The TaFIM1 gene mediates wheat resistance against Puccinia striiformis f.sp.tritici and responds to abiotic stress
2021-06-02SHIBeibeiWANGJuanGAOHaifengZHANGXiaojuanWANGYangMAQing
SHI Bei-bei,WANG Juan, ,GAO Hai-feng,ZHANG Xiao-juan,WANG Yang,MA Qing
1 State Key Laboratory of Crop Stress Biology for Arid Areas,Northwest A&F University,Yangling 712100,P.R.China
2 School of Life Science,Shanxi Datong University,Datong 037009,P.R.China
3 Institute of Plant Protection,Xinjiang Academy of Agricultural Sciences/Key Laboratory of Integrated Pest Management on Crop in Northwestern Oasis,Ministry of Agriculture and Rural Affairs,Urumqi 830091,P.R.China
Abstract Fimbrin,a regulator of actin cytoskeletal dynamics that participates in numerous physiological and biochemical processes,controls multiple developmental processes in a variety of tissues and cell types.However,the role of fimbrin in pathogen defense of wheat and the mechanisms have not been well studied.Here,we investigated that the expression of TaFIM1 gene of wheat was significantly induced in response to avirulent race of Puccinia striiformis f.sp.tritici (Pst) and silencing of TaFIM1 by virus-induced gene silencing method.The results show that silencing of TaFIM1 resulted in a reduction of resistance against the stripe rust indicated by both phenotypes and a histological examination of Pst growth.Additionally,the expression level of TaFIM1 gene was up-regulated under abiotic stresses.These findings suggest that TaFIM1 functions as a positive regulator of pathogen resistance of wheat plants and response to abiotic stress.Our work may show new light on understanding the roles of fimbrin in wheat.
Keywords:wheat,Puccinia striiformis f.sp.tritici,fimbrin,disease resistance,abiotic stress
1.Introduction
The common wheat (TriticumaestivumL.) is a major global cereal grain essential to human nutrition,with a cultivated acreage of about 220 million hectares around the world(Singhet al.2016).During the growing season,the wheat is seriously damaged by the wheat stripe rust,an airborne fungal disease caused byPuccinia striiformisf.sp.tritici(Pst),in most countries of the world.Among various control tactics,breeding resistant cultivars is the most economical,safe and effective approach to control the disease (Wellingset al.2011;Beddowet al.2015),since the plants rely on an innate immune system to resist pathogens infection (Dodds and Rathjen 2010;Jones and Dangl 2006).For defending themselves,firstly,plants pattern recognition receptors(PRRs) recognize pathogen-associated molecular patterns(PAMPs) and induce basic defense response,known as PAMPs-triggered-immunity (PTI),is a major and first line of plant-based disease resistance (Galá and Collmer 1999).In order to overcome the defense response induced by PTI,pathogens secrete effectors into plant cells to destroy host PTI and enhance the virulence of pathogens.Effector proteins are specifically identified by plant resistance proteins and activate downstream resistance,which is known as effector-triggered-immunity (ETI) (Yipinget al.2011;Macho and Zipfel 2015).Later,ETI can trigger hypersensitive response (HR) reaction of host plant cells(Jones and Dangl 2006;Minsavageet al.1990;Niirnbergeret al.1994),and involve the signal cascade reaction behind HR:influx of Ca2+ions from the extracellular space and/or anion flux results in an oxidative burst producing reactive oxygen intermediates (ROIs) (Danglet al.2013).
Fimbrin/plastin,a kind of actin-binding proteins (ABPs),is one of the important factors regulating the dynamics of actin cytoskeleton which involved in numerous physiological and biochemical processes,such as cell polar growth,cell morphogenesis,vesicle transport,stomatal opening and closure,and signal transductionin vivo(Dayet al.2011;Liu 2011;Shinomiya 2012).Fimbrin protein contains two repetitive acting-binding domains,including two calponin homology-domains,and a Ca2+-binding domain on N-terminal (Miyakawaet al.2012;Wanget al.2016),and it can cross-link actin cytoskeleton into higher aggregates,such as bundles,through a pair of acting-binding domains(Shinomiyaet al.2012).Fimbrin was cloned in plant first from wheat (Cruz-Ortega 1997).Recently,fimbrin protein was found to participate in the regulation of actin cytoskeleton dynamics and plant growth (Zhanget al.2016).For examples,apical actin structures are disorganized to different degrees in the pollen tubes offim5loss-offunction mutants inArabidopsis thaliana(Kovaret al.2001);in lily pollen tube,fimbrin1 participates in pollen tube growth by stabilizing actin (Suet al.2012).In mammals,fimbrins/plastins participate in immune response,neuronal differentiation and intestinal epithelial development (Adamset al.1995).In a few fungal fimbrin,the function is related to endocytosis,cytokinesis and polarization (Adamset al.1991;Zhenget al.2014).However,fimbrin has not been reported to participate in immune regulation in plants so far.
