Pyrolysis behaviors of coal-related model compounds catalyzed by Ni-modified HZSM-5 zeolite
2021-06-02HOUMengyingLIGangJINLijunHUHaoquan
HOU Meng-ying ,LI Gang,JIN Li-jun ,HU Hao-quan
(State Key Laboratory of Fine Chemicals,School of Chemical Engineering, Dalian University of Technology, Dalian 116024,China)
Abstract:The phenyl ethyl ether(PEE)and benzyloxybenzene(BOB)were selected as coal-related model compounds,and their catalytic pyrolysis behaviors in the presence of Ni-modified HZSM-5 zeolite were studied in a fixed-bed reactor. Nickel was introduced on HZSM-5 zeolite by wet impregnation.The catalysts were characterized by XRD,FT-IR,H2-TPR and NH3-TPD,and the effect of hydrogen reduction pretreatment on the catalytic performance was studied. NiO/HZSM-5 can significantly improve the yield of phenols in the liquid phase products of the two model compounds. Compared with Ni/HZSM-5, NiO/HZSM-5 exhibits superior pyrolysis conversion of PEE.
Key words:coal-related model compounds;nickel;HZSM-5;catalytic pyrolysis
Pyrolysis of coal is one of the important ways for its direct conversion and efficient utilization.There are many high value-added chemical products in the pyrolysis process.Phenols are one kind of the main products extracted from coal[1−3].The structure,composition and weak bonding structure of coal are closely related to the direct transformation process.However,the structure of coal is very complex.The organic portion of coal is mainly composed of polycyclic aromatic, hydroaromatic and heterocyclic clusters, which are linked together by short aliphatic and ether bonds to form a cross-linked threedimensional network[4].Many researches have used simple coal-like model compounds to explore the pyrolysis mechanism of coal[5−8].Since the amount of phenolic substances produced during coal pyrolysis is closely related to monohydric phenolic carbon and aromatic ether carbon[9],the selection of ether model compounds for study can reflect the pyrolysis properties of coal to some extent from the molecular level.Compounds containing ether bonds have been widely used in the study of coal structure and reactivity.Kong et al[10]studied the pyrolysis behavior of benzyloxybenzene(BOB) between 500 and 700°C without catalyst,and found that free radical reaction dominated the pyrolysis of the compound.Li et al[7,11]found that the free radical reaction of phenyl ethyl ether(PEE)was very obvious at high temperature,and the non-free radical reaction was dominant at low temperature due to the existence of β-H.
Although there are many researches on the pyrolysis of model compounds,the influence of catalysts on the pyrolysis is less studied.At present the oil yield of coal pyrolysis process still needs to be improved.The application of catalyst can improve the conversion of coal pyrolysis and adjust the composition and distribution of pyrolysis products.Studies of coal pyrolysis catalyst mainly focus on metal oxide catalyst,metal salt catalyst,supported-catalyst etc.[12,13].The supported catalyst can be supported by molecular sieves,and the active components are usually transition metals such as Co,Mo, Ni or their oxides[14].Amin et al[15]investigated the catalytic effect of NiO/ZSM-5 catalysts on low-level coal pyrolysis,and found that the NiO supported by ZSM-5 had the ability to reduce the gas yield and improve the liquid products yield,and the fraction of light pyrolysis oil(light oil, phenol oil and naphthalene oil)and H/C ratio were improved,while the phenolic compounds in pyrolysis oil increased with the use of catalysts.Similarly,Liu et al[16]prepared the NiO/HZSM-5 catalyst by wet impregnation and studied its effect on the yield and tar properties for pyrolysis of Shengli lignite,and found the tar with aromatics content of 94.2%could be produced by NiO/HZSM-5,which could be used as a potential candidate for catalytic upgrading of pyrolysis oil.In a word, NiO/HZSM-5 plays an effective role in coal pyrolysis.
Herein a series of nickel-related catalysts were prepared.PEE and BOB with typical ether bonds were selected as coal-related model compounds.The distribution of catalyzed pyrolysis products for the two ether model compounds was studied using NiO or Nimodified HZSM-5 zeolite.
1 Experimental
1.1 Materials
PEE and BOB as coal-related model compounds were purchased from ACROS and TCI, respectively.ZSM-5 zeolite powder(20−50 nm,SiO2/Al2O3=26)was purchased from Dalian Ligong Qiwangda Chemical Technology. Ammonium chloride(AR),nickel nitrate(AR)and absolute ethyl alcohol(AR)were purchased from Tianjin Bodi,Tianjin Damao and Sinopharm Chemical Reagent Co.,Ltd.,respectively.
