Receptor-like kinase OsASLRK regulates methylglyoxal response and content in rice
2021-06-02LINFamingLIShenWANGKeTIANHaoranGAOJunfengDUChangqing
LIN Fa-ming,LI Shen,WANG Ke,TIAN Hao-ran,GAO Jun-feng,DU Chang-qing
Collaborative Innovation Center of Henan Grain Crops/Henan Key Laboratory of Rice Biology/College of Agronomy,Henan Agricultural University,Zhengzhou 450002,P.R.China
Abstract Receptor-like kinases (RLKs) are essential for plant abiotic stress responses.Methylglyoxal (MG) is a cellular metabolite that is often considered to be a stress signal molecule.However,limited information is available about the relationship between RLKs and MG.Here,we addressed the function of a receptor-like kinase,OsASLRK,in the MG response and content in rice.A typical MG-responsive element (AAAAAAAA) exists in the promoter region of the OsASLRK gene.RTqPCR analysis indicated thatthetranscript level of OsASLRK was significantly increased by exogenous MG in a time-and dosage-dependent fashion.GUS staining also confirmed that the expression of OsASLRK in rice root was enhanced by exogenous MG treatment.Genetic analysis suggested that the Osaslrk mutant displays increased sensitivity to MG and it showed higher endogenous MG content under exogenous MG treatments,while OsASLRK-overexpressing rice plants showed the opposite phenotypes.Diaminobenzidine (DAB) staining,scavenging enzyme activities and GSH content assays indicate that OsASLRK regulates MG sensitivity and content via the elevation of antioxidative enzyme activities and alleviation of membrane damage.Therefore,our results provide new evidence illustrating the roles that receptor-like kinase OsASLRKplaysin MG regulation in rice.
Keywords:receptor-like kinase (RLK),OsASLRK,methylglyoxal (MG),rice
1.Introduction
Methylglyoxal (MG),also known as acetylformaldehyde,is an ubiquitous endogenous byproduct metabolite in the biosphere which is mainly produced by glycolysis,and is usually toxic at higher than physiological levels in living organisms (Salem 1957;Cooper 1984;Thornalleyet al.1999;Murata-Kamiya 2001;Yadavet al2008).It can react readily with biological macromolecules such as DNA,RNA and proteins,resulting in modulation of their expression and activity (Kalapos 1999;Thornalley 2008).Given that MG is a mutagen and genotoxic agent,high concentrations of MG will inhibit cell growthviainactivation of the antioxidant defense system (Rayet al.1994).The toxicity mechanism of MG has been well studied in yeast and animals.For example,the role of MG as a signal initiator involved in oxidative stress in yeast has been well-defined (Maetaet al.2005;Sun and Liao 2017).In animals,an increased level of cellular MG is believed to be one of the causative factors of aging as well as of a number of diseases,such as cancer and diabetes (Royet al.2003;Ramasamyet al.2006;Jack and Wright 2012).Compared with yeast and animals,the mechanisms of MG production and action in plants are not yet clear.In plants,endogenous MG content can also rapidly rise to toxic concentrations in response to abiotic and biotic stresses,and elevated MG can exert inhibitory effects on plant growth and development,suggesting a potentially important role of MG as a signal molecule in plants (Yadavet al.2005;Singla-Pareeket al.2006;Ghoshet al.2014).For example,the endogenous MG content in rice rises 2-to 6-fold under drought,high salinity and cold conditions (Yadavet al.2005;Ghoshet al.2014).It is crucial for plants to eliminate MG in a timely and effective manner under normal or stressed conditions.In order to maintain control over the MG concentration,the antioxidant defense system,including the glutathione (GSH)-dependent enzyme system,is believed to be the major detoxification route for MG in biological systems (Thornalley 2003).In plants,some studies have suggested that multiple gene families,such as receptor-like protein kinase (RLK),are involved in the MG response (Kauret al.2015).However,the detailed functions of the RLK members and the molecular mechanisms underlying the MG response are still unknown.
RLK members in plants usually consist of three domains:a N-terminal extracellular domain,a single or multiple transmembrane domain(s) and a C-terminal intracellular Ser/Thr kinase domain (Shiu and Bleecker 2001a,b).The RLK protein family is considered to be the largest gene family inArabidopsis(Arabidopsis thaliana) and rice (Oryza sativa),with more than 610 and 1 131 family members,respectively (Shiu and Bleecker 2001a,b,2003;Shiuet al.2004).However,the functions of the vast majority of them are still unknown.RLK members have been shown to play critical roles in almost all cellular signal processes,such as in the responses to external stress,hormones,pathogens,etc.For example,analogous to the well-studied BR receptors(BRI1 and BAK1) (Nam and Li 2002;Tanget al.2008;Penget al.2018),CLAVATA1 (Clark 2001;Huet al.2018) and PSY1 (Oehlenschlægeret al.2017) control plant growth and development under normal growth conditions.In addition,substantial efforts have been devoted to examining the important roles of RLKs in optimizing plant responses to abiotic stresses such as salinity (Chenet al.2013;Zhouet al.2018;Linet al.2020).For instance,an S-domain receptor-like kinase (OsSIK2) confers abiotic stress tolerance and delays leaf senescence in rice (Chenet al.2013).Another receptorlike kinase,STRK1,improves rice salt toleranceviaregulation of H2O2homeostasis (Zhouet al.2018).Although a great deal of research has focused on the roles of RLK in abiotic stress regulation,direct evidence indicating a function of RLK members in MG responses and content regulation has so far not been obtained.Our objective in this study is to determine whether the RLKs are involved in the MG response,in order to provide new insights for better understanding the abiotic stress regulationviaRLKs.
