Synthesis of ZSM-22/ZSM-23 intergrowth zeolite as the catalyst support for hydroisomerization of n-hexadecane
2021-06-02SONGChengyeMENGJipengLIChuangZEYAODONGPahaerLIANGChanghai
SONG Cheng-ye,MENG Ji-peng,LI Chuang,*,ZEYAODONG Pahaer ,LIANG Chang-hai,*
(1.State Key Laboratory of Fine Chemicals& Laboratory of Advanced Materials and Catalytic Engineering,School of Chemical Engineering, Dalian University of Technology, Dalian 116024,China;2. Dushanzi Petrochemical Company, PetroChina Company Limited, Karamay 833699,China)
Abstract:ZSM-22/ZSM-23 intergrowth zeolite was successfully synthesized by hydrothermal method with diethylamine and dimethylamine as co-structure directing agents at a dimethylamine/diethylamine molar ratio of 24.The physicochemical properties of ZSM-22/ZSM-23 intergrowth zeolite including the crystallinity,crystal morphology, texture and acidity were determined by XRD,FE-SEM,TEM, N2-physisorption, NH3-TPD,Py-FTIR,and so on;the performance of Pt/ZSM-22/ZSM-23 catalyst prepared by impregnation in the hydroisomerization of n-hexadecane was then investigated.The results indicate that the ZSM-22/ZSM-23 intergrowth zeolite displays the needle-like morphology with the topological structures of both ZSM-22 and ZSM-23,which is rather different from pure ZSM-22 and ZSM-23 and their mechanical mixture.After loading 0.5%Pt,the bi-functional Pt/ZSM-22/ZSM-23 catalyst exhibits excellent performance in the hydroisomerization of n-hexadecane,with a much higher yield of i-C16 products(dominated by mono-branched isomers)than those obtained over Pt supported on ZSM-22 and ZSM-22 and their mechanical mixture.
Key words:ZSM-22/ZSM-23;intergrowth zeolite;n-hexadecane;hydroisomerization CLC number:O643.32 Document code: A
Nowadays,hydroisomerization has become one of the most important catalytic conversion processes in the petrochemical industry due to the increasingly stringent environmental regulations.By convertingn-alkanes toiso-paraffins, hydroisomerization can increase the octane number of gasoline,lower the pour point of diesel and improve the viscosity-temperature properties of lubricant base oils[1].Bifunctional catalysts with acid sites for isomerization and metal centers for hydrogenation/dehydrogenation are widely used for the hydroisomerization ofn-alkanes[2].In this regard,zeolites have been extensively used as the supports for the bifunctional catalysts[3].Recently, plentiful studies have shown that various zeolites such as ZSM-48[4],ZSM-22[5], SAPO-11[6]and ZSM-23[7]exhibited excellent catalytic performance in the hydroisomerization ofn-alkane,as their one-dimensional 10-membered ring elliptical straight pore channels are comparable in size to the kinetic diameter ofn-alkane[8].In general,the zeolites used as the catalyst supports have a single structure[9].However,given the better adaptability of complex fractions,composite[10],mixed and intergrowth zeolites in particular are nowadays considered as favored catalyst supports for various applications[11].
ZSM-22 zeolite has TON topology, with a pore size of 0.46 nm×0.57 nm[12], whilst ZSM-23 of MTT topology has a pore size of 0.45 nm×0.52 nm[13].Since both ZSM-22 and ZSM-23 zeolites have identical basic structural units,the intergrowth zeolites could be synthesized under specific conditions.Cocrystallization could change the cell parameters and the pore channel structure of the original single zeolites,thus affecting the catalytic performance.ZSM-22/ZSM-23 intergrowth zeolite was first reported in 1999 with different pore structures and acidities[14].Lateef et al[15]successfully synthesized the ZSM-22/ZSM-23 intergrowth zeolite and named it as SSZ-54, with the composition of 30%ZSM-22/70%ZSM-23.Wang et al[16]proposed a method to synthesize ZSM-22/ZSM-23 with a fixed proportion of 40%ZSM-22/60%ZSM-23.Burton et al[17]reported that single template agents and dual template agents can be both successfully used in the synthesis of ZSM-22/ZSM-23.However,most of the above researches have been confined to the synthesis of the intergrowth zeolite materials[18],whereas their catalytic performance in the hydroisomerization of long chainn-alkanes remains unclear.
In this work, two small amines,dimethylamine(DMA)and diethylamine(DEA),were adopted as structure-directing agents(SDA)for the synthesis of ZSM-22/ZSM-23 via dynamic hydrothermal method.The synthesis conditions for ZSM-22/ZSM-23 were optimized. Various characterization techniques such as XRD,SEM,TEM, N2-physisorption,Py-FTIR and NH3-TPD were employed to determine the crystallinity,morphology and textural and acidic properties.The hydroisomerization ofn-hexadecane were then comparatively carried out over various Pt catalysts supported on ZSM-22,ZSM-23,ZSM-22/ZSM-23 intergrowth and their mechanical mixture,to reveal the catalytic advantage of ZSM-22/ZSM-23 intergrowth.
1 Experimental
1.1 Chemical and materials
Silica sol(40%,LUDOX HS-40)and fumed silica(SiO2,%,Wacker)were used as the silicon sources and aluminum sulfate(Al2(SO4)3·18H2O,AR,Aladdin)and sodium aluminate(NaAlO2,AR,Macklin)as the aluminum sources.The rest chemicals used in this work include sodium hydroxide(NaOH, AR,Tianjin Kermel), potassium hydroxide(KOH,AR,Tianjin Kermel),dimethylamine(DMA,C2H7N,33%,Sinopharm),diethylamine(DEA,C4H12N,AR,Sinopharm),pyrrolidine(PY,(CH2)4NH,AR,Sinopharm),isopropylamine(IPA,C3H9N,CP,Sinopharm),1,6-diaminohexane(DAH,C6H15N2,AR,Sinopharm), andn-hexadecane (C16H34, GC, Sinopharm).
1.2 Zeolite synthesis
1.2.1ZSM-22/ZSM-23intergrowth zeolite
The initial gel molar composition was 1 SiO2:(0.005−0.0125) Al2O3:aDMA :bDEA:(30−70) H2O:(0.05−0.4)OH−,wherea/b=24,a+b=0.4−1.Typically,9.26 g silica sol,0.25 g NaOH and 21.40 g deionized water were mixed to form gel A.Solution B formed by dissolving 0.41 g Al2(SO4)3·18H2O with deionized water was dropwise added to gel A.After half an hour of intense stirring,0.14 g DEA and 5.00 g DMA were added in sequence. When all ingredients in the three-necked flask were evenly dispersed, the mixture was transferred to an autoclave for dynamic crystallization(40 r/min)at 180°C for 66 h. After that,the product was dried,filtered and washed to obtain the Na-ZSM-22/ZSM-23 sample. After calcining at 550°C for 4 h to dislodge the structure directing agents, the zeolite sample was placed in a 0.5 mol/L NH4NO3aqueous solution (30 mL per 1 g zeolite) at 80 °C for 2 h for ammonium ion exchange and the ion-exchange process repeated for three times.Lastly, the zeolite sample was calcined at 550°C for 3 h, to obtain the H-form H-ZSM-22/ZSM-23 intergrowth zeolite.