In this paper,we investigated the role ofTaFIM1in disease resistance responses in wheat.First,qRT-PCR was used to analyze the disease resistance ofTaFIM1gene.Then the function ofTaFIM1in wheat resistance to stripe rust was verified by virus-induced gene silencing technology(BSMV-VIGS).Finally,TaFIM1was also response to abiotic stress.Our work may shed new light on understanding the roles of fimbrin in wheat in response to pathogen infection and immunity.
2.Materials and methods
2.1.Strains and plant growth
The common wheat (Triticum aestivum) cultivar Suwon 11(Su11) was used in this experiment.The cultivar expresses an incompatible interaction withPstrace CYR23,triggering a typical hypersensitive reaction (HR) after inoculation,and exhibit a compatible interaction with race CYR31 on the wheat leaves which a large number of uredia were attached.CYR23 and CYR31 were grown on wheatcv.Mingxian 169.Wheat plants were grown at 23°C,light intensity of 8 000–10 000 lx,and light period of L:H=16 h:8 h.
2.2.Cloning,sequencing and phylogenetic analysis of TaFIM1
The total RNA was extracted following the BIOZOL/phenol/chloroform method (Invitrogen,Carlsbad,CA,USA).Reverse transcription of first-strand cDNA was performed following the manufacture protocols of FastKing RT Kit(TIANGEN,Beijing,CA,China).The full-length primer ofTaFIM1(Table 1) was designed according to theAegilops tauschiifimbrin gene sequence (GenBank accession no.XM_020337393.1) with Primer Premier 6.0,andTaFIM1DNA sequence was obtained through PCR technology (Qiet al.2017).
The amino acid sequence of TaFIM1 was deduced using the online NCBI tool ORF finder (http://www.ncbi.nlm.nih.gov/gorf/gorf.html).The molecular weight and protein isoelectric point of TaFIM1 were calculated using Protparam(http://web.expasy.org/protparam/).The phylogenetic analysis was performed with the MEGA Software (v.6.0,http://www.megasoftware.net/).Analysis of the homeologs ofTaFIM1in wheatcv.Chinese Spring (CS) genome was used in URGI Database (https://urgi.versailles.inra.fr/blast/?dbgroup=wheat_survey&program=blastn).The intron/exon structure of the three homeologs ofTaFIM1in CS genomewere analyzed by Gene Structure Display Server (GSDS) (http://gsds.cbi.pku.edu.cn/).The multiple protein sequence alignment of TaFIM1 and three homeologs were analyzed by DNAMAN,and the prediction conserved domain ofTaFIM1was carried out by InterPro (http://www.ebi.ac.uk/interpro/).Sequences of fimbrin in the wild emmer wheat (Triticum dicoccoides)cv.Zavitan andcv.CS were downloaded from GrainGenes (http://wheat.pw.usda.gov/GG2/index.shtml) using BLAST tool and other sequences of fimbrin were downloaded from NCBI Database (http://www.ncbi.nlm.nih.gov/) (Sunet al.2019).
2.3.Pathogen inoculation and chemical treatments
Pathogen inoculation and culturing was assessed following Kang and Li (1984).The wheat leaves were sampled at 0,12,24,36,48,72,96 and 120 h post-inoculation (hpi).For hormone treatment,10-d-old Su11 were sprayed with 100 mmol L–1ethylene (ET),100 mmol L–1abscisic acid (ABA),100 mmol L–1methyl jasmonate (MeJA) and 2 mmol L–1salicylic acid (SA) and were sampled at 0,0.5,3,6,12 and 24 h post-treatment (hpt).For high-salinity and drought-stress treatments,roots of 10-d-old seedlings were submerged in 200 mmol L–1NaCl and 20% (w/v) PEG6000.For low-and high-temperature treatments,seedlings were placed in 4 and 37°C incubators,respectively.Wheat leaves were harvested at 0,1,3,6,12 and 24 hpt.Leaves inoculated with water were used as control.For tissuespecific expression analysis ofTaFIM1,seeds of Su11 and the roots,stems and leaves of 10-d-old Su11 were selected.Three biological replicates were performed for each treatment at each time point (Zhanget al.2017).