Given the importance of RLKs in abiotic stress responses,we selected some putative RLK members in the rice genome as objects and investigated their functions in abiotic stress.In a previous study,we found thata candidate ABA Sensitivity-related LRR-RLK (LOC_Os01g53920,unpublished),designated asOsASLRK,also responds to exogenous MG treatment,so here we selected theOsASLRKgene for further MG analysis and attempted to uncover the link betweenOsASLRKand the MG response.Our results further confirmed thatOsASLRKobviously responds to MG in a time-and dosage-dependent fashion,and the growth phenotype and physiological status of theOsaslrkmutant andOsASLRK-overexpressing rice plants were significantly alteredviathe antioxidative enzyme system under exogenous MG treatments.In summary,our findings provide new evidence that RLKs play important roles in the MG response and highlight the importance of receptor-like kinaseOsASLRKin MG regulation in rice.
2.Materials and methods
2.1.Plant materials
Rice seeds were surface-sterilized in 15% (v/v) H2O2for 10 min,and washed five times with sterilized water to induce germination.Germinated seeds were normally cultured in 1/2 MS liquid medium in an incubator with a 16-h light/8-h dark cycle at 28°C for the indicated times.Then the seedlings were treated with a series of MG concentrations (0,1,5,10,and 20 mmol L–1) for 12 h or with 5 mmol L–1MG for 0,1,2,4,6,and 12 h.After the MG treatments,rice leaf samples were harvested at the indicated times,immediately frozen in liquid nitrogen and stored at–80°C for RNA isolation.
2.2.RNA extraction,cDNA synthesis and RT-qPCR
Total RNA isolation was performed using the Trizol reagent(Thermo Fisher,USA) according to the manufacturer’s protocol.The isolated RNA was treated with DNaseI to eliminate genomic DNA contamination.First-strand cDNA was prepared from 1 μg of total RNA with reverse transcriptase (Thermo Fisher,USA) and Oligo (dT18)primers following the manufacturer’s instructions.Real-time quantitative PCR (RT-qPCR) was conducted using SYBR premixEx TaqII (TaKaRa,Japan) in a total volume of 20 μL on a CFX96™ Real-Time PCR Detection System(Bio-Rad,USA).Data were normalized based on the internal riceACTINgene (OsACTIN1).Relative fold expression changes were calculated using the relative 2−ΔΔCT method(Livak and Schmittgen 2001).All of the samples were tested in three biological replicates.The primers used in this study are shown in Appendix A.
2.3.GUS histochemical staining assay
For histochemical detection of GUS expression,2-wkold young roots of T3progeny of theProOsASLRK::GUStransgenic rice lines were collected and treated with 5 mmol L–1MG for 0,12 and 24 h,and then stained following the protocol of Jeffersonet al.(1987).
2.4.Exogenous MG sensitivity and endogenous MG content assays
For the MG sensitivity assay,rice seeds of theOsaslrkmutant (PFG_1A-24919.R,Hwayong (HY) background),OsASLRK-overexpressing rice lines (T2generation,Kitaake background) and their corresponding wild types (WT) were surface-sterilized as described above,and the germinated seeds were then cultured in 1/2 MS medium supplemented with either 0 or 5 mmol L–1MG under a 16-h light/8-h dark photoperiod at 28°C.After 2 days,the growth status of these seedlings was photographed,and shoot and root lengths were measured.
The MG content assay was performed mainly as described by Mustafizet al.(2010) as follows:about 0.3 g flag leaves were collected and homogenized in 3 mL of 0.5 mol L–1trichloroacetic acid (TCA) in an ice-cold mortar for 15 min.The homogenate was centrifuged at 13 000×g for 15 min at 4°C,and centrifugation was repeated once more.Next,1 mL supernatant was transferred into a new 1.5 mL centrifuge tube and titrated with saturated K2CO3to neutrality for subsequent analyses.The total volume of 1 mL reaction buffer contained 250 μL 1,2-diaminobenzene (7.2 mmol L–1),100 μL trichloroacetic acid (5 mmol L–1) and 650 μL neutralized supernatant above,and was maintained at room temperature for 30 min.Absorbance of the supernatant was monitored at 336 nm and the MG content was expressed as μmol g–1FW protein.
2.5.Diaminobenzidine (DAB) staining
For the ROS content assay,2-wk-old rice seedlings of theOsaslrkmutant,OsASLRK-overexpressing rice lines(T2generation) and their corresponding WT grown in normal conditions were first soaked in 1/2 MS medium supplemented with either 0 or 5 mmol L–1MG for 24 h,and then flag leaves were excised and immersed in a 1%solution of DAB in Tris-HCl buffer (pH 6.5) for DAB staining at room temperature for 2 h in the dark.When brown spots had clearly appeared,the leaves were decolorized by boiling with a mixture of absolute ethyl alcohol,glacial acetic acid and glycerol (3:1:1,v/v) for 20 min to visualize the brown spots.Brown regions or spots on leaves indicate the presence of H2O2.