1.2.2ZSM-22zeolite
11.57 g silica gel was added to the solution of 2.42 g DAH,0.324 g KOH and 0.51 g Al2(SO4)3·18H2O,stirring to get the precursor synthesis gel with a molar composition of 1 SiO2∶0.01 Al2O3∶0.27 DAH∶36 H2O∶0.075 KOH.The products were transferred into a stainless steel reactor and the hydrothermally treated at 160°C for 48 h under rotational conditions.Following the same calcination and ion-exchange processes,HZSM-22 zeolite was then obtained.
1.2.3ZSM-23zeolite
The synthesis gel with the composition of 1 SiO2∶0.01 Al2O3∶0.25 PY∶0.75 IPA∶38 H2O∶0.08 NaOH was created in the alkaline environment with 0.14 g NaOH,4.37 g fumed silica,50 g deionized water,1.21 g PY,3.23 g IPA and 0.17 g NaAlO2. After crystallization for 96 h at 180°C and necessary calcination and ion-exchange processes,H-ZSM-23 zeolite was then produced.
1.2.4Mechanicalmixtureof ZSM-22andZSM-23 zeolites
ZSM-22 and ZSM-23 with a mass ratio of ZSM-22/ZSM-23 being 40/60 were manually mixed to produce the mechanical mixture,donated as MZSM-22/ZSM-23.
1.3 Preparation of the supported Pt catalysts
The bifunctional supported Pt catalysts with a platinum loading of 0.5%were prepared via the impregnation method, with abovementioned zeolites in proton form as the supports.The support was impregnated with H2PtCl6solution(15 mL per 1 g zeolite)and then stirred for 7 h.After rotational drying,the products were heated at 400°C with a heating rate of 4°C/min in a mixed flow of Ar(30 mL/min)and O2(30 mL/min)for 3 h.
1.4 Catalyst characterization
Powder X-ray diffraction was carried out in the 2θrange of 5°−70°on a Rigaku Smartlab-9 kW(CuKα,40 kV,100 mA),to determine the purity and the relative crystallinity(RC).After spraying of gold,the morphology of the as-synthesized zeolites and catalysts was observed with a QUANTA 450 field emission scanning electron microscope(FE-SEM) produced by FEI Company of America.Transmission electron microscope(TEM)was conducted on Tecnai G2F30 STWIN.The actual molar ratio of SiO2/Al2O3(SAR)and the platinum loading were analyzed by using a Perkin-Elmer Optima 2000DV plasma atomic emission spectrometer (ICP-AES).
NH3-TPD and Py-FTIR profiles were measured on CHEMBET-3000 and EQUINOX 55,respectively,to determine the acidity of different zeolites.For NH3-TPD, the zeolite sample,after pretreated in a He flow at 350°C for 2 h,were exposed to 10%NH3/He(40 mL/min)flow at 120°C for 30 min;after that,the sample was purged in a He atmosphere for 1 h and then heated with the heating rate of 10°C/min from 120 to 700 °C in a He flow. For Py-FTIR, the background spectra were recorded at room temperature after purging at 400°C for 40 min;allowing absorbing pyridine for 10 min,the samples were heated to 150,300 and 450°C,to collect the Py-FTIR spectra.
The specific surface area, pore size distribution and pore volume of the prepared catalysts and zeolites were obtained on the Quantachrome Autosorb-iQ physisorption apparatus.All samples were calcined at 300°C for 12 h before the test.To calculate the dispersion and size of platinum particles on the bifunctional catalyst,CO-chemisorption was carried out on Micromeritics Autochem II 2920.Solid-state29Si and27Al MAS NMR investigations were carried out on Agilent DD2-500 MHz spectrometer to examine the surroundings of zeolite framework.
1.5 Catalytic tests
The catalytic tests ofn-hexadecane hydroisomerization were conducted in a fixed-bed reactor.0.15 g bifunctional platinum catalyst was diluted with 2 mL of 60−80 mesh quartz sand and placed in the center of reaction tube.3 mL of 40−60 mesh quartz sand was filled at each end of the tube to get a uniform flow without increasing the bed-side pressure drop.The catalyst was first reduced at 400°C in a hydrogen atmosphere for 3 h.After that,the reaction unit was adjusted to the desired conditions and the feedstock was pumped into the reactor; the catalytic performance was evaluated by varying the reaction temperature at a specific contact time.The reaction products condensed by the cold trap were collected for offline analysis,with an Agilent 7890A gas chromatograph (USA, HP-5 column, FID). The products were quantified by using an Agilent 7890B-5977A gas chromatography-mass spectrometer on an HP-5 column.
2 Results and discussion
2.1 Synthesis of ZSM-22/ZSM-23 zeolite with the dual-SDA of DMA and DEA
The effects of various factors on the synthesis of ZSM-22/ZSM-23 intergrowth zeolite were investigated,such as the gel compositions(DMA/DEA,OH−/SiO2,H2O/SiO2),alkali sources,crystallization time and temperature.In general,an increase in the temperature could accelerate the crystallization[19].In the actual industrial production process,relatively higher crystallization temperatures were often used to shorten the crystallization time and to increase productivity.Therefore,a temperature of 180°C as reported in literature[20]was chosen to investigate the effect of crystallization time.Similarly,to provide more suitable acidic sites for the bifunctional catalysts[21],a SiO2/Al2O3ratio of 100 was selected[22],which also facilitated the synthesis of ZSM-22,ZSM-23 and their mechanical mixture.
As presented in Table 1 and Figure 1,the XRD results indicate that the proportion of SDAs was a crucial factor in the synthesis of ZSM-22/ZSM-23 intergrowth zeolite.The ZSM-22/ZSM-23 intergrowth zeolite can only be synthesized directionally when the molar ratio of DMA/DEA is 24 and total SDAs/SiO2is 0.75.As ZSM-22 is a metastable substance,only a suitable SDA amount cannot guarantee the synthesis of intergrowth zeolite with a high crystallinity.As shown in Figure 1(b),an excessively long crystallization time leads to sufficient growth of cristobalite(21.7°)and zeolite with a wide synthetic interval such as ZSM-5[23].However,a short reaction time cannot allow a complete crystallization to form the ZSM-22/ZSM-23 intergrowth zeolite and highest relative crystallinity can be archived in the synthesis interval of 60−66 h.As a result,a crystallization time of 66 h was used subsequently for the synthesis of high purity ZSM-22/ZSM-23 intergrowth zeolite
The type and amount of alkali source also have a significant influence on the synthesis of ZSM-22/ZSM-23 intergrowth zeolite. Moderate alkalinity can promote the formation of Si−O−Al fragments and accelerate both the nucleation and crystallization processes. At the same time, the metal cations act as the charge balance components for the zeolite framework, whereas the OH−anions play the role of mineralizer. As given in Table 1 and Figure 1(c), the relative crystallinity reaches a peak at an OH−/SiO2ratio of 0.1. A lower alkalinity may not sufficiently dissolve the silicon and aluminum sources, leading to the formation of amorphous and low-crystallinity samples, whereas excessive alkalinity may inhibit the growth of zeolite crystals, resulting in a lower yield of the desired product or a large amount of quartz phase. NaOH as the alkali source can get high crystallinity products,although KOH has a slightly wide synthesis interval.The amount of water is also an important factor for the crystallization, influencing the crystallinity and the yield of the as-synthesized zeolites. Less water leads to a decline in the purity of synthetic samples and the agglomeration of crystals, whereas too much water may reduce the concentration of the reactants, affecting the reaction rate and the yield of the single-vessel.Figure 1(d) and Table 1 demonstrate that an H2O/SiO2ratio of 45 is suitable. As a result, the ZSM-22/ZSM-23 intergrowth zeolite of high crystallinity was synthesized with an initial gel composition of 1 SiO2∶0.01 Al2O3∶0.72 DMA : 0.03 DEA∶45 H2O∶0.1 NaOH at 180 °C for 66 h.