2.4.Quantitative real-time PCR analysis and statistical analyses
Based on the cloned sequence,the qRT-RCR fragment was obtained (Appendix A),and wheatTaEFgene (GenBank accession no.Q0303033) was selected as the internal reference gene.The qRT-PCR Reaction System was added following the protocol of ChamQTM SYBR qPCR Master Mix Kit (Vazyme,Nanjing,China).The relative transcript expression was assessed by the 2–ΔΔCtmethod (Wanget al.2011).The SPSS 23.0 Software was used to determine the statistical significance (P<0.05).
2.5.BSMV-induced gene silencing
A 119-bp fragments ofTaFIM1(Appendix A) was inserted into the original barley stripe mosaic virus (BSMV) vector for gene silencing.The transcripts of BSMV:α,BSMV:β and BSMV:γ-TaFIM1were mixed equallyin vitroand transfected into the second leaf of two-leaf Su11 seedling.Buffer,BSMV:γ and BSMV:γ-TaPDS(phytoene desaturase enzyme) was used as negative control,blank control and positive control,respectively (Heinet al.2005).The phenotype was observed at 14 d after inoculating the virus,then the fourth leaves ofTaFIM1silencing group and the control plants were inoculated with urediospores ofPstraces CYR23 and CYR31,respectively.The fourth leaves were collected at 0,24,48,and 120 hpi for qRT-PCR analysis(Pettyet al.1990).
2.6.Histological observations of fungal growth in silenced plants
In the virus-mediated gene silencing experiment,the fourth leaves of control andTaFIM1-silenced plants were sampled at 24,48 and 120 hpi with inoculating urediospores ofPstrace CYR23.At each time point four–six leaves were collected for histological observations.After fluorescent staining (WGA AF488),the number of mycelium branches,hypha length and colony area were measured with an Olympus BX-51 microscope (Olympus Corp,Tokyo).Only the sites where a substomatal vesicle had formed over a stoma were treated as successful penetration.A minimum of 50 infection sites were examined for each treatment(Wanget al.2012).
3.Results
3.1.Isolation and sequence analysis of TaFIM1
Based on the sequence data of a wheat fimbrin (GenBank accession no.XM_020337393.1),a 2 238-bp homolog of fimbrin was isolated from wheatcv.Su11 by homologybased cloning.The gene obtained was named asTaFIM1.Blast analysis ofTaFIM1nucleotide sequence in theTriticum aestivum cv.CS genome sequence revealed that three copies were present,with 99,95,and 98% nucleotide sequence identity,which were located on chromosomes 6A,6B and 6D,respectively (Appendix A),and the intron/exon structure of the three homeologs ofTaFIM1in CS were shown in Fig.1-A.The predicted open reading frame (ORF)region encodes an inferential protein consisting of 745 amino acids with an isoelectric point molecular of 5.65.TaFIM1 protein was composed of four repetitive CH-domains,belonging to the calponin homology (CH) superfamily(Appendix A).An evolutionary tree constructed from DNA sequences of 12 fimbrins shows thatTaFIM1has the closest relationship withAtFIM1 AAegilops tauschiicv.AL8/78(XM_020337393.1) (Fig.1-B).These results indicate thatTaFIM1is a typical member of the fimbrin in wheat.
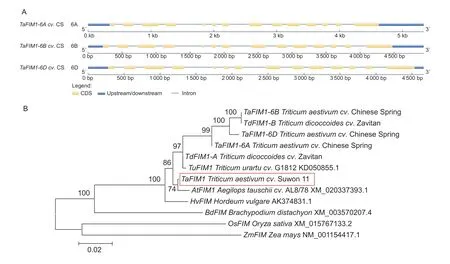
Fig.1 Sequence and structure analysis of wheat fimbrin (TaFIM1).A,the intron/exon DNA structure of wheat cv.Chinese Spring(CS) TaFIM1-6A,TaFIM1-6B and TaFIM1-6D.B,phylogenetic analysis of TaFIM1 and other fimbrin proteins,AtFIM1 Aegilops tauschii cv.AL8/78 (XM_020337393.1),TuFIM1 Triticum urartu cv.G1812 (KD050855.1),TaFIM1-6A/B/D Triticum aestivum cv.Chinese Spring,TdFIM1-A/B Triticum dicoccoides cv.Zavitan,HvFIM Hordeum vulgare (AK374831.1),BdFIM Brachypodium distachyon (XM_003570207.4),OsFIM Oryza sativa (XM_015767133.2),and ZmFIM Zea mays (NM_001154417.1).
