Residual stress and precipitation of Mg-5Zn-3.5Sn-1Mn-0.5Ca-0.5Cu alloy with different quenching rates
2021-05-21CongWngTinjioLuoYuntengLiuQiuynHungYunshengYng
Cong Wng, Tinjio Luo, Yunteng Liu, Qiuyn Hung, Yunsheng Yng,*
a Institute of Metal Research, Chinese Academy of Sciences, Shenyang 110016, China
b School of Materials Science and Engineering, Northeastern University, Shenyang 110819, China
c Shandong Key Laboratory for High Strength Lightweight Metallic Materials, Advanced Materials Institute, Shandong Academy of Sciences, Shandong 250014, China
Received 13 November 2019; received in revised form 20 January 2020; accepted 13 February 2020
Available online 16 February 2021
Abstract
Keywords: Mg-5Zn-3.5Sn-1Mn-0.5Ca-0.5Cu alloy; Solution treatment; Quenching rate; Residual stress; Precipitation; Age-hardening.
1. Introduction
Heat treatment can be employed to improve the mechanical properties of some sand cast or wrought magnesium alloys that are heat-treatable, like Mg-Zn [1], Mg-Sn [2] and Mg-RE [3,4] alloys. Before aging treatment, magnesium alloys are solution treated followed by fast cooling to suppress dynamic precipitation and obtain a supersaturated solid solution that decomposes to form fin precipitates during aging. However, high quenching rate gives rise to temperature gradient,which leads to thermal stress. If the thermal stress is higher than the local yield strength, inhomogeneous plastic deformation happens and residual stress forms [5,6]. Although the compressive residual stress can be beneficia to the fatigue and corrosion resistance [7,8], it has an undesirable effect on dimensional stability [5]. Since the quenching rate is a key factor that affects both residual stress and the solution treated microstructure, it is desirable to fin a cooling method that reduces the residual stress while maintaining supersaturation of alloying elements for precipitation, the study on the 2A14 aluminum alloy showed that this could be achieved with appropriate cooling method [9]. So information on the evolution of residual stress and precipitation behavior of magnesium alloy with the quenching rate is necessary to determine the optimal cooling method that balances the residual stress and the strength of magnesium alloys.
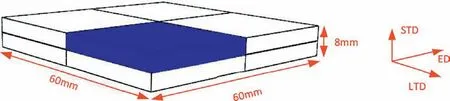
Fig. 1. Schematic illustration of the specimen used for modeling (blue part).
However, the effect of cooling rate on age hardening ability, which is known as quench sensitivity, is not clear for magnesium alloys. Wang et al. [10] studied the quench sensitivity of a LPSO-phase containing magnesium alloy through comparing the mechanical properties and microstructure of water cooled and air cooled alloy and found that the long basal precipitates formed during air cooling led to significan quench sensitivity and reduced the strength of the aged alloy. However, the quench sensitivity of other magnesium alloys is still not clear due to the dependence of quench sensitivity on alloy composition [11,12]. High quenching rate is usually used in the study of quench-induced residual stress in magnesium alloys [5,6], without considering the relationship between the quenching rate and the residual stress, so the effect of cooling condition on residual stress is still not clear.
Mg-Zn-Sn magnesium alloys can be heat treated to gain high strength and are promising to be used as load-bearing components. So in the present study, a newly developed heattreatable high strength Mg-5Zn-3.5Sn-1Mn-0.5Ca-0.5Cu alloy is studied [13], it is subjected to cold water cooling, hot water cooling and air cooling following solution treatment. The residual stress formed after quenching and the precipitation behavior are investigated to explore the possibility of simultaneously decreasing the quench-induced residual stress and maintaining the age-hardening ability of the alloy, the results would be beneficia to both application and composition optimization of the alloy.
2. Experimental
Pure Mg, Zn, Sn, Cu, Mg-10wt.% Mn and Mg-25wt.%Ca master alloys were used to prepare the Mg-5Zn-3.5Sn-1Mn-0.5Ca-0.5Cu alloy in a resistance furnace protected by a mixture of CO2and SF6. The alloy was melted at 760°C and the temperature was lowered to 700°C before casting.The composition of the alloy was Zn: 4.9wt.%, Sn: 3.8wt.%,Mn:1.1wt.%,Ca:0.4wt.%,Cu:0.4wt.%and balanced by Mg according to X-ray fluorescenc spectroscopy. The cast ingot was homogenized at 440°C for 4h followed by extrusion into plate with cross section of 60 mm×8mm. Plate specimens with a dimension of 60 mm×60 mm×8mm in extrusion direction(ED), long transverse direction(LTD) and short transverse direction(STD)were cut from the extruded plate and solution treated at 440°C for 4h followed by cold water cooing,hot water cooling or air cooling. The solution treated specimens were cut into cuboid measuring 8 mm×8 mm×5mm and aged at 75°C for 24h followed by aging at 175°C for 3h to achieve peak hardness. The hardness of the solution treated and double aged alloy was measured with a load of 50g with a dwell time of 15s and a minimum of three points were measured for each specimen. Tensile test specimens with a gage length of 15mm and a gage diameter of 3mm were machined from the aged specimen and tested with an initial strain rate of 1×10-3s-1. The solution treated specimens were ground with SiC paper followed by polishing and etching with aceticpicral solution for optical microscope and scanning electron microscope(SEM) characterization. Disks with 3mm in diameter were punched from thin foils with 40μm thickness and dimpled to 10μm before ion milled to electron transparent and characterized with transmission electron microscope(TEM).
