Additive manufacturing of biodegradable magnesium implants and scaffolds: Review of the recent advances and research trends
2021-05-21NurettinSezerZaferEvisMuammerKo
Nurettin Sezer, Zafer Evis, Muammer Koç
a Division of Sustainable Development, College of Science and Engineering, Hamad Bin Khalifa University, Qatar Foundation, Education City, Doha, Qatar b Engineering Sciences, Middle East Technical University, PO Box 06800, Ankara, Turkey c University of Karabük, Turkey
Received 27 December 2019; received in revised form 17 May 2020; accepted 29 September 2020
Available online 6 November 2020
Abstract
Keywords: Magnesium; Alloy; Implant; Tissue scaffold; Additive manufacturing; Biodegradation.
1. Introduction
With the self-healing ability, bone tissue undergoes remodeling, maturation, differentiation, and controlled resorption through a dynamic process [1]. This process contains osteoclasts and osteoblasts, which are responsible for maintaining tissue health. However, the ability of functional regeneration is considerably limited when the affected tissues are either completely destroyed by traumatic injuries or degenerated by age-related or inflammator diseases [2]. In such cases, the repair of bone tissue defects is very difficult and usually external treatment such as autograft or allograft is required to restore previous form and functions [3]. In autograft, a bone taken from the same person’s body is used, while in allograft,a bone taken from a deceased donor is used. Harvesting autograft tissue increases the morbidity to the patient associated with an additional surgical site, and allograft tissue carries the risk of infectious disease from the donor and depends on the availability of the donor and logistics. Thus, inadequacies of autograft and allograft tissue have led to greater research efforts towards developing biomimetic materials that are suitable for tissue repair without any intrinsic problems. In this context, tissue engineering is an emerging area of research,which typically involves the use of scaffolds to provide structural support to a newly forming tissue [4]. Scaffolds also serve as temporary templates to aid the diffusion of nutrients and oxygen to and excretion of the metabolic waste from the cells,as well as to form the extracellular matrix(ECM)in defected region. The quest for new developments in the design of these scaffolds can be realized by computer-aided designing using the patient-specifi anatomic data. Suitable design of porosity, size and topology of each specifi defect can be selected by computational modeling. Then, the designed scaffold can be materialized by AM technology.
Scaffolds can be produced using various types of materials such as polymers, ceramics, and metals [5-7]. Polymers possess good plasticity and biocompatibility but low strength, poor hydrophilicity, and aseptic inflammatio risk restrict their applications in hard tissue repair [8,9]. Ceramics combine excellent bioactivity and biocompatibility but are usually limited by their high brittleness. Compared to ceramics and polymers, the high mechanical strength and fracture toughness of metals make them more suitable for load bearing applications such as joint replacement, bone plates and screws, tissue scaffolds, as well as dental root implants [10].Currently, stainless steels, titanium, and cobalt-chromiumbased alloys are the most commonly used metallic biomaterials [11,12]. However, the release of toxic and carcinogenic metal ions or particles during degradation process causes inflammatio in human body that weakens the biocompatibility[13]. Also, the mismatch between the elastic moduli of metal and bone leads to stress shielding that deteriorates new bone stimulation and remodeling[14].Further,after the completion of the healing process, the metallic biomaterials are removed by secondary surgical operation(s),which in turn increases the cost and morbidity to the patients [15-17]. Even in the case that metallic biomaterial is permanently fi ed in the body and no additional surgery is required, bone regeneration may not be complete. Thus, biodegradability is considered one of the essential requirements of an ideal implant and scaffold [18].
1.1. Biodegradable metals
Biodegradable metals such as iron (Fe), zinc (Zn), and magnesium (Mg) have attracted special interest in biomedical research. Numerous studies have been performed to prepare biodegradable materials using the alloys of these metals.Pure iron has several advantages to be used as biodegradable metal such as good mechanical properties, biocompatibility,and biodegradability. It is also one of the essential elements for human life [19]. It has favorable mechanical properties.For example, the high elastic modulus of Fe leads to a high radial strength. However, the degradation rate of Fe is too slow to meet the requirements of tissue repair [18,20]. Higher degradation rate is desired for biodegradable Fe [20]. Thus,without modifying its corrosion rate, Fe is not suitable to be used in biomedical applications.
Zinc is another essential element of human life. It is present in several body tissues such as skin, muscle, liver,and bone. Infants and adults need around 2 mg·day-1and 6.5-15 mg·day-1of Zn, respectively for the basic biological functions such as DNA synthesis, nucleic acid metabolism,enzymic reactions, and apoptosis regulation etc. [21-23]. Besides, Zn corrodes at a moderate rate that can be controlled by adding several alloying elements or by applying forming processes. In the literature, it has been shown that the degradation rate of Zn is slower than Mg and faster than Fe, which makes Zn a good candidate to be used as degradable biomaterial. Therefore, Zn has recently attracted significan attention.Yet, the poor strength and ductility still restrict its use in load-bearing applications [5].
Mg is the prominent biodegradable material with exceptional performance [24-26]. It is also an essential element in human body [27], with daily intake of around 300-400mg[25]. 25mg Mg is stored in the body, half of which is stored in bone tissue [13], and the redundant magnesium cations can be effectively excreted in the urine [24]. It has been reported that the presence of Mg in the bone tissue promotes bone strength and stimulate bone growth [13,28]. With a density and Young’s modulus of 1.7g/cm3and 42GPa, very similar to that of human bone;1.8-2.1g/cm3and 3-20GPa,respectively, Mg is a promising material in biomedical applications[25].
The environment of a human body is very aggressive to metallic products. Despite many favorable intrinsic properties of Mg, its rapid degradation in body flui or blood plasma causes uncontrollable hydrogen evolution and formation of gas pockets in scaffold-tissue interface, which delays or prevents the healing of the surgery region [29,30]. In addition,the local alkalization around the Mg-scaffold with increased OH-ions due to rapid degradation can alter the physiological microenvironment and even lead to alkaline poisoning of the surrounding tissues when pH reaches above 7.8. Thus, controlling the degradation rate of Mg, so the generation rate of hydrogen gas and OH-ions would be a good strategy to allow the human body to progressively adjust or deal with the biodegradation byproducts [20,24].
1.2. Biodegradable Mg alloys
Mg alloys are known as the lightest structural alloys [31].These alloys are composed of Mg, the lightest structural metal, and some other elements, such as silicon, aluminum,rare-earth elements, zinc, manganese, zirconium, and copper,so as to enhance the physical properties [32]. The exceptional properties of Mg alloys such as high strength-to-weight ratio, high stiffness-to-weight ratio, lightweight, machinability,castability, and great damping allow them to be used in variety of industries including automotive, electronics, defense,and aerospace.
Cast Mg alloys are widely used in the interior and powertrain components of the automotive industry and constitute the major magnesium alloys used today. Whereas, wrought Mg alloys are less used because of the poor formability and high cost [33].
Rare earth elements, such as Nd, Y, Ce, and Gd, are used as major alloying elements in cast magnesium alloys.These elements possess relatively high solubility in Mg and are effective in precipitation hardening and creep resistance[33]. The prevailing cast magnesium alloys can be listed as: AZ63, AZ81, AZ91, AM50, ZK51, ZK61, ZE41, ZC63,HK31, HZ32, QE22, QH21, WE54, WE43, and Elektron 21,whereas typical wrought magnesium alloys are AZ31, AZ61,AZ80, Elektron 675, ZK60, M1A, HK31, HM21, ZE41, and ZC71. The prefi letters defin two principal alloying metals in magnesium alloys that were developed according to ASTM B275 where A: Aluminum, B: Bismuth, C: Copper, D: Cadmium, E: Rare earths, F: Iron, H: Thorium, K: Zirconium, L:Lithium, M: Manganese, N: Nickel, O: Silver, R: Chromium,S: Silicon, T: Tin, W: Yttrium, and Z: Zinc [34].
In biomedical applications, alloying is the commonly practiced method for controlling the degradation rate of Mg[32,35]. Careful selection of the alloying elements can significantl enhance the degradation resistance and mechanical properties of Mg [13,35]. Mg alloys can be classifie in two main groups. First group contains 2-10 wt% Al with trace amount of Mn and Zn, which exhibits enhanced mechanical properties and moderate degradation resistance. Second group contains mixture of rare earth elements together with a different metal such as Y, Zn, or Ag, and a lesser amount of Zr,which leads enhanced mechanical performance, fine grain structure, and improved degradation resistance [13,36,37].
