Enhancement of corrosion resistance of AZ31 Mg alloys by one-step in situ synthesis of ZnAl-LDH film intercalated with organic anions (ASP, La)
2021-05-21YuhnSongYnTngLingFngFngWuXinGungZengJiHu
Yuhn Song, Yn Tng, Ling Fng, Fng Wu, XinGung Zeng, Ji Hu,
Shu Fang Zhange, Bin Jiangd, HaiJun Luof,*
aState Key Laboratory of Power Transmission Equipment & System Safety and New Technology, Chongqing Key Laboratory of Soft Condensed Matter Physics and Smart Materials, College of Physics, Chongqing University, Chongqing 400044, PR China
b School of Chemical &Environmental Engineering, China University of Mining & Technology, Beijing 100083, PR China
cMaterial Corrosion and Protection Key Laboratory of Sichuan Province, Institute of Materials Science and Engineering, Sichuan University of Science and Engineering, Zigong, Sichuan Province 643000, PR China
d National Engineering Research Center for Magnesium Alloys, College of Materials Science and Engineering, Chongqing University, Chongqing 400044, PR China
e College of Software, Chongqing College of Electronic Engineering, Chongqing 401331, PR China
fKey Laboratory on Optoelectronic Functional Materials, College of Physics and Electronic Engineering, Chongqing Normal University, Chongqing 401331,PR China
Received 22 December 2019; received in revised form 5 March 2020; accepted 21 March 2020
Available online 10 October 2020
Abstract
Keywords: ZnAl-LDHs; Magnesium alloy; Organic anions; Corrosion resistance; Aspartic acid; Lauric acid.
1. Introduction
Magnesium alloys are widely used in automobile manufacturing, electronics industry, aerospace and other field due to their high stiffness ratio, excellent electrical and thermal conductivity, good cast and process ability, and ease of recycling
[1]. However, the low standard potential (-2.36V relative to a standard hydrogen electrode) of Mg alloys cause them being prone to corrosion in humid air,sea water,acids and salts,greatly limiting their widely application[2-5].So a lot of surface modificatio methods have been proposed to improve its corrosion protection property. Among these various protective coatings approaches, layered double hydroxides (LDHs) have got increasingly attention on their prevention Mg alloys from corrosion in recent years [6-9].
LDHs are a class of an anionic intercalated compounds with a special laminated structure which can be expressed by a general formula: [M2+1-xM3+x(OH)2]x+(An-)x/n·yH2O. The hydrotalcite layer consists of M2+and M3+cationslocating in the octahedral pores, An-is interlayer anions, x is the molar ratio of M3+/(M2++M3+), and m is the number of water molecules in the interlayer [10-13]. The unique inter-layer anion exchange ability makes LDHs can capture aggressive anions such as Cl- and retard corrosion reactions. Furthermore, the corrosion inhibitors exchanged with the LDHs laminates may protect the substrate from local corrosion [14]. Therefore,encapsulating corrosion inhibitor into the hierarchical LDHs was proposed to a new approach to achieve high corrosion resistance or obtain a self-healing protection for metallic substrates [14-21]. But the proper types of LDH and the corrosion inhibitor maybe quite different for various substrates, so it is worth findin out the most suitable one for the protection of Mg alloy.
Up to now, two kinds of typical LDHs, MgAl-LDHs and ZnAl-LDHs, have been mainly explored to encapsulate corrosion inhibitor. For example, Montemor et al. fabricated MgAl-LDH inserted with mercaptobenzothiazolate(MBT)anions and got an enhanced corrosion resistance to chloridecontaining solution[15].Chen et al.deposited MgAl-PA-LDH by embedding organic phytic acid (PA) into the synthesized MgAl- CO32--LDH laminate [16]. Zeng, et al. intercalated the molybdate into MgAl hydrotalcite coating on AZ31 Mg alloy[17].Wang et al.found their MgAl-LDHs film prepared using a hydrothermal method consist of graded nanosheets(NSs) and uniformly cover the substrate surface [18], thus effectively improve the corrosion resistance of Mg alloys. But the graded LDH NSs structure was destroyed after immersion in a 3.5 wt.% NaCl solution for 6 h, indicating that the MgAl-LDH was not so stable in corrosion solution.
Besides MgAl-LDH, due to its excellent structural stability and ion exchange capacity, ZnAl-LDHs have been also employed as a matrix container to load corrosion inhibitors to protect Mg alloys [19-21]. The electrochemically deposited ZnAl-NO3-LDH [19], and the superhydrophobic ZnAl-CO3-LDH [20], ZnAl-LDH, respectively intercalated with Cl-and VOx-anions [21] were tried to increase the anti-corrosion performance of AZ91D Mg alloy.
