Extracellular superoxide dismutase VdSOD5 is required for virulence in Verticillium dahliae
2021-06-02TIANLiHUANGCaiminZHANGDandanLIRanCHENJieyinSUNWeixiaQIUNianweiDAIXiaofeng
TIAN Li,HUANG Cai-min,ZHANG Dan-dan,LI Ran,CHEN Jie-yin,SUN Wei-xia,QIU Nian-wei,DAI Xiao-feng
1 College of Life Science,Qufu Normal University,Qufu 273165,P.R.China
2 State Key Laboratory for Biology of Plant Diseases and Insect Pests/Institute of Plant Protection,Chinese Academy of Agricultural Sciences,Beijing 100193,P.R.China
Abstract Plants produce reactive oxygen species (ROS) to defend pathogens.To counteract this attack,certain pathogens express superoxide dismutases (SODs) to scavenge host-derived ROS.However,the roles of SODs in Verticillium dahliae,an important vascular pathogen,are not clear.Our previous study has shown that a putative extracellular SOD (VdSOD5)of V.dahliae is significantly induced by culturing in cotton tissues,suggesting that VdSOD5 may play an important role in host–pathogen interactions and virulence.Here,we showed that VdSOD5 encoded a superoxide dismutase with a cofactor copper-binding site and a functional signal peptide that can conduct protein secretion in an invertase-mutated yeast strain.The mutations in VdSOD5 (ΔVdSOD5) did not change the normal vegetative growth and conidial production but reduced the virulence of V.dahliae on susceptible host cotton.Further studies showed that the transcription of VdSOD5 was significantly up-regulated during the early stage of infection,and the loss-of-function of VdSOD5 decreased culture filtrate and fungal tissue SOD activities of V.dahliae by 74 and 28%,respectively.Compared to the wild-type strain Vd991,the ΔVdSOD5 showed the same sensitivity to the intracellular ROS generator menadione.Furthermore,nitroblue tetrazolium(NBT) staining demonstrated that VdSOD5 functioned in the detoxification of superoxides generated by host roots during infection.These results suggest that VdSOD5 of V.dahliae is an important virulence factor,secreted out of cells to combat host-derived ROS.
Keywords:Verticillium dahliae,superoxide dismutase,secretion,virulence,ROS detoxification
1.Introduction
SODs are metalloenzymes that use a redox-active metal to disproportionate two molecules of superoxide to oxygen and hydrogen peroxide,the latter of which is removed by catalase and peroxidase enzymes (Apel and Hirt 2004).SODs can be classified into four groups by the type of the metal co-factor:iron SOD (FeSOD),manganese SOD(MnSOD),copper-zinc SOD (Cu/Zn-SOD) and nickel SOD(NiSOD) (Fridovich 1995;Abreu and Cabelli 2010).These SODs exist in various species and are localized in different cellular compartments.Mn-SOD and Fe-SOD are evolved from a common ancestor and recently assigned to the same family.Mn-SOD and Fe-SOD are prevalent in prokaryotes and generally intracellular/cytosolic.Consistent with the mitochondrial endosymbiosis theory,Mn-SOD and Fe-SOD have been retained in eukaryotic mitochondria and chloroplast,equivalent to bacterial cytosol (Narasipuraet al.2005;Miller 2012).Cu/Zn-SODs are extracellular in bacteria and mainly distributed in the cytosol in eukaryotes,equivalent to bacterial extracellular spaces according to endosymbiosis theory (Benovet al.1995;Okado-Matsumoto and Fridovich 2001).Besides,extracellular Cu/Zn-SODs can also be found in eukaryotes (Adachiet al.2001;Frohneret al.2009).The rare Ni-SOD family has only been found in cyanobacteria and streptomycetes so far (Priyaet al.2007).The compartmentalization of different versions of SODs throughout organisms allows them to counteract various ROS locally (Schatzman and Culotta 2018).
Cu/Zn-SODs are usually the most abundant SOD and the main contributor to the SOD activity.Currently,Cu/Zn-SODs are divided into two classes (Zelkoet al.2002).One type is cytosolic Cu/Zn-SOD1,which is the first characterized SOD existing almost exclusively in intracellular cytoplasmic spaces.The other group consists of extracellular Cu/Zn-SODs (EC-SODs),which contain a signal peptide that directs this enzyme to extracellular spaces or is attached to the cell wall by a glycophosphatidyl inositol (GPI) anchor.Numerous studies have indicated that Cu/Zn-SOD1 plays an essential role in pathogenesis.For example,Cu/Zn-SOD1 deletion mutants ofBotrytis cinerea(Rolkeet al.2004),Sclerotinia sclerotiorum(Xu and Chen 2013) andFusarium graminearum(Yaoet al.2016) have reduced pathogenicity for their host.However,Cu/Zn-SOD1s inClaviceps purpurea(Mooreet al.2002) andAspergillus fumigatus(Lambouet al.2010) are not essential for pathogenicity,suggesting that Cu/Zn-SOD1s possess various functions in different fungal pathogens.
Unlike EC-SODs in plants and animals,which function as bimetalloenzymes and require both Cu2+and Zn2+for catalysis,fungal EC-SODs harbor only Cu2+but not Zn2+.This unusual form of SOD is termed as Cu-only SOD(Robinettet al.2018).Until recently,Cu-only SODs are considered the only type of extracellular SODs in the fungal kingdom.Extracellular Cu-only SODs have been shown to combat host’s oxidative burst and are virulence factors for human pathogenic fungi.The most extensively studied ECSOD is SOD5 in the opportunistic fungal pathogenCandida albicans.InC.albicans,three signal peptide-containing Cu-only SODs (SOD4,SOD5,and SOD6) are secreted and attached to the cell wall through GPI anchors.Of these,SOD5 has been shown to directly degrade host cell-derived ROS to escape innate immune surveillance in animal models(Martchenkoet al.2004;Frohneret al.2009).The Cuonly EC-SOD of pulmonary fungal pathogenHistoplasma capsulatumis the major extracellular superoxide dismutase,which is essential against the superoxide-dependent killing of macrophages for fungal survival in a lung infection mouse model (Youseffet al.2012).Furthermore,Cu-only ECSODs are widely distributed throughout the fungi kingdom,which are required for the virulence of other fungi,including the human pulmonary fungal pathogenParacocidiodes brasiliensis(Tamayoet al.2016) and the filamentous entomopathogenBeauveria bassianal(Liet al.2015).Interestingly,plant pathogenic fungiPuccinia striiformis,the causal agent of wheat stripe rust,encodes a novel ECSOD (PsSOD1),which only contains the Zn2+binding sites and lacks the Cu2+binding sites (termed as Zn-only SOD).PsSOD1 is secreted into the host–pathogen interface to scavenge host-derived ROS and functions as an important pathogenicity factor (Liuet al.2016).However,the functions of the ubiquitous Cu-only EC-SODs in phytopathogenic fungi have not been understood.