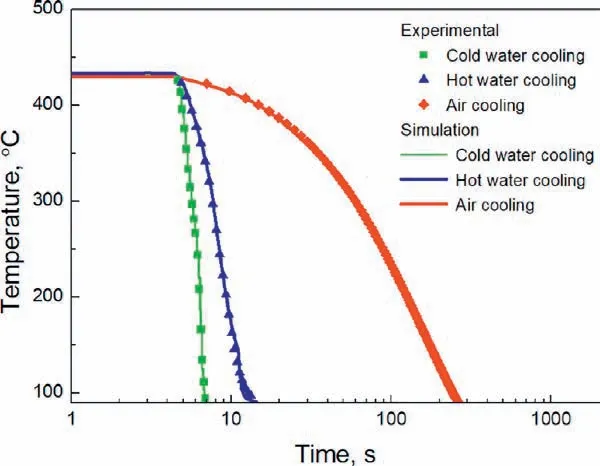
Fig. 2. Measured data and simulated cooling curves under different cooling conditions after solution heat treatment.
Parallel specimens were instrumented with thermocouples with a diameter of 1mm to track the temperature evolution around 4mm away from the top surface under different cooling conditions. The temperature history was used to calculate the heat transfer coefficien of the surface with the inverse heat transfer method using Deform. The formation of residual stress during cooling was modeled with the obtained heat transfer coefficient The model is one eighth of the size of the specimen used in the experiment due to its symmetry as shown in Fig. 1. A homogeneous temperature fiel of 440°C for the specimen was set as the initial condition and the specimen was set to be residual stress free before quenching considering the high solution heat treatment temperature.Elasto-plastic material model with von Mises yield criterion was selected. The constitutive behavior of the alloy was obtained from compression tests [14] and the thermophysical parameters from [15] were used. The residual stress after solution treatment was measured with X-ray diffraction with parameters similar to those used in [6] to verify the simulation results.
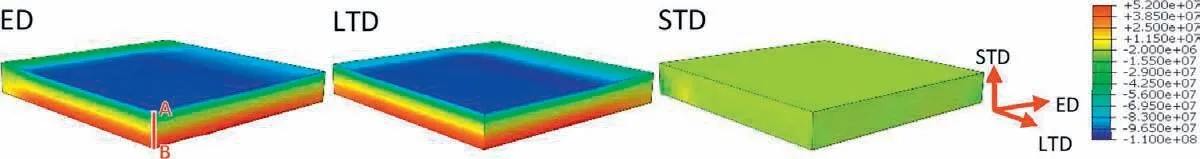
Fig. 3. Simulation results of the distribution of residual stress in the specimen in ED, LTD and STD.
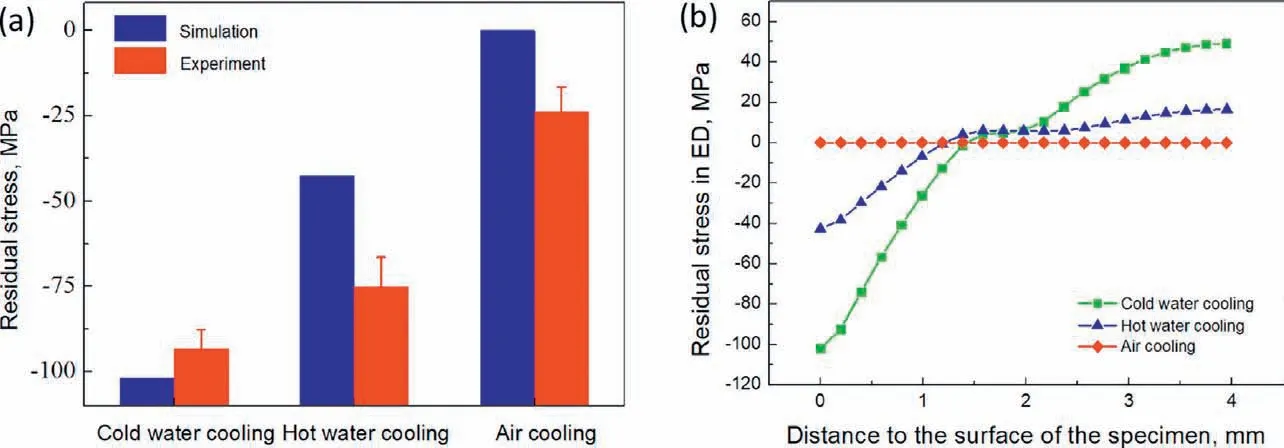
Fig. 4. (a) Simulation and experimental ED residual stress on the surface and (b) simulation result of the distribution of ED residual stress of Mg-5Zn-3.5Sn-1Mn-0.5Ca-0.5Cu alloy under different cooling conditions after solution treatment.