However, inorganic compounds are generally employed as the corrosion inhibitor on MgAl-LDH or ZnAl-LDH[17,19-21]. Due to special functional groups and containing nitrogen, oxygen, phosphorus, sulfur and multiple bonds or aromatic rings, the organic compounds may have better inhibition effect than the inorganic ones [22], so surface treatments with organic coating can be adopted to protect metal surface from corrosion.
Aspartic acid (ASP) and lauric acid (La), two natural environmentally friendly organic compounds, were found to have the ability to improve the anti-corrosion of Al alloys [22-23].For instance, in order to increase the corrosion resistance of Al alloys, the ZnAl-LDH coating intercalated with lauric acid(La) [23], and ZnAl- and MgAl-LDHs loaded with quinaldate(QA) and 2-MBT anions were explored [24]. Zeng et al. synthesized the ZnAl-LDH/poly(lactic acid) composite coating on AZ31 Mg alloy [25]. But to the best of our knowledge,there are few reports about the application of ZnAl-LDHs with ASP or La for the protection of Mg alloy. Meanwhile,most of the LDH-based anti-corrosion coatings were obtained by a complicated two-step process (the corrosion inhibitors are usually inserted into the LDH layers by ion-exchange after the formation of LDH), so some facile and more costeffective approaches, especially one-step methods are urgent to develop. In our previous work, ZnAl-LDHs encapsulated with different inorganic anions(NO3-, Cl-, VO43-, PO43-,and MoO42-) [26] and rose-like MgAl-LDH coating with organic ASP [27] were synthesized on AZ31 Mg alloys with a simple hydrothermal method, and their corrosion resistance or self-healing were observed.
Therefore,in this work,the ASP and La were chosen as the corrosion inhibitor and embedded into the ZnAl-LDH laminate to form ZnAl-ASP-LDHs and ZnAl-La-LDHs coating on AZ31 Mg alloy by a simple hydrothermal method.The effects of ASP or La organic anions on the morphologies, structures and corrosion resistance of ZnAl-LDHs were investigated by XRD, FTIR, SEM and electrochemical test systems.
2. Experimental method
2.1. Materials
AZ31 Mg alloy sheets (Al:8.5-9.5 wt%, Zn:0.45-0.9 wt%, Mn: 0.17-0.4 wt%, Si <0.08 wt%, Fe<0.004 wt%, Ni<0.001 wt% and balanced Mg) with a size of 20mm×25mm×2.0mm were employed as the substrates.All the chemical reagents used in this experiment, including Zn(NO3)2·6H2O, Al(NO3)3·9H2O, C4H7NO4, C12H23NaO2,NaCl and NaOH, were analytical grade.
2.2. Experimental Details
Both the ZnAl-ASP-LDHs and ZnAl-La-LDHs film were synthesized by a hydrothermal method. First, the AZ31 Mg alloys were ground with 400, 800, 1200, and 2000 grit SiC sand paper, ultrasonically cleaned in ethanol for 15 minutes and dried by cold air, respectively. Then, in order to remove impurities and oxides on the substrate surface, the Mg sheets were immersed in 0.2 M NaOH solution for ultrasonic cleaning for 1 minute, and placed in a 333K oven for drying after ultrasonically washed with ethanol for 10 minutes. In a typical synthesis, 0.04 M Zn(NO3)2·6H2O and 0.02 M Al(NO3)3·9H2O were dissolved in deionized(DI)water to form a homogeneous solution under nitrogen atmosphere, then the above mixture was added dropwise to 0.04 M C4H7NO4and 0.04 M C12H23NaO2solution, respectively.The pH was adjusted to 10.0 by addition 2 M NaOH solution during the synthesis process. The above two turbid liquids obtained and the pretreated Mg sheets were separately transferred to PTFE autoclave and heated to 393K for 12h. After the reaction, the Mg sheets were washed with DI water and vacuum dried at 333 K for 12h. Finally, the ZnAl-La-LDHs and ZnAl-ASP-LDHs film were obtained.
2.3. Characterization and measurements
The morphology of the samples was characterized by scanning electron microscopy (SEM, JEOL JSM-7800F) equipped with an energy dispersive X-ray spectrometer (EDS). The crystal structure of the film was tested using a powder Xray diffraction (XRD).The XRD instrument is Rigaku D/max 2500 type X-ray diffractometer (Cu Ka, λ=0.1540598nm,50kV/250MA) at a scanning rate of 0.02s-1in the scanning range of 5°-75°. The functional groups on the film surface were analyzed using a Fourier transform infrared spectrometer(Nicolet iS50).