Most studies indicated that alloying is a promising method for controlling the degradation rate of Mg without compromising its favorable intrinsic properties. For instance, AZ31B,produced by alloying Mg with Al and Zn, improved the in vivo degradation resistance inside the femora of rabbits [38].Hampp et al. [39] studied LANd442 alloy with a composition of 90 wt% Mg, 4 wt% Li, 4 wt% Al, and 2 wt%Nd. An improved corrosion resistance with formation of new bony tissue was observed in a rabbit model. However, small amount of subcutaneous gas was detected in the implant region. Li et al. [40] showed that addition of <2wt% Sr considerably improves corrosion resistance for Mg-Zr-Sr and Mg-Sr alloys. Incorporation of Sr is also found to improve the corrosion resistance of Mg-5Al alloys [41]. In vitro studies by Bornapour et al. [42] showed the formation of a Srhydroxyapatite (HAp) layer on the surface of the binary Mg-Sr alloy after immersion in simulated body flui (SBF). This bioactive surface layer improved the corrosion resistance. After investigating the corrosion behavior and mechanical properties of MgNd2alloy, Seitz et al. [43] suggested Nd2as a suitable alloying element of Mg for bioresorbable applications but not for load bearing applications.
Bone growth can be promoted by incorporation of Ca in Mg alloy,and the mechanical and corrosive properties of Mg-Ca alloys can be manipulated by changing the Ca content. Li et al.[44]prepared binary Mg-Ca alloy with Ca content varying from 1 to 20wt%. Mg-1Ca alloy induced no toxicity to cells. Apatite layer was formed on the surface of Mg-1Ca alloy,which improved corrosion resistance.Similarly,Rad et al.[45] observed enhanced corrosion after adding 0.5% Ca in Mg.
Biodegradable Mg alloys with various compositions investigated thus far revealed favorable results. Careful selection of the type and content of the alloying element(s) can significantly improve the biodegradation resistance of Mg without compromising its biocompatibility and mechanical function.
Mg and its alloys have attracted special interest for temporary implant applications such as bone plates and screws in orthopedics and as stents in cardiovascular implantology.Biodegradable fixatio hardware made of Mg alloy can be a good alternative to today’s stiff bone fixatio hardware [32].Mg alloy can provide adequate support to the bone through the healing period and degrade gradually.
For cardiovascular applications, CE-approved biodegradable Mg-based vascular closure device,named as“Velox CD”,has been developed through transluminal technologies [46].Ureteral stents and coronary scaffolds are other examples to the application of Mg alloys in biomedicals [47,48]. Wang et al. [49] used Mg alloy (Mg-Zn-Y-Nd) stent for the treatment of esophageal cancer. Mg alloy inhibited the growth of esophageal cancer cells and possessed good biodegradability and milder hardness than 317L stainless steel.
Zhao et al. [50] investigated the potential of applying pure Mg screws for the fixatio of vascularized bone graft in osteonecrosis of the femoral head (ONFH) patients. According to the results, Mg screw is found to be biocompatible and effective in stabilizing bone flap Its degradation rate is tolerable by the tissue healing rate, and the released Mg ions stimulate new bone formation.
Various types of materials are available for airway stenting, including silicone, metallic, and hybrid tubes. Though,none of these stents provides satisfactory long-term effectiveness. Non-degradable silicone tubes interfere with the mucociliary clearance and metallic stents cause undesirable tissue ingrowth and formation of severe granulation.These complications often necessitate secondary surgical procedures for removal of the stents. Due to the obvious deficiencie of currently available stents, there is a significant clinical need for a biodegradable airway stents that would maintain airway patency and totally degrade overtime after fulfillin the desired objectives. Wu et al. [51] studied the feasibility of biodegradable magnesium alloys for tracheal stent application. The study identifie Mg alloys(magnesium-aluminum-zinc-calcium-manganese (AZXM)) to show excellent cytocompatibility and found AZXM to be a promising candidate in tracheal stent applications.
Yuan et al. [51] developed a biodegradable magnesium alloy (Mg-Nd-Zn-Zr) nerve conduit for peripheral nerve defect repair and implanted it into the defect area of sciatic nerve of adult SD rat. 2 months after implantation, good nerve regeneration, no scar tissue or inflammator response around, and no bubble formation were observed.
The above-summarized literatures are few examples to the application of Mg alloys in biomedical field It is believed that Mg and its alloys are a new generation of biomaterials that will play a significan role in revolutionizing biomedical applications through suitable design and manufacturing [52].
1.3. Tissue scaffolds
The capacity of the functional tissue regeneration is very limited in the case of severe destruction or degeneration as mentioned before. Scaffolds are engineered to restore or replace the damaged or diseased tissue with a healthy and wellfunctioning tissue. Ideal tissue scaffolds should have the following fi e essential characteristics:
1. To have mechanical properties matching to that of the surrounding tissues to meet anatomic loading conditions in order to eliminate stress shielding and loosening of the scaffold [35].
2. To have suitable surface features such as wettability and roughness to enable attachment, proliferation, and differentiation of new cells [53].
3. To have highly porous, well-define interconnected networks to provide sufficien permeability for the ingrowth of cells in three-dimensional architecture,diffusion of oxygen and nutrients to and excretion of metabolic wastes from the cells [20,54,55].
4. To be biodegradable or bioresorbable with a controllable degradation and resorption rate to match the cell/tissue growth rate [56,57].
5. To be biocompatible to prevent immunological or foreign body reactions [35,56].
Considering these essentials, development of an ideal tissue scaffold necessitates careful material selection, model design, and manufacturing. A suitable material can be selected based on its biocompatibility, biodegradability, and mechanical properties according to the clinical requirements. Computational modeling can be used to design optimum porous scaffold topology consistent with the patient-specifi anatomic data. The applied manufacturing method should be capable of building models generated by computer design and using the selected material with high dimensional accuracy.
1.4. Additive manufacturing
AM, solid free-form fabrication, and 3D printing are synonymous [58], and have been considered an ideal solution for manufacturing complex 3D porous architectures with a precise control over pore topology [58-60]. AM is capable of fabricating versatile scaffolds with intricate geometrical shapes, which can promote uniform cell distribution and mimic the ECM. It employs a layer-by-layer fabrication process from computer aided design (CAD) models [61]. There are several types of AM processes depending on the feedstock materials (powder or wire) and heat sources (laser, electron beam or arc) used. ASTM Standard F2792 classifie the AM processes in two groups as Powder Bed Fusion (PBF) and Directed Energy Deposition (DED) [62]. PBF is considered as one of the near net shape fabrication methods [63], and the mostly preferred AM method for the manufacturing of metallic scaffolds. It employs thermal energy through different technologies such as selective laser melting (SLM) and electron beam melting (EBM) to selectively melt and fuse metal powders together in layers in a powder bed to form a solid pattern [14,60,64]. A layer of powder is spread over the building platform and selectively melted in a desired pattern.Then, the building platform is lowered to a preset distance by a piston to spread and selectively melt the next layer of powder on the preceding layer [65]. These steps are repeated with successive layers of powder until the desired material is completely built [28]. Typical thickness of each powder layer ranges from 20 to 100μm. The powder that remains unaffected by the laser serves as a natural support for the model and remains in place until the model is complete. A shielding gas such as nitrogen or argon continuously fl ws through the build chamber to ensure high purity by minimizing oxygen and hydrogen in the environment, as well as to dissipate the heat and provide rapid cooling in the build chamber [60,64].
This review aims to compile and analyze the available literature on AM of biodegradable Mg alloys and composites for biomedical implants and scaffolds. Recent research on AM of biomedical Mg implants and scaffolds, and their formation quality and microstructural,mechanical and biological properties are reviewed and discussed. Opportunities and challenges of AM of Mg are presented. Briefl , this paper is a comprehensive review that compiles, analyzes, and critically discusses the recent literature on the important aspects of AM of biodegradable implants and scaffolds made of Mg, its alloys and composites.