2.6.Determination of malondialdehyde (MDA) and relative electrolyte leakage
For the MDA assay,about 0.1 g flag leaves from 2-wk-oldOsaslrkmutant,OsASLRK-overexpressing rice lines (T2generation) and their corresponding WT grown in normal conditions were collected and homogenized in 1 mL of 10% trichloroacetic acid (TCA) in an ice-cold mortar.The homogenate was centrifuged at 8 000×g for 10 min at 4°C.After transferring 1 mL supernatant into a new 1.5 mL centrifuge tube,it was mixed thoroughly with 1 mL of thiobarbituric acid (TBA) (prepared in 10% TCA).Finally,the mixture was boiled for 30 min and then quickly cooled on ice.Absorbance of the supernatant was monitored at 532 nm and the MDA content was expressed as nmol g–1FW protein.
For the relative ion leakage assay,0.3 g of leaves was cut into 0.5–1 cm pieces which were immersed in 10 mL ddH2O for 2 h to determine the conductivity before boiling(S1) value on a DDS-11A conductometer.The solution was boiled for 20 min and then quickly cooled on ice to determine the conductivity after boiling and cooling (S2) value.Relative ion leakage was calculated as S1/S2.
2.7.Measurement of the superoxide dismutase (SOD),peroxidase (POD) and catalase (CAT) activities
2-wk-old seedlings of the Osaslrk mutant,OsASLRKoverexpressing rice lines (T2generation) and their corresponding WT grown in normal condition were soaked in half-strength Murashige and Skoog (MS) medium (0 mmol L–1exogenous methylglyoxal (MG)) or MS supplemented with 5 mmol L–1exogenous MG for 24 h,then about 0.1 g flag leaves were collected and homogenized (1:10,m/v) in an ice-cold mortar using 50 mmol L–1sodium phosphate buffer(pH 7.8) containing 1% polyvinylpyrrolidone and 10 mmol L–1β-mercaptoethanol.After centrifugation (8 000×g,10 min,4°C),the supernatant was used for the determination of SOD,POD and CAT activities,mainly following the method of Ouyanget al.(2010).
For the SOD activity assay,the inhibition of the photochemical reduction of nitro blue tetrazolium (NBT)chloride was measured.The enzyme activity was expressed as U min–1mg–1FW protein.
For the POD activity assay,the increase in absorbance resulting from the oxidation of guaiacol to tetraguaiacol was monitored at 470 nm.The enzyme activity was expressed as U min–1μg–1FW protein.
For the CAT activity assay,1 U of the CAT activity was defined as a 0.01 absorbance decrease per minute at 240 nm.Enzyme activity was expressed as U min–1mg–1protein.
2.8.Measurement of glutathione (GSH) content
GSH content was measured using the GSH Test Kit(Solarbio,China) according to the manufacturer’s protocol.The detailed procedure is as follows:about 0.1 g flag leaves from 2-wk-old seedlings of theOsaslrkmutant,OsASLRK-overexpressing rice lines (T2generation) and their corresponding WT grown in normal conditions were collected and homogenized in an ice-cold mortar using 1 mL Solution I (Code:BC1175).The homogenate was centrifuged at 8 000×g for 10 min at 4°C.The supernatant was stored at 4°C until the determination of GSH content by monitoring the absorbance of the supernatant at 412 nm,and the GSH content was expressed as μg g–1FW protein.
2.9.Statistical analysis
Statistical analyses were carried out using Microsoft Excel and SPSS 16.0 for Windows (SPSS Inc.,Chicago,IL,USA).All the experiments were repeated at least three times.Data are presented as mean±SD (standard deviation).Data were compared using Student’st-tests.Differences were considered to be significant if*,P<0.05 or**,P<0.01.
3.Results
3.1.OsASLRK responds to exogenous MG treatment in a time-and dosage-dependent fashion
A query of the public Rice Genome Annotation Project database (http://rice.plantbiology.msu.edu/) showed that the full-length cDNA sequence ofOsASLRK(LOC_Os01g53920) is 4 398 bp long and contains a 3 201-bp open reading frame (ORF),with a 72-bp 5´-untranslated region (UTR) and a 995-bp 3´-UTR.OsASLRKgenomic DNA contains two exons and one intron,and encodes a 1 067-amino-acid protein (Fig.1-A).Promoter analysis showed that theOsASLRKpromoter region contains a typical MG responsive element,MGRE-motif (AAAAAAAA,–2 065–(–2 058) bp) (Fig.1-A),suggesting thatOsASLRKmay play an important role in the MG response in rice.To confirm our hypothesis thatOsASLRKmay respond to MG treatment,we performed theOsASLRKexpression level assays after exogenous MG treatments involving the indicated time or dosage courses using RT-qPCR.We found that the time-and dosage-gradient treatments both largely inducedOsASLRKexpression relative to its levels in the 0 h or 0 mmol L–1controls (Fig.1-B and C).To further test whetherOsASLRKaccumulation is regulated by MG treatment,we also generatedproOsASLRK::GUStransgenic rice plants in the WT background and analyzed the GUS expression in the absence or presence of MG.As shown in Fig.1-D,in either the absence or presence of MG treatment,GUS staining was very weak in rice root.In contrast,strong GUS activity was observed when the roots were treated with 5 mmol L–1MG for 12 or 24 h under darkness before GUS staining,which also indicates that theOsASLRKpromoter is induced by MG treatment.Taken together,our results unambiguously show thatOsASLRKexpression responds to MG treatment in a time-and dosage-dependent manner.