2.4. Electrochemical test
The polarization curves and electrochemical impedance spectroscopy (EIS) were used to judge the corrosion resistance of the coating, which were performed by an electrochemical workstation (CHI760E, Shanghai Chenhua) in a 3.5 wt.% NaCl solution at room temperature (RT). A classic three-electrode system was applied, in which the sample (area 1cm2) is used as the working electrode, a platinum plate as the counter electrode and saturated calomel electrode (SCE)as a reference electrode. Prior to measurement, the working electrode was immersed in 3.5 wt.% NaCl solution until the open circuit voltage was stable. In order to obtain stable output electrical signal, the electrochemical test usually starts after the samples have been immersed the solution for 30 minutes. The potentiodynamic polarization curve was recorded at a scan rate of 2mV/s. The EIS measurements were carried out from 100mHz to 100kHz with an amplitude disturbance of 5mV.
3. Results and discussion
3.1. Morphology, composition and structure of the ZnAl-LDHs films intercalated with ASP and La anions
The SEM images and EDS spectra of ZnAl-ASP-LDHs and ZnAl-La-LDHs film are given in Fig. 1. It shows that both the two LDHs film densely grow on Mg alloy(Fig. 1a and 1b), and exhibit a uniform and dense NSs structure (Fig. 1c and 1d). Both the NSs of the ZnAl-ASP-LDHs and ZnAl-La-LDHs film are alternately staggered on Mg alloy, but the NSs of the latter film (Fig. 1d) are larger in size, slanted stagger, more uniform and denser, which can more effectively block the penetration of aggressive ions and prevent the substrate from being exposed to aggressive environments, thereby improving the corrosion resistance of the Mg alloy. From the cross-section SEM diagrams (Fig. 1e, f),the thickness of ZnAl-ASP-LDHs film and ZnAl-La-LDHs film were obtained to be 15.65 and 17.39μm, respectively.The thicker the films the more resistant to the infiltratio of aggressive Cl- to the substrate, consequently ZnAl-La-LDHs film have better ability to protect the Mg alloy.
The chemical composition of ZnAl-LDHs film intercalated with ASP and La anions was analyzed by EDS and the results were shown in Fig.1e and 1f. It can be seen that the main components of the both two ZnAl-LDHs film (Fig. 1e)are Zn, Al, C, N and O elements, the content of Mg is mainly derived from the substrate. The organic anions are mainly composed of C, O and H elements. Although there are a large number of these elements in the air, the C and O peak intensities of ZnAl-LDHs film intercalated with organic ASP and La are significantl stronger than those of the corresponding ZnAl-LDHs film intercalated with inorganic anions in our previous work [26]. This indicates that the ASP and La were successfully embedded in ZnAl-LDHs film on Mg alloys.
The XRD patterns of the Mg alloy substrate, ZnAl-ASPLDHs film and ZnAl-La-LDHs film were displayed in Fig. 2. It reveals that the typical (003) and (006) layered characteristic peaks of LDHs appear in the XRD pattern of ZnAl-ASP-LDHs films but only (006) (2θ≈5.2°) and (009)(2θ≈7.8°) peaks occur in ZnAl-La-LDHs films According to the report of Y.H. Cao and F.Z. Zhang, the characteristic peak of (003) is estimated to be between 0~5° [28,23]. Due to the limitation of the detection range of our XRD instrument,it is impossible to accurately measure the diffraction peak of 0~5°, so the (003) peaks for ZnAl-La-LDHs film was not observed. Our results of (006) and (009) characteristic peaks of ZnAl-La-LDHs film are consistent with the reports of Yarger and Whilton [29,30], indicating that the layered ZnAl-La-LDHs were successfully prepared on Mg alloy.
According to the Bragg equation, the crystalline interplanar spacing of d(003)of ZnAl-ASP-LDHs film was calculated to be 0.824nm. Since the (003) peak of ZnAl-La-LDHs film shifts to a smaller 2θ angle, its d(003)spacing should be much larger than 0.824nm, meaning that the ZnAl-LDHs intercalated with different anions will have different layer spacing and the one intercalated with La has larger spacing than that with ASP, this may be related to the order of the interlayer anions and the size of the anions [31].