Verticillium dahliaeis a soil-borne plant pathogen that causes Verticillium wilt diseases in over 200 hosts worldwide,including many economically important crops such as cotton and potato (Fradin and Thomma 2006;Klostermanet al.2009).ROS were detected in cotton roots after infection withV.dahliae,suggesting host andV.dahliaeinteraction is also accompanied by the increased ROS accumulation (ZhangYet al.2017).Furthermore,the ROS production in tomato plants expressing theVe1(Verticillium wilt resistance gene 1)resistance gene is faster than that observed in a susceptible variety inoculated withV.dahliae,indicating host ROS levels are associated with disease resistance (Gayosoet al.2010).Studies on the pathogenesis mechanism have shown that some secreted proteins ofV.dahliaecan increase host ROS accumulation,such as necrosis-inducing protein VdNLP1 and VdNLP2(Zhouet al.2012),hypersensitive-like response-inducing elicitor PevD1 (Wanget al.2012),pathogen-associated molecular patterns VdEG1/3 and VdCUT11 (Guiet al.2017,2018) and putative effector protein VdSCP7,27,113 and 126 (ZhangLet al.2017;Wanget al.2020).However,the mechanisms by whichV.dahliaecopes with these oxidative challenges and the factors enablingV.dahliaeto survive the host-derived ROS in the host–pathogen interactions are still unclear.
To identify new factors facilitatingV.dahliaeinfection,we examined the extracellular proteome ofV.dahliaecells culturing in a cotton tissue powder containing C’zapek-Dox medium (Chenet al.2016;Wanget al.2018).We identified a predicted EC-SOD (VdSOD5),whose abundance was upregulated by 4.47 folds compared to that cultured in C’zapek-Dox medium (Chenet al.2016),suggesting VdSOD5 may function in host–pathogen interactions and pathogenicity.Therefore,the main objectives of the current study were:1) to explore the secretion characteristic of VdSOD5;2) to investigate the role of VdSOD5 in contributing toV.dahliaeSOD activity and detoxifying host-derived ROS during plant–pathogen interaction;and 3) to determine the role of VdSOD5 in pathogenicity.
2.Materials and methods
2.1.Growth of microbial and plant materials
The highly virulent isolateV.dahliaewild-type strain Vd991 was cultured on PDA (potato dextrose agar,potato,200 g L–1;glucose,20 g L–1;agar,15 g L–1) or in liquid CM(C’zapek-Dox medium,NaNO3,3.0 g L–1;MgSO4·7H2O,0.5 g L–1;KCl,0.5 g L–1;FeSO4·7H2O,0.01 g L–1;K2HPO4,1.0 g L–1;sucrose,30.0 g L–1) with shaking at 200 r min–1at 25°C.Agrobacterium tumefaciensAGL-1 strain (used for fungal transformation) was cultured in YEB (yeast extract and beef medium:beef extract,5 g L–1;yeast extract,1 g L–1;peptone,5 g L–1;sucrose,5 g L–1;MgSO4·7H2O,0.04 g L–1) at 28°C.Escherichia colistrain DH5α was used to propagate all plasmids in LB (Luria-Bertani broth:yeast extract,5 g L–1;tryptone,10 g L–1;NaCl,10 g L–1) with constant shaking at 250 r min–1at 37°C.Cotton (Gossypium hirsutum cv.Junmian 1) was grown with a 14-h light/10-h dark photoperiod in a greenhouse at 28°C for 2 weeks (until the development of the first euphylla) for pathogenicity assays.
The two-week vacation at the end of summer school ended yesterday. Kevin had gone nowhere during his vacation. He had the money, but he hated to travel alone. He used to take vacations with Gary, his youngest brother, but they had a big argument at the end of their last vacation. Each of them had spent the last three years waiting for the other to apologize.
2.2.Gene cloning and bioinformatics analysis
TheVdSOD5gene (VEDA_04232) was amplified from the genomic DNA and cDNA samples of Vd991 with the primer VdSOD5-F/R (Appendix A),based on theVdSOD5sequence in theVerticillium dahliaegenome database (Chenet al.2018).The physicochemical properties of the protein sequence of VdSOD5 were predicted by the ProtParam tool of ExPASy (http://www.expasy.org).The protein domain and active site were predicted based on NCBI’s conserved domain database.Homologous proteins of VdSOD5 were searched usingSaccharomycesGenome Database.Clustal X2 was used for multiple sequence alignment of SOD5 orthologs in other fungi (Larkinet al.2007).The signal peptide of VdSOD5 was predicted using SignalP 5.0(Armenteroset al.2019).The presence of the GPI-anchor and the position of the ω-site of VdSOD5 were predicted by GPI-anchor predictor (Pierleoniet al.2008).
2.3.Yeast signal sequence trap system
To validate the secretion function of the putative N-terminal signal peptide ofVdSOD5,the yeast invertase secretion assay was performed as previously described (Jacobset al.1997).Briefly,the predicted signal peptide of VdSOD5 was amplified using the primer SP-VdSOD5-F/R and fused in-frame to the secretion-defective invertase gene in the pSUC2 vector.The resulting plasmid,pSUC2::SOD5SP,was transformed into the yeast strain YTK12 using the lithium acetate method.Positive clones were selected by PCR using the vector-specific primer SP-VdSOD5-F/R(Appendix A).Invertase secretion was assayed by plating pSUC2::SOD5SPcontaining YTK12 on YPRAA medium (1%yeast extract,2% peptone,2% raffinose,and 2 mg mL–1antimycin A) plates.YTK12 transformed with an empty pSUC2 or pSUC2:Avr1bSPvector was used as the negative and positive control,respectively.