3.2.TaFIM1 is differentially induced following perception of abiotic and biotic stress
Quantitative real-time PCR was used to analyze the interaction between wheat and the stripe rust forTaFIM1.The results show that the expression ofTaFIM1was upregulated in the incompatible interaction and reached the peak at 48 hpi,while in the compatible interaction,there was no variation except at 36 hpi.The differential expression ofTaFIM1between the compatible and incompatible interactions was prominent at 48 hpi,significantly higher in the incompatible interaction than in the compatible interaction approximately by six-fold (Fig.2-A),indicating thatTaFIM1might be involved in the wheat–Pstinteraction.
To verify the expression dynamics ofTaFIM1involving in abiotic stress,we next assessed the effect of environmental stressors such as salt,high temperature (37°C),low temperature (4°C) and simulated drought (PEG6000) on accumulation ofTaFIM1.The results show thatTaFIM1was up-regulated under all four abiotic stresses (Fig.2-B),suggesting thatTaFIM1responds to abiotic perception.
To clarify the role ofTaFIM1in broader stress signaling responses in wheat,the expression patterns ofTaFIM1were investigated following perception of hormone treatments(ET,ABA,MeJA and SA).Under the MeJA treatment,the expression level ofTaFIM1was significantly induced in 6–24 hpt,with an approximate 4.3-fold change in expression at 24 hpt.After treatment with ET,TaFIM1transcript levels were greatly induced at 0.5–6 hpt.When seedlings were treated with ABA,TaFIM1was up-regulated at 24 hpt.Treatment with SA,however,had no significant effect on the expression (Fig.2-C).These results indicated thatTaFIM1responded positively to the regulation of ET,ABA and MeJA.
TaFIM1was differentially expressed in wheat tissues(Fig.2-D).The expression ofTaFIM1in the leaves was 6.1 folds as high as in the roots.
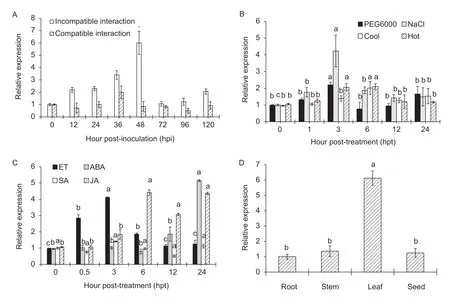
Fig.2 Quantitative real-time PCR expression analyze of wheat fimbrin (TaFIM1).A, TaFIM1 was rapidly induced in incompatible reaction between the wheat–Pst interaction.B,the transcription of TaFIM1 responded to abiotic stress.C,the expression level of TaFIM1 in wheat answered to ethylene (ET),abscisic acid (ABA),methyl jasmonate (MeJA),and salicylic acid (SA) treatments.D,transcript level of TaFIM1 in the root,stem,leaf and seed issues.Error bars represent the standard deviation of expression from three independent biological replicates.Wheat leaves treated with distilled water were included as the control.Asterisks (*)indicate significant difference (P<0.05) between the incompatible interaction and compatible interaction plants at the same time points using Student’s t-test.Different letters in the same column mean significant difference (P<0.05) between time-course points using Student’s t-test.
3.3.Silencing of TaFIM1 results in a decrease in resistance against Pst
Barley stripe mosaic virus-induced gene silencing (BSMVVIGS) was used to determine the role ofTaFIM1in wheat infection byPst.At 14 dpi,symptoms of the barley stripe mosaic virus (BSMV) appeared on the fourth leaves of BSMV:γ and BSMV:γ-TaFIM1,and photo-bleaching was evident on leaves infected with BSMV:γ-PDS,confirming the induction of BSMV-VIGS-mediated silencing (Fig.3-A).