3. Results
3.1. Cooling rate
The cooling curves measured by the thermocouples under different cooling conditions are shown in Fig. 2. Cold water provides the highest cooling speed with an average value of 147.6 °C/s, which is followed by hot water cooling with an average cooling speed of 38.2 °C/s and the cooling speed is the lowest for air cooling with an average value of 1.3°C/s. The simulated cooling curves using the heat transfer coefficien obtained from the inverse heat transfer simulation are also shown in Fig. 2 and are in good agreement with the experimental ones.
3.2. Quench-induced residual stress
The simulation results of the distribution of residual stress in ED,LTD and STD of the specimen after solution treatment and cold water cooling are shown in Fig. 3. Residual stress in ED and LTD is similar, which are compressive on the surface and tensile in the center, the residual stress in STD is relatively small and is compressive in the center and negligible on the surface. The measured residual stress and simulation result of ED residual stress on the surface of the specimen is shown in Fig. 4(a). Although discrepancies can be observed under hot water cooling and air cooling, where simulation results underestimate the residual stress on the surface, the simulation results are in qualitative agreement with the experimental ones and lower residual stress is generated under lower cooling speed. Changing from cold water cooling to hot water cooling and air cooling results in a decrease of the experimental measured surface residual stress by 13% and 43%, respectively. To study the evolution of residual stress along the thickness of the specimen, the simulation results of residual stress in ED along line AB shown in Fig. 3 are plotted in Fig. 4(b). Under cold water cooling, compressive residual stress around -100MPa develops on the surface and firs decreases and then increases in magnitude towards the center with tensile residual stress around 50MPa generated in the center. This is similar to the result of ZK60 magnesium alloy after quenching [6]. Similar pattern of residual stress can be observed for the specimen after hot water cooling, but the magnitude is lower. The residual stress is very low after air cooling.
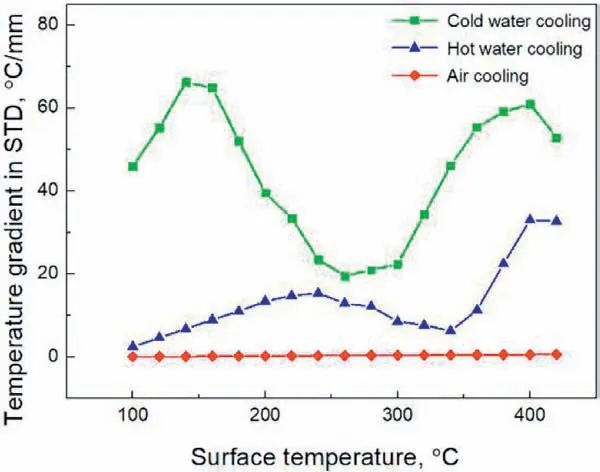
Fig. 5. Simulation results of surface temperature gradient in STD.
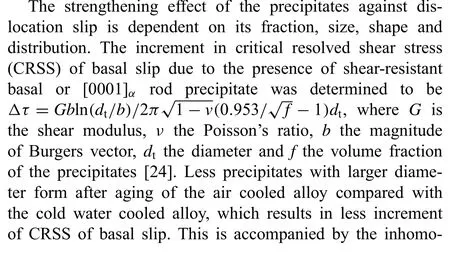
The residual stress formed during cooling is mainly resulted from the inhomogeneous plastic deformation caused by the temperature gradient in the specimen. Fig. 5 shows the simulation results of the evolution of the temperature gradient on the surface of the specimen. Higher temperature gradient can be observed for the cold water cooled alloy at surface temperatures ranging from 100 °C to 440 °C and the difference is more apparent at high temperature when the fl w stress of the material is low and the specimen deforms easily. At the start of cooling, contraction caused by the faster cooling of the surface leads to positive plastic strain on the surface to maintain the compatibility of geometry and more plastic deformation happens for the cold water cooling condition due to its higher temperature gradient. As the temperature drops, the cooling speed in the center outpaces that on the surface, although the temperature gradient for cold water cooling is also higher than that for hot water cooling and air cooling under low temperature, which should produce higher negative plastic strain on the surface and positive plastic strain in the center, partially negating the strain produced at high temperature,however,the strain increment is low compared with that at high temperature due to the increased resistance to plastic deformation with decreasing temperature[16], so the plastic strain produced at high temperature is dominant and high average cooling speed corresponds to high magnitude of plastic strain as shown in Fig. 6(a). Since the residual stress is proportional to the inhomogeneous plastic strain, higher residual stress forms under cold water cooling.The plastic strain changes more sharply near the surface of the specimen compared with that in the center as shown in Fig. 6(a), so higher magnitude of residual stress forms on the surface. A schematic illustration of the distribution of ED plastic strain along the whole thickness of the specimen is shown in Fig. 6(b).