2. Additive manufacturing of Mg scaffolds
Despite many potential advantages of biodegradable metal scaffolds, their manufacturing in three-dimensional porous architecture with customized micro- and macro-scale geometry is the major challenge.The conventional manufacturing methods, such as powder metallurgy, sintering, foaming, casting,chemical vapor deposition, and electrodeposition fall short of controlling the pore dimensions and the customized geometry of the scaffolds that must match the anatomy of the patient[20]. Thus, conventional manufacturing methods can process only randomly organized porous structures, which do not allow precise control over pore architecture,and are not suitable for optimizing scaffold properties such as porosity, permeability, mechanical strength, and stiffness [20,59]. The random porous structures cannot possess homogenous biological and mechanical properties especially at the micro scale [59],which may cause undesirable material behavior such as strut bending and twisting[66].However,AM allows fabrication of interconnected porous metal scaffolds with the customized external shape and internal architecture [20,55]. These scaffolds possess much superior mechanical and biological properties than those processed through conventional methods [36,55].
A firs ever study on AM of Mg was reported by Ng et al. [67] in 2010. They reported successful melting of single tracks of Mg layer. Later, many articles have been published on AM of Mg, its alloys and composites using mainly SLM process, as well as other processes such as Selective Laser Sintering (SLS), binder-jetting, and indirect processes. Today,owing to its ability to build customized shapes with intricate structures in high dimensional accuracy, AM is attracting more interest in fabricating Mg scaffolds. This section summarizes the present researches on AM of biodegradable Mg-based implants, and scaffolds based on the applied manufacturing processes.
2.1. Selective laser melting
SLM process involves rapid heating and cooling cycles that typically reaches above 105K/s [68,69]. Thus, the grain growth is inhibited through a rapid solidificatio at such high cooling rates[70].It also reduces the composition segregation,allowing a homogeneous microstructural architecture through the scaffold.Dense and homogeneous microstructure,in terms of phase morphology and grain size favors enhanced densifi cation,mechanical properties,and degradation resistance[71].Liu et al. [72] studied manufacturing of porous Mg-Ca alloys by SLM. Due to the grain refinemen and solid solution strengthening, microhardness of SLM-produced sample was superior to that of as-cast pure magnesium.
WE43 is a biodegradable Mg alloy with addition of Y and rare earth elements, and it has been clinically verifie [73].Zumdick et al. [74] investigated the properties of SLM produced WE43 in comparison to the traditional manufacturing methods. The SLM-produced samples showed an extremely fin grains and homogeneous microstructure with grain sizes of around 1μm and very fin secondary phases whereas the as-cast sample possessed much larger grain size 44.3μm and different phases. Tensile tests of the additively manufactured samples possessed an improved ultimate tensile strength of 308MPa and 12% elongation to failure. Another investigation of SLM-produced WE43 alloy was conducted by B¨ar et al.[36], which showed an improved biodegradation resistance in comparison to the as cast counterparts. The studies suggested that SLM is a suitable process to fabricate Mg parts with enhanced microstructure, mechanical properties, and biodegradation performance due to the rapid heating and cooling inherent to the SLM process.
Zn-containing magnesium alloy with satisfactory mechanical properties can be developed into a biodegradable material[24]. In this regard, Chen et al. [71] fabricated binary Mg-Zn alloys by SLM, and studied the mechanical and corrosive properties. The SLM processed alloy exhibited homogenous grains, with an average size of 15μm. Grain growth was effectively inhibited by the precipitation of the MgZn phase and rapid solidification Finer grains reduced the degradation rate and enhanced the microhardness. Mg-Zn binary alloys with varying Zn content was studied by Wei et al. [75] using SLM process. Near full dense parts were obtained at Zn content 1 wt%. Mg-1Zn sample possessed comparable mechanical properties to that of the as-cast counterpart.
The corrosion resistance of Mg-Zn can be enhanced by alloying with Al. AZ31, AZ61, and AZ91 are the common Mg-Al-Zn alloys with moderate corrosion rates. He et al.[76] studied fabrication of Mg-Al-Zn (AZ61) alloy using SLM. Uniformed equiaxed grains were achieved at laser input energy 80W, which revealed minimum mass loss 12.26 mg·cm-2after immersion in SBF for 144h, and maximum microhardness 93.00 HV. Further, apatite formation was observed on the surface of AZ61 alloy. In the study of Liu et al.[77], AZ61 magnesium alloys (Mg-6Al-1Zn) with the addition of Y (0-4wt%) were fabricated using SLM. The degradation resistance of the AZ61 alloy was improved by the addition of Y element. The optimal degradation and hardness were achieved at 2wt% Y. Zhang et al. [78] manufactured Mg-9wt%Al alloy with >99.9% relative density using SLM.A hardness value of 75 HV was reported as compared to that of as-cast AZ91 alloy 60 HV. Wei et al. [63] built AZ91D alloys without obvious macro-defects using SLM. Mechanical properties of the SLM-processed AZ91D were superior to that of the die-cast AZ91D due to the grain refinemen and solid solution strengthening.
Another way of improving the corrosion resistance of Mg-Zn alloys is incorporating HAp (Ca10(PO4)6(OH)2) [79,80].In this respect, Shuai et al. [81] prepared Mg-3Zn/xHA composites using SLM process. Relative density of the as-built samples ranged from 95.5%-97.9%.Rapid solidificatio prevented agglomeration of HA particles and promoted homogeneous dispersion.Increasing HA content lead to the formation of fine grains.Finer grains together with the formation of apatite coating layer lead to improved biodegradation resistance.Further,hardness of Mg-3Zn alloy was improved through fin grain strengthening and second phase strengthening.
Zr is another alloying element for Mg-Zn alloys to reduce the grain size and degradation rate [82-84]. Zhang et al.[85] prepared porous Mg-Zn-Zr (ZK61) alloys at varying Zn content using SLM. A relatively high surface quality was acquired at laser energy density ranging from 1019J/mm3to 1146J/mm3. Shuai et al. [16] used SLM process to prepare ZK60-Cu alloy, which possess excellent antibacterial properties and favorable corrosion in body fluid ZK60-0.4Cu alloy had an improved compressive strength due to grain refinemen strengthening, precipitate strengthening, and dispersion strengthening. The cell culture experiments revealed that ZK60-Cu alloy has good cytocompatibility.
Long et al. [86] prepared Mg-3Zn-xDy (x=0 - 5wt%) alloys using SLM process. Degradation and hydrogen evolution rate of the Mg-3Zn-1Dy alloys was remarkably reduced due to the combined effect of smaller grain size, homogeneous microstructure, and the presence of the second phase. Shuai et al. [87] prepared Mg-Sn-Zn alloys at varying Zn content using SLM. The SLM processed Mg-Sn alloys showed fine grains than as-cast and as-rolled Mg-Sn alloys. With increasing Zn content up to 4wt%, degradation resistance of the alloys improved due to grain refinemen and the formation of Zn(OH)2protective layer. Zn also increased the hardness of the alloys owing to the grain refinemen strengthening and solid solution strengthening.
Li et al. [88] fabricated topologically ordered porous magnesium (WE43) scaffolds based on the diamond unit cell by SLM process.Scaffolds were designed with strut size 400μm,pore size 600μm to yield 67% porosity, while the as-built scaffolds possessed an average strut size 420μm and porosity 64%. Mechanical properties of the porous WE43 (E=0.7-0.8GPa) scaffolds were found to fall into the range of the values reported for trabecular bone (E=0.5-20GPa) even after 4 weeks of biodegradation. The actual topology of the porous structures closely matched the designed topology including a fully interconnected porous structure, high porosity, and precisely controlled geometry of the unit cells. Additively manufactured porous Mg specimens showed a satisfactory biodegradation behavior with about 20% volume loss after 4 weeks. Limited cytotoxicity of less than 25% was reported. In a follow up study, fatigue behavior of the same scaffold was investigated in r-SBF and in air [89]. Biodegradation affected the fatigue resistance of the additively manufactured WE43 magnesium alloy scaffolds and reduced the fatigue strength from 0.3σyto 0.2σy. Cyclic loading accelerated the biodegradation process in r-SBF. The study pointed that the fatigue properties of the biodegradable scaffolds can be improved by optimizing the topological design and laser processing parameters that greatly affect the microstructure of the porous scaffolds. Qin et al. [73] mechanically mixed WE43 and pure Zn powders to fabricate porous Zn-xWE43(x=0%, 2%, 5% and 8%) scaffolds by SLM. High densifica tion of above 99.9%was reported for the as-manufactured Zn-WE43 scaffold. Rapid cooling rate and the addition of WE43 remarkably refine the grain size. Molten Zn easily took a capillary action among powders and sintered the surrounding powders together. Therefore, a lot of powders attached to the molten pool, which increased the strut diameter compared with the design value. Thus, there was a big difference between the geometrical porosity of as-designed 67% and asbuilt 45% scaffolds. The fracture behavior of the porous scaffold was found to be directly related to the structural design.