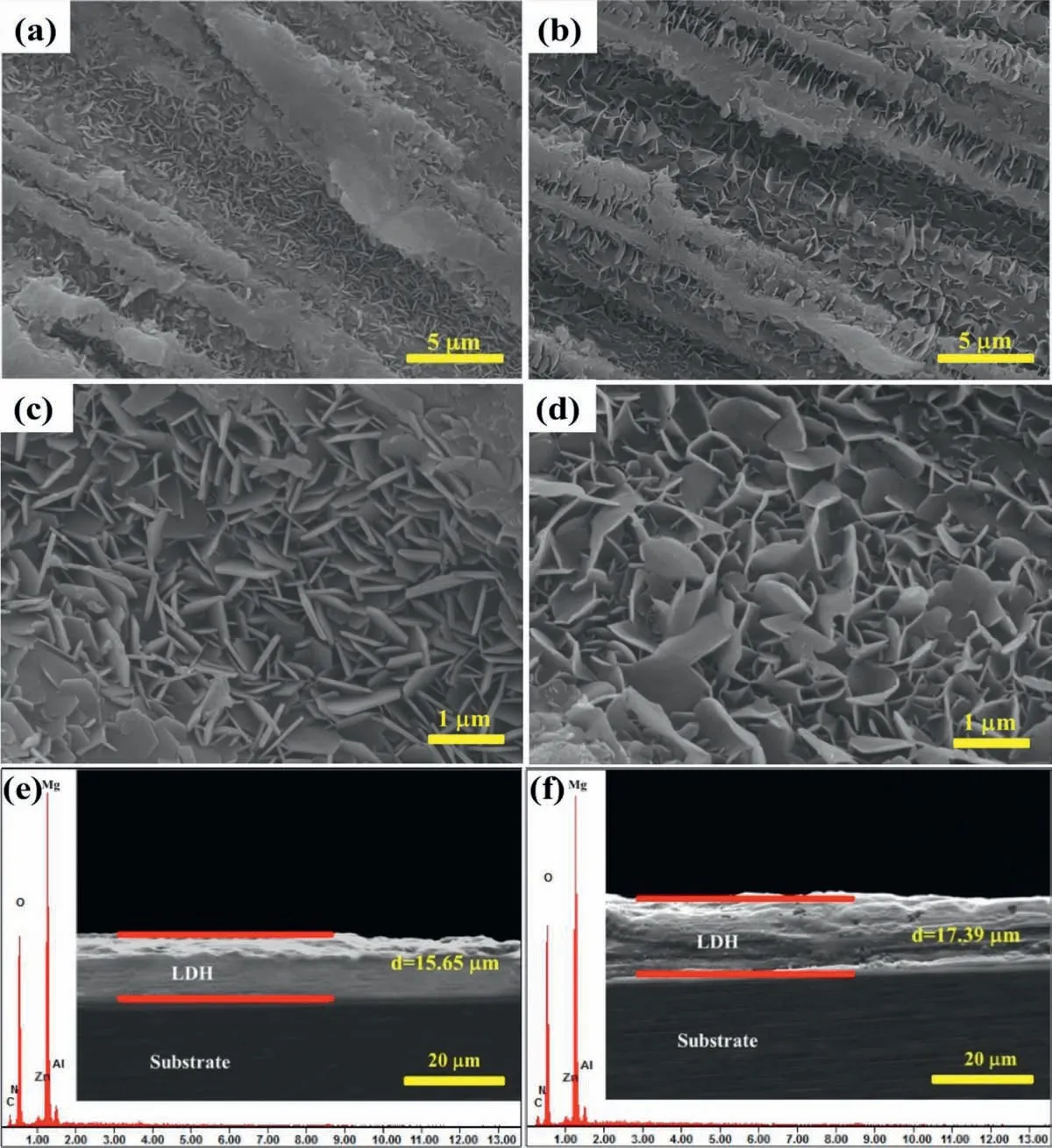
Fig. 1. SEM images, cross-sectional views and the corresponding EDS spectra of ZnAl - ASP - LDHs (a, c and e) and ZnAl-La-LDHs(b, d and f) films
The FT-IR spectra of ZnAl-ASP-LDHs and ZnAl-La-LDHs film are shown in Fig. 3. The absorption band at 3694cm-1is attributed to the lattice vibration of M-OH in the LDHs laminate (e.g. Zn-OH, Al-OH, and Mg-OH) [32]. The band at about 3450cm-1corresponds to O-H due to water molecules absorbed on the surface and interlayer water molecules. The band at 1637cm-1can be ascribed to the bending vibration of water molecules. The characteristic absorption band at 1378cm-1of ZnAl-ASP-LDHs film is caused by the asymmetric and symmetric tensile vibration of -COOH group [33].In the ZnAl-La-LDHs films the spectra at 2849cm-1and 2918cm-1are attributed to the tensile vibration of the alkyl C-H group, and the absorption peak at 1409cm-1is related to the symmetrical and asymmetric vibration of the COOgroup [28]. These results show that the ASP and La anions are successfully intercalated into the ZnAl-LDHs films In addition, there are some absorption peaks at 472 cm-1, which may be related to the lattice vibration of the metal (e.g.M-O, M-O-M) [34]. From the above FT-IR spectra, it was further confirme that the ZnAl-LDHs film intercalated with ASP and La anions have been successfully prepared on Mg alloy.