2.4.Construction of VdSOD5 deletion mutants and complemented strains
The targeted gene deletion plasmid was generated based on a previously described method with modifications (Liuet al.2013).Briefly,a 1 028-bp upstream sequence and a 1 189-bp downstream sequence ofVdSOD5were amplified from the genomic DNAofV.dahliaeVd991 with the primers VdSOD5-Up-F/R and VdSOD5-Down-F/R,respectively.The~1.8 kb hygromycin phosphotransferase gene cassette was amplified from the pUC-Hygvector with the primer HYG-F/R.The above three amplicons were fused to one DNA fragmentviafusion PCR with the primer VdSOD5-Nest-F/R.Subsequently,nested PCR was carried out to obtain an internal amplicon,which was then cloned intoHindIII/XbaI-linearized pGKO2-Gateway vector to generate the targeted gene deletion plasmid using a standard homologous recombination reaction (Khanget al.2005).The complementation fragment,containing theVdSOD5wild-type gene with approximate 1 kb native promoters and 0.5 kb downstream terminator regions,was amplified from the genomic DNAofVd991 with the primer VdSOD5-C-F/R and fused intoEcoRI/HindIII-linearizedpCOMwith geneticin resistance to generate a complementation plasmid by homologous recombination (Liet al.2019).All primers used for vector construction are listed in Appendix A.Agrobacterium tumefaciens-mediated fungal transformation was performed as described previously (Mullinset al.2001).Gene knockout mutants were selected on PDA medium supplemented with 50 μg mL–1of hygromycin,200 μg mL–1of cefotaxime,and 200 μg mL–1of 5-fluoro-2´-deoxyuridine,while complemented strains were selected in the presence of 200 μg mL–1of cefotaxime and 50 μg mL–1of geneticin.Single spore isolations were performed for all transformants followed by PCR verification with primers HYG-Test-F/R and VdSOD5-Test-F/R,respectively.
2.5.Growth and conidiogenesis assays
For the radial growth rate assay,2-μL conidial suspension with a concentration of 2×106conidia mL–1of the wild-type strain Vd991 and ΔVdSOD5-1/2strain were placed in the center of a PDA plate and incubated at 25°C for 9 d.Different carbon source containing plates were prepared by incorporating sucrose (30 g L–1),starch (15 g L–1),pectin (10 g L–1),and sodium carboxymethyl cellulose (10 g L–1) individually into Czapek-Dox agar medium (2 g L–1NaNO3,0.5 g L–1MgSO4·7H2O,0.5 g L–1KCl,100 mg L–1FeSO4·7H2O,and 1 g L–1K2HPO4,pH 7.2).Fungal growth was tested in the medium mentioned above in the same way.Mycelial growth rates were measured by placingV.dahliaestrains on PDA plates amended with 0,10,30,and 50 μmol L–1menadione for 12 d,respectively.
For evaluating the conidial production of the ΔVdSOD5strain,agar plugs were collected from the edge of 5-and 7-d-old fungal colonies using a 5-mm-diameter cork borer.Each plug was then shaken in 1 mL sterile water,and the number of conidia was quantified using a hemocytometer.Experiments were conducted in triplicate for each strain.
2.6.Gene expression analysis at different stages of infection
The cotton root samples at different stages of infection were prepared by the root-dip method (Liuet al.2013).After RNA extraction and cDNA synthesis,the expression levels ofVdSOD5were measured by qRT-PCR using FastFire qPCR premix (SYBR Green,TianGen,Beijing,China).The real-time PCR program consisted of an initial denaturation step at 94°C for 10 min,followed by 40 cycles of 94°C for 15 s and 60°C with the primer qPCR-F/R for 1 min.The relative gene expression levels were calculated using the 2−ΔΔCTmethod (Livak and Schmittgen 2001) with elongation factor-1α (EF-1α) as an internal control.Each reaction was carried out in triplicate.
2.7.Pathogenicity assays
Pathogenicity assays were carried on cotton seedlings using a root-dip method as previously described (Guiet al.2017).Briefly,2-week-old cotton seedlingswere gently uprooted,washed free of soil,and immersed in 15-mL of 5×106conidia mL–1suspension from the wild-type strain and each transformant for 5 min.The seedlings were then replanted into new pots and maintained at 28°C (14 h/10 h,day/night cycle).Three replicates consisting of 30 cotton seedlings were used for each transformant.Verticillium wilt disease symptoms and vascular discoloration of inoculated plants were assessed 3 weeks after inoculation.Forin plantabiomass quantification,roots of three plants were harvested 3 weeks after inoculation and grounded to a powder for genomic DNA extraction.The fungal biomass was quantified by RT-qPCR withV.dahliaeEF-1α quantifying fungal colonization and cotton18Sgene serving as the endogenous plant control (Santhanamet al.2013).
2.8.Measurement of enzyme activity in fungal tissue and culture filtrate
Mycelia of the wild-type strain,ΔVdSOD5-1/2and complemented strainswere cultured in liquid C’zapek-Dox medium for 7 d.To prepare the culture filtrate,the supernatant was separated from the fungal tissue by centrifugation and ultrafiltration.To prepare the protein fraction,fungal tissues were collected by configuration and then grounded in liquid nitrogen.The total SOD activity of each sample was measured following the nitroblue tetrazolium (NBT) reduction method of Beauchamp and Fridovich (1971).The SOD unit was defined as the amount of crude enzyme required to inhibit the reduction of NBT by 50% and expressed as units per mg protein (U mg–1protein).All assays were performed in triplicate,and the average values were reported.The protein content was measured using the Bradford procedure (Bradford 1976).