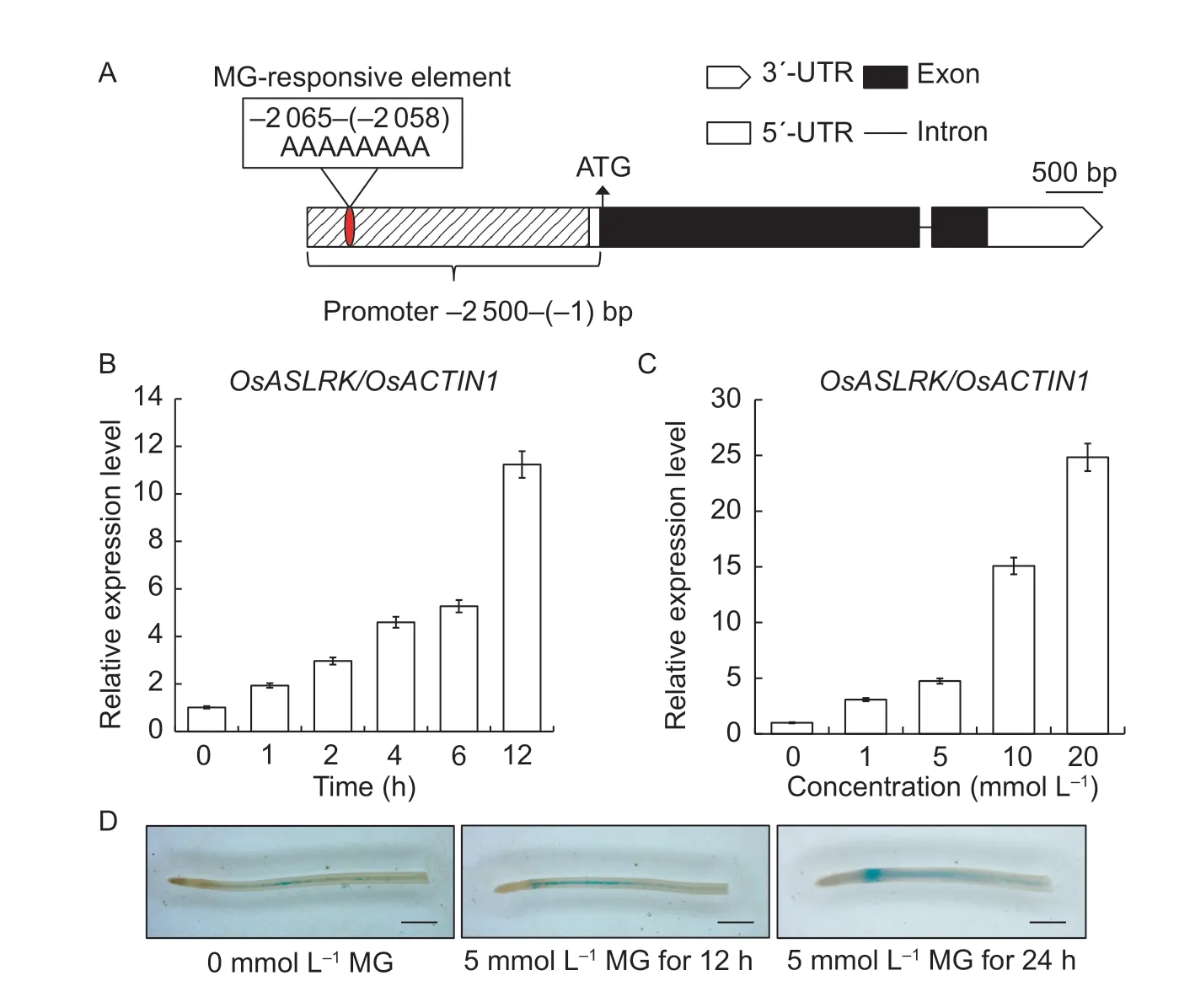
Fig.1 Gene structure and relative expression analysis of OsASLRK by RT-qPCR following treatment with exogenous methylglyoxal(MG) as indicated for the time-or dosage-courses in 14-d-old Nipponbare seedlings.A,exon/intron structure predictions of OsASLRK from the public Rice Genome Annotation Project database (http://rice.plantbiology.msu.edu/).Exons and introns are represented by black boxes and black lines,respectively.The MG responsive-motif (AAAAAAAA) was searched using PLANTCARE Software in the promoter region (–2 500–(–1) bp) and is marked by a red ellipsoid.B,expression analysis of OsASLRK/OsACTIN1 at 0,1,2,4,6,and 12 h under 5 mmol L–1 MG treatment.C,expression analysis of OsASLRK for a series of treatments with varying MG concentrations (0,1,5,10,and 20 mmol L–1) for 12 h.RT-qPCR data were normalized using the OsACTIN1 gene and are shown relative to the corresponding 0 h or 0 mmol L–1 treatments.Error bars represent the mean±standard deviation (SD) of three biological replicates.D,GUS staining of ProOsASLRK::GUS rice roots treated with 5 mmol L–1 MG for 12 and 24 h,using ProOsASLRK::GUS rice root without MG treatment as a negative control.Bars,1 mm.