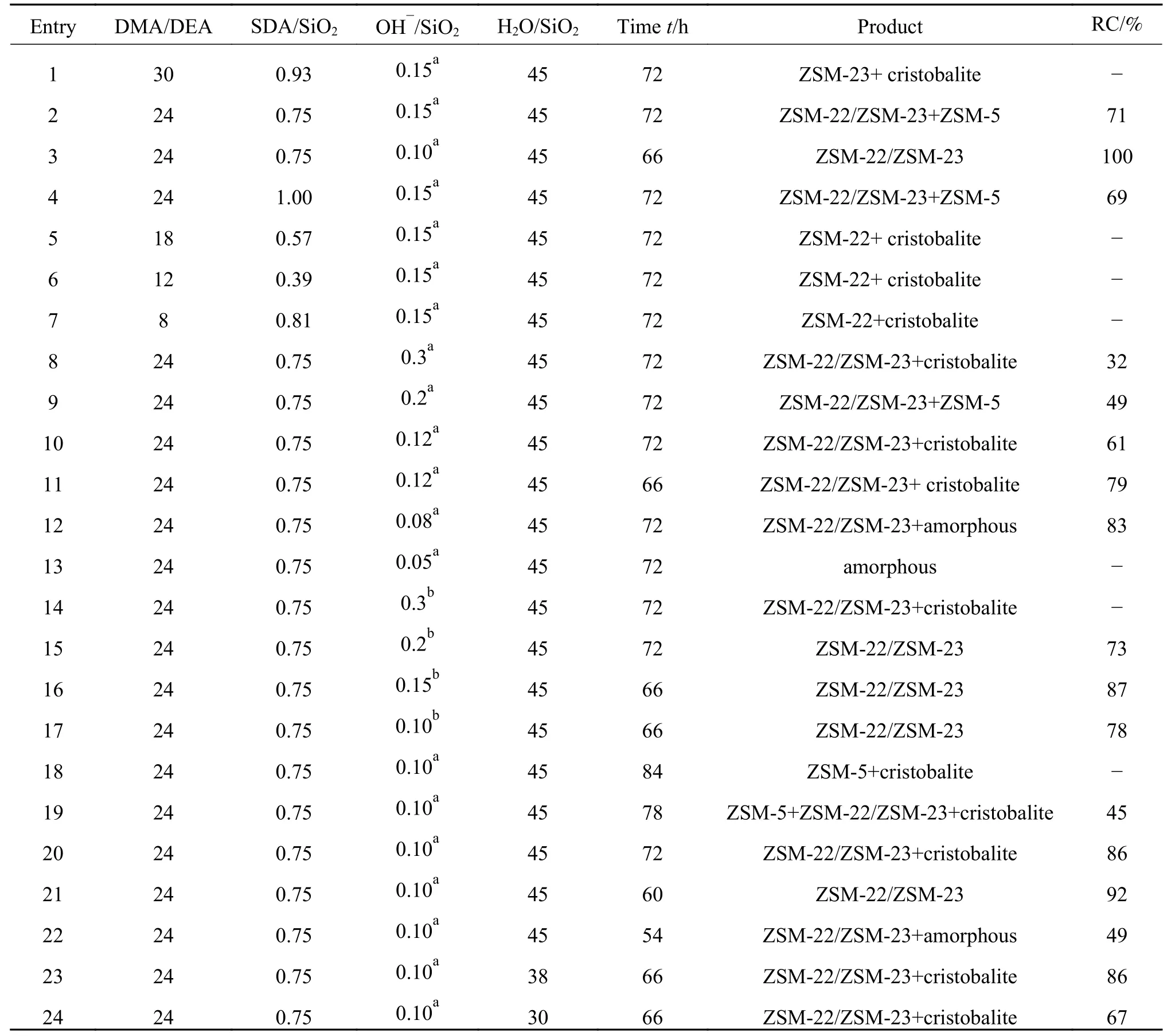
Table 1 Effect of gel composition and synthetic conditions on the crystallization products
ZSM-22 zeolite belongs to the TON topology[24],whereas ZSM-23 to the MTT topology[25], both take the one-dimensional pore structure with the ten-membered ring. As shown in Figure 2,the XRD pattern of Pt/ZSM-22/ZSM-23 intergrowth zeolite is notably different from those of Pt/ZSM-22,Pt/ZSM-23 and Pt/MZSM-22/ZSM-23, where the characteristic diffraction peaks of ZSM-22 and ZSM-23 can be found at 20.32°,22.96°,24.1°,24.6°,and 25.76°.Even so,the ZSM-22/ZSM-23 intergrowth is not a simple addition of ZSM-22 and ZSM-23,nor is a hybrid zeolite that made up of arbitrary proportions.It has a new topology structure, with the basic structural unit of ZSM-22 and ZSM-23 in accordance with the specific crystallization way of intergrowth.By comparison with the Diffax simulations of random intergrowths of TON and MTT,it is ascertained that the as-synthesized intergrowth zeolite has a specific ratio of 40%TON+60%MTT[17].There is no typical peaks for metallic platinum at 39°,46°and 67°,indicating that the platinum particles are small and homogeneously dispersed on the zeolite supports[26].