3.2. Corrosion resistance of the ZnAl-LDHs films intercalated with ASP and La anions
The potentiodynamic polarization curves of AZ31 Mg alloy, ZnAl-ASP-LDHs and ZnAl-La-LDHs film immersed in 3.5 wt.% NaCl aqueous solution are demonstrated in Fig.4, and the corresponding results are listed in Table 1.It shows that the corrosion potential (Ecorr) of AZ31 Mg alloy bare substrate,ZnAl-ASP-LDHs and ZnAl-La-LDHs film are -1.511V, -1.503V and -1.074V (vs. SCE), respectively, indicating that ZnAl-La-LDHs film have the highest Ecorr, then the best corrosion resistance. The corrosion current density (icorr) of bare AZ31 Mg alloy, ZnAl-ASPLDHs and ZnAl-La-LDHs film decreases from 7.472×10-5to 2.772×10-7A/cm2. Compared with the icorrof the substrate, the icorrof the ASP and La -contained ZnAl-LDHs film are reduced by two orders of magnitude.It is known that the less icorr, the slower the interface reaction, the better corrosion resistance, means that the samples coated with ZnAl-ASP-LDHs or ZnAl-La-LDHs have better anti-corrosion performance. Meanwhile, their icorrvalues were compared with that of ZnAl-NO3--LDHs film reported in our previous work[26], and it is found (from in Table 1) that the corrosion resistance of the film ZnAl-LDHs film intercalated with organic ASP/La anion is much better. These results illustrate that the ZnAl-LDHs film intercalated with ASP/La prepared by one-step in situ method can effectively improve the corrosion resistance of the AZ31 Mg alloy.

Table 1 The polarization measurements(corrosion potential (Ecorr) and corrosion current densities (icorr)) of the magnesium alloy substrate, ZnAl-ASP-LDHs, ZnAl-La-LDHs and ZnAl-NO3--LDHs films

Fig. 2. XRD patterns of the magnesium alloy substrate, ZnAl-ASP-LDHs and ZnAl-La-LDHs films

Fig. 3. FT-IR spectra of ZnAl-ASP-LDHs and ZnAl-La-LDHs films
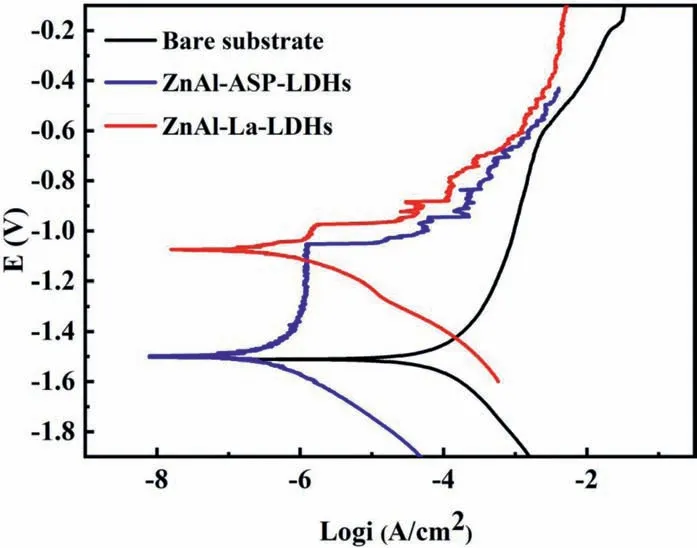
Fig. 4. Potentiodynamic polarization curves of the magnesium alloy substrate, ZnAl- ASP- LDHs and ZnAl-La-LDHs films
Electrochemical impedance spectroscopy (EIS) was also used to evaluate the anticorrosion behaviors of the LDHs films The Nyquist plot is shown in Fig.5a, from which one clear loop could be observed for our three samples and the order of the radius of curvature is ZnAl-La-LDHs >ZnAl-ASP-LDHs >AZ31 alloy. Usually the larger the curvature radius, the better the corrosion resistance, so it indicates that the two ZnAl-LDHs increase the corrosion property of AZ31 alloy, and the ZnAl-La-LDHs has the better anti-corrosion performance than ZnAl-ASP-LDHs.
The Bode plot is displayed in Fig.5b, from which a clear peak at high-mid frequency and a weak peak at low frequency were found, indicating that two time constants exist in the phase angle plot, which are corresponding to two capacitive loops.Similar results about the bare substrate and LDHs film were also reported in previous researches [21,27].