2.9.Histochemical detection of O2–
2.10.Statistical analyses
Significance analysis was performed by Student’st-test.*and**in the figures denote significant differencesP<0.05 andP<0.01,respectively.All the statistic calculations were processed by the Excel 10.0 Software.
3.Results
3.1.VdSOD5 encodes a Cu-only superoxide dismutase containing a signal peptide
Proteomic analysis ofV.dahliaeextracellular proteins mimicking host–pathogen interaction identified a putative extracellular superoxide dismutase VdSOD5 (Chenet al.2016).The full-length of theVdSOD5gene (VEDA_04232)contains an 801-bp open reading frame (ORF) with one intron of 71 bp,which is confirmed by PCR and RT-PCR analyses using genomic DNA and cDNA as templates,respectively (Fig.1-A).TheVdSOD5ORF is predicted to encode a peptide of 266 amino acids with a molecular weight of 27.50 kDa and an isoelectric point of 4.56.VdSOD5 has one typical superoxide dismutase motif (pfam00080,from N-62 to H-180),four Cu2+binding residues (H-97,H-99,H-114,and H-180),two disulfide cysteines (C-108 and C-190),and one active arginine (R-187),indicating VdSOD5 is a functional SOD enzyme (Fig.1-B).However,we noticed that VdSOD5 only had two,instead of four,potential Zn2+binding residues (H114 and D138) in the SOD motif,indicating VdSOD5 cannot bind Zn2+and is probably a Cuonly SOD.These features are not unique to VdSOD5,but also can be found in most of the other aligned EC-SODs of phytopathogenic fungi.However,Puccinia striiformisEC-SOD lacks two Cu2+-binding residues (equivalent to VdSOD5 residue H-97 and H-180),indicating that it is a Zn-only SOD enzyme (Fig.1-B).All these results showed unique characteristics in the selection of ECSOD metal co-factors in different phytopathogenic fungi.Interestingly,although the EC-SOD from a closely related speciesVerticillium alfalfaedisplays high conservation with VdSOD5 (62% identity to VdSOD5),it lacks one of the two highly conserved cysteine residues at position 190,thus destroying the disulfide crosslinking vital for the formation of the active catalytic center (Fig.1-B).In addition,the N-terminus of VdSOD5 (20 amino acids)was predicted as a signal peptide using SignalP 5.0.We also identified a potential glycophosphatidyl inositol (GPI)anchor with a putative omega site at residue 243 at the C-terminus.These two motifs were potentially involved in the extracellular distribution of VdSOD5 (Fig.1-B).
To verify the active function of the predicted signal peptide,the yeast signal trap assay was used for the functional analysis of the N-terminus of VdSOD5.Yeast cells need a secreted invertase to grow on media with raffinose as the sole carbon source.The 20 amino acids of the N-terminus were fused to the vector pSUC2,which carries a signal-peptide-lacking invertase gene,to generate pSUC2::VdSOD5SP,which was then transformed into the invertase negative yeast strain YTK12.The signal peptide of Avr1b,which is a secreted protein inPhytophthora sojae,was fused to pSUC2,which was then transformed into YTK12 as the positive control.In the CMD-W medium with sucrose as the sole carbon source,YTK12 carrying emptypSUC2(negative control),pSUC2::Avr1bSP(positive control) and pSUC2::VdSOD5SPall grew well (Fig.1-C).In the YPRAA media with raffinose as the sole carbon source,YTK12 strain carryingpSUC2did not grow,but pSUC2::Avr1bSPand pSUC2::VdSOD5SPcontaining YTK12 grew normally,indicating that the invertase can be successfully secreted in pSUC2::VdSOD5SPcontaining YTK12 (Fig.1-C).These results demonstrated that the N-terminal peptide of VdSOD5 was a functional signal peptide and could conduct the protein secretion.
3.2.VdSOD5 does not affect vegetative growth,carbon utilization and conidiospore production
To study the function ofVdSOD5,gene knockout mutant ofVdSOD5was constructed by replacing the coding sequence of the wild-type strain with the hygromycin resistance cassetteviahomologous recombination.Five independent transformants were verified by PCR,of which two (ΔVdSOD5-1/2) were selected randomly for further research.Complemented strains (EC-1/2) were generated by reintroducingVdSOD5with its native promoter as well as the neomycin resistance gene to ΔVdSOD5-1andΔVdSOD5-2,respectively (Appendix B).The colony morphology of theVdSOD5deletion mutants did not change on PDA compared to the wild-type strain.All retained white mycelial development without the generation of additional pigmentation (Fig.2-A).The growth rates of the wild-type strain and mutants were also comparable,indicating the loss-of-function of VdSOD5 did not interfere with the vegetative growth ofV.dahliae(Fig.2-A).To test the capabilities of utilizing different carbon sources by ΔVdSOD5,the growth rate was monitored using four carbon sources,including sucrose,starch,pectin and cellulose.The growth rates of the mutants were also identical in four types of carbon source containing medium compared with those of the wild-type strain (Fig.2-A).In addition,the number of conidia produced by twoVdSOD5deletion strains was similar to that of the wild-type strain (Fig.2-B).Taken together,these results indicated that the deletion ofVdSOD5did not alter vegetative growth,carbon source utilization capacity and conidial production inV.dahliae.