We inoculated thePstraces CYR23 and CYR31 on the fourth leaves of wheat,and detected the silencing efficiency ofTaFIM1at 0,24,48 and 120 hpi.The results show that the expression ofTaFIM1in silent plants was significantly reduced compared with BSMV:γ (Fig.3-D).The silencing efficiency ofTaFIM1was nearly 60% in CYR23-inoculated plants,and was 60–88% in CYR31-inoculated plants indicating thatTaFIM1gene silencing was effective in wheat.
Based on silence effectiveness,a visible necrosis can be observed on the surface of the fourth leaves of BSMV:γ,BSMV:γ-TaFIM1and Mock at 15 dpi (Fig.3-B),indicating that the plants had hypersensitive reaction (HR) after inoculation with urediospores ofPstrace CYR23.The necrotic leaf area of BSMV:γ-TaFIM1was larger than those of the negative and blank control,with a small amount of uredia produced,when the phenotypes of BSMV:γ-TaFIM1was“2+”and the control was“0;”.After inoculating with CYR31,the leaves produced considerable uredia at 15 dpi.Compared with the control,the uredia onTaFIM1-silenced leaves were denser,with all the phenotypes as“4”(Fig.3-C).These results show that inhibiting the expression ofTaFIM1reduced the disease resistance and made plants more susceptible to pathogens.
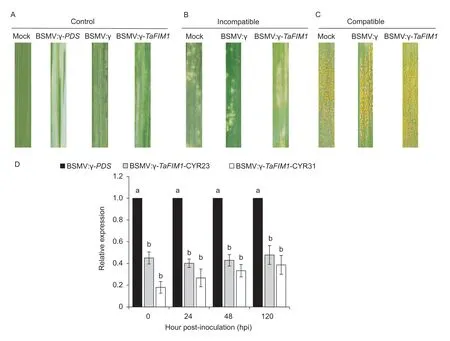
Fig.3 The function of wheat fimbrin (TaFIM1)gene resistant to stripe rust using barley stripe mosaic virus-induced gene silencing(BSMV-VIGS).A,the phenotype in wheat leaves at 14 d post-inoculation (dpi) with BSMV.Photo-bleaching was evident on leaves infected with BSMV:γ-PDS (phytoene desaturase enzyme),while mild chlorotic mosaic symptoms was obvious on leaves after inoculating with BSMV:γ/TaFIM1 at 14 dpi.B and C,photos of fourth leaves inoculated with urediospores of Puccinia striiformis f.sp.tritici (Pst) race CYR23 (B) and CYR31 (C).Typical leaves were photographed at 15 dpi.D,silence efficiency detection of TaFIM1 gene.The expression of TaFIM1 was detected by qRT-PCR,and the data were normalized using the TaEF-1a gene.Error bars represent the standard deviation of expression from three independent biological replicates.Analysis of significance was calculated according to Student’s t-test method (P<0.05).
3.4.Histological observation in silent plants
In order to further clarify the effect ofTaFIM1gene on wheat resistance to stripe rust infection,the number of hyphal branches,hyphal length and colony area of gene silenced plants were observed at 24,48 and 120 hpi with CYR23 (Table 2).Histopathological observation shows that substomatal vesicles (SV),primary mycelia (IH)and haustorial mother cells (HMC) were visible under fluorescence microscopy inTaFIM1-silenced and control plants (Fig.4).
At 24 hpi,there was no significant difference in hyphal branches and hyphal length between the silenced and blank control plants.With the prolongation of time,the infection of stripe rust deepened,and the number of infected hyphal branches,hyphal length and colony area ofTaFIM1increased significantly (Table 2).TaFIM1-silenced plants showed increased susceptibility compared with control plants,further indicating thatTaFIM1participated in plant disease resistance.