3.2.Loss-of-function of OsASLRK in rice leads to increases in sensitivity and content to MG under exogenous MG treatment
To preliminarily confirm the biological function ofOsASLRKin the rice response to MG,we first used the homozygous mutant ofOsASLRK(Osaslrk,PFG_1A-24919.R) (Appendix B) and its corresponding WT (HY) for phenotypic observations at the germination stage.In the absence of MG treatment (0 mmol L–1),no significant phenotypic differences between theOsaslrkmutant and HY were detected(Fig.2-A).When treated with 5 mmol L–1endogenous MG for 2 days,however,theOsaslrkmutant showed significant growth retardation compared to the HY control,and the shoot and root lengths were shorter than in the HY control(Fig.2-B and C).Moreover,we further measured the endogenous MG contents of theOsaslrkmutant and the HY control under exogenous MG treatment.The results showed that no significant differences of MG content between theOsaslrkmutant and HY were observed under 0 mmol L–1exogenous MG treatment;however,theOsaslrkmutant had an increased endogenous MG content compared to the HY control when treated with 5 mmol L–1exogenous MG for 2 days (Fig.2-D).
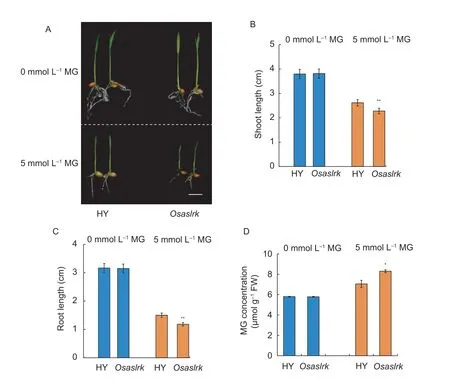
Fig.2 Methylglyoxal (MG) sensitivity and content analysis of wild type (WT,Hwayong (HY)) and the Osaslrk mutant rice plants at the germination stage.A,phenotypic comparison of WT (HY) and the Osaslrk mutant after germination and grown for 2 days in half-strength Murashige and Skoog (MS) medium (0 mmol L–1 exogenous MG) or MS supplemented with 5 mmol L–1 exogenous MG.Bars,1 cm.B,shoot length comparison of the plants.C,root length comparison of the plants.D,endogenous MG content of WT (HY) and the Osaslrk mutant after germination and grown for 2 days in half-strength MS medium (0 mmol L–1 exogenous MG)or MS supplemented with 5 mmol L–1 exogenous MG treatments.Data are shown as the mean±SD (n=30) of 20–30 seedlings.Asterisks indicate significant differences between WT (HY) and the Osaslrk mutant (* and **,P<0.05 and P<0.01,respectively) by Student’s t-test.
Next,we further analyzed the MG sensitivity of theOsaslrkmutant and WT (HY) rice plants at the 4-wk-old seedling stage.As shown in Fig.3-A,the phenotypic changes were consistent with the above germination stage results,in that theOsaslrkmutant showed stronger sensitivity than the HY control in the response to MG.The shoot length,root length and dry weight were also diminished relative to the HY control when treated with 5 mmol L–1endogenous MG (Fig.3-B–D).Collectively,these results suggest that disruption of theOsASLRKgene enhances sensitivity to MG and increases the endogenous MG content under exogenous MG treatment in rice.
3.3.OsASLRK-overexpressing rice plants exhibit decreases in sensitivity to exogenous MG and endogenous MG content under exogenous MG treatment
To further investigate the biological role of theOsASLRKresponse to exogenous MG in rice,we selected three independentOsASLRK-overexpressing rice lines (OE-15,OE-28 and OE-33) (Appendix C) and their corresponding WT(Kitaake) for exogenous MG sensitivity and endogenous MG content analyses.We first treatedOsASLRK-overexpressing and WT (Kitaake) rice plants with exogenous 0 or 5 mmol L–1MG according to the same procedure as described in theOsaslrkmutant analysis.Next,we measured shoot and root lengths of theOsASLRK-overexpressing rice lines (OE-15,OE-28 and OE-33) and the WT (Kitaake) under these 0 and 5 mmol L–1MG stress conditions.There were no significant differences in the shoot and root lengths betweenOsASLRK-overexpressing and WT (Kitaake) plants without an exogenous MG stress treatment.However,the shoots and roots ofOsASLRK-overexpressing plants exhibited greater increases in length than those of WT (Kitaake)plants under 5 mmol L–1exogenous MG stress (Fig.4-A–C).Furthermore,theOsASLRK-overexpressing plants had a decrease in endogenous MG content compared to the WT(Kitaake) control under 5 mmol L–1exogenous MG treatment(Fig.4-D).Meanwhile,we also selected one T3progeny of anOsASLRK-overexpressing rice line (OE-28) to re-confirm that for theOsASLRKresponse to MG,the result was consistent with the T2progeny ofOsASLRK-overexpressing rice lines (Appendix D).From the above test,our finding indicates that theOsASLRKgene does indeed participate in the exogenous MG response and endogenous MG content regulation in rice.