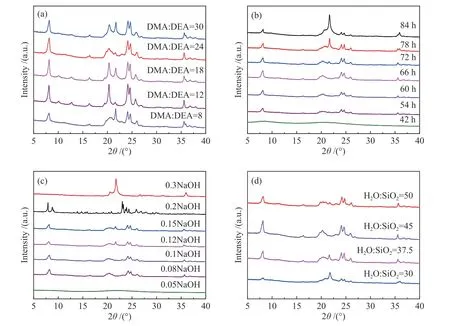
Figure 1 XRD patterns of the as-synthesized zeolite samples for the gel of 1 SiO2 :0.01 Al2O3:w DMA : x DEA : y H2O:m NaOH at 180°C for z h:(a) varying DMA/DEA ratio;(b) varying crystallization time;(c)varying NaOH amount;(d) varying water quantity
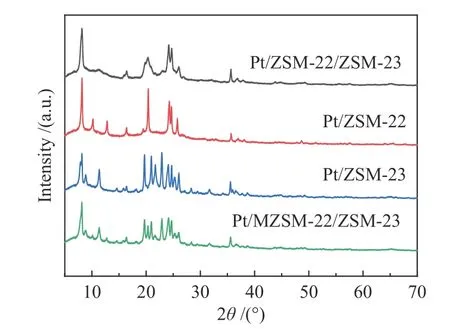
Figure 2 XRD patterns of Pt/ZSM-22/ZSM-23,Pt/ZSM-22,Pt/ZSM-23 and Pt/MZSM-22/ZSM-23
2.2 Physical properties of the zeolite samples
Figure 3(a)demonstrates that the ZSM-22/ZSM-23 intergrowth zeolite has a uniformly distributed n eedle-like crystal morphology with a length of 0.5−1μm and a width of 0.08−0.12μm,consistent with those reported in the literature[27].In contrast,ZSM-22 displays homogeneous rod-shaped crystals with a length of 1−1.25μm and a width of 0.25μm(Figure 3(b))[28],whereas ZSM-23 is sheet-like with a length of 0.75−1.75μm(Figure 3(c))[29]. As an artificial mixture of ZSM-22 and ZSM-23 at a ratio of 40%−60%,its crystal morphology is uniformly interwoven with ZSM-22 and ZSM-23.Agglomeration was also observed in ZSM-22/ZSM-23,displayed with spheres with a diameter of 9−14μm;in contrast, the spheres in ZSM-23 are slightly smaller with a diameter of 8μm.The mechanical mixture of MZSM-22/ZSM-23 is just simply clumped together and has no fixed shape,whereas ZSM-22 is simply stacked together.

Figure 3 FE-SEM images of (a)ZSM-22/ZSM-23,(b)ZSM-22,(c)ZSM-23 and (d)MZSM-22/ZSM-23
The N2adsorption-desorption isotherms and pore size distribution of the as-synthesized zeolites and catalysts are depicted in Figure 4 and the corresponding textural properties are given in Table 2.The adsorption quantity of all the samples increases rapidly at low relative pressures,consistent with type I isotherms,typical for microporous materials[30,31]; the appearance of a typical platform period is due to the saturation of the microporous adsorptions[32].The pore size distribution of all zeolite samples(Figure 4(b))is mainly concentrated in the microporous region (< 2 nm).Except for ZSM-22,other three samples are relatively close in the specific surface areas(198−209 m2/g),probably due to their small particle size.All zeolite samples have a pore size of around 0.5 nm,corresponding to the typical orifice size of a zeolite with ten-membered ring channels[33].Compared to ZSM-22 and ZSM-23, the ZSM-22/ZSM-23 intergrowth has a larger total pore volume and microporous volume, proving the superior pore channel properties of the composite zeolites.After loading the metal Pt,the properties except total pore volume of all catalyst samples do not have significant change because of the high dispersion of the metal component;as measured by CO-chemisorption, the platinum particle sizes for Pt/ZSM-22/ZSM-23,Pt/ZSM-22,Pt/ZSM-23 and Pt/MZSM-22/ZSM-23 are 1.15,2.19,1.81 and 1.58 nm,respectively.The TEM images shown in Figure 5 further illustrate that the platinum particles are evenly dispersed on the zeolite supports and the platinum particle sizes measured by TEM remain consistent with the CO-chemisorption results.
2.3 Chemical structural and acidic properties of the zeolite samples
Figures 6 and 7 show the27Al NMR and29Si NMR spectra of four zeolite samples. All zeolites display two similar peaks, with a sharp peak at chemical shift 52 attributed to the tetracoordinated framework aluminum and a signal peak at chemical shift 0 to the extraframework six-coordinated aluminum[34].By calculating the signal intensities of the corresponding species, the non-skeletal aluminum species contents of the ZSM-22/ZSM-23,ZSM-22,ZSM-23 and MZSM-22/ZSM-23 samples are presented in Table 3.It demonstrates that most of the aluminum atoms are located in the zeolite framework[35,36].The distribution of aluminum atoms also has an influence on the acid properties of the zeolite.As shown in Figure 7,four distinct signal peaks are observed in the29Si NMR spectra at chemical shift−116,−113,−108 and−104.As reported in the literature[37],the peaks from chemical shift−113 to−116 are attributed to tetrahedral framework silicon atoms,connected to other silicon atoms by an oxygen bridge(4Si,0Al).The peak around chemical shift −108 is associated with the framework silicon interacting with three silicon and one aluminum atom(3Si,1Al),where the Brönsted acid site forms[38].Lower intensity resonance at chemical shift−104 corresponds to silicone hydroxyl,(2Si,2OH)or(3Si,1OH).ZSM-22 shows a significant shift for the peaks in the29Si NMR spectra compared to other samples,indicating a different chemical environment for Si in the zeolite structure[39].
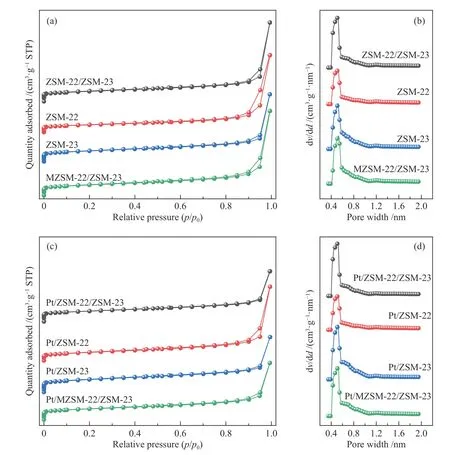
Figure 4 N2 adsorption-desorption isotherms and pore size distribution of various zeolites and catalysts

Table 2 Textural properties of as-prepared zeolites and catalysts
Meanwhile,the SiO2/Al2O3ratios of the four zeolites are essentially the same,slightly lower than that derived from the initial gel composition.It was propoably ascribed to the fact that when HF was used to dissolve the samples,a small part of silicon turned into gaseous SiF4and spilled out,resulting in a lower SiO2/Al2O3ratio measured by ICP.