1.2 Catalysts preparation
The NiO/HZSM-5 catalysts were prepared by wet impregnation.Firstly,the Na-ZSM-5 zeolite was calcined at heating rate of 1°C/min to 550°C and then held for 4 h in air.Then it was placed in 1%ammonium chloride solution for ions exchange,which was repeated twice.After the above treatment,the sample was put in a muffle furnace and calcined in air for 4 h,thus HZSM-5 was obtained.A certain amount of Ni(NO3)2·6H2O was dissolved in water which was 6 times that of saturated inhalation of HZSM-5.The catalysts were impregnated for 2 h at 80°C,dried for 12 h at 100°C and then calcined at 550°C.The NiO/HZSM-5 samples with metal mass of 1.26%,3.14%and 4.40%were obtained and coded as NiO/HZ-1,NiO/HZ-2 and NiO/HZ-3, respectively. For comparison,NiO was also prepared and used in catalytic pyrolysis experiment.
NiO/HZ-1, NiO/HZ-2 and NiO/HZ-3 wereinsitureduced and referred as Ni/HZ-1, Ni/HZ-2 and Ni/HZ-3,respectively.The furnace was purged at room temperature with high purity N2,then switched to H2(~60 mL/min),heated to the desired temperature by 2°C/min,reduced for 3 h,finally cooled to room temperature in the N2to obtain Ni/HZSM-5 containing nickel species.
1.3 Catalysts characterization
Powder X-ray diffraction(XRD) patterns were recorded on a Smart Lab 9 diffractometer equipped with CuKαradiation(40 kV and 40 mA).The Fourier transform infrared spectroscopy(FT-IR)spectra were recorded on a Bruker EQUINOX55 spectrometer,scanned from 4000 to 400 cm−1.The temperatureprogrammed reduction(TPR)profiles by H2for the catalysts were completed on a self-built device.The sample was loaded into the U quartz reaction tube,placed in the reaction furnace and pretreated for 2 h at 300 °C in 40 mL/min nitrogen atmosphere.The reaction furnace was heated at 10°C/min to desired temperature under H2/N2(volume ratio 1∶9) mixture. A TCD detector was used to detect the change of gas signal. Temperatureprogrammed desorption(TPD) profiles of NH3for catalysts were carried out on the same instrument.
1.4 Pyrolysis
The pyrolysis experiments of model compounds were carried out in a fixed-bed reactor(shown in Figure 1).A certain amount of compounds(0.3 mL or 0.3 g)and catalysts(0.05 g)were placed in the quartz boat,which was placed in a fixed position inside the stainless steel reaction tube.After purging the tube with high purity N2(50 mL/min)and keeping the temperature of the furnace constant,the stainless steel reaction tube was placed in the constant temperature area of the furnace for pyrolysis.The pyrolysis was carried out for 10 min.The gas products were collected by air bags and analyzed by GC(TECHCOMP GC 7890II),and the liquid products in the cold trap were analyzed by GC-MS(HP6890/MS5973)and GC(Agilent 6890N).The amount of char was obtained by weighing the mass of the reaction tube before and after the reaction.The calculation method of conversion and product yield is as same as our previous work[17].
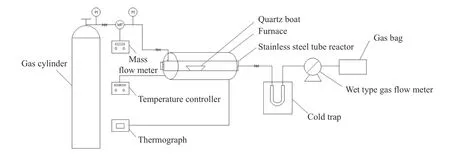
Figure 1 Flow sheet of experimental apparatus
2 Results and discussion
2.1 Characterization of catalysts
XRD patterns of NiO,HZSM-5 and NiO/HZSM-5 with different nickel loading are presented in Figure 2.NiO displays the characteristic diffraction peak at37.2°,43.2°,62.7°,76.5°and 79.9°,corresponding to(111),(200),(220),(311)and(222)crystal planes,respectively[18].The XRD pattern of the modified HZSM-5 is basically consistent with that of the parent HZSM-5,indicating that the modification has no obvious effect on the structure of the parent zeolite[19].The crystallinity of HZSM-5 decreases slightly with the increase of nickel load. No NiO peaks are found in the diffraction pattern indicating that it is highly dispersed on the HZSM-5 catalyst.