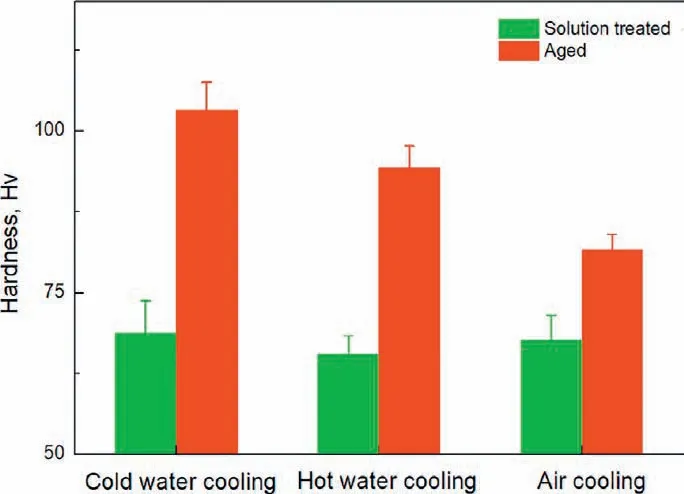
Fig. 7. Hardness of the solution treated and aged alloy with different cooling rates after solution treatment.
3.3. Age hardening and precipitation behavior
The hardness of the alloy in the solution treated and aged states is shown in Fig. 7. The hardness is very similar for the solution treated alloy irrespective of the quenching rate,they are 69 Hv, 66 Hv and 68 Hv for the cold water cooled,the hot water cooled and the air cooled alloy. However, the cooling method has a significan effect on the age hardening ability, with the maximum hardness of 103 Hv for the cold water cooled alloy, 94 Hv for the hot water cooled alloy and 82 Hv for the air cooled alloy. This is consistent with the evolution of the tensile properties of the aged alloy, as shown in Fig. 8, yield strength and ultimate tensile strength is the highest for the cold water cooled alloy, they decrease a little for the hot water cooled alloy and drop sharply for the air cooled alloy, the effect of cooling condition on elongation is modest with a relatively low value for the hot water cooled alloy. So the age-hardening behavior and tensile properties of the alloy are dependent on the cooling speed after solution treatment, which means the alloy exhibits quench sensitivity.A decrease of the average cooling speed from 147.6 °C/s of the cold water cooled alloy to 38.2 °C/s of the hot water cooled alloy and to 1.3 °C/s of the air cooled alloy results in 8.7% and 20.3% decrease in hardness, 1.5% and 17.3%decrease in yield strength and 0.1% and 12.7% decrease in ultimate tensile strength after aging treatment.
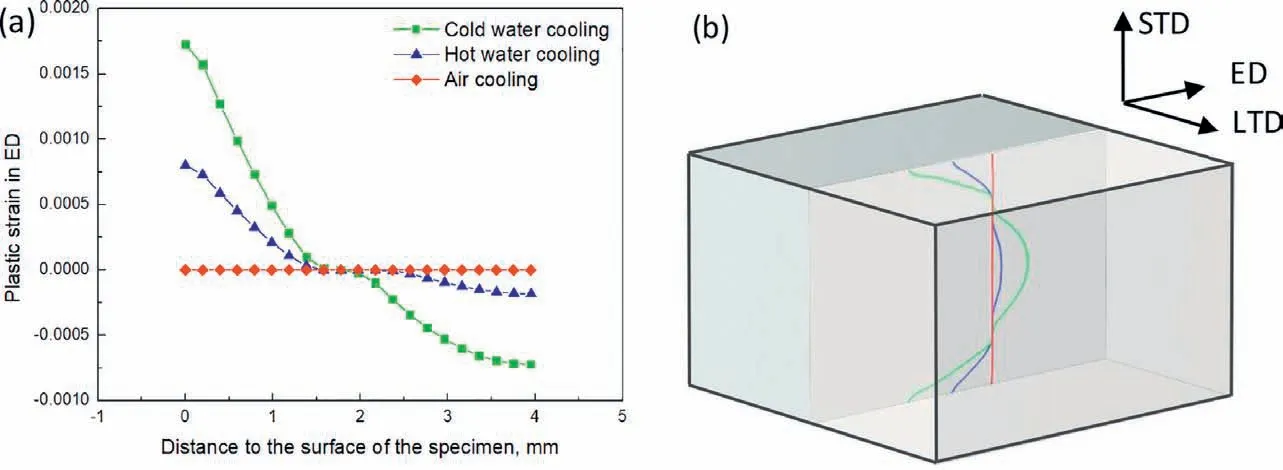
Fig. 6. (a) Simulation results of the evolution of ED plastic strain with the distance to the surface of the specimen and (b) schematic illustration of the ED plastic strain along the whole thickness of the specimen.