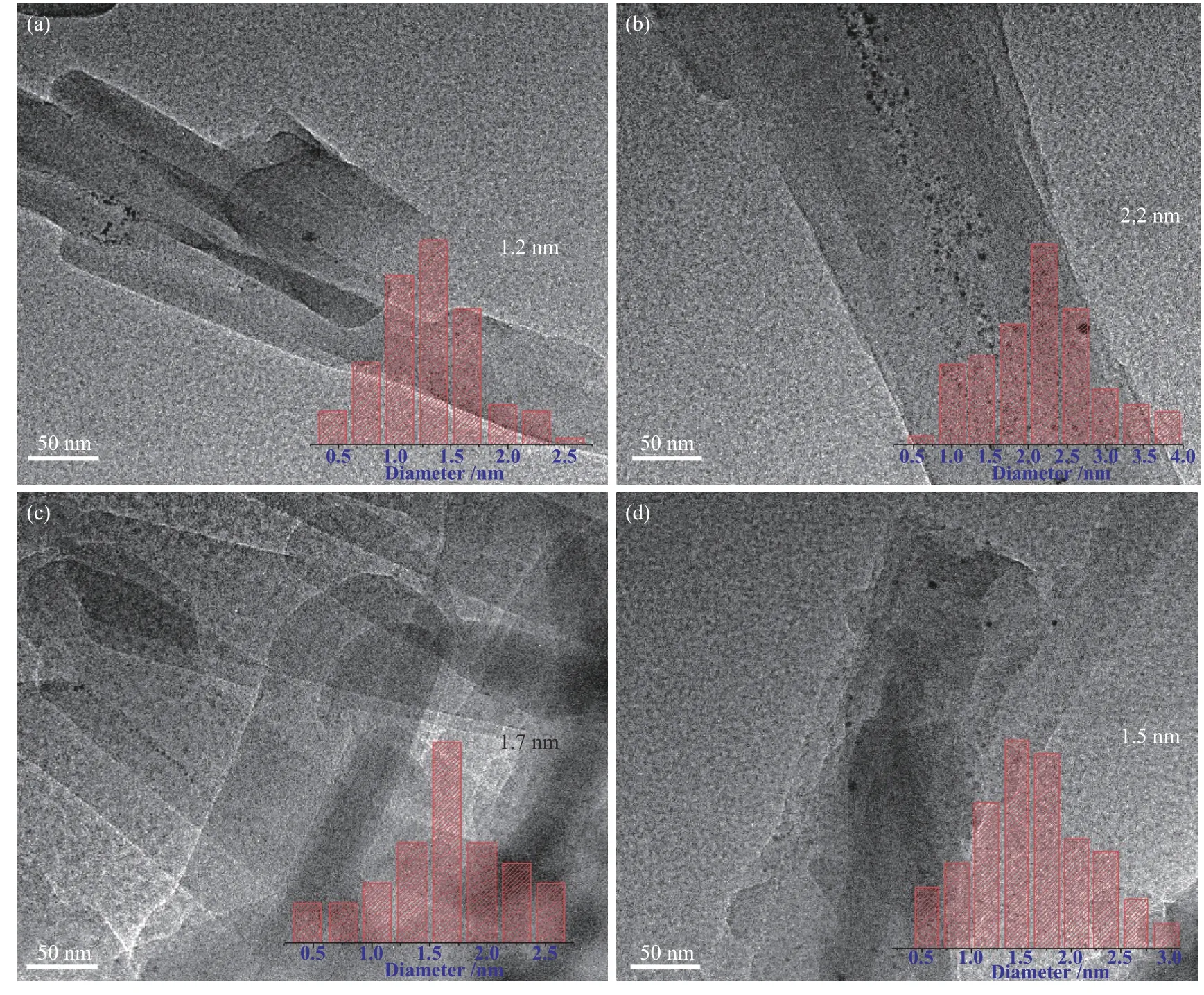
Figure 5 TEMimages of (a)Pt/ZSM-22/ZSM-23,(b)Pt/ZSM-22,(c)Pt/ZSM-23 and (d)Pt/MZSM-22/ZSM-23
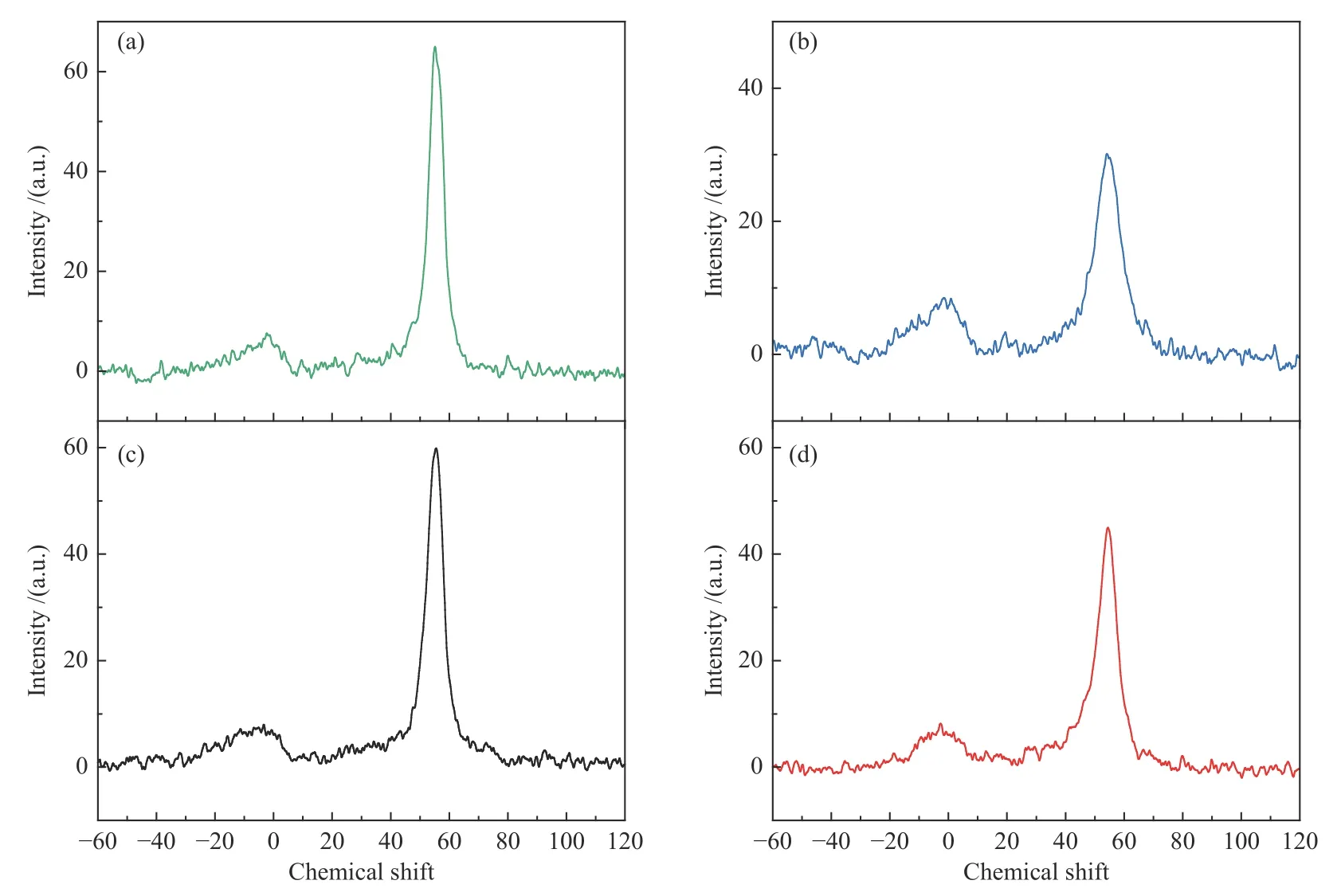
Figure 6 27Al MAS NMR spectra of (a)ZSM-22/ZSM-23,(b)ZSM-22,(c)ZSM-23,and (d)MZSM-22/ZSM-23
When the zeolites are used as the catalyst support,as an important factor,acidity is closely associated with the catalytic activity and lifetime[40].The acidic characteristics can be determined by NH3-TPD[18],as shown in Figure 8.The NH3desorption curves can be divided into three peaks centered at 250−270,330−350 and 450−470°C,corresponding to the weak,medium and strong acid centers, respectively[41].All four zeolite samples display a large peak at low temperature,proving their abundant weak acid sites;the quantity of acid sites according to their strength can be estimated by the NH3desorption signals,as given in Table 3.Obviously, by the total acid amounts,four samples follows the order of ZSM-23>MZSM-22/ZSM-23>ZSM-22/ZSM-23>ZSM-22.Strong acidity may exacerbate the excessive cracking,whereas medium acidity can encourage isomerization by promoting the rearrangement of the framework carbon chain. Although four zeolites show essentially the same medium acidity,ZSM-22/ZSM-23 intergrowth and ZSM-22 display significantly weaker acidity than ZSM-23 and MZSM-22/ZSM-23,in terms of less strong acid sites for the former two zeolite samples.