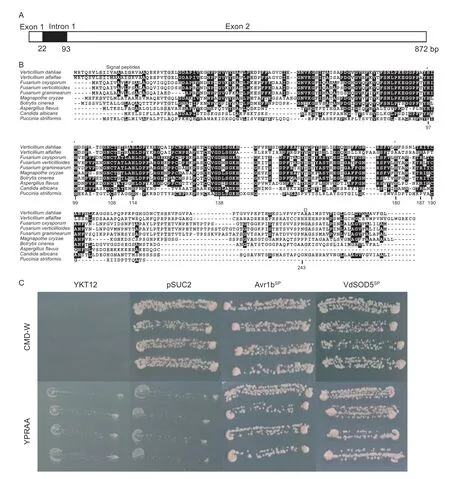
Fig.1 Bioinformatics and secretion analysis of VdSOD5.A,a schematic of VdSOD5.B,the protein sequence of VdSOD5 was aligned with other known extracellular Cu/Zn-SODs (EC-SODs) of phytopathogenic fungi using CLUSTAL 2.1.The strongly conserved residues are highlighted in black background and the weakly conserved residues are in gray.The Cu2+ binding sites of VdSOD5 are labeled with *.The putative omega-site for the GPI anchor is marked as Ω.The signal peptide of VdSOD5 is underlinedGenBank accession numbers:Verticillium dahliae (VEDA_04232);Verticillium alfalfa (XP_003004902.1);Fusarium oxysporum (EMT61982.1);Fusarium verticillioides (EWG37413.1);Fusarium graminearum (CEF77131.1);Magnaporthe oryzae(XP_003710682.1);Botrytis cinereal (EMR88570.1);Aspergillus flavus (XP_002380367.1);Puccinia striiformis (KI515837.1);Candida albicans (XP_719507.1).C,secretion function validation of the putative signal peptide of VdSOD5 using the yeast signal trap assay.The yeast strain YTK12 could not grow on the CMD-W medium without tryptophan,while all the strains containing the pSUC2 vector grew on CMD-W based on the function of the Trp operon in the backbone of pSUC2.Both the yeast strain YTK12 and the strain containing the empty vector of pSUC2,which lacks a functional invertase gene,could not grow on YPRAA media.In contrast,the YTK12 strain carrying pSUC2::VdSOD5SP grew normally.The known functional signal peptide Avr1b was used as a positive control.
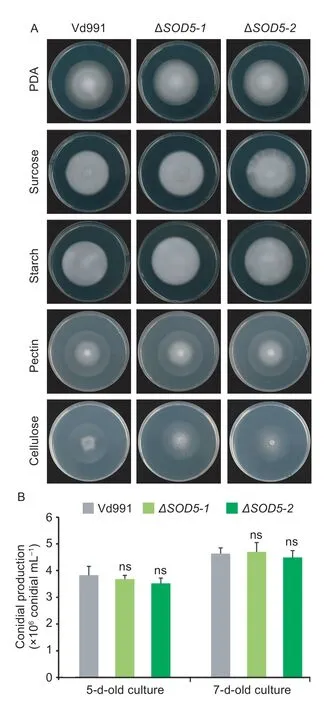
Fig.2 Analysis of fungal growth,carbon source utilization and conidial production of VdSOD5 deletion strains in vitro.A,the wild-type strain and ΔVdSOD5-1/2 grew on plates containing PDA and different carbon sources (sucrose,starch,pectin and cellulose),respectively.A total of 2 μL conidial suspension(5×106 conidia mL–1) were dropped on the center of the plate and incubated at 25°C for 9 d.B,quantification of conidial production was based on a 5-mm-diameter PDA agar plug from the edge of 5-and 7-d-old culture fungal colonies in 1.0 mL water.The error bars represent the standard deviations of the mean (n=3).Significance analysis was analyzed by Student’s t-test and ns means no significance.
3.3.VdSOD5 is required for virulence in V.dahliae
To test whetherVdSOD5involves in pathogenicity,theVdSOD5transcript levels were measured duringV.dahliaeinfection on susceptible cotton plants by RT-qPCR.The results showed thatVdSOD5was expressed in all tested root samples harvested from 6 to 144 hpi (Fig.3).In CM medium (Mock),theVdSOD5transcripts were detected at low levels.However,the expression ofVdSOD5was significantly increased,reaching the maximum at 12 hpi(Fig.3).During the late stages of infection,the expression level ofVdSOD5returned to a relatively low level.These results indicated thatin-plantainduction ofVdSOD5was present in the early stage ofV.dahliaeinfection,suggesting that VdSOD5 may play an important role in pathogenicity during infection on host plants.
To assess the possible role ofVdSOD5to virulence inV.dahliae,the susceptible cotton was inoculated with ΔVdSOD5,complemented strains andthe wild-type strain Vd991 using a root-dip method.As expected,the cotton inoculated with the wild-type strain showed typical Verticillium wilt symptoms including leaf wilting,necrosis and vascular discoloration.In contrast,the plant seedling inoculated with ΔVdSOD5significantly alleviated the symptoms of the disease (Fig.4-A).Quantification of fungal biomass by qPCRin plantarevealed approximately 30% less biomass inVdSOD5deletion mutant treatments relative to the wild-type strain (Fig.4-B).The virulence and fungal biomassin plantaof the complemented strains were comparable to those of the wild-type strain (Fig.4-A and B).These results suggest thatVdSOD5is required for virulence ofV.dahliaeon cotton.
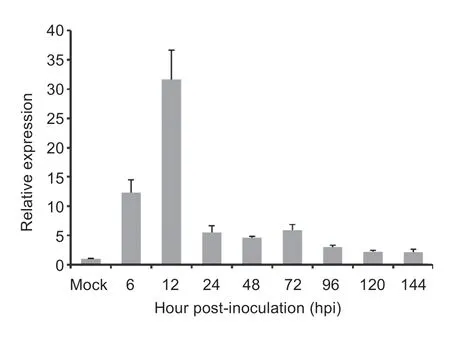
Fig.3 Transcript levels of VdSOD5 during infection of cotton.The expression levels of VdSOD5 were calculated by the comparative Ct method with EF-1α of V.dahliae as an endogenous control.Relative quantifications are compared with the expression levels of fungal tissue culturing in CM medium.The values are averaged,and the error bars represent the standard deviations of the mean (n=3).Asterisks ** and* represent significant differences at P<0.01 and P<0.05 by Student’s t-test,respectively.
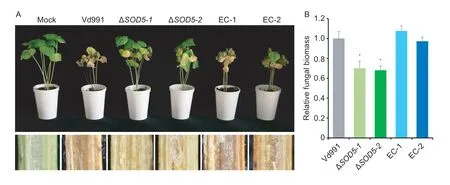
Fig.4 VdSOD5 is required for full virulence of Verticillium dahliae.A,the disease symptoms of cotton seedlings inoculated with sterile water (Mock) and the indicated strains at 21 d post-inoculation (top).The discoloration of the inoculation shoot longitudinal sections is shown at the bottom.B,the fungal biomasses of each fungal strain in cotton plants were determined by qPCR.Error bars represent standard deviations (n=3).Asterisks * indicate significant differences (P<0.05) by Student’s t-test.
