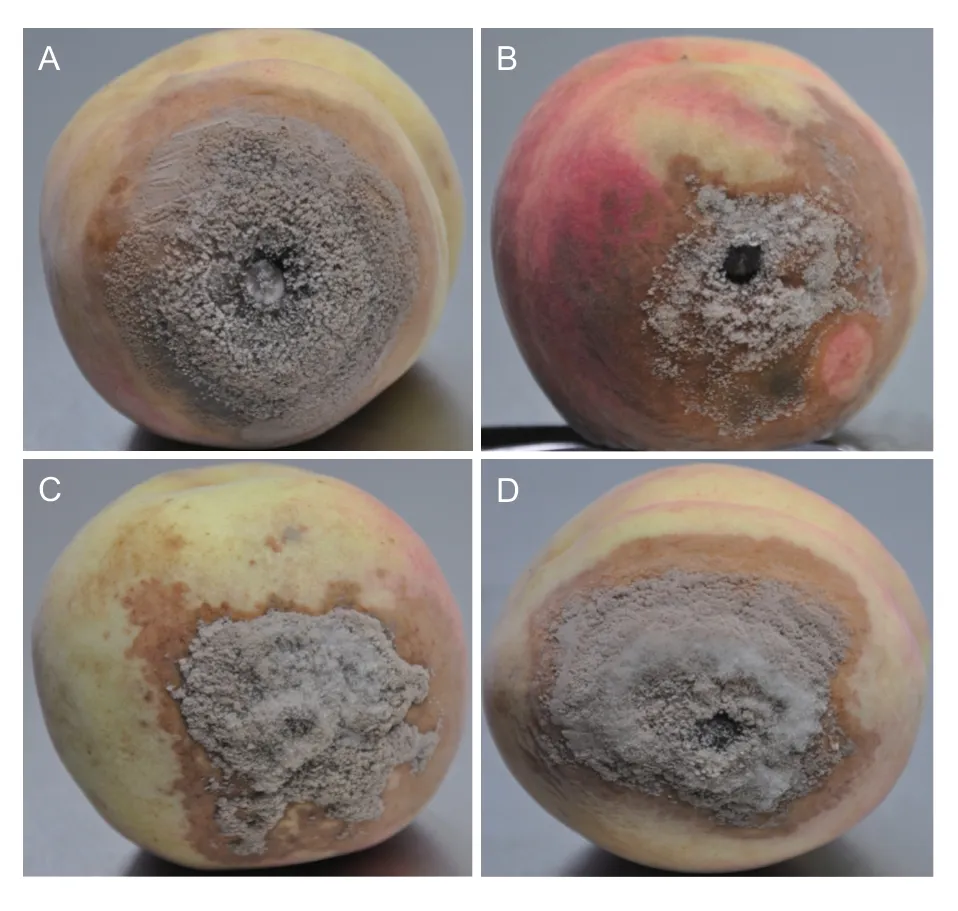
Fig.4 Symptoms on peach fruits.Monilinia fructicola isolates were from peach (A) and loquat (B,C,and D).Inoculated fruits were incubated at 23°C for 4 d under a 12 h light/12 h dark regime.
The reason why brown rot disease caused byMoniliaspecies on loquat has not been reported so far is unclear.It is possible that loquat is not easily infected byMoniliaspecies.We have noticed this at the time when the loquat brown rot samples were collected.Three areas of loquat trees were planted in the sampling orchard,where peach,plum and apricot trees were also planted in the same orchard.Loquat brown rot disease was not observed in the area which was far from infected stone fruits,and few loquat brown rot samples were collected in the area which was near the peach trees with a low infection rate.However,loquat trees were severely infected in the third area which was near the highly infected peach and plum trees.These observations suggested that loquat brown rot under natural conditions depended on the presence of sufficient inocula.Nevertheless,when the pathogenicity test was conducted on detached loquat fruits withMoniliaspecies from peach,typical brown rot symptoms could be observed.At the same time,M.fructicolaisolates from loquat can produce a similar lesion size on detached peach fruits asM.fructicolaisolates from peach,indicating that there is no significant difference in virulence between isolates from these two hosts.
5.Conclusion
Brown rot symptoms were observed on loquat fruits in an orchard in Wuhan,Hubei Province.The causal agent was identified asM.fructicolabased on morphological characteristics and molecular analysis.This is the first report of loquat brown rot disease caused byMoniliaspecies in the world.Furthermore,inoculation tests suggested that loquat could be infected by otherMoniliaspecies andM.fructicolaisolates from loquat also have the potential to damage other Rosaceae family fruits in practice.
Acknowledgements
This work was financially supported by the earmarked fund for China Agriculture Research System (CARS-30) and the National Natural Science Foundation of China (31401686 and 31872934).
Declaration of competing interest
The authors declare that they have no conflict of interest.
杂志排行
Journal of Integrative Agriculture的其它文章
- Receptor-like kinase OsASLRK regulates methylglyoxal response and content in rice
- Heredity and gene mapping of a novel white stripe leaf mutant in wheat
- Construction of a high-density adzuki bean genetic map and evaluation of its utility based on a QTL analysis of seed size
- Effects of temperature and solar radiation on yield of good eatingquality rice in the lower reaches of the Huai River Basin,China
- Difference in corn kernel moisture content between pre-and postharvest
- The effect of elevating temperature on the growth and development of reproductive organs and yield of summer maize