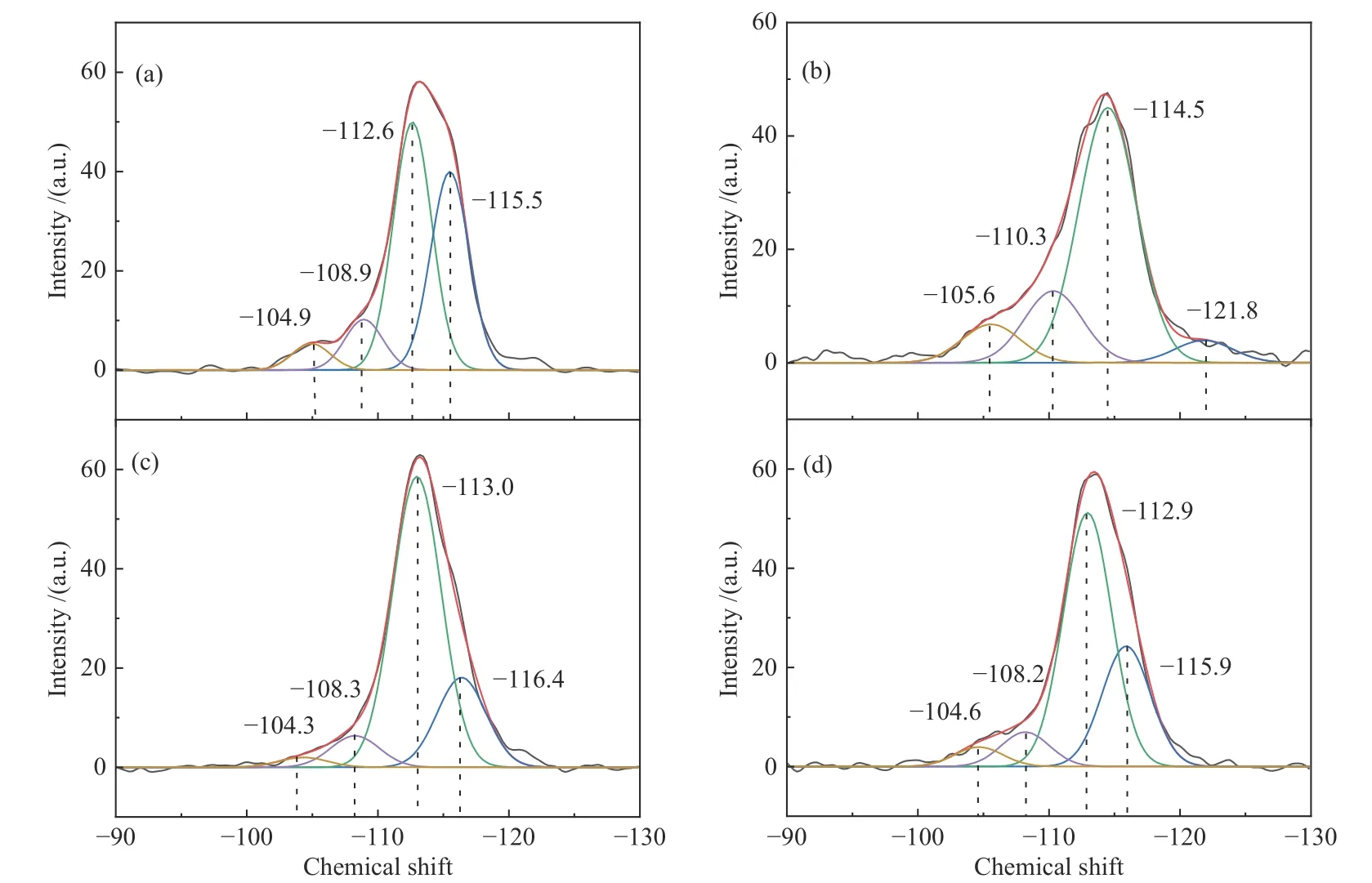
Figure 7 29Si MAS NMR spectra of (a)ZSM-22/ZSM-23,(b)ZSM-22,(c)ZSM-23,and (d)MZSM-22/ZSM-23

Table 3 SiO2/Al2O3 ratios,27Al NMR data and NH3-TPD analysis results of various zeolites
Figure 9 shows the Py-FTIR spectra of as-prepared zeolites at different desorption temperatures;the absorption peaks at 1545 and 1454 cm−1are attributed to Brönsted and Lewis acid sites,respectively[42],whereas the adsorption quantities at 150 and 450°C are deemed to the total acidity and strong acidity,respectively.As given in Table 4,most of the Brönsted acid sites belong to strong acid sites.In particular,the number of Brönsted acid sites in ZSM-23 far exceeds the number of Lewis acid centers,whereas ZSM-22 has more Lewis acid sites.Compared to MZSM-22/ZSM-23,ZSM-22/ZSM-23 has a higher amount of Brönsted acid sites.Therefore,ZSM-22/ZSM-23 should be catalytically more active for the hydroisomerization reaction which occurs primarily on the Brönsted acid sites[43].