3.4.VdSOD5 mainly contributes to extracellular SOD activities of V.dahliae
To verify the biochemical activity of VdSOD5,the total SOD activity was tested in the wild-type strain Vd991,two independent mutants (ΔVdSOD5-1and ΔVdSOD5-2) and two complemented strains (EC-1 and EC-2) by the NBT reduction method.As shown in Fig.5,the SOD activity in both fungal tissue and culture filtrate decreased as a consequence ofVdSOD5deletion,indicating VdSOD5 possesses the enzymatic activity of superoxide dismutase.Compared with the wild-type strain,the SOD activity of the fungal tissue decreased moderately by 28% due toVdSOD5deletion.In contrast,the SOD activity of the culture filtrate decreased significantly by 74% inVdSOD5deletion mutants.These results demonstrate that VdSOD5 is the major contributor to the extracellular SOD activity,which is in accordance with the extracellular localization of VdSOD5 directed by a signal peptide.Complemented strains restored the SOD activities of cell-free culture filtrates and cell-associated fungal tissues,which were comparable to those of the wild-type strain.
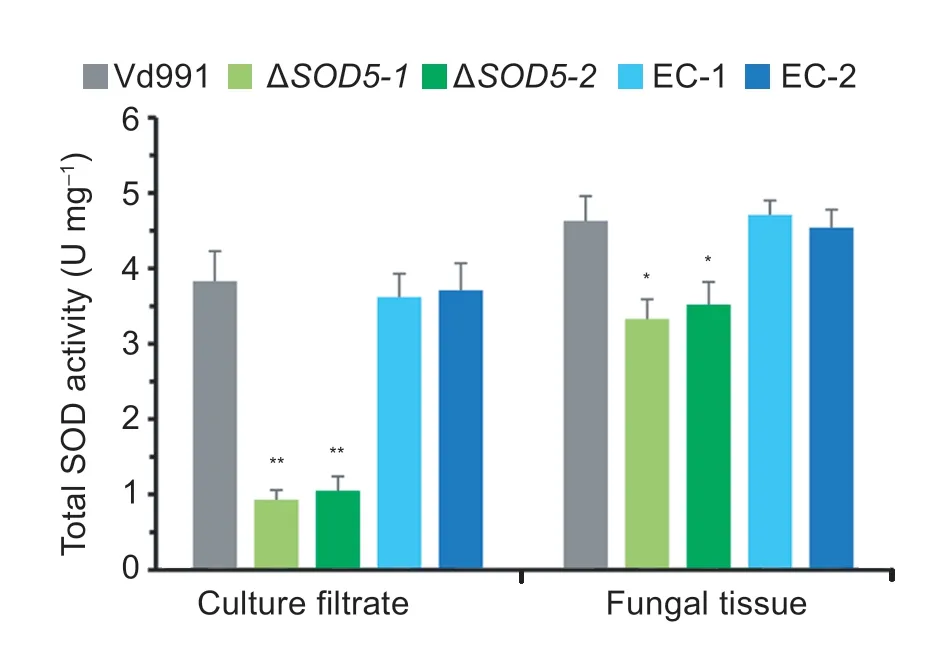
Fig.5 Total superoxide dismutases (SODs) activities of soluble extracellular culture filtrate and fungal tissue.Verticillium dahliae culture filtrate and fungal tissue were collected from 7-day-old culture in CM medium.SOD activities were determined by the nitroblue tetrazolium (NBT) reduction method.Error bars represent standard deviations (n=3).Asterisks ** and * represent significant differences P<0.01 and P<0.05 by Student’s t-test,respectively.
3.5.VdSOD5 is involved explicitly in detoxifying extracellular ROS
We further investigate the role of SOD enzymatic activity of VdSOD5 in detoxification of intracellular and extracellular ROS.Menadione,an intracellular superoxide generating agent (Kawamuraet al.2006),was supplemented to PDA medium to evaluate whether VdSOD5 plays roles in degrading intracellular ROS.As shown in Fig.6-A and B,the wild-type strain Vd991 tolerated well on PDA plates supplemented with 10 μmol L–1menadione,but formed colonies with remarkable smaller sizes under the treatment of 30 and 50 μmol L–1menadione.The colony diameters were merely about 85 and 55% of those grown without menadione at 12 d,respectively.The phenotype and colony diameter of ΔVdSOD5were similar to those of the wild-type strain in all of the tested concentrations of menadione (Fig.6-A and B),indicating VdSOD5 deletion does not increaseV.dahliaesensitivity to menadione,i.e.,VdSOD5 is not responsible for scavenging intracellular superoxide radicals.
We next determined whether VdSOD5 could scavenge extracellular ROS generated by the host during infection.NBT can react withto form an insoluble blue formazan compound.Roots of susceptible cotton infected with the wild-type strain,VdSOD5 deletion and complemented strains were stained with NBT.The superoxide production was visualized as a blue formazan deposit within root tissues.The results showed that more superoxides were accumulated in roots inoculated with theVdSOD5deletion mutant than those inoculated with the wild-type strain at 24 hpi.Cotton roots inoculated with complemented strains restored the low intensity of staining,which was comparable to that of the wild-type strain (Fig.6-C).These results demonstrate thatVdSOD5deletion led to increasedaccumulation in cotton roots upon infection,and VdSOD5 played an important role in scavenging extracellular host-derived ROS.
4.Discussion
Cu-only EC-SODs of animal-associated pathogens play an important role in scavenging host-derived ROS and function as virulence factors.However,only a few studies have conducted on the role of Cu-only EC-SODs in phytopathogens.In the present study,we found that VdSOD5 possessed the extracellular SOD activity in scavenging host-derived ROS,and significantly contributed to virulence during infection on host plants.