Figure 2 XRD patterns of different catalysts
Figure 3 shows the infrared spectra of HZSM-5 and NiO/HZSM-5. Absorption peaks at 800 and 1100 cm−1respectively belong to the symmetric and anti-symmetric stretching vibration of T−O−T bond of ZSM-5,which are sensitive to the changes of zeolite framework[20].It can be seen that there is not new absorption peak and significant band position shifts after the modification of HZSM-5,indicating the absence of isomorphous substitution[21]and little influence on the zeolite framework of HZSM-5 with NiO introduced by the impregnation method.

Figure 3 FT-IR spectra for catalysts
The H2-TPR profiles of NiO/HZSM-5 are shown in Figure 4.It can be considered that most of the nickel oxide can be reduced to Ni0after 500°C.With the increase of nickel load on HZSM-5, the low temperature reduction characteristics of surface NiO become more obvious.
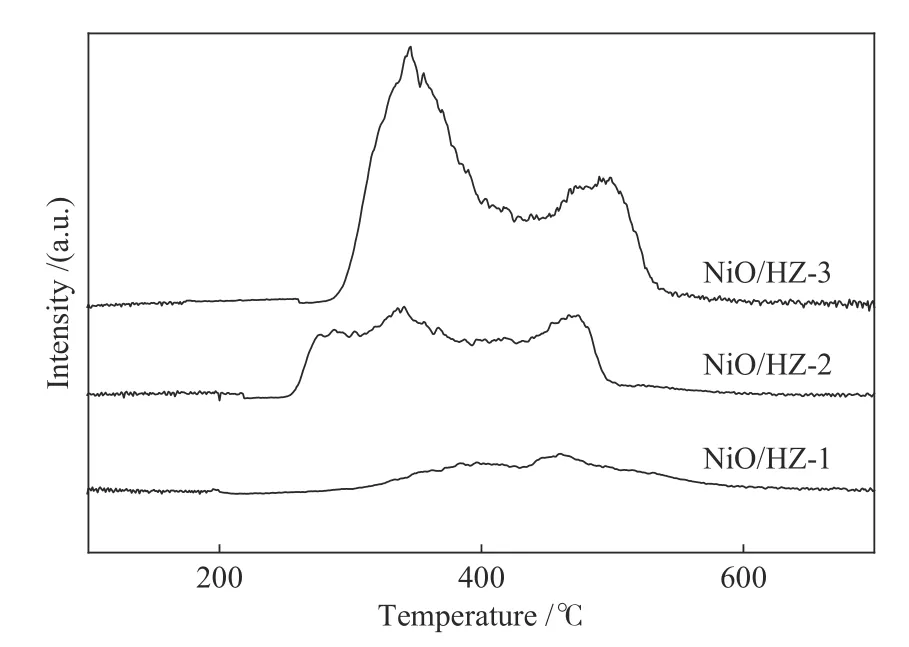
Figure 4 H2-TPR profiles for catalysts
Figure 5 illustrates NH3-TPD profiles obtained from the catalysts. New strong acid sites are found in the NiO/HZSM-5,which are attributed to the formation of Lewis acid sites induced by NiO,as previously discovered[21−23].These reports suggest that the emergence of new acid sites is due to aluminum with low coordination or dehydroxylation processes in the thermal treatment above 500°C[21−23].It is notable that the strong acid amounts(centered at 400−600°C)of NiO/HZ-1 and NiO/HZ-2 are much lower than that of NiO/HZ-3.