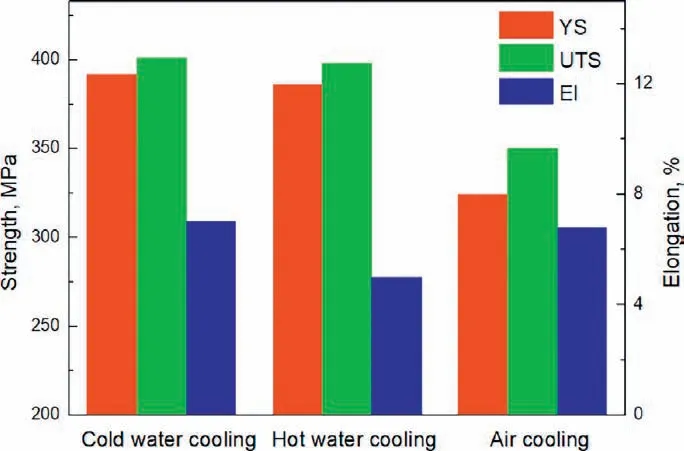
Fig. 8. Tensile properties of the aged alloy with different cooling rates after solution treatment (The results for cold water cooling condition is obtained from [13]).
The microstructure of the alloy after solution and aging treatment is studied to clarify the origin of the quench sensitivity. The optical micrographs of the ED-STD plane of the alloy after solution treatment followed by cold water cooling or air cooling are shown in Fig. 9. The grain size is around 21μm in both states. Some undissolved coarse particles can be observed which are MgZn2and MgSnCa phase according to our previous research [13]. Some relatively fin particles(arrowed) can be observed in Fig. 9(c)(d), they are Mn particles as will be shown later, the Mn particles on the grain boundary (arrowed) inhibit grain growth during solution treatment through Zener pinning effect.
The TEM micrographs of solution treated Mg-5Zn-3.5Sn-1Mn-0.5Ca-0.5Cu alloy after cold water cooling and air cooling are shown in Fig.10.After cold water cooling,there is no second phase preferably precipitates on the grain boundary as shown in Fig. 10(a), but some second phases around 100nm can be observed inside the grain as shown in Fig. 10(b), the selected area diffraction result in Fig. 10(c) shows that they are Mn particles formed during solution treatment due to the low solubility of Mn in Mg [17]. No other precipitates can be observed suggesting that cold water cooling is fast enough to suppress the dynamic precipitation. Fig. 10(d) shows that no second phase preferably precipitates on the grain boundary even under the low cooling speed of 1.3 °C/s. Moreover, The EDS result of the grain boundary area in Fig. 10(e)shows no apparent segregation of alloying elements along the grain boundary. This is in contrast with what was found in aluminum alloys where grain boundary precipitation was observed under low cooling speed [12,18]. In the grain interior,some precipitates located near the Mn phase (arrowed) can be observed as shown in Fig. 10(f), showing signs of heterogeneous nucleation. The EDS result shows that the precipitate is enriched in Zn, so it is postulated to be Mg-Zn phase.
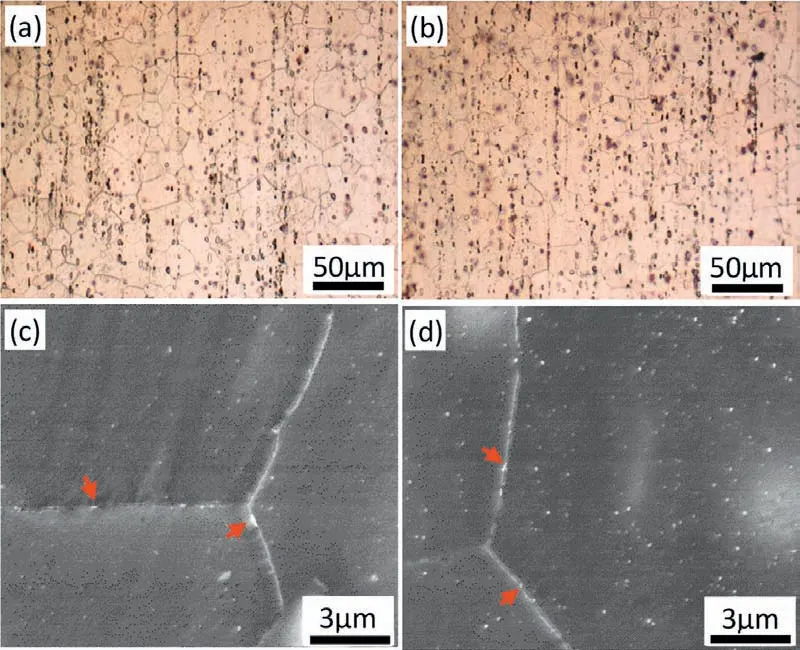
Fig. 9. Micrographs of solution treated Mg-5Zn-3.5Sn-1Mn-0.5Ca-0.5Cu alloy after (a)(c) cold water cooling and (b)(d) air cooling.