The reviewed literature shows that SLM is a promising method to fabricate Mg-based parts using Mg powders of a wide variety of chemical compositions. A number of different Mg alloys have been successfully built by SLM. Researches also show that the mechanical and corrosive properties of the as-built SLM parts are superior to the counterparts fabricated by traditional methods that is discussed in detail in Section 3.3 and Section 3.4. In addition to building bulk Mg cubes, SLM also allows for building porous Mg scaffold models with a well-define interconnected network in high dimensional accuracy.
2.2. Selective laser sintering
Selective laser sintering (SLS) is another type of powderbased AM that uses laser to sinter powder particles, thereby binding the material together to form a solid structure [90].The major difference between the SLS and SLM processes is that laser scanning in SLM involves complete melting of powder particles, whereas they are partially melted and resolidifie in SLS. Tsai et al. [91] incorporated magnesiumcalcium silicate (Mg-CS) powder into poly-ε-caprolactone(PCL) to manufacture 3D porous bioactive Mg-CS/PCL scaffolds by SLS process.The wettability and degradation rates of PCL were improved by the incorporation of Mg-CS powder.Mg-CS content significantl stimulated the new bone growth in SBF. Biocompatibility of the scaffold samples was confirme by in vitro studies with human mesenchymal stem cells (hMSCs).
Poly-l-lactic acid (PLLA) is a promising bone repair material owing to its good biocompatibility and natural degradability. However, the poor mechanical properties due to its poor crystallinity and local inflammator response due to its acid degradation byproducts limit its application in tissue scaffold. To eliminate these limitations, Shuai et al.[92] incorporated magnesium oxide nanoparticles (nMgO)into PLLA to manufacture PLLA/3nMgO scaffold using SLS method. nMgO exhibited a good affinit to PLLA and served as a nucleating agent during crystallization process to enhance the crystallinity of PLLA. The tensile strength, Elastic modulus, and Vickers hardness of PLLA/3nMgO was enhanced by 38%, 24% and 11%, respectively, as compared with PLLA. Further, nMgO neutralized the acid degradation byproducts of PLLA that can prevent local tissue inflammatio after implantation. Improved biocompatibility of PLLA/nMgO scaffolds was observed in vitro cell culture.Another study incorporated nMgO into poly(3-hydroxybutyrateco-3-hydroxyvalerate) (PHBV) to manufacture 3D porous PHBV/nMgO scaffolds by SLS [93]. The SLS processed scaffold exhibited a well-ordered interconnected microporous structure. The presence of nMgO imparted strong antibacterial activity to the scaffold and enhanced the compressive strength of the PHBV scaffolds by 96% at 5 wt% nMgO loading. nMgO similarly neutralized the acid degradation byproducts of PHBV and promoted biodegradation of the scaffolds. Further, nMgO stimulated cellular adhesion, proliferation, and differentiation. Sun et al. [94] incorporated wollastonite (CaSiO3) into Mg2SiO4to fabricate MgO-CaOSiO2scaffolds via SLS. The as-built scaffold exhibited a well interconnected porous network, favorable cell adhesion and proliferation. It was noted that addition of CaSiO3enhanced the densification degradability, and bioactivity of the scaffold.
There are limited studies on the manufacturing of biodegradable Mg parts using SLS process. Generally, SLS processed scaffolds exhibit poor formation quality due to partial melting of particles that cannot fully infiltrat into the voids, thereby leave formation pores inside bulk metal and struts. Thus, SLS processed implants and scaffolds possess relatively low densificatio and poor mechanical properties,which restricts their use in load bearing orthopedic applications [1].
2.3. Binder jetting
Binder jetting is one of the AM methods, which employs a two-step process. The firs step includes binding of powder through adhesion and chemical reaction mechanisms through a specifie region in each progressive layer [95]. For adhesion, a polymeric binder material either in a liquid or solid form is used to glue particles [96-98], while in the case of chemical reaction, particles react with a selectively deposited solution to bond together in specifie regions [99,100]. As with other powder-based manufacturing methods, the object being printed is self-supported within the powder bed and is removed from the unbound powder once the process is complete. In the second step of binder jetting, the binder is removed through a debinding process, followed by posttreatment processes such as sintering [101-103], infiltratio with a second material [104], and HIP [105] for densifica tion.
Magnesium phosphate Mg3(PO4)2is a bioceramic with exceptional biocompatibility, bioactivity, and biodegradability[106]. Farag and Yun [107] developed a gelatin/Mg3(PO4)2composite scaffold via binder jetting. The addition of up to 6 wt% gelatin into Mg3(PO4)2induced the formation of dense struts, thus significantl improved the mechanical properties of the scaffolds.In addition,the composite scaffolds exhibited good wettability and cell affinit .Vorndran et al.[108]applied binder jetting on Mg3(PO4)2powder with a binder liquid containing 2M K2HPO4, 0.5M (NH4)2HPO4or 20% H3PO4to form a matrix of either struvite-(K) (MgKPO4·6H2O), struvite (MgNH4PO4·6H2O) or newberyite (MgHPO4·3H2O) by a hydraulic setting reaction. Post-hardening process increased the compressive strength of struvite and newberyite scaffolds to 10MPa and 35MPa, respectively compared to the initial strengths of the as-built samples in a range of 1.3-2.8MPa.Despite the good mechanical properties achieved with the newly developed scaffolds, the study lacks cell viability and biocompatibility tests to assess their application potential in tissue engineering. Meininger et al. [109] applied binder jetting process to fabricate Mg3(PO4)2and Sr-substituted Mg3(PO4)2-based biodegradable scaffolds with pure water as the binder. Sintering and hardening processes were applied to the additively manufactured samples. Highly interconnected porous topology with an average pore size of 20μm was achieved. No compromise of mechanical strength was observed after substituting Sr in magnesium phosphate cement.In vitro tests showed a reduced release of Mg2+and improved corrosion resistance with the incorporation of Sr.
Salahi et al. [110] developed a capillarity-driven bridging method which aided in rapid assembling of Mg particles into 3D structures with least metallurgical complexity. This method converted the MgO fil on the outermost layer of Mg powder into inter-particle bridges. The concept was based on relating the physical properties of Mg powder feedstock to the amount of liquid required for the formation of capillarydriven bridges. Capillary-mediated assembly of powder particles eliminated the use of polymeric binders and maintained the overall composition of powder materials constant. Mg-5.9Zn-0.13Zr powder and a single-phase solvent were used to manufacture Mg alloy scaffold through binder jetting by Salahi et al. [111]. The interactions between the solvent and superficia particle layer in the powder bed enabled capillarymediated binderless assembling of particles in each layer. After completion of printing, the as-built scaffold was sintered to decompose interparticle networks.
Binder-jetting employs a two-stage process for fabricating 3D parts. The use of sacrificia binders for binding the powders together and the requirements of an additional process to remove the binder are the drawbacks of this method. Parts produced with the binder-jetting technology are basically particles glued together resulting in fragile structures with limited mechanical performance. Thus, post-processing is needed for densification In binder jetting, due to the method of binding, the material characteristics are generally not suitable for structural parts and despite the relative speed of printing, additional post processing can add significan time to the overall process.
2.4. Indirect additive manufacturing
Infiltratio can be employed as an indirect method for 3D manufacturing of Mg scaffolds. Firstly, a 3D CAD model with desired architecture is developed. Then, a positive polymeric template of the CAD model is manufactured by AM.This polymeric template is infiltrate with NaCl paste. The template is then removed by heating, and NaCl is sintered to form a negative NaCl template. Liquid Mg is casted into this template with an applied pressure, followed by dissolving NaCl to finall obtain the Mg scaffold [112].