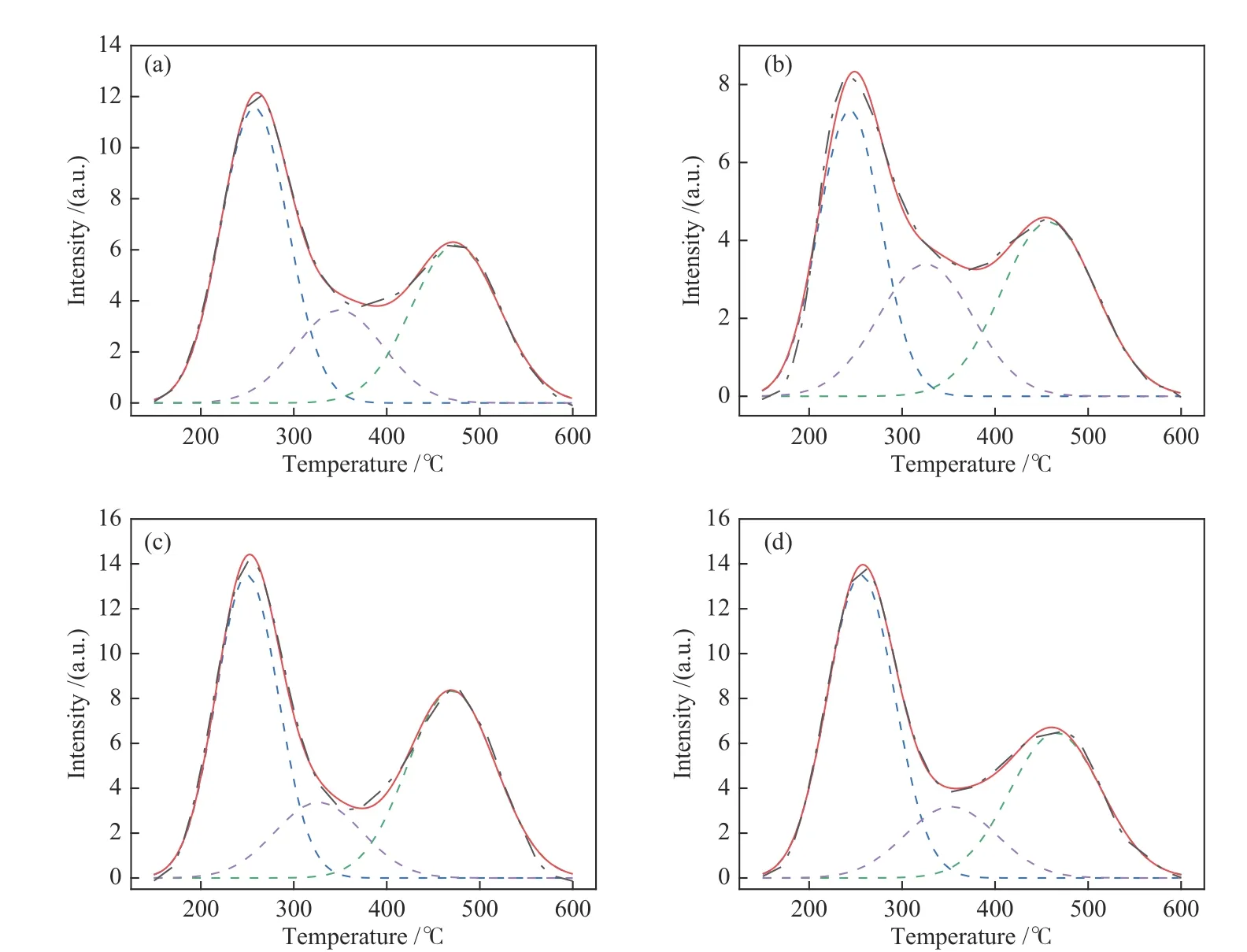
Figure 8 NH3-TPD profiles of (a)ZSM-22/ZSM-23,(b)ZSM-22,(c)ZSM-23,and (d)MZSM-22/ZSM-23
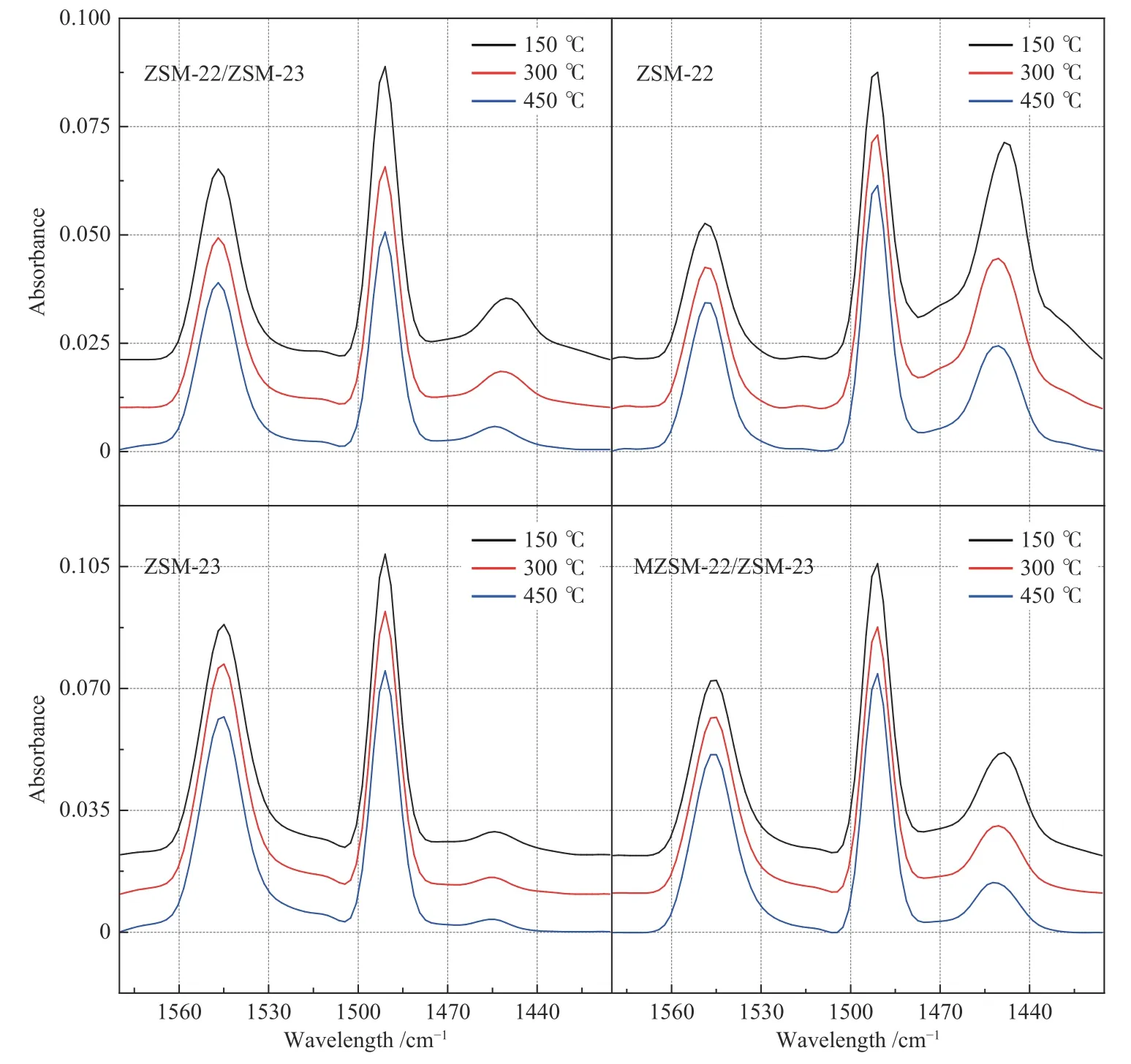
Figure 9 Py-FTIR spectra of as-prepared zeolites at different desorption temperatures

Table 4 Acidity distribution of as-synthesized zeolites derived from Py-FTIR
2.4 Catalytic performance in hydroisomerization
In general,the hydroisomerization process follows the traditional bifunctional catalytic reaction mechanism[44]:n-alkane is first dehydrogenated at the metal centers to form olefin and olefin is then protonated at the Brönsted acid sites and successively rearranged to mono-branched and multi-branched carbenium ions,which are further hydrogenated at the metal centers to give the corresponding isomeric products or cracking products.The activity difference of various catalysts was mainly related to the nature of the zeolite supports,because approximately the same active metal loading was used by the same preparation method.