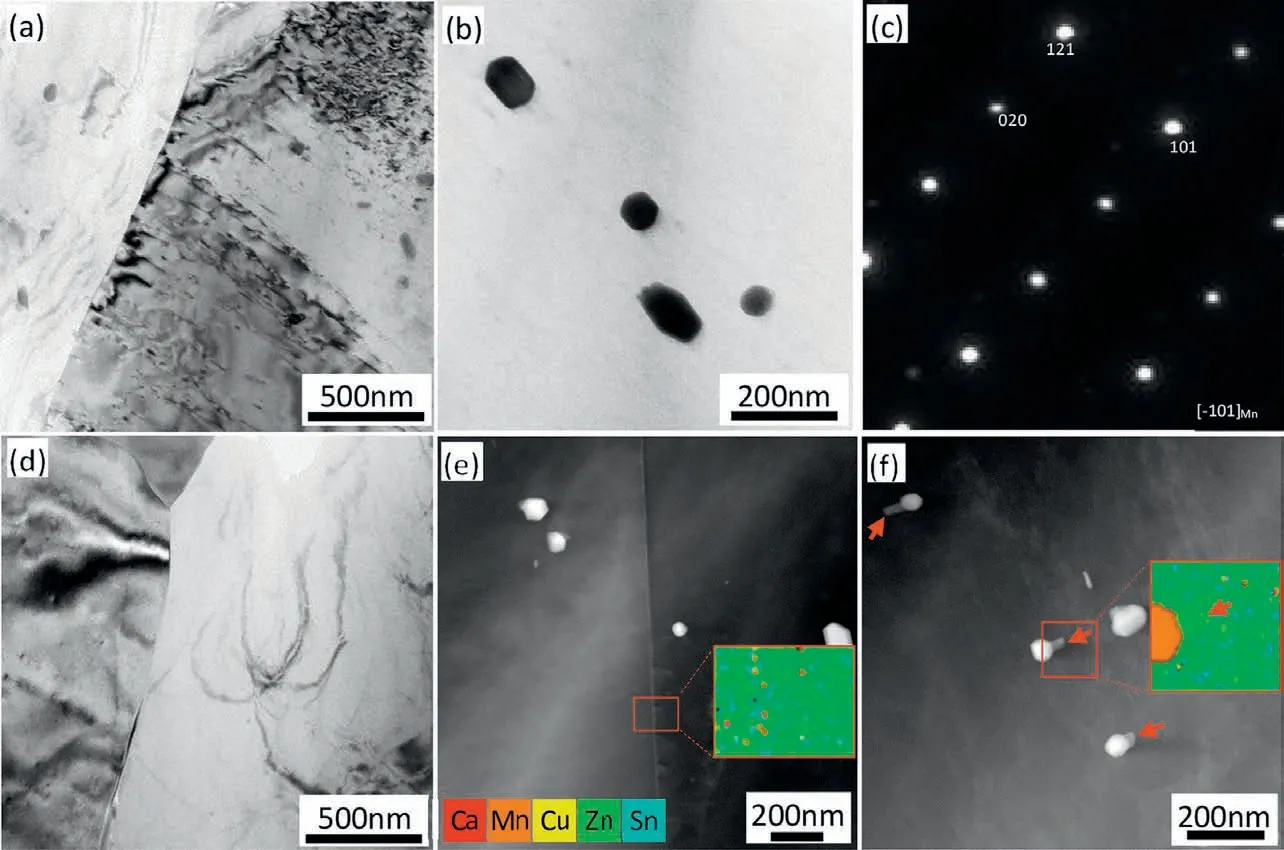
Fig. 10. Microstructure of solution treated Mg-5Zn-3.5Sn-1Mn-0.5Ca-0.5Cu alloy after (a)(b) cold water cooling, (d)(e)(f) air cooling with inset of EDS results and (c) diffraction pattern of particles in (b).
The microstructure of the cold water cooled and air cooled alloy after aging treatment is shown in Fig. 11. Fine and homogeneously distributed precipitates can be observed after aging treatment for the cold water cooled alloy as shown in Fig. 11(a)(c), the precipitates show dotted contrast under the[11-20]Mgzone axis,the high resolution transmission electron micrograph in the inset of Fig. 11(c) shows mainly two kinds of precipitates,the firs is perpendicular to the basal plane and the second is parallel to the basal plane, they are postulated to be β1’ and β2’, respectively, according to the precipitate formed in Mg-Zn-Sn magnesium alloys [19,20]. These fin precipitates contribute to the age hardening of the cold water cooled alloy. Fig. 11(b)(d) show the air cooled alloy after the same aging treatment, coarser precipitates with lower density compared with the cold water cooled alloy can be observed,the distribution of the precipitates is inhomogeneous and is affected by the Mn particles near which a low-density precipitate zone is formed as shown in Fig. 11(d).
4. Discussion
The simulation result is in qualitative agreement with the experimental results in the prediction that the quench-induced residual stress decreases with decreasing cooling rate. The lower residual stress obtained from simulation on the surface as compared to the experimental result should be attributed to the constitutive equation used in the simulation. The plastic deformation during quenching is driven by the evolution of the temperature field which leads to plastic deformation under changing temperature and strain rate, this process is not accurately described by the constitutive equation that does not take into account the strain history. So the simulation result provide a qualitative prediction of residual stress and more sophisticated constitutive equation is needed to simulate the quenching process more accurately.