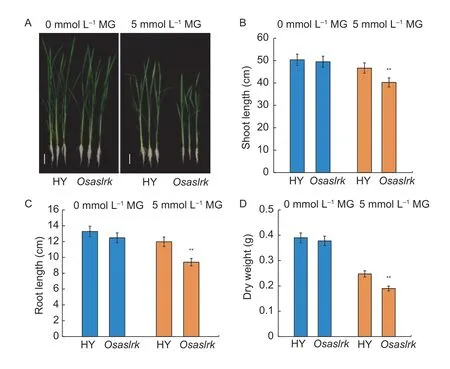
Fig.3 Methylglyoxal (MG) sensitivity analysis of wild type (WT,Hwayoung (HY)) and the Osaslrk mutant rice plants at the 4-wk-old seedling stage.A,phenotypic comparison of 4-wk-old WT (HY) and the Osaslrk mutant seedlings grown for 2 days in half-strength Murashige and Skoog (MS) medium (0 mmol L–1 exogenous MG) or MS supplemented with 5 mmol L–1 exogenous MG.Bars,5 cm.B,shoot length comparison of the plants.C,root length comparison of the plants.D,dry weight comparison of the plants.Data are shown as the mean±SD (n=30) of 20–30 seedlings.Asterisks indicate significant differences between WT (HY) and the Osaslrk mutant (**,P<0.01) by Student’s t-test.
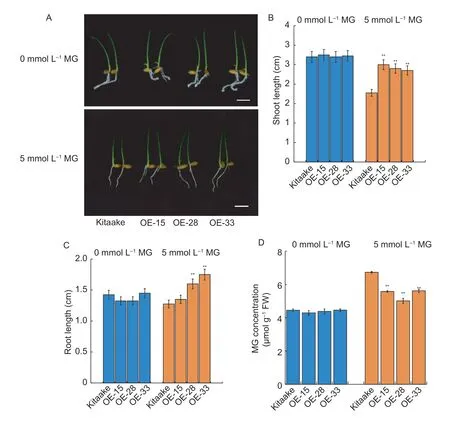
Fig.4 Methylglyoxal (MG) sensitivity and MG content analysis of WT (Kitaake) and OsASLRK-overexpressing rice plants (OE-15,OE-28 and OE-33) at the germination stage.A,phenotypic comparison of WT (Kitaake) and OsASLRK-overexpressing rice plants after germination and grown for 2 days in half-strength Murashige and Skoog (MS) medium (0 mmol L–1 exogenous MG) or MS supplemented with 5 mmol L–1 exogenous MG.Bars,1 cm.B,shoot length comparison of the plants.C,root length comparison of the plants.D,endogenous MG content of WT (Kitaake) and OsASLRK-overexpressing rice plants (OE-15,OE-28 and OE-33)grown for 2 days in half-strength MS medium (0 mmol L–1 exogenous MG) or MS supplemented with 5 mmol L–1 exogenous MG.Data are shown as the mean±SD (n=30) of 20–30 seedlings.Asterisks indicate significant differences between WT (Kitaake) and OsASLRK-overexpressing rice plants (**,P<0.01) by Student’s t-test.
3.4.ROS accumulation is altered in the Osaslrk mutant and OsASLRK-overexpressing plants
In general,the reactive oxygen species (ROS) level and membrane damage in plants are often triggered under abiotic stress conditions (You and Chan 2015;Zhuet al.2020).Since MG is a stress signal,we conducted a series of experiments on ROS content and membrane damage in the context of exogenous MG treatment conditions.First,we detected the ROS accumulation changes in theOsaslrkmutant,OsASLRK-overexpressing rice lines and their corresponding WT under 0 and 5 mmol L–1MG stress conditions using 3,3´-diaminobenzidine(DAB) staining.The results showed that there were no significant differences in the ROS contents between theOsASLRK-overexpressing rice lines or theOsaslrkmutant and their corresponding WT in the treatment without exogenous MG (Fig.5-A and B).However,ROS content increased significantly in theOsaslrkmutant,but decreased significantly inOsASLRK-overexpressing rice lines under 5 mmol L–1exogenous MG treatment (Fig.5-A and B).Next,the MDA content and relative ion leakage(which reflects the degree of membrane damage) were also determined in this study.As shown in Fig.5-C–F,upon exogenous MG stress,the MDA content and relative ion leakage were obviously decreased inOsASLRKoverexpressing rice lines when compared with their corresponding WT (Kitaake) (Fig.5-C and D).By contrast,there was a noticeable increase of ROS content in theOsaslrkmutant compared with its corresponding WT (HY)under MG treatment (Fig.5-E and F).Collectively,these findings indicated that the ROS system was significantly changed in both theOsaslrkmutant andOsASLRKoverexpressing rice lines,suggesting a vital role ofOsASLRKin modulating the cellular ROS homeostasis under MG treatment conditions.