Table 2 Histological observations during the incompatible interaction between Su11 and CYR231)
4.Discussion
4.1.TaFIM1 gene is a typical member of the fimbrin in wheat
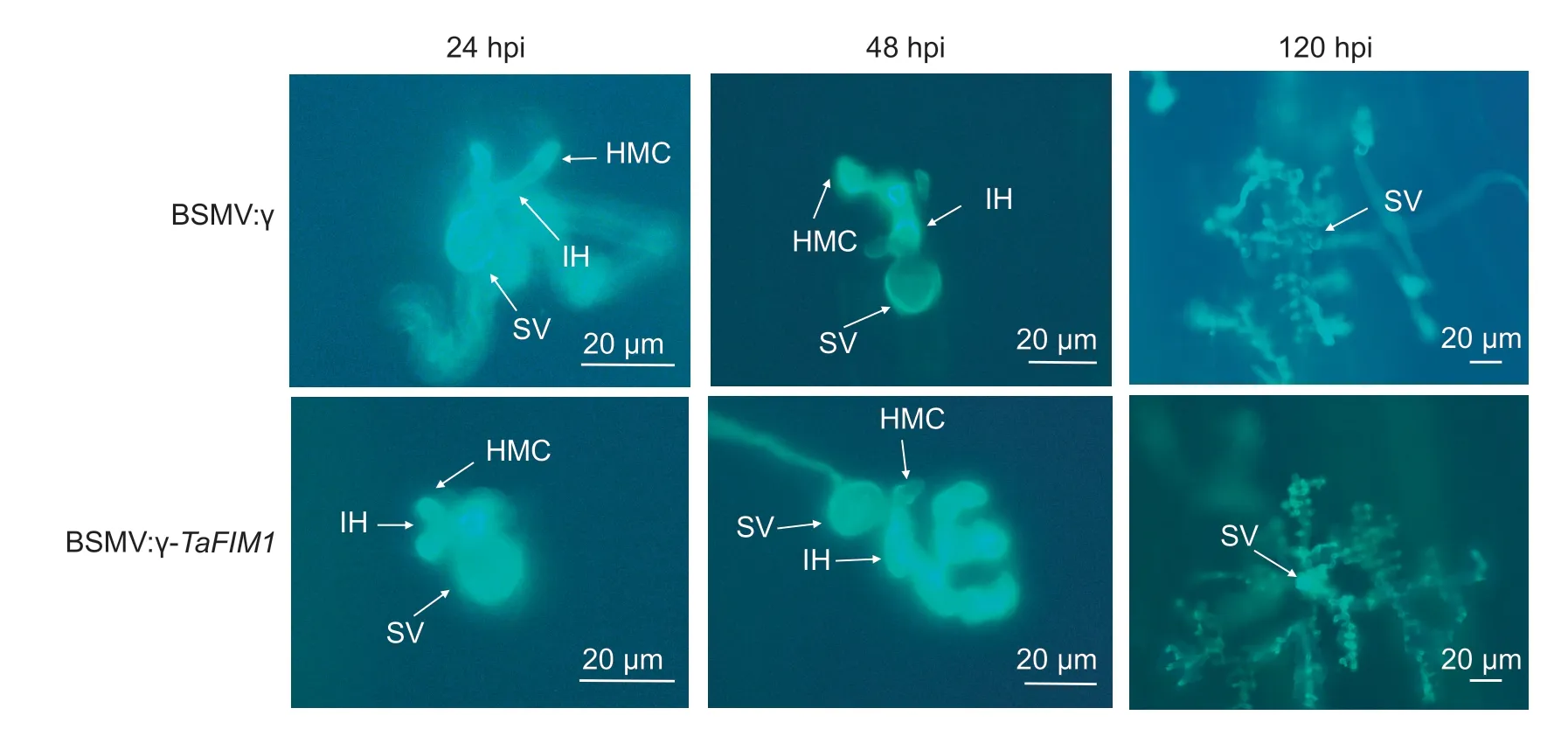
Fig.4 Expansion of Pst race CYR23 in wheat fimbrin (TaFIM1)-silenced plants.The fungal structures were stained with wheat germ agglutinin (WGA).The fungal growth of Pst race CYR23 in wheat leaves inoculated with BSMV:γ and BSMV:γ-TaFIM1 at 24,48,and 120 h post-inoculation (hpi) was observed under a fluorescence microscope.SV,substomatal vesicle;HMC,haustorial mother cell;IH,initial hyphae.