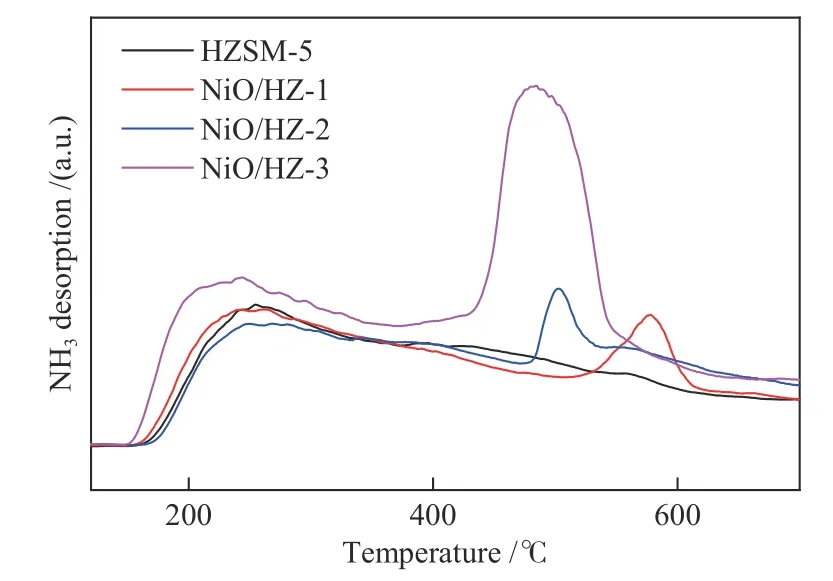
Figure 5 NH3-TPD profiles for catalysts
2.2 Catalytic pyrolysis of PEE
PEE was pyrolyzed in a fixed bed reactor at 650°C in the presence of different catalysts and the products were analyzed by GC.The pyrolysis conversion,liquid,gas and char yield are shown in Figure 6.Figures 7 and 8 show the distribution of liquid and gas products of PEE pyrolysis.In the absence of catalyst,the pyrolysis conversion of PEE is significantly lower,only 32.02%.The Caliphatic−O bond(step 3)has been shown to be the weakest bond in PEE(Figure 9),so phenol is the dominant liquid phase product[7,24].Compared with the pyrolysis results without catalysts,although the pyrolysis conversion of PEE is improved when NiO catalyst is added,the yield of liquid decreases,while the yields of gas phase products and char increase.In terms of product distribution, there is not new product under the action of NiO,and the yield of benzofuran increases,while the amount of methane and ethane in the gas phase increases significantly,and the amount of ethylene decreases. NiO has the function of dehydrogenation and dealkylation.It can generate active intermediate[NiO−H] with PEE to generate a large amount of H·and improve the pyrolysis conversion(Figure 10).H·can combine with alkyl radicals to release methane and ethane,and the cross-linking of the remaining macromolecular radicals result in the increase of char.At the same time intermediate radical is cyclized to benzofuran.It is considered that the non-free radical reaction(step 4)is not easy to occur in the presence of NiO.

Figure 6 Conversion, yield of gas,liquid and char from PEE pyrolysis

Figure 7 Distribution of liquid products from PEE pyrolysis
When HZSM-5 is used as catalyst,it not only improves the conversion of PEE pyrolysis, but also ensures the yield of liquid at a certain level,while the yields of gas phase products and char only increase slightly.Under this condition, the yield of phenol increases greatly.Methyl and ethyl radicals have the tendency to bond with phenyl radicals, thus toluene and ethylbenzene appear in the products.
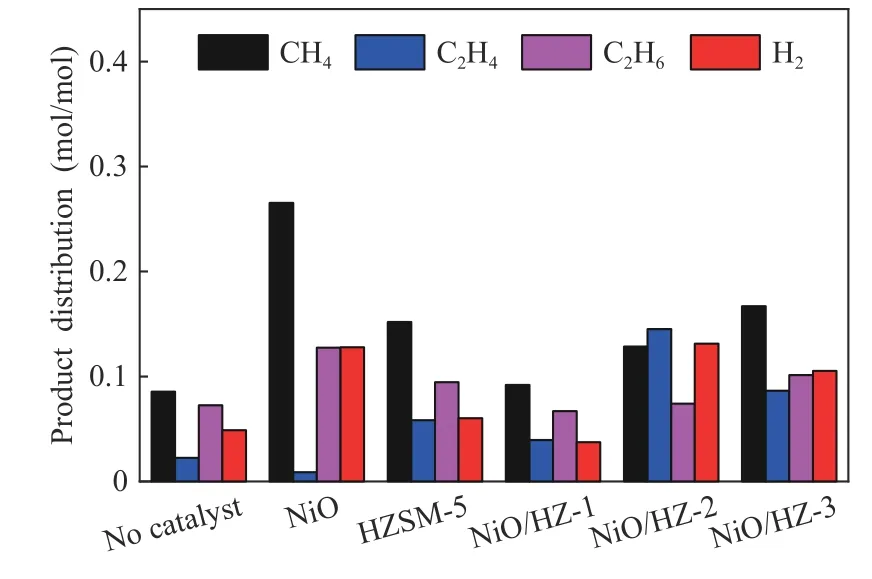
Figure 8 Distribution of gas products from PEE pyrolysis
Under the action of NiO/HZSM-5 catalyst,pyrolysis conversion increases. NiO/HZ-2 containing 3.14%Ni has a significant catalytic effect with a pyrolytic conversion of 62.49%.With the further increase of metal content in the catalyst,the conversion of PEE decreases.With the addition of NiO/HZ-2, the yield of phenol increases obviously and the amount of ethylene and hydrogen also increases significantly.By comparison,the product types of PEE under the action of NiO/HZSM-5 are consistent with those of HZSM-5.Because both ethylene and phenol increase under the action of NiO/HZ-1 and NiO/HZ-2,it is believed that NiO/HZSM-5 mainly promotes the non-free radical reaction when the Ni loading is low.In conclusion,NiO/HZSM-5 greatly improves the pyrolysis conversion of PEE,and increases the yield of phenol,the main liquid phase product.