An open-cell porous Mg with a dimensional accuracy 88-95% and fin macroscopic features 0.8mm was successfully manufactured using infiltratio method by Staiger et al.[113].Nguyen et al. [59] also manufactured topologically ordered porous Mg, which showed a smaller difference of 2.5% -8.3%in dimensions from CAD models to Mg scaffolds.Compared to the initial design, there was a maximum of 6.1%reduction in porosity in Mg scaffolds. Owing to the rough surface topography (10.2mm) of the manufactured scaffolds,the surface area increased by 70%.
Oosterbeek et al. [114] studied a topologically ordered magnesium-biopolymer composite. First, a porous Mg cylinder with 2mm square pores and 1.5mm-thick struts was fabricated to yield a theoretical porosity of 59.6% through infil tration method. Then, porous Mg structure was molded with polylactic acid (PLA) through injection to produce Mg-PLA composite. Mg/PLA composite showed reduced lower yield strength and corrosion resistance than Mg. It was suggested that PLA is not a suitable biopolymer to be used in this composite system. Lin et al. [115] manufactured CoCr scaffolds (porosity: 80 vol%, pore size: 700μm, inner diameter:24mm) using SLM method, and infiltrate the scaffolds with Mg-3Al-1Zn (AZ31) alloy at 7500C in inert atmosphere, using pressure-less infiltratio method.The infiltrate composite was almost completely dense and demonstrated superb mechanical properties. The degradation of magnesium fille lead to a progressive reduction in the mechanical strength of the composite. However, it was observed that the degradation rate of the composite was much greater than pure AZ31 alloys due to galvanic corrosion, composite interface, and surface area.The degradation of AZ31 resulted in reduced stiffness and strength.
Despite 3D printing of the polymer mold then infiltratio of salt paste inside the printed mold, Kleger et al. directly printed NaCl templates with a structured porosity [116].Stable NaCl paste with suitable fl w behavior was prepared with the aid of surfactants. Printed NaCl paste was sintered,then infiltrate with Mg melt. Later, the NaCl template was removed by leaching in aqueous NaOH solution. The strut size of the NaCl template and the respective pore size of the Mg scaffold were found to correlate closely with a maximum mismatch of 0.03mm. This corresponds to a difference less than 7%. Dimensional accuracy of the overall process was therefore found to be limited by the 3D-printing precision of the NaCl template rather than by the infiltratio capabilities of the Mg melt.
Infiltratio method holds the advantage of eliminating the use of Mg powder, which exhibits a volatile and explosive nature that are discussed in Section 4. However, in infil tration method, the geometric features such as pores and struts are limited to macro-scale, which may not be suitable in biomedical applications since open porous structures with micron-scale pores and struts are required for good biological performance. Also, topological discrepancies between the as-designed and as-built scaffolds, reported in the indirect manufacturing of Mg scaffolds, result in different pore size and strut thickness, which substantially impact cell ingrowth and mechanical integrity [117-121]. The advantages and challenges for AM of Mg are summarized in Table 1.
3. Properties of additively manufactured Mg
A number of materials and processes for AM of biodegradable Mg has been investigated. The mechanical and biological performance of the fina products are greatly influence by microstructure, design geometry, and dimensional accuracy of the manufacturing method, as well as the formation quality.In this section, the performance of additively manufactured Mg-based biodegradable materials is extensively reviewed and discussed.
3.1. Formation quality
Densificatio is a term used for the assessment of formation quality and optimization of the process parameters. Densificatio is the prevention of defects, which are random and separated pores, so-called processing pores, resulted by improper processing conditions. The undesired processing pores inside the struts can deteriorate the biological and mechanical performance of the Mg-based scaffolds [28,73]. Thus, a better formation quality can be attained by a more stable process and increased densificatio [122,123]. In the literature, relative densities greater than 99.5% have been achieved in SLM processed bulk metals [60,124]. The recent literature on the formation quality of additively manufactured Mg is given in Table 2. AM has been able to build Mg alloys with a relative density as high as above 99.9%.
3.2. Microstructure
The microstructure significantl effects the physical properties such as strength, toughness, ductility, hardness, as well as the wear and corrosion resistance of a material. The microstructure of the additively manufactured Mg parts is determined by the chemical composition of the powder material and processing conditions. High cooling rates involved in SLM results in much fine grains than those achievable with conventional methods. Ng et al. [125] reported enhanced microstructure by SLM of single track Mg due to high cooling rate and rapid solidification SLM-produced WE43 exhibited much fine grain size 0.4 - 2.9μm, as compared to as-cast counterparts 44μm [74]. Similarly, Wei et al. [63] achieved an average grain size 1.2μm with SLM produced AZ91D,whereas die-cast counterparts possessed much coarser grain size 57μm.
The recent literature on the microstructural studies of additively manufactured Mg is summarized in Table 3. High cooling rates favored improvement of microstructure and physical properties in manufacturing of Mg alloys[126,127].The grain size of the additively manufactured Mg-based parts ranged from sub-micron to 30μm, much fine than the counterparts (40 - 300μm) produced through the traditional methods. Besides, typical microstructural characteristics of additively manufactured Mg parts are refine grains, enhanced solid solution, and homogenized microstructure owing to the high cooling rate and rapid solidificatio during SLM process. These factors have dominant effect on the mechanical and corrosive properties of Mg. Thus, improved microstructure favors superior biological and mechanical functions that are discussed in the following sections.
3.3. Biodegradation
Biodegradation performance of the additively manufactured Mg depends on the selected material, design, and manufacturing process. In addition, processing conditions have dominant effect on the biodegradation characteristics of the as-built parts. Niu et al. [128] investigated the corrosion behavior of bulk pure Mg fabricated by SLM. The study emphasized the importance of selecting suitable parameters for SLM processing of Mg to minimize processing pores and corrosion rate. The corrosion rate (r) is calculated by the following equation:

where M1and M2are the material mass before and after corrosion, respectively, and tiis the immersion time.
Previous studies show that corrosion rate of Mg alloys can be reduced through grain refinemen [129]. As mentioned earlier, SLM process involves rapid cooling, which lead to the formation of fin grains and homogenized microstructure [77] that favors enhanced corrosion resistance.Li et al. [88] successfully fabricated WE43 scaffolds using SLM method. The as-built scaffolds exhibited enhanced biodegradation resistance 0.17ml/cm2·day compared to theas-cast and as-extruded counterparts 0.3-2ml/cm2·day. Shuai et al. [130] employed SLM process to enhance the corrosion resistance of ZK60 for biodegradable implant application. The grain refinement homogenized microstructure, and extended solid solution due to the rapid solidificatio during SLM process enhanced corrosion resistance of ZK60 alloy. Cu-containing Mg alloys (AZ61) are aimed to combine the benefit of biodegradation of Mg and antibacterial functions of Cu. However, their clinical application is restricted by fast degradation. He et al. [76] improved the degradation resistance of AZ61 using SLM process. Likewise, Xu et al.[70] improved biodegradation resistance of ZK30-Cu alloy,through grain refinemen owing to rapid solidificatio during SLM process. The SLM-processed Mg-Sn alloys exhibited fine grains in comparison to as-cast and as-rolled Mg-Sn alloys [87], which lead to an improved biodegradation resistance.

Table 1 Benefit and drawbacks of technologies for AM of biodegradable Mg.
Bioceramics exhibit low mechanical strength, while Mg exhibits suitable mechanical strength but poor corrosion resistance. Thus, bioceramic reinforced Mg alloys can provide the combination of adequate mechanical strength and corrosion resistance.However, agglomeration of β-TCP in Mg matrix during conventional manufacturing processes inhibits successful fabrication of β-TCP/Mg alloy composites [131-133].Hence, Deng et al. [134] aimed to employ SLM process to prepare the composites of β-tricalcium phosphate (β-TCP)and Mg-6Zn-1Zr (ZK60). Rapid solidificatio allowed a homogeneous distribution of β-TCP along grain boundaries of α-Mg that promoted the formation of apatite fil on the surface, thus hindered corrosion. Also, ZK60/8β-TCP composite possessed improved mechanical strength and cytocompatibility. Shuai et al. [135] incorporated Nd into ZK60 alloy via SLM process. Fine α-Mg grains and intermetallic phases along the grain boundaries were observed in the as-built sample. Degradation resistance was improved with the formation of a dense surface layer promoted by the presence of Nd2O3,as well as the 3D honeycomb structure of intermetallic phases,which formed a tight barrier to inhibit corrosion.