Fig. 5. Nyquist (a) and Bode (b) plots of the magnesium alloy substrate, ZnAl-ASP-LDHs and ZnAl-La-LDHs films

Table 2 Fitting results of EIS spectrum for magnesium alloy substrate, ZnAl-ASP-LDHs and ZnAl-La-LDHs films
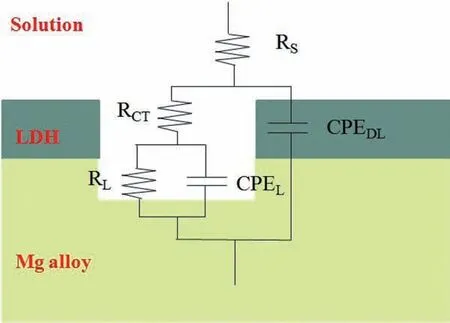
Fig. 6. The equivalent electrical circuit of ZnAl-LDHs films
Based on the above results, the proposed equivalent electrical circuit of ZnAl-LDHs film is exhibited in Fig. 6, in which Rsis the solution resistance, RCTis the charge transfer resistance and the constant phase element (CPEDL) is the electric double layer capacity and RLrepresents the fil resistance and CPELrepresents the fil capacity. Based on the equivalent circuits, the EIS spectra were fitte and the data fittin results listed in Table 2. It shows that the RCTvalue of the bare AZ31 substrate is only 61.36Ωcm2, which is much lower than that of the ZnAl-ASP-LDHs (9841Ωcm2)and ZnAl-La-LDHs (1.409×104Ωcm2). The CPEDLvalues of the bare AZ91substrate is 22.76μFcm-2, which is higher than that of the ZnAl-ASP-LDHs(0.7179μFcm-2)and ZnAl-La-LDHs (0.0813μFcm-2). It indicated that the ZnAl-LDHs film play an important role in improving the protection performance of the Mg alloy.
It is generally believed that the higher RCT, the lower CPEL, the better anticorrosion performance [21,27]. Compared with the RCTand CPEDLvalues of the LDHs film with different anions, it also suggested that their corrosion resistance should be: ZnAl-La-LDHs >ZnAl-ASP-LDHs >AZ 31 alloy, which is consistent with the results of the Nyquist plot (Fig.5a).
3.3. Morphology and composition of the ZnAl-LDHs films after immersion
The ZnAl-ASP-LDHs and ZnAl-La-LDHs film were immersed in a 3.5 wt.% NaCl solution for 168h. Then the morphology and composition of the samples were measured. The SEM images were shown in Fig.7. From the photos with lower magnificatio (Fig.7a and b), it can be seen that the surface of ZnAl-LDHs film intercalated with ASP and La on the Mg alloy looks very flat no obvious corrosion marks were observed after corrosion. At larger magnification the surface of ZnAl-ASP-LDHs film (Fig.7c and e) seems still very smooth,which is attributed to the dissolution of the LDH NSs, and the dissolved product are evenly covered on the surface of Mg alloy, which can effectively slow down the corrosion of Mg alloy. Compared with the SEM imagines of ZnAl-La-LDHs film before corrosion (Fig.1b and d), it can be seen from Fig.7d and f that the complete NSs structure still exists and uniform and densely covers on Mg alloy after corrosion, the only change is the NSs structure transforming from oblique staggered to vertical staggered, resulting from the anion exchange of Cl-during the corrosion process, indicating that the ZnAl-La-LDHs film can absorbs Cl-in the corrosion solution to protect the Mg alloy.
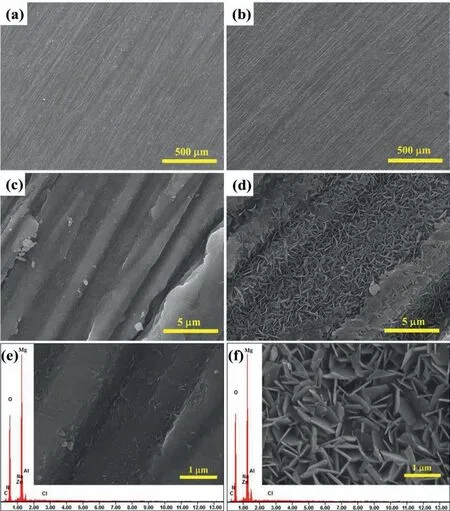
Fig. 7. SEM images and the corresponding EDS spectra of the ZnAl-ASP-LDHs (a, c and e) and ZnAl-La-LDHs (b, d and f) film after corrosion.
The chemical compositions of the samples after the immersion experiment were shown in Fig. 7e and f. Compared with the pre-etched films the immersed ZnAl-LDHs film have Cl element after corrosion, which is attributed to the fact that the organic anion contained ZnAl-LDHs can effectively absorb and retain the aggressive Cl-between the interlayer, this result is consistent with the findin of the previous experiments[26].
As shown in Fig. 8, the FT-IR spectrum of the samples after immersion reveals that after the corrosion, the absorption band of the ASP/La anions is still present, which indicates that the structure of film can effectively resist corrosion, but the intensity of absorption band at 3694cm-1of ZnAl-La-LDHs film is obviously stronger than that of ZnAl-ASP-LDHs films The 3694cm-1band is usually ascribed to the precipitation of Mg(OH)2,so the weaker intensity of 3694cm-1band of ZnAl-La-LDHs film means that the Mg(OH)2precipitate formed from ZnAl-La-LDHs film after corrosion is less than that of ZnAl-ASP-LDHs films which implying that the La contained ZnAl-LDHs film has better corrosion resistance.