Previous work stated that the fimbrin/plastin is required for actin-crosslinking and the formation of branched actin networks (Kleinet al.2004).In the present study,we demonstrated thatTaFIM1gene was putatively involved in the interaction between wheat andPst.The wheatTaFIM1gene,a typical member of the fimbrin,belongs to the CH-superfamily.TheTaFIM1nucleotide sequence of theTriticum aestivum cv.CS revealed that at least three copies were present,localized on chromosomes 6A,6B,and 6D,respectively.In addition,some stripe rust resistance genes,such asYr20in 6D (Chenet al.1995),Yr23in 6D (Chenet al.1995),Yr35in 6BS (Maraiset al.2003),Yr36in 6BS(Uauyet al.2005),Yr38in 6AS (Maraiset al.2006),Yr42in 6AL (Maraiset al.2009),Yr77in 6DS (Donget al.2017),Yr78in 6BS (Donget al.2017) andYr81in 6AS (Gesseseet al.2019) were also mapped on chromosomes 6A,6B and 6D,and the relationship between these stripe rust resistance genes and fimbrin needs further study.
4.2.TaFIM1 participates in wheat resistance to stripe rust
Plant growth environment is complex and changeable.Under adverse stresses,plants can sense the dangerous signal in time,and regulate the immune system quickly to improve the adaptability (Lazniewskaet al.2012).Actin cytoskeleton is ubiquitous in eukaryotic cells with continuous dynamic changes (Fujiwaraet al.2002).It not only plays a vital role in maintaining cell morphology,but also is closely related to material transport,energy conversion,information exchange and gene expression (Henty-Ridillaet al.2013).Moreover,they are tightly interrelated with plant disease resistance (Vidaliet al.2001;Wanget al.2009).Actinbinding proteins (ABPs) regulate actin activity and control filament assembly,including profilin (Sunet al.2018),cofilin/ADFs (Wanget al.2011),capping proteins (Liet al.2015),Arp2/3 complex (Qiet al.2017;Sunet al.2019),Rho of plants GTPases (Sugiyamaet al.2019),which are involved in plant disease resistance.Fimbrin,a kind of ABPs,regulates the dynamic changes of actin filaments and plays a significance role in plant growth (Zhanget al.2016).However,its role in fungal defense is less defined.Our data revealed that the expression ofTaFIM1was significantly up-regulated in the incompatible interaction,and theTaFIM1-silenced plantsdecreased in resistance againstPst.However,it is still unknown whetherTaFIM1patriciates in either ETI,PTI,or both layers of immunity.Moreover,the specific signaling pathways and regulatory mechanisms of cytoskeletal function remains to be fully clarified.
4.3.Differential induction of TaFIM1 by abiotic stress
Previous work shows that the actin cytoskeleton responds to abiotic perception (Ouelletet al.2001;Huanget al.2012).We found that wheat fimbrin gene showed differential abiotic stresses responsive expression patterns,in which the expression level ofTaFIM1gene was up-regulated at high-temperature,low-temperature,salt and drought stress,suggesting thatTaFIM1may enhance adaptability of wheat to various stresses through complex regulatory mechanisms.Hormones can mediate plant signaling pathways and play a crucial role in the whole growth period of plants (Alazem and Lin 2015).The expression ofTaFIM1was significantly up-regulated under ET,ABA and MeJA treatments,indicating that it may play an important role in different hormone-mediated signaling pathways.
5.Conclusion
TaFIM1was a typical member of the fimbrin proteinby sequencing and phylogenetic analysis.TaFIM1involved in the wheat–Pstinteraction and response to abiotic stresses.In addition,the role ofTaFIM1is verified by barley stripe mosaic virus-induced gene silencing technology (BSMVVIGS).These results may shed new light on understanding the diverse roles ofTaFIM1thatlikely plays a key role in the host response to pathogen infection and abiotic perception.
Acknowledgements
The research was supported by the National Natural Science Foundation of China (31571960),the NSFC-Xinjiang Joint Fund,China (U1903110) and the 111 Project from the Ministry of Education of China (B07049).
Appendixassociated with this paper is available on http://www.ChinaAgriSci.com/V2/En/appendix.htm
杂志排行
Journal of Integrative Agriculture的其它文章
- Receptor-like kinase OsASLRK regulates methylglyoxal response and content in rice
- Heredity and gene mapping of a novel white stripe leaf mutant in wheat
- Construction of a high-density adzuki bean genetic map and evaluation of its utility based on a QTL analysis of seed size
- Effects of temperature and solar radiation on yield of good eatingquality rice in the lower reaches of the Huai River Basin,China
- Difference in corn kernel moisture content between pre-and postharvest
- The effect of elevating temperature on the growth and development of reproductive organs and yield of summer maize