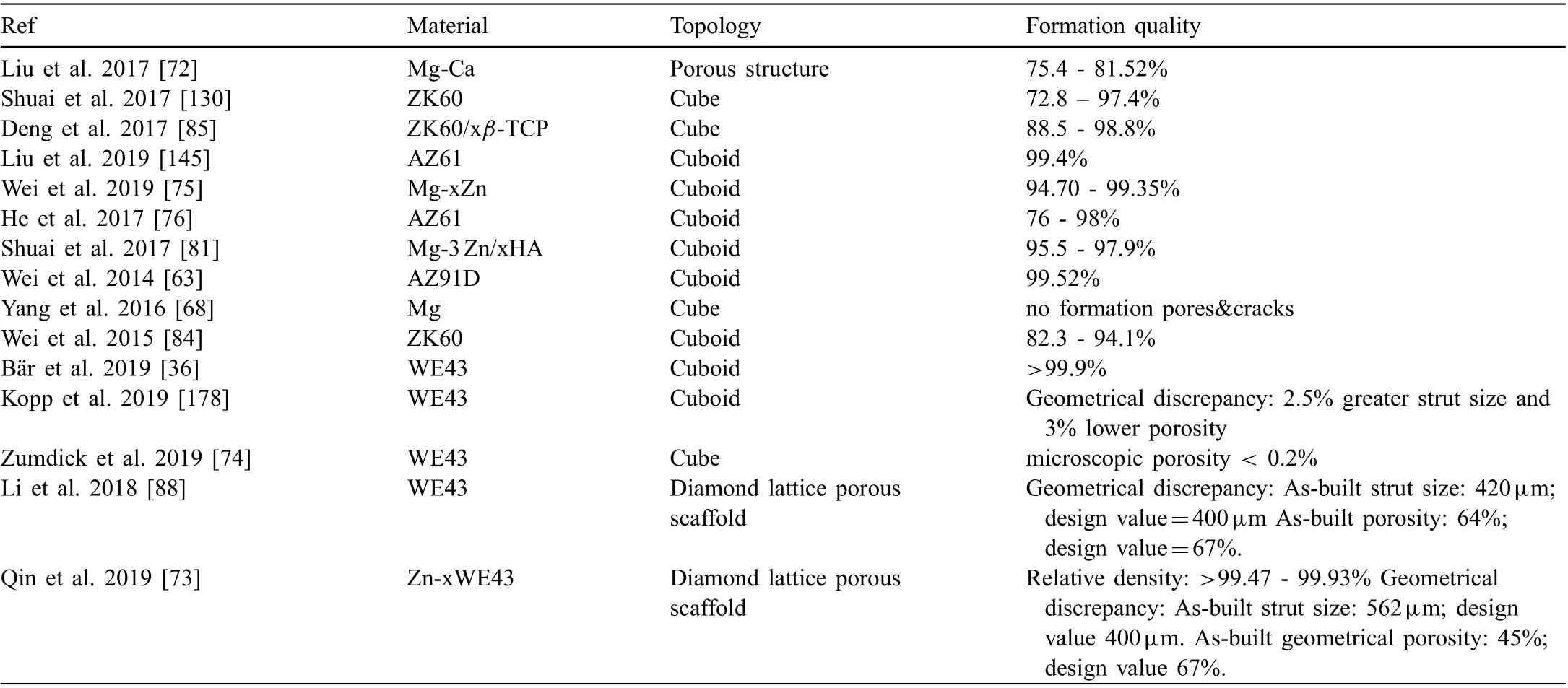
Table 2 Formation quality of the additively manufactured Mg-based biomaterials.
In addition to grain size, intermetallic phase is also an important factor affecting the biodegradation behavior of Mg alloys[136,137].Shuai et al.[138]studied different grain size and intermetallic phase volume fraction by introducing varying concentrations of Al into Mg-Zn alloy. ZK30-xAl cubes(5mm×5mm×5mm) were fabricated via SLM. According to the results, grain size refine and fraction of intermetallic phase volume increased with Al content. When Al content was below 3 wt%, the grain refinemen was the major factor affecting the degradation behavior. The fine grain created numerous grain boundaries, making the alloy passivate readily and lead to an improved degradation resistance. However,with Al further increasing, the intermetallic phase became the main factor affecting the degradation behavior even though grain size was further refined In another study, Yang et al.[139] incorporated mesoporous silica into ZK60 alloy to improve the degradation resistance via SLM process. Owing to the fast heating and cooling during SLM process, mesoporous silica particles homogeneously distributed inside Mg matrix and formed a decent interface binding. Having a more positive corrosion potential, mesoporous silica served for surface passivation of the Mg matrix. Also, the mesoporous structure and exceptional bioactivity of silica promoted the deposition of apatite layer, which served as a protection fil against corrosion of Mg matrix.
The available literature on the biodegradation performance of additively manufactured Mg is summarized in Table 4. Improved microstructure, owing to the fast cooling and rapid solidificatio in SLM process, leads to enhanced biodegradation resistance.
3.4. Mechanical properties
Implants and scaffolds should endure anatomic loads [46].Therefore, their mechanical properties such as hardness, compressive and tensile strength, elasticity of stiffness, ductility,and toughness need to be analyzed before clinical applications. A significan mismatch between the elastic moduli of an implant/scaffold and the surrounding tissue can cause the so-called stress shielding, which occurs when the physical stresses are taken up by the implant rather than by the bone.Stress shielding causes bone atrophy that result in the loosening of implant/scaffold and eventually the premature failure of the implant/scaffold. The elastic modulus of cancellous and cortical bones are in the range of 22.4 - 132.3MPa and 7.7 - 21.8GPa, respectively. Implants/scaffolds should exhibit an elastic modulus mimicking that of natural human bone[140]. Thus, adjusting the elastic modulus to a right value is essential for implant/scaffold design. In that sense, several approaches can be used to prevent mismatch of mechanical properties between the implant/scaffold and bone. Alloying with β stabilizers is one of the approaches to reduce the elastic moduli of the implant/scaffold through the introduction of a β phase in the microstructure. Elastic moduli canalso be effectively reduced with porosity. According to the model of Gibson and Ashby [141], relative density is the most important structural characteristic of a porous material that influence Young’s modulus. Relative density is the ratio of the density of the porous material (ρ) to that of the solid material (ρs). The relationships among the elastic modulus,plastic collapse strength, and relative density is define by the following formulas:

Table 3 Microstructural characteristics of the additively manufactured Mg.