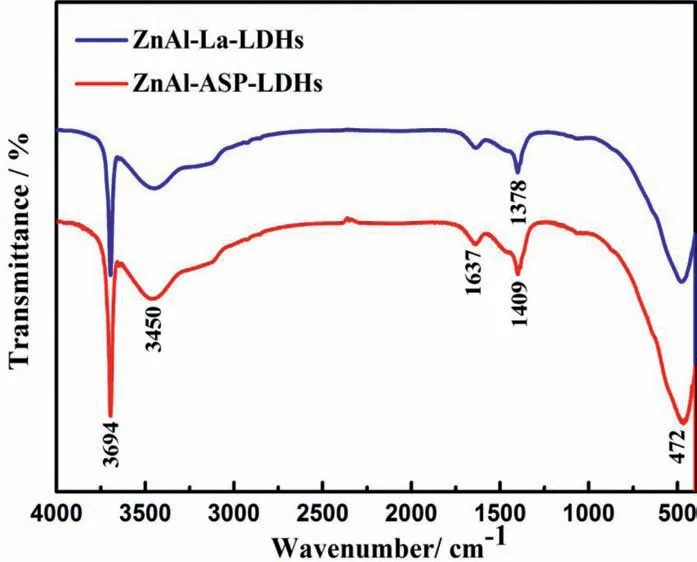
Fig. 8. FT-IR spectra of the ZnAl-ASP-LDHs and ZnAl-La-LDHs film after corrosion.
3.4. Anti-corrosion mechanism of ZnAl-LDHs films with ASP and La anions
Based on the above analysis, the anticorrosion mechanism of the Zn-Al LDHs film with different anions could be depicted as follows: (1) the ZnAl-LDHs film with high density of nanosheets act as a protective barrier coating to block the direct contact of the chloride anions to Mg alloys,which delay the initiation of the corrosion; (2) due to the anion-exchange of LDHs, the intercalated ASP or La anions exchange with the aggressive chloride anions, means, the harmful chlorides are entrapped and its concentration will decrease; (3) the released ASP or La anions in the aggressive environment can form a corrosion inhibitor protective layer on the surface of Mg alloy. This process is usually also called as self healing. In a short word, the ASP or La-intercalated Zn-Al LDHs film play three roles to enhance the improve corrosion resistance: barrier coating, the entrapment of harmful chlorides and the formation of inhibition protective layer. Our proposed mechanism is accordance with the results of Tedim,et al.[35].Schematic diagram of ion exchange of ASP or La anions with Cl-and formation of corrosion inhibition protective layer on Mg alloy is demonstrated in Fig. 9.
Eddy [36,37] and Sorkhabi et al. [38] reported that corrosion inhibition efficien y of organic compounds is dependent on the molecule structure (molecular size, molecular mass,internal structure, the nature and adsorptive tendencies of the hetero-atoms) and the number of adsorption-active centers usually originating from some functional groups. The ASP molecular (C4H7NO4) has three polar groups (one -N2H and two -OH), means, it has three active adsorption sites. The structural formula of the La molecule (C12H24O2) is HOOC-10CH2-COOH, which has two active adsorption sites (two polar groups: one -CH3 and one -OH), implying that La have one less active adsorption sites than ASP. On the other hand, the molecular weight (MW) of La (200.32) is larger than that of ASP (MW:133.1). Usually the larger the MW or the more adsorption sites, the better the corrosion inhibition efficien y. So it is hard to judge ASP or La, which one have a better the corrosion inhibition efficien y, since the effect of the more active adsorption sites of ASP offsets against its lighter MW.
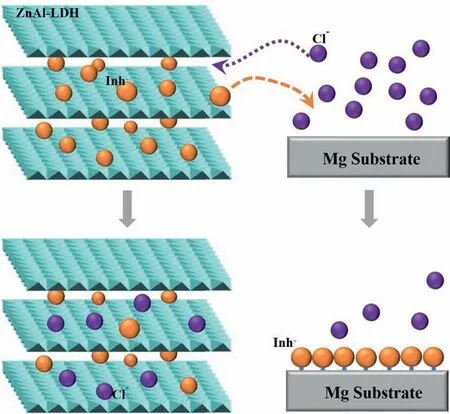
Fig. 9. Schematic diagram of ion exchange of organic ions and formation of corrosion inhibition protective layer on Mg alloy (Inh=ASP or La).