Since the specimens were cut into small cuboid before aging treatment, the residual stress is believed to be relieved and does not affect the precipitation behavior during aging,the quench sensitivity is attributed to the change in solution treated microstructure under different cooling conditions. The activation of Mn particles as heterogeneous nucleation site for dynamic precipitation may be resulted from the higher interfacial energy between Mn particles and the matrix, which lowers the energy barrier for dynamic precipitate nucleation. Two reasons are accountable for the coarsening of the precipitate for the air cooled alloy, the firs reason is that the dynamic precipitation lowers supersaturation of alloying elements and leads to lower driving force for the nucleation of precipitates;the second reason is the lower concentration of vacancies,it was postulated in the study of age hardening of Mg-Zn and Mg-Zn-Al alloys containing Cu that the presence of Cu enhanced the interaction between vacancies and alloying element [21,22], which was useful in maintaining a high concentration of vacancies to aid the nucleation by forming clusters with alloying element and promoting diffusion. The low cooling speed leads to decreased concentration of quenched-in vacancies, which also lowers the nucleation rate of precipitates, these nuclei grow faster due to the lack of competition from other nuclei in its vicinity and form coarse precipitates with low density after aging. These two reasons also apply to the decreased density of precipitates near Mn particles.The lower driving force for precipitation during aging due to the reduced concentration of alloying element caused by dynamic precipitation near Mn and the growth advantage of these precipitates (arrowed in Fig. 11(d)) that absorb more alloying element during aging treatment results in lower nucleation rate for precipitates, moreover, since the interface between Mn and the matrix acts as sink to vacancies [23],more vacancies near Mn particles are lost due to the presence of the interface under low cooling speed, the positive effect of vacancies on precipitate nucleation is reduced. So the reduced concentration of alloying element and vacancies lead to the formation of a low-density precipitation zone around Mn particles.
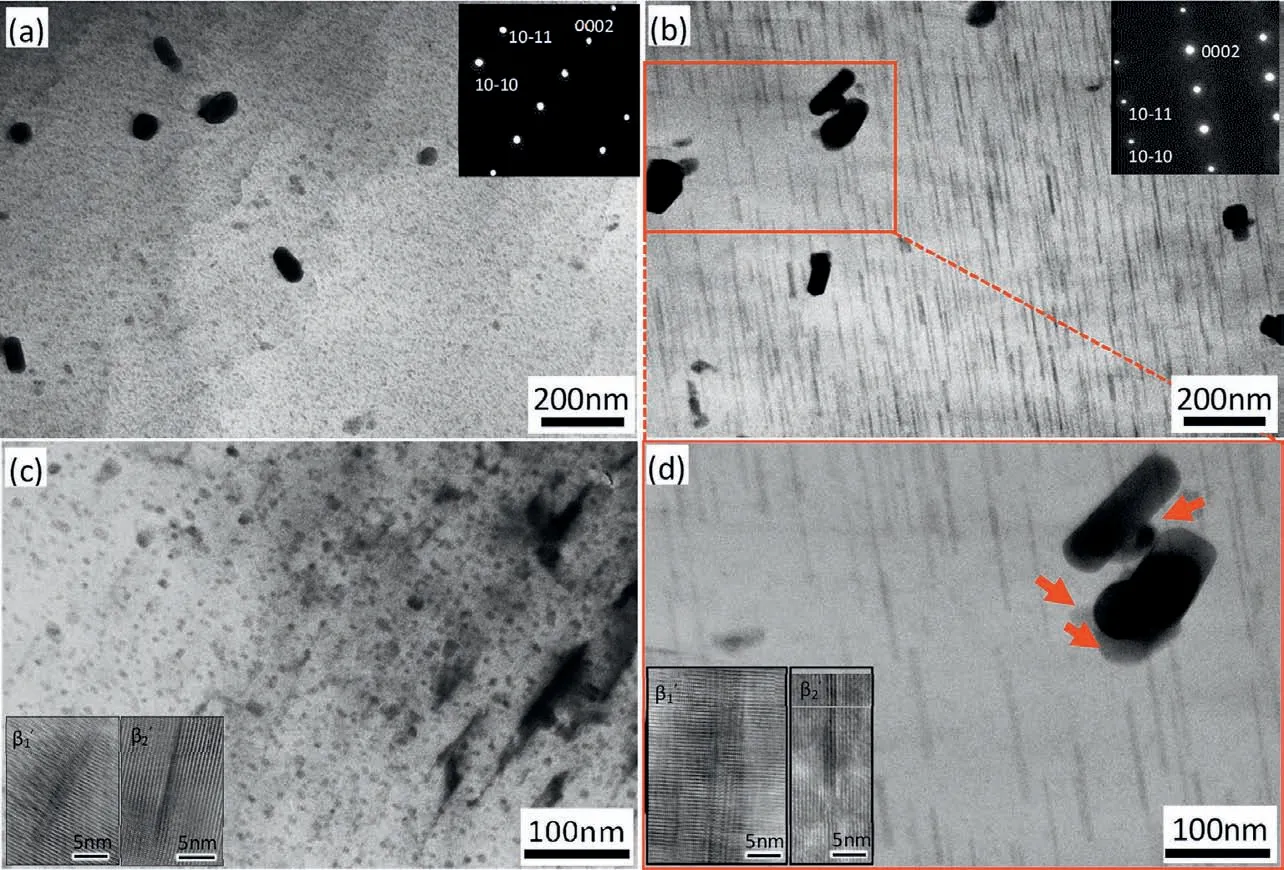
Fig. 11. Microstructure of aged Mg-5Zn-3.5Sn-1Mn-0.5Ca-0.5Cu alloy with (a)(c)cold water cooling and (b)(d) air cooling.