The bimetallic Cu2+and Zn2+containing Cu/Zn-SODs are widely distributed in nature from bacteria to mammals,requiring Cu2+for catalysis and Zn2+for stabilization (Potteret al.2007).So for a long time,fungal EC-SOD has also been described as Cu/Zn-SOD (Martchenkoet al.2004).However,recent studies have shown that fungalC.albicansEC-SOD (SOD5) is not a canonical Cu/Zn-SOD as revealed by three-dimensional structures and biochemical analyses.AlthoughC.albicansSOD5 shares the overall characteristics of Cu/Zn-SODs,it misses two histidine residues necessary for coordinating zinc,resulting in a more exposing copper binding site,which may enhance Cu2+capturing from the extracellular environment (Gleasonet al.2014).This unique feature is an adaptation ofC.albicansto the harsh environment in host-microbe interaction.During infection,the host can attack invadingC.albicansthrough Cu2+toxicity,and the microbe may,in turn,use host Cu2+to activate its extracellular SODs for antioxidant defense (Hodgkinson and Petris 2012).In this study,we found that Cu-only SODs were widely distributed among phytopathogenic fungi includingV.dahliae(Fig.1-B).Copper has also been shown to play a role in protecting certain plants against Verticillium wilt(Chmielowskaet al.2010).However,whether using host copper against host oxidative attack applies to VdSOD5 still needs further investigation.EC-SODs of plant pathogenic fungi are distinguished from conventional EC-SODs (Cu/Zn-SODs) of plants in co-factor selection,making them a possible target for the treatment of infections caused by phytopathogenic fungi.Interestingly,EC-SOD ofV.alfalfaelacks one of the two highly conserved cysteine residues in the active catalytic center,indicating EC-SOD ofV.alfalfaemay not be a functional SOD enzyme (Fig.1-B),which may reflect different host adaptation betweenV.alfalfaeandV.dahliae.
Different types of SODs are localized in various cellular compartments,allowing them to cope with various ROSin situ(Schatzman and Culotta 2018).For instance,mitochondrial Mn-SODs are involved in removing superoxides produced by mitochondrial respiration (Gileset al.2005),while chloroplast Fe-SODs are critical for scavenging superoxides generated from photosystems (Kuoet al.2013).EC-SODs are appropriately positioned to specifically combat hostderived ROS in human pathogens.For example,C.albicansSOD5 is important for fungal survival against the oxidative attack of macrophages and neutrophils in a systemic model of infection (Frohneret al.2009).Histoplasma capsulatumSOD3 facilitates pathogenesis by detoxifying host phagocyte cell-derived reactive oxygens (Youseffet al.2012).Similarly,studies have shown that knockdown of the extracellular Zn-only SOD inP.striiformisleads to ROS accumulation in the host (Liuet al.2016).In this study,sequence analysis suggestedV.dahliaeVdSOD5 protein had the functional signal peptide,which is confirmed by yeast trap assay(Fig.1-C).Not surprisingly,VdSOD5 contributed to the extracellular SOD activity of fungi (Fig.5) and functioned in detoxification of ROS generated by cotton roots during infection (Fig.6-B).To our knowledge,this is the first study to demonstrate that Cu-only EC-SOD of plant fungal pathogens plays an important role in eliminating host-derived oxidative stress,which is similar to the extensively studied Cu-only EC-SODs in human pathogens and Zn-only EC-SOD in plant pathogenP.striiformis.Interestingly,we found the SOD activity of fungal tissues also reduced in VdSOD5 mutants(Fig.5).The GPI signal has been proven to promote cellwall-association of a portion of EC-SOD in fungal pathogenHistoplasma capsulatum(Youseffet al.2012).We speculate that another part of VdSOD5 may be associated to fungal cell wall though C-terminal GPI anchoring and does not reside inside the cell,since VdSOD5 is not responsible for scavenging intracellular superoxide radical menadione(Fig.6-A).Thus,both soluble and cell-associated VdSOD5 are considered to be extracellular.
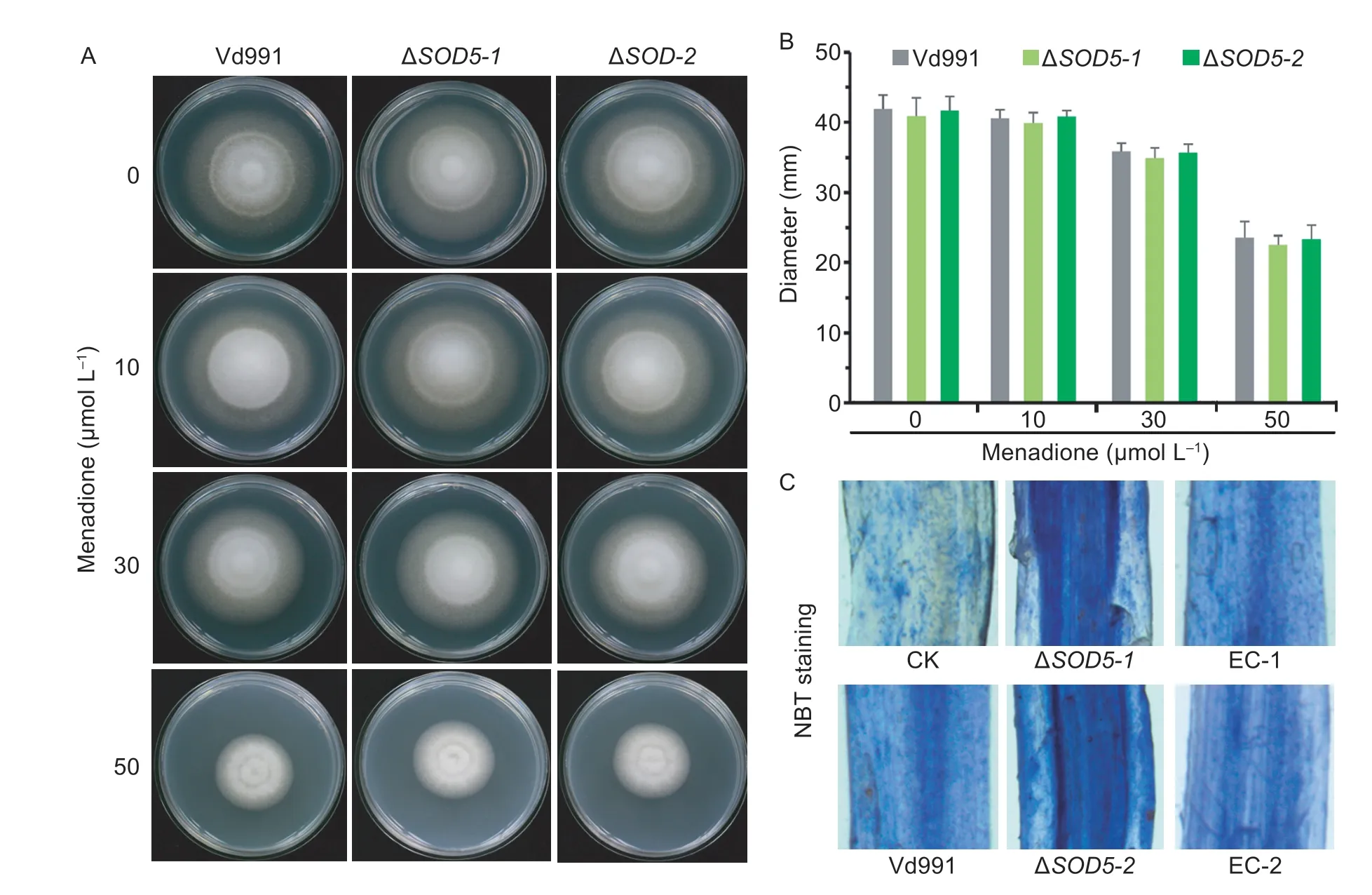