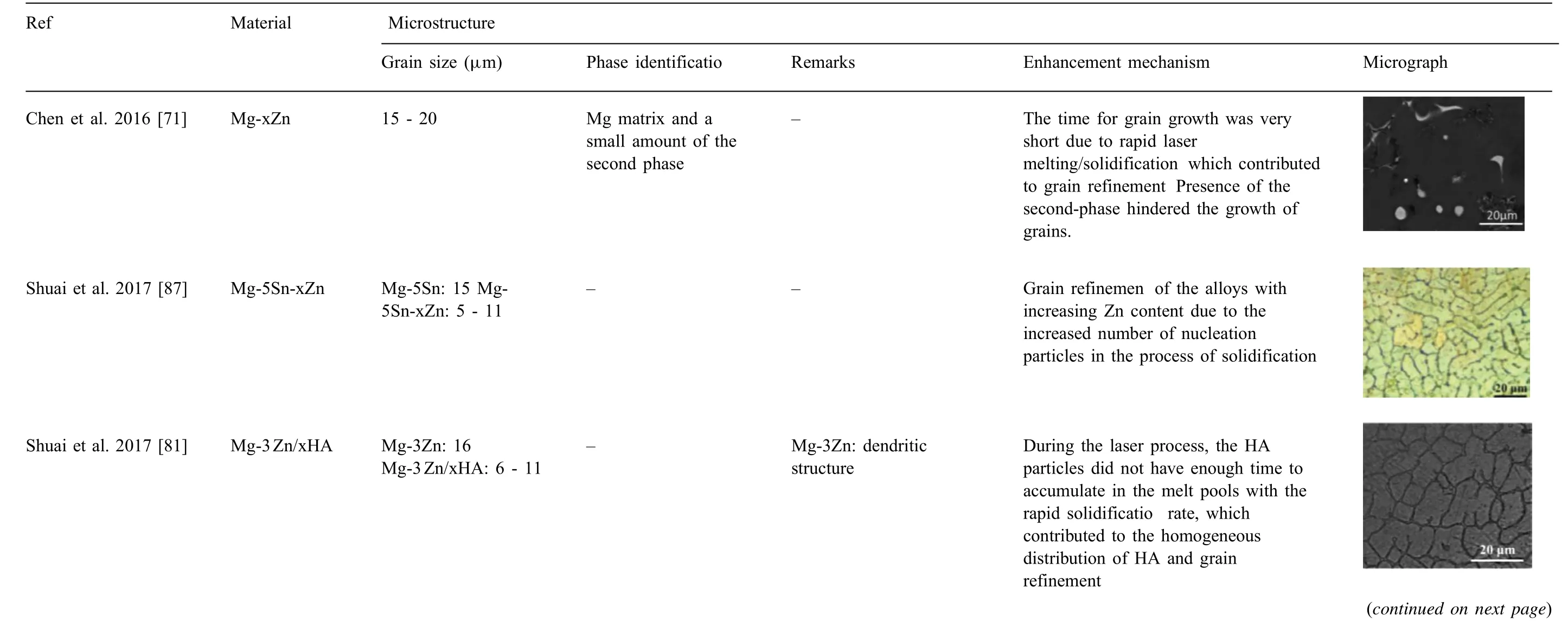
Table 3 (continued)
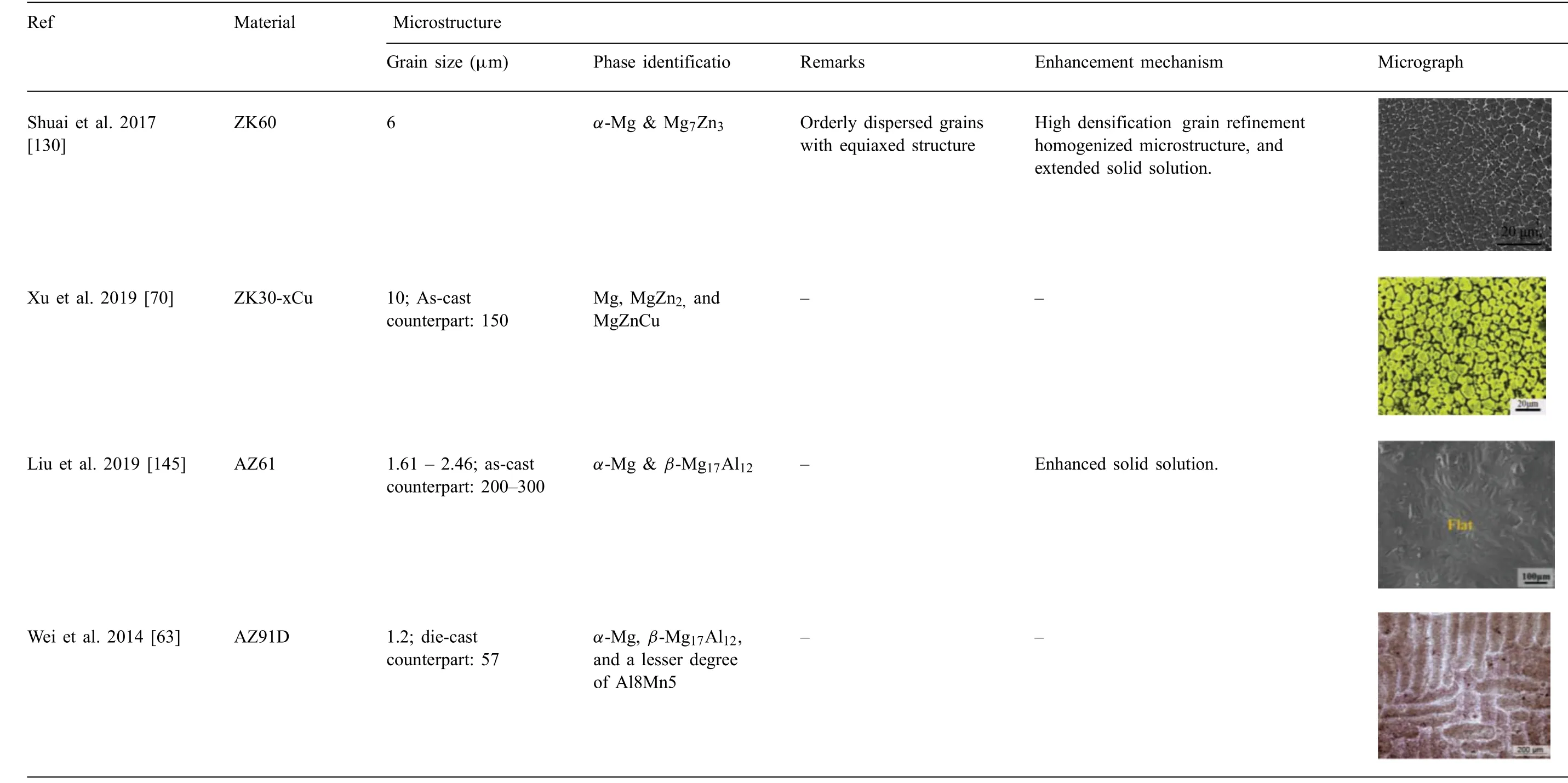
Table 3 (continued)
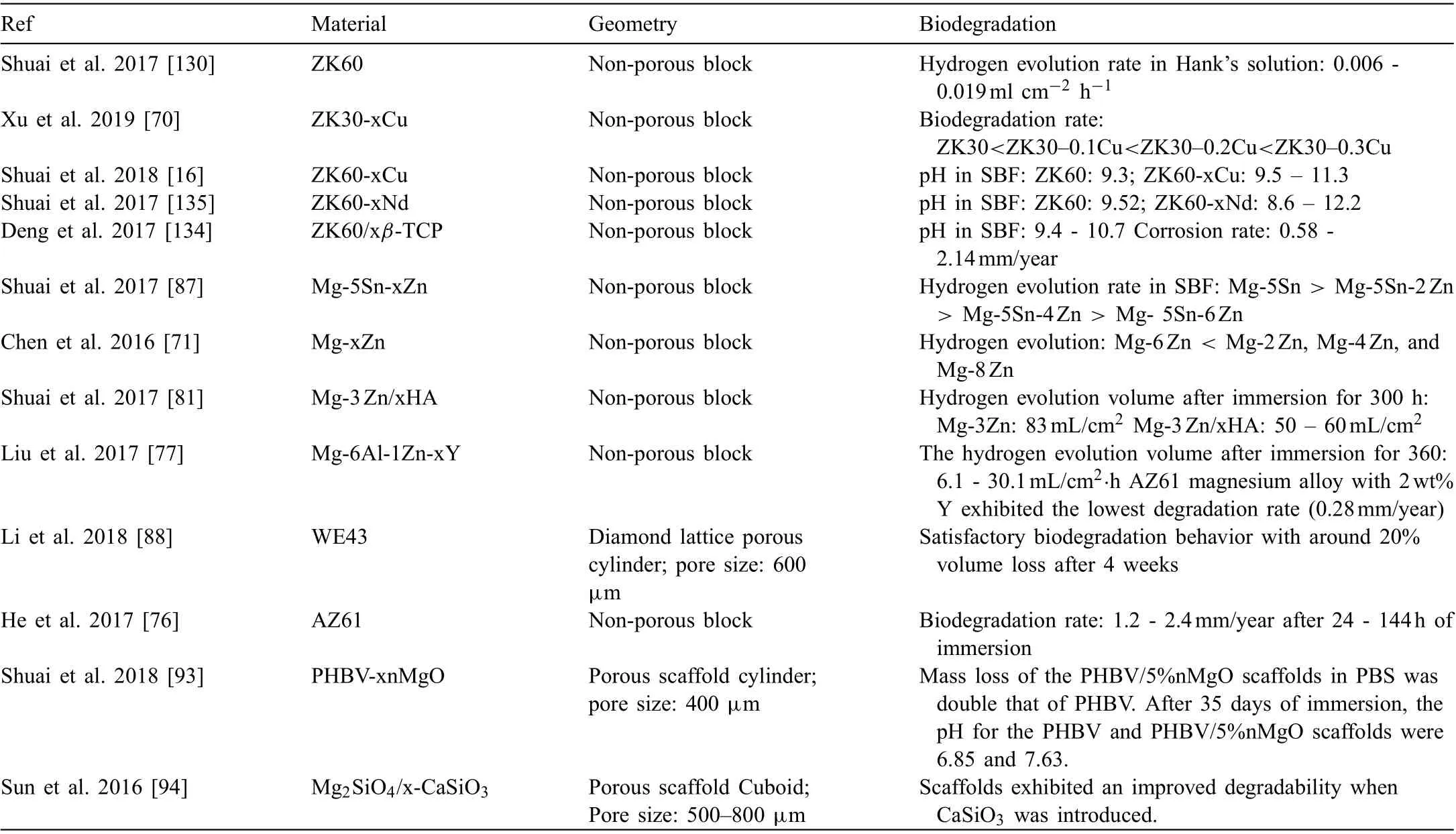
Table 4 Biodegradation performance of the additively manufactured biodegradable Mg parts.