More importantly, the larger molecule size of La caused the larger d(003)spacing of the ZnAl-La-LDHs, resulting in a better ability to absorb Cl-and release interlayer anions than ZnAl-ASP-LDHs.Meanwhile the NSs of ZnAl-La-LDHs film stagger slanted and are thicker, denser and more uniform, which can more effectively block the penetration of aggressive ions and prevent the substrate from being exposed to aggressive environments.
Therefore, owing to the total effect of the above comprehensive factors, the ZnAl-La-LDHs film shows better anticorrosion property than the ZnAl-ASP-LDHs films
3.5. Comparison of corrosion resistance of ZnAl-DHs films with different organic anions
The corrosion resistance of ZnAl -LDHs film intercalated with different organic anions is briefl compared and the result is given in Table 3. It is found that the corrosion potential of LDH coating is in the range of -1.50— 0.90V, and the corrosion current density of the metal substrate is tens of μA/cm2, ie, at the 10-5A/cm2order of magnitude; and the corrosion current density of the LDH coating is usually at the 10-5~10-9A/cm2order of magnitude, indicating LDH coating can greatly increase the corrosion resistance. Among the LDHs intercalated with organic anions,the La-intercalated ZnAl-LDH coatings fabricated by Zhang et al.[23]on Al alloy got the lowest corrosion current density (~10-9A/cm2). The

Table 3 Comparison of corrosion resistance of ZnAl-DHs film intercalated with different anions (corrosion solution: 3.5wt.% NaCl).
corrosion current density of the ZnAl-ASP-LDH and ZnAl-La-LDH prepared in this work is lower than that of the MgAl-PA-LDH deposited by Chen et al.[16],but near that of MgAl-ASP-LDH reported in our previous work [27], implying that our ASP- or La-intercalated ZnAl-ASP-LDH have better corrosion resistance than the PA-intercalated MgAl-LDH and almost the same corrosion resistance to the ASP-intercalated MgAl-LDH.
4. Conclusions
In this paper, the ZnAl-ASP-LDHs and ZnAl-La-LDHs film were prepared by the hydrothermal method on AZ31 Mg alloy. The effects of different organic anions on the morphology, structure and corrosion resistance of ZnAl-LDHs film were investigated. It was found that both ZnAl-ASP-LDHs and ZnAl-La-LDHs film grow uniformly and densely on AZ31 Mg alloy. The ZnAl-ASP-LDHs film exhibited a vertical growth of NSs structure, while the ZnAl-La-LDHs film showed mutual interlaced tilted NSs structure, which is more conducive to block the penetration of corrosive liquid into the Mg alloy. The ZnAl-La-LDHs has a much larger d(003)spacing value than ZnAl-ASP - LDHs, indicating that ZnAl-La-LDHs film have better ability to absorb Cl-and release interlayer anions. The corrosion resistance of ZnAl-La-LDHs film is obviously better than that of ZnAl-ASP-LDHs films and the corrosion current density (2.772×10-7A/cm2) is reduced by two orders of magnitude compared to that of bare Mg alloy (7.482×10-5A/cm2). The morphology still shows a uniform and dense complete NSs structure after corrosion,which indicates that the ZnAl-LDHs film intercalated with organic La anions can significantl improve the corrosion resistance of Mg alloy.
Acknowledgments
This work was supported by the Natural Science Foundation of Chongqing (cstc2019jcyjmsxmX0566, cstc2018jcyjAX0450, cstc2018jcyjA2923,cstc2017jcyjAX0393), Projects of President Foundation of Chongqing University (2019CDXZWL002), Fundamental Research Funds for the Central Universities (2019CDXYWL0029, 2018CDJDWL0011,106112017CDJQJ328839, 106112016CDJZR288805), Science and Technology Research Program of Chongqing Municipal Education Commission (KJKJQN201800102,KJQN201800619, KJ1703042), NSFC (11544010, 11374359,11304405 and 1155305),the Sharing Fund of Large-scale Equipment of Chongqing University (201903150094). We also thank Analytical and Testing Center of Chongqing University for performing XRD, XPS, TEM tests.
杂志排行
Journal of Magnesium and Alloys的其它文章
- Mechanism of Mn on inhibiting Fe-caused magnesium corrosion
- An efficien and comparative adsorption of Congo red and Trypan blue dyes on MgO nanoparticles: Kinetics, thermodynamics and isotherm studies
- Twin recrystallization mechanisms in a high strain rate compressed Mg-Zn alloy
- Corrosion behaviour and cytocompatibility of selected binary magnesium-rare earth alloys
- Correlation between test temperature, applied load and wear transition of Mg97Zn1Y2 alloy
- Residual stress and precipitation of Mg-5Zn-3.5Sn-1Mn-0.5Ca-0.5Cu alloy with different quenching rates