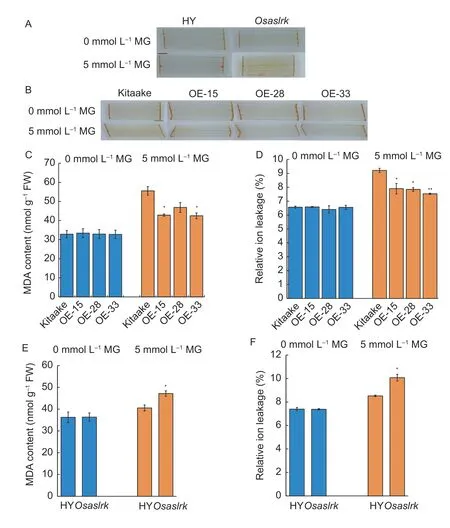
Fig.5 3,3-Diaminobenzidine (DAB) staining,malondialdehyde (MDA) content and relative ion leakage assays.A,2-wk-old rice seedlings of the Osaslrk mutant and its corresponding wild type (WT,Hwayoung (HY)) grown for 24 h in normal conditions were soaked in half-strength Murashige and Skoog (MS) medium (0 mmol L–1 exogenous MG) or MS supplemented with 5 mmol L–1 exogenous MG.B,2-wk-old rice seedlings of OsASLRK-overexpressing rice plants (OE-15,OE-28 and OE-33) and its corresponding WT (Kitaake).Brown regions or spots on leaves indicate the presence of H2O2.C,MDA content in 2-wk-old WT rice (Kitaake) and ASLRK-overexpression rice lines (OE-15,OE-28 and OE-33).D,relative ion leakage in 2-wk-old WT rice (Kitaake) and ASLRKoverexpression rice lines (OE-15,OE-28 and OE-33).E,MDA content in the 2-wk-old Osaslrk mutant and its corresponding WT(HY).F,relative ion leakage in the 2-wk-old Osaslrk mutant and its corresponding WT (HY).Bars mean SD (n=30).Asterisks indicate a significant difference (* and **,P<0.05 and P<0.01,respectively) by Student’s t-test.
3.5.Scavenging enzyme (POD,SOD and CAT)activities and GSH content assays in the Osaslrk mutant and OsASLRK-overexpressing plants
Changes in ROS level are often associated with changes in the activities of ROS-scavenging enzymes (SOD,POD and CAT).To examine the potential effects ofOsASLRKon ROS-scavenging enzyme activities,we first measured the changes in their activities in 2-wk-oldOsASLRKoverexpressing rice lines under 0 and 5 mmol L–1MG stress conditions.Under 0 mmol L–1MG conditions,no significant differences in the SOD,POD and CAT activities were found between theOsASLRK-overexpressing rice lines or theOsaslrkmutant and their corresponding WT(Fig.6).However,SOD,POD and CAT activities increased significantly in theOsASLRK-overexpressing rice lines compared with those of the corresponding WT (Kitaake)control under 5 mmol L–1exogenous MG treatment (Fig.6-A,C and E).In contrast,SOD,POD and CAT activities decreased significantly in theOsaslrkmutant compared with those of the corresponding WT (HY) control under 5 mmol L–1exogenous MG treatment (Fig.6-B,D and F).
In addition,as an important antioxidant,glutathione (GSH)also plays important roles in the antioxidant defense system in plants,so we next measured the GSH content in 2-wk-oldOsASLRK-overexpressing rice lines and theOsaslrkmutant under 0 and 5 mmol L–1MG stress conditions.The results here also showed that there was no significant difference in GSH content betweenOsASLRK-overexpressing rice lines and their corresponding WT (Kitaake) without exogenous MG treatment.However,GSH content increased significantly inOsASLRK-overexpressing rice lines (Fig.6-G),but decreased significantly in theOsaslrkmutant under 5 mmol L–1exogenous MG treatment (Fig.6-H).Thus,we concluded thatASLRKmay enhance MG-tolerance by elevating the activities of the antioxidative defense system enzymes and alleviating membrane damage.
4.Discussion
Faced with a series of environmental stress conditions,higher plants often exhibit growth retardation in adapting to multiple abiotic stress factors by integrating a range of membrane-located proteins such as receptor-like protein kinases (RLK).Plant RLKs have well-established roles in abiotic stress responses (Ouyanget al.2010;Shiet al.2014;Wuet al.2015;Dievartet al.2016).Methylglyoxal(MG) is a toxic metabolite which has also been inextricably linked with abiotic stresses (Yadavet al.2005;Kauret al.2014).However,the relationship between plant RLKs and MG has not been reported to date.In the present study,we investigated the role of an RLK gene,OsASLRK,on the MG response in rice.
4.1.Receptor-like protein kinase OsASLRK is an important regulator for the MG response in rice
Given the toxicity of MG at high levels,mechanisms must exist for sensing an elevation in its level in cells,which may occur through MG-mediated signal transduction.Studies have shown that RLK may be involved in the MG response as an important signal response factor (Kauret al.2015).However,little is known regarding the functions of RLK members in the MG response.In our research,we found a predicted MG-responsive element (AAAAAAAA) in theOsASLRKpromoter sequence (Fig.1-A),implying thatOsASLRKmay potentially be a gene involved in the MG response in rice.qRT-PCR results further showed that the transcript level ofOsASLRKwas significantly induced in a dose-or time-dependent manner upon exogenous MG application (Fig.1-B and C),similar to the results earlier reported (Kauret al.2015).GUS staining also confirmed thatOsASLRKexpression can be substantially enhanced upon exogenous MG treatment (Fig.1-D).These results preliminarily demonstrated thatOsASLRK indeed participates in the MG response in rice.