The pre-reduction of nickel in the catalyst was also studied.Referring to the characterization results of H2-TPR, NiO/HZSM-5 with different metal loads werein-situreduced using H2at 500°C.It was believed that most of the nickel oxide was reduced to Ni0. The catalysts were applied to the pyrolysis of PEE at 650°C and were compared with the non-reduced catalyst.It is found that PEE exhibits higher pyrolysis conversion under the action of several Ni/HZSM-5,and the yields of gas phase products and char increase(Figure 11).With the increase of nickel loading,methane becomes the main pyrolysis product in gas phase(Figure 12).The dealkylation of nickel is very obvious and causes macromolecules to condense and form a large amount of char.In general,reduction has an adverse effect on the catalytic effect.The NiO/HZSM-5 catalysts do not need to be reduced in practical use.
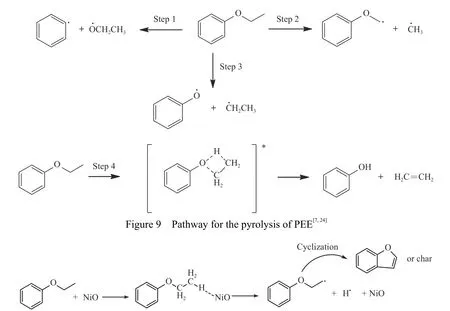
Figure 10 Pathway for the pyrolysis of PEE on NiO catalyst
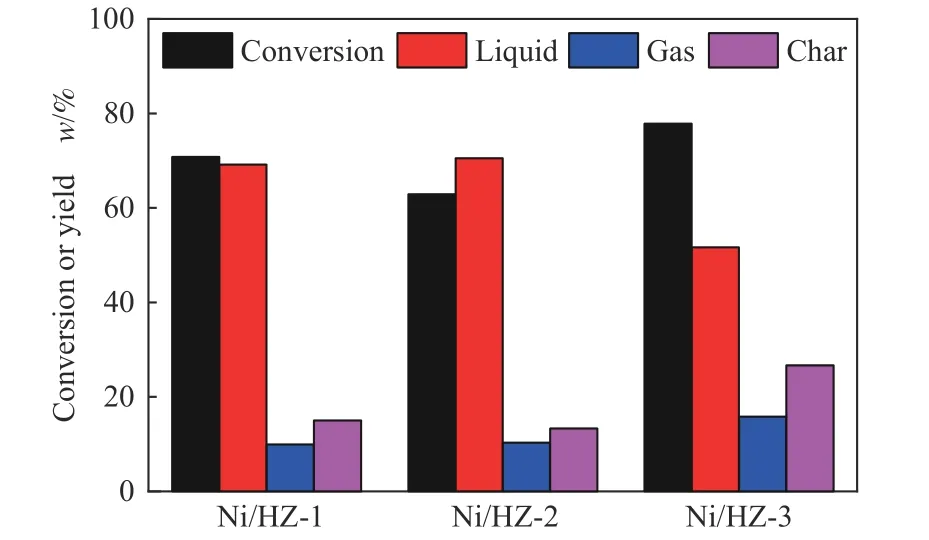
Figure 11 Conversion,yield of gas,liquid and char from PEE pyrolysis under the catalytic action of Ni/HZSM-5

Figure 12 Distribution of gas products from PEE pyrolysis under the catalytic action of Ni/HZSM-5
2.3 Catalytic pyrolysis of BOB
NiO,HZSM-5 and NiO/HZ-1 were also added as catalysts during the pyrolysis of BOB at 650°C.Compared with the pyrolysis results without catalyst,the pyrolysis conversion,liquid,gas and char yield are shown in Figure 13.Only methane and hydrogen are detected in the gas phase products.When NiO catalyst is added, both the pyrolysis conversion of BOB and the yield of char decrease. NiO tends to rob theα−H of aliphatic carbon to produce stable conjugate intermediates(Figure 14).This hinders the pyrolysis of BOB to some extent, reducing the pyrolytic conversion and the yield of char.Under the catalytic action of HZSM-5, the conversion is greatly improved, but a large part of BOB is converted to char instead of liquid phase products.Compared with HZSM-5,when the NiO/HZSM-5 is applied to the pyrolysis reaction,the conversion is further improved.Under the action of supported NiO,the yield of liquid increases and the yield of char is effectively reduced.Yung et al[23]found that nickel helps prevent coke formation and keeps the catalyst active,which is consistent with our results.Higher conversion and product oil/gas ratio can be obtained in BOB pyrolysis catalyzed by NiO/HZSM-5.