Fig.6 The role of VdSOD5 in the degradation of reactive oxygen species (ROS).A,the effect of menadione on fungal growth.The wild-type strain and ΔVdSOD5-1/2 were cultured on PDA medium supplemented with menadione at indicated concentrations for 12 d.B,colony diameters of the wild-type strain and ΔVdSOD5-1/2 on PDA plates containing different concentrations of menadione after growing 12 d.Error bars represent standard deviations (n=3).C,accumulation of superoxide in infected cotton roots.Detection of O2 accumulation in cotton roots inoculated with sterile water,wild-type strain,ΔVdSOD5-1/2 and complemented strainsat 24 h post-inoculation (hpi) by NBT staining.
Cu/Zn-SODs are important virulence factors in nearly all plant pathogenic fungi.However,most of the identified Cu/Zn-SODs are intracellular SOD1,the loss of which increases the sensitivity to intracellular oxidative molecules.For example,deletion ofSsSOD1inSclerotinia sclerotiorumincreases the sensitivity to a cytosolic superoxide generator paraquat and reduces the virulence against peas (Xu and Chen 2013).Loss-of-function of FgSOD1 inFusarium graminearumexhibits increased sensitivity to the intracellular ROS generator menadione and reduced virulence against wheat (Yaoet al.2016).These studies suggest that,with the loss of intracellular SOD1,virulence attenuation of fungi may owe to their impaired ability to alleviate ROS stress arising from altered fungal metabolism imposed by growth in hosts,rather than their inability of scrubbing host-derived superoxides.In contrast,great progress of another type of Cu-SOD,EC-SOD,has been achieved in human fungal pathogens,likeC.albicans,H.capsulatumandP.brasiliensis(Frohneret al.2009;Youseffet al.2012;Tamayoet al.2016).EC-SODs in these fungi are specifically extracellular and have extracellular SOD activities to combat ROS produced by the hosts.All the EC-SODs of human pathogens have been shown to be virulence factors.In this study,we determined the pathogenic role of EC-SOD(VdSOD5) inV.dahliae,a destructive phytopathogenic fungus.VdSOD5was clearly induced in the early stage of infection (Fig.3),when ROS accumulation reached the highest level in susceptible cotton (ZhangYet al.2017),suggesting thatVdSOD5may play an important role in host–pathogen interactions andV.dahliaepathogenicity.Deletion ofVdSOD5did not affect the growth or viability ofV.dahliae(Fig.2),but alleviated disease symptoms and reduced fungal biomass in cotton,suggesting that EC-SOD was also required for the virulence ofV.dahliae(Fig.4).This may be mainly due to the function of VdSOD5 in scavenging extracellular host-derivedThus,VdSOD5 may help pathogen confront host oxidative burst,which can directly damage pathogen macromolecules.In addition,recent studies have shown that the extracellularproduced by fungal NOX enzymes also serves as a substrate for ECSOD (Robinettet al2018).InV.dahliae,VdNoxB/VdPls1-mediated ROS has shown to regulate penetration peg differentiation within the hyphopodium,which is essential for redirecting fungal growth toward host cells to penetrate cotton roots and to colonize the host vascular system (Zhaoet al.2016).Thus,ROS generated by VdNoxB may also be the substrate for VdSOD5 inV.dahliae,and VdSOD5 may function in facilitating penetration peg formation during the initial colonization of cotton roots.
5.Conclusion
VdSOD5 is a Cu-only EC-SOD with a signal peptide,which possesses the extracellular SOD activity and plays an important role in detoxifying host-derived ROS during infection.While being nonessential for growth or viability,VdSOD5 contributes to the virulence ofV.dahliaeinfection on host plant of cotton.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (31501588,31972228,and 31970142).
Declaration of competing interest
The authors declare that they have no conflict of interest.
Appendicesassociated with this paper are available on http://www.ChinaAgriSci.com/V2/En/appendix.htm
杂志排行
Journal of Integrative Agriculture的其它文章
- Receptor-like kinase OsASLRK regulates methylglyoxal response and content in rice
- Heredity and gene mapping of a novel white stripe leaf mutant in wheat
- Construction of a high-density adzuki bean genetic map and evaluation of its utility based on a QTL analysis of seed size
- Effects of temperature and solar radiation on yield of good eatingquality rice in the lower reaches of the Huai River Basin,China
- Difference in corn kernel moisture content between pre-and postharvest
- The effect of elevating temperature on the growth and development of reproductive organs and yield of summer maize