Next,we further analyzed the function of theOsASLRKgene from a genetic perspective.Overexpression or knock-out mutation phenotypic analysis is an effective way to verify gene function,so the growth status of theOsASLRK-overexpressing rice lines and theOsaslrkmutant were evaluated to confirm the role ofOsASLRKin the rice response upon exogenous MG application.Consistent with the reported observations that excess MG seriously affects the growth of plants (Hoqueet al.2012;Kauret al.2015),our results indicated thatOsASLRKpositively regulates MG tolerance.This may have occurredviareducing the endogenous MG content under exogenous MG stress in rice (Figs.2–4),and thus reducing the degree of growth retardation.Based on the results above,we speculated that receptor-like protein kinaseOsASLRKis likely a repressor of MG uptake or biosynthesis through some unknown pathway.To shed light on the underlying mechanisms,further studies will be required to determine how the MG signal is perceived and transmitted.
4.2.Receptor-like protein kinase OsASLRK enhances MG tolerance via regulation of the antioxidant defense system
Reactive oxygen species (ROS) content changes are often associated with a variety of abiotic stress conditions,so we determined the ROS changes of theOsASLRKoverexpressing rice lines and theOsaslrkmutant in response to exogenous MG treatment.As expected,ROS accumulation and membrane damage were significantly inhibited in theOsASLRK-overexpressing rice lines and enhanced in theOsaslrkmutant in response to exogenous MG treatment (Fig.5)viaregulation of antioxidative enzyme(SOD,POD and CAT) activities (Fig.6).Additionally,some reports have indicated that the glutathione (GSH)-dependent enzyme system is the major detoxification route for MG in biological systems (Thornalley 2003),so we also determined the change in GSH content in theOsASLRK-overexpressing rice lines and theOsaslrkmutant in response to exogenous MG treatment.These results suggested that the GSH content was also altered,and increased in theOsASLRKoverexpressing rice lines but decreased in theOsaslrkmutant upon exogenous MG treatment (Fig.6),implying thatOsASLRK also can affect GSH content under MG stress.Collectively,our results suggested thatOsASLRKenhances MG-tolerance in rice,perhaps by regulating cellular redox homeostasis under MG stress.
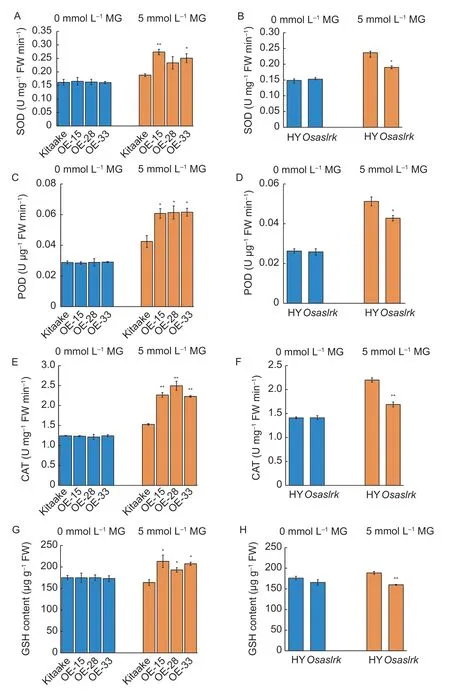
Fig.6 Scavenging enzyme (superoxide dismutase (SOD),peroxidase (POD) and catalase (CAT)) activity and glutathione (GSH)content assays.A,C and E,SOD,POD and CAT activities in the 2-wk-old wild type (WT) rice (Kitaake) and ASLRK-overexpression rice lines (OE-15,OE-28 and OE-33).B,D and F,SOD,POD and CAT activities in the 2-wk-old Osaslrk mutant and its corresponding WT (Hwayoung (HY)).G,GSH content in 2-wk-old OsASLRK-overexpressing rice lines.H,GSH content in the 2-wk-old Osaslrk mutant.Bars mean SD (n=30).Asterisks indicate significant differences (*,P<0.05;**,P<0.01,respectively) by Student’s t-test.
5.Conclusion
In this study,we provide the first report on the relationship between an RLK member and MG response.We found that a rice RLK gene,OsASLRK,responded to MG in a time-and dosage-dependent fashion,thereby causing phenotypic and physiological changes in plant tolerance.Genetic analysis suggested thatOsASLRK negatively regulates MG sensitivity and endogenous MG content under exogenous MG treatment in rice.In summary,our findings offer the new evidence which enriches the roles of RLK and illustrates the functions of receptor-like kinaseOsASLRKin MG regulation in rice.
Acknowledgements
This research was financially supported by the National Natural Science Foundation of China (U1704106,3190142),the Doctoral Scientific Research Fund of Henan Agricultural University,China (30500561) and the Open Innovation Project of Undergraduate Laboratory of Henan Agricultural University,China (KF1902).
Declaration of competing interest
The authors declare that they have no conflict of interest.
Appendicesassociated with this paper are available on http://www.ChinaAgriSci.com/V2/En/appendix.htm
杂志排行
Journal of Integrative Agriculture的其它文章
- Heredity and gene mapping of a novel white stripe leaf mutant in wheat
- Construction of a high-density adzuki bean genetic map and evaluation of its utility based on a QTL analysis of seed size
- Effects of temperature and solar radiation on yield of good eatingquality rice in the lower reaches of the Huai River Basin,China
- Difference in corn kernel moisture content between pre-and postharvest
- The effect of elevating temperature on the growth and development of reproductive organs and yield of summer maize
- High plant density increases seed Bt endotoxin content in Bt transgenic cotton