Figure 10 displays the conversion ofn-hexadecane,product selectivity and yield of isomeric products with the reaction temperature for the hydroisomerization ofn-hexadecane over different catalysts.Obviously, the conversion ofn-hexadecane increases with the increase of reaction temperature(Figure 10(a))[45].As the Brönsted acid sites are responsible to the protonation reactions and the rearrangement of the framework of carbenium ions,the conversion ofn-hexadecane over of various catalysts follows the same sequence of the amount of Brönsted acid sites,viz.,Pt/ZSM-23>Pt/ZSM-22/ZSM-23 > Pt/MZSM-22/ZSM-23 > Pt/ZSM-22.Pt/ZSM-22 shows much lowern-hexadecane conversion than other three catalysts,which may also be related to the greater mass transfer resistance due to the larger grains.
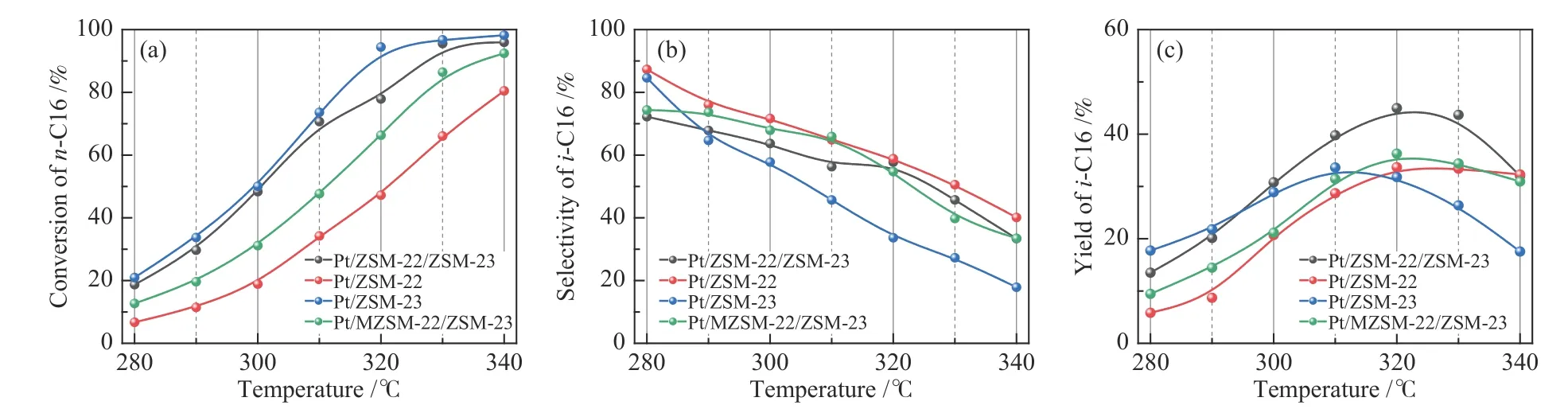
Figure 10 Conversion of n-C16 (a),selectivity to i-C16 (b),and yield of i-C16(c)as a function of temperature over different catalysts
Meanwhile,as shown in Figure 10(b),various catalysts are rather different in the product selectivity:Pt/ZSM-22 exhibits the highest selectivity to the isomeric products,whereas Pt/ZSM-23 the lowest selectivity;in contrast,Pt/ZSM-22/ZSM-23 and Pt/MZSM-22/ZSM-23 display approximately the same selectivity to isomeric products.It is probably explained by the fact that excessive amounts of strong acid sites are favorable to the hydrocracking side reactions. In practice,an ideal bifunctional catalyst in the hydroisomerization ofn-hexadecane is characterized by the high yield of isomeric hexadecanes, that is, both good conversion and high selectivity.As illustrated in Figure 10(c),the yield of isomeric hexadecanes all increases first and then decreases with increasing temperature.At first,a gradual increase in temperature may promote the hydroisomerization reaction and increase the conversion;however,overly high temperature is also more conducive to the cracking reaction,leading to a decrease in the yield ofi-C16.Although Pt/ZSM-22/ZSM-23 is not the most outstanding catalyst in terms of then-hexadecane conversion or the selectivity to isomeric products,it exhibits the highest yield of isomeric hexadecanes.Owing to the inheritance of both Pt/ZSM-22 and Pt/ZSM-23,Pt/ZSM-22/ZSM-23 shows ann-hexadecane conversion of 58%and a selectivity of 78%to isomeric hexadecanes,resulting in a high yield ofi-C16(45%).
Table 5 gives the product distribution ofn-hexadecane hydroisomerization over different catalysts at 320°C with a contact time of 1.12 min.The products are abbreviated asn-Cx,i-CxandyM-Cx,wheren-Cxandi-Cxrefer to straight chain alkanes and branched chain alkanes,respectively andxdenoted the number of carbon atoms.The isomer productsyM-Cxmeans that methyl appears at theyposition of isomeric hexadecane. As mentioned above,Pt/ZSM-23 exhibits more hydrocracking products due to its more strong Brönsted acid sites[46].The high proportion of 7Me-C15(with the branched chain at the center of the chain)in the products over Pt/ZSM-22/ZSM-23 and Pt/MZSM-22/ZSM-23 confirms the“lock-key”form selection mechanism.In contrast,the high content of both 7MC15and 2M-C15in the product of Pt/ZSM-22 proves the existence of both “pore mouth”and “key-lock” reaction routes[47].

Table 5 Product distribution of the n-hexadecane hydroisomerization over different catalysts
3 Conclusions
ZSM-22/ZSM-23 intergrowth zeolite was successfully synthesized by hydrothermal method with diethylamine(DEA)and dimethylamine(DMA)as dual structure directing agents at a DMA/DEA molar ratio of 24,from the gel of 1 SiO2∶0.01 Al2O3∶0.72 DMA∶0.03 DEA∶45 H2O∶0.1 NaOH and crystallized at 180°C for 66 h.
In comparison with ZSM-22 with large grain size,small surface area and insufficient acid quantity and ZSM-23 with excessive strong Brönsted acid sites,ZSM-22/ZSM-23 intergrowth shows more balanced morphological and acidic properties. After loading 0.5%Pt,the bi-functional Pt/ZSM-22/ZSM-23 catalyst exhibits excellent performance in the hydroisomerization ofn-hexadecane,with a much higher yield ofi-C16products(dominated by mono-branched isomers)than those obtained over Pt supported on ZSM-22 and ZSM-22 and their mechanical mixture.Such a discovery may provide helpful perspectives to the development of efficient bifunctional catalysts for the hydroisomerization of long-chain alkanes.