The experimental surface residual stress and hardness of the specimen corresponding to different cooling conditions are normalized with respect to those corresponding to cold water cooling and the results are shown in Fig. 12. Residual stress drops faster than the hardness under decreasing cooling speed,a decrease of 13%and 43%in residual stress and 8.7%and 20.9% decrease in hardness are observed after changing from cold water cooling to hot water cooling and air cooling,which suggests that decreasing the cooling speed to certain extent that produces acceptable strength can be used to reduce the residual stress even without changing the aging treatment conditions for this alloy. The sample used for quenching is 60 mm×60 mm×8mm, an increase in the thickness will lead to higher temperature gradient, higher residual stress but lower cooling speed under constant cooling condition.The effect of sample thickness on the residual stress can be further studied with simulation. The hardness of the specimen with other dimension can be determined by comparing the cooling speed obtained from simulation with the cooling speedhardness curve shown in Fig. 12. Since the magnitude of the quench induced residual stress is dependent on factors like thermal conductivity, thermal expansion coefficien and constitutive behavior of the alloy, all of which are determined by the composition and microstructure of the alloy, the quenchinduced residual stress is composition and microstructure dependent, the same also applies to the quench sensitivity due to the dependence of dynamic precipitation during cooling and controlled precipitation during aging on the composition,microstructure and even the aging treatment condition. The effect of alloying element and aging treatment condition on the quench sensitivity of magnesium alloy could be the subject of further study.
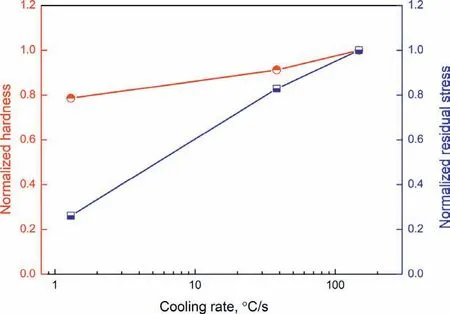
Fig. 12. Evolution of normalized residual stress and hardness with average cooling rate.
The addition of Mn leads to formation of particles with high thermal stability, it was beneficia to refin the grain size of extruded alloy and solution treated alloy by Zener pinning of grain boundary [25-27], the addition of Mn also enhanced the corrosion resistance by depleting the matrix of Fe [28].However, the presence of Mn causes quenching sensitivity,so the addition of Mn as an alloying element should be used with caution in Mg-Zn-Sn alloy whenever quench sensitivity is important. Limiting the content of Mn to the scale that do not form Mn particles or other phase that induces dynamic precipitation under low cooling rate can be one way to exploiting the beneficia effect of Mn and minimizing its negative effect on quench sensitivity at the same time.
5. Conclusions
The effect of quenching rate after solution treatment on the residual stress and age hardening of a high strength Mg-5Zn-3.5Sn-1Mn-0.5Ca-0.5Cu alloy is studied by simulation and experiment and the following conclusions are drawn:
1. The temperature gradient along the thickness of the plate decreases with decreasing cooling speed, which leads to weakened inhomogeneous plastic deformation and a decrease of residual stress.
2. Dynamic grain boundary precipitation does not happen even after air cooling, but Mg-Zn phases precipitate dynamically on Mn particles during air cooling, which leads to the formation of a low-density precipitate zone around the Mn particles after aging.
3. The low-density precipitate zone near Mn particles and the coarsening of precipitates lead to the quenched sensitivity of the alloy.
4. The residual stress drops faster than the hardness, tensile yield strength and ultimate tensile strength with decreasing quenching rate, which makes it possible to lower the residual stress without sacrificin too much age-hardening ability of the alloy by decreasing the cooling speed.
Acknowledgments
This work was supported by the National Key Research and Development Program of China [2016YFB0301105]and the National Key Research and Development Plan[2017YFB0103904].
杂志排行
Journal of Magnesium and Alloys的其它文章
- Mechanism of Mn on inhibiting Fe-caused magnesium corrosion
- An efficien and comparative adsorption of Congo red and Trypan blue dyes on MgO nanoparticles: Kinetics, thermodynamics and isotherm studies
- Twin recrystallization mechanisms in a high strain rate compressed Mg-Zn alloy
- Corrosion behaviour and cytocompatibility of selected binary magnesium-rare earth alloys
- Correlation between test temperature, applied load and wear transition of Mg97Zn1Y2 alloy
- Preclinical in vivo research of magnesium-based implants for fracture treatment: A systematic review of animal model selection and study design