Figure 13 Conversion,yield of gas,liquid and char from BOB pyrolysis
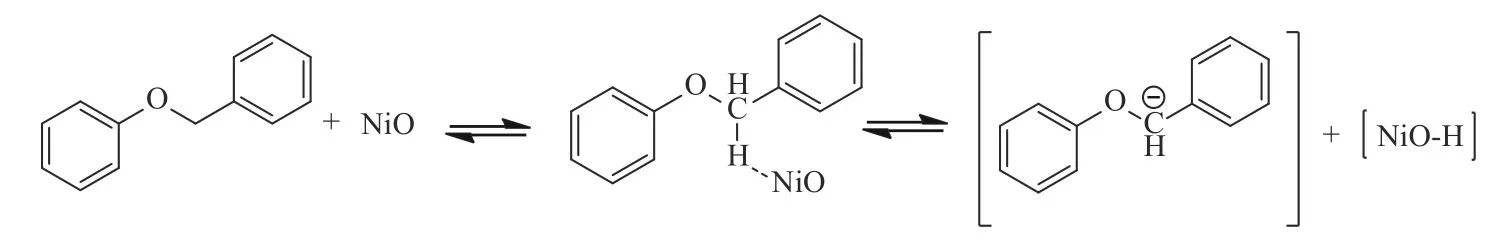
Figure 14 Pathway for the pyrolysis of BOB on NiO catalyst
Figure 15 shows the distribution of products in liquid phase during the pyrolysis of BOB. NiO does not change the type of product.However,in the presence of HZSM-5, the original toluene, bibenzyl, phenanthrene and stilbene disappear.A new product, 4-benzyl phenol,is formed. At the same time,the yield of 2-benzyl phenol increases significantly. As bibenzyl is generated from benzyl radicals and is a prerequisite for the formation of stilbene and phenanthrene,HZSM-5 seems to promote the binding of benzyl with phenoxy,rather than benzyl coupling.Under the catalysis of NiO/HZSM-5,the amount of phenol and 2-benzyl phenol is maximum in liquid phase products and the selectivity of phenolic products is greatly improved.It is proposed the pyrolysis free radical reaction path of BOB(Figure 16),and the initial steps include three kinds of bond dissociation Caliphatic−O(step 5),Caryl−O(step 6), and (−O−) Caliphatic−Caryl(step 7)[10]. NiO/HZSM-5 especially promotes the cleavage of Caliphatic−O bond in BOB and generates a large amount of phenoxy radical.These phenoxy radicals can be hydrogenated to produce large quantities of phenol,or combine with benzyl radical to produce 2-benzyl phenol by coupling reaction[10].
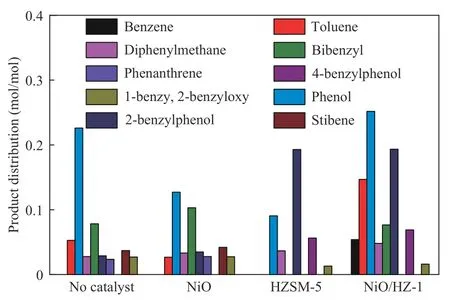
Figure 15 Distribution of liquid products from BOB pyrolysis

Figure 16 Pathway for the pyrolysis of BOB on NiO/HZSM-5 catalyst
3 Conclusions
The catalytic pyrolysis of coal-related model compounds(PEE and BOB) by Ni-modified HZSM-5 zeolite was studied in a fixed-bed reactor,and the catalytic effect was compared with that of NiO.The results of pyrolysis show that nickel oxide has a dehydrogenating effect on PEE. Under the appropriate nickel loading, NiO/HZSM-5 can effectively promote the non-free radical reaction of PEE and the cleavage of BOB"s Caliphatic−O bond,thus more phenolic compounds can be produced.In addition, NiO species on HZSM-5 is more beneficial to the pyrolysis of PEE than Ni species.