where σplis the plastic collapse strength, ρ/ρsis relative density, ρysis yield strength. E represent the Young’s modulus;subscript (s) represents the substrate material property. These equations describe that increasing porosity reduces the elastic modulus and plastic collapse strength. On the other hand,mechanical strength of an implant/scaffold can be improved through grain refinemen according to the Hall-Petch equation as follows [142];

where σyis yield stress, σ0is a material constant for the starting stress for dislocation movement, K is another material constant; so-called strengthening coefficient and d is grain size. In a similar way, the grain refinemen signifi cantly improves the hardness of the materials according to the Hall-Petch equation as follows [142];

where H is the hardness of the material, H0and k are appropriate constants associated with the material hardness, and d is the grain size.
As mentioned before, SLM-produced materials possess refine grains due to high cooling rates and rapid solidification Researchers studied manufacturing of biodegradable Mg alloys of different compositions using SLM. In the study of Ng et al. [143], single track of pure Mg showed grain refinemen and enhanced hardness in accordance with the Hall-Petch equation. Yang et al. [68] built cubes of pure Mg through SLM process and confirme the same enhancement mechanism. Later, variety of Mg alloys were built by SLM and exhibited improved hardness owing to the high cooling rates and rapid solidificatio [16,71,72,87,144,145].
Mechanical properties such as compressive strength, tensile strength, yield strength, and elastic modulus of the SLM produced Mg samples outperformed traditionally produced counterparts and favored orthopedic applications. Researchers agreed that grain refinemen improves the hardness of the biodegradable Mg parts in accordance with the Hall-Petch equation.The hardness of the SLM produced Mg ranged from 0.4 to 1.2GPa. The recent literature on the mechanical properties of additively manufactured Mg biomaterials is summarized in Table 5.

Table 5 Mechanical properties of the additively manufactured biodegradable Mg.

Table 5 (continued)
3.5. Topology design
Human bone tissues are two types: cortical and cancellous.Cortical bone, also called compact bone, is the hard outer layer of bones with low porosity, while cancellous bone, also called trabecular bone, is the internal tissue of the skeletal bone with an open cell porous network and is much lighter than cortical bone. The porosity of cancellous bone is in the range of 30% - 95% and the pore size ranges from 200 to 1000μm.Porous scaffolds are designed to mimic human bone structure. The delicate pores allow for new tissue ingrowth and body flui circulation. Moreover, the porous scaffolds can provide the necessary support for cells to proliferate and maintain their differentiated functions, and their structure define the ultimate shape of the new bone[140].The formation and regeneration of new tissues are enabled by the biologic activities such as the attachment and proliferation of the cells.Thus, a well-designed porous structure is necessary for a decent biologic function. Porosity can also be used to adjust the mechanical properties of the designed structures, such as the strength and Young’s modulus.
In the AM of a biomedical implant or scaffold,the patient’s own bone is scanned before the model design.To this end,different scanning methods such as magnetic resonance imaging(MRI) and X-ray computed tomography (CT) can be used to collect an accurate image data [62]. This data is corrected by using a biomaterial software such as Biobuild(Anatomics,Australia) or Mimics(materialise, Belgium) and transferred to 3D CAD models. After the design is complete, the model is materialized via AM processes. In addition to the anatomical shape of the patient, scaffolds should be designed in a porous structure to mimic human bone tissue. The intricate pores inside the scaffold promote tissue ingrowth by providing pathways for effective diffusion of nutrients and oxygen to the cells and excretion of waste from the cells. Moreover, the biodegradation rate of the scaffolds is significantl influence by porosity due to the change in the surface area [140].
The influenc of geometric features on biological and mechanical properties is interrelated and needs to be wellunderstood [28]. For example, an increase in porosity can lead to better cell ingrowth but at the cost of poor stiffness,strength, and corrosion resistance [14,20,146,147]. Thus, the biodegradable scaffolds should be carefully designed and manufactured with customized geometry and porosity to meet the multiple requirements such as sufficien stiffness and permeability for a decent mechanical and biological function [148]. In this regard, findin the optimal topologies for scaffolds is of critical importance. Topology optimization is a design method that provides optimal geometry to achieve multiple goals such as maximum stiffness and strength with specifi porosity under the given boundary conditions. Eventually,the optimal unit cell is designed, and based on that,the fina periodical architecture is attained to meet the predefine design goals. Dong-ming et al. [148] employed topology optimization to achieve a microstructure with maximum stiffness under the constraint of specifi pore volume fraction. In the study, algorithms and programs were built to obtain 2D and 3D optimized microstructure, then they were converted to CAD models.The 3D geometry and pore shape in the metallic scaffolds were relatively precisely reproduced at a minimum mean pore size of 231μm. Arabnejad et al. [118] identifie the design limits of different lattice structures fabricated by SLM process by taking into consideration of the bone ingrowth requirements (i.e. pore size and porosity), mechanical function, and manufacturing limitations (i.e. strut thickness).Similar strategies can be applied to obtain design limits of other unit cell topologies and other manufacturing methods.
Porosity is define as the percentage of void space in a solid structure,and the gravimetric method is used to calculate the porosity (P) as follows;
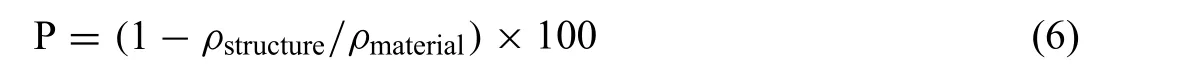
where ρmaterialand ρstructureare the density of raw material and porous structure, respectively.
In natural tissues, minimum distance of cells to the nearest capillary is 200μm, which is the effective diffusion distance of oxygen and nutrients [149]. Thus, a scaffold should be designed with a minimum pore size of a few hundred micrometers for the delivery of oxygen and nutrients to the cells[150].In the literature,the mostly used pore size varies between 100 and 500μm [151-155]. Quantity of cells that can penetrate and grow in scaffold, as well as the amount of biofunctions that can be transported through the scaffold depend on the size and interconnectivity of pores [156]. Therefore, a porous structure with small pore size and poor interconnectivity may not provide sufficien space for bone ingrowth, and permeability for material transport through the scaffold. Interconnectivity of pores is also essential for the matrix deposition and vascularization [156]. Besides the porosity and pore size,other geometrical features such as the surface curvature and pore shape have also significan influenc on the cellular response and tissue regeneration [157].It has been reported that tissue regeneration occurs preferentially on concave surfaces rather than convex and planar surfaces[158-160],and the rate of tissue regeneration improves with curvature [161-163].
3.6. Process parameters

The properties of the additively manufactured scaffolds strongly depend on process parameters, such as laser spot diameter, input power, scanning speed, hatch spacing, and layer thickness [152,164-166]. Therefore, understanding the effects of these parameters during the building process and selection of the optimal conditions can allow attaining near full density parts. The range of suitable processing parameters is also dependent on the chemical composition of the primary alloys[167]. Generally, it has been reported that an optimal laser energy density is required to produce parts with maximum density [168,169]. The optimal energy density can be set by adjusting the laser power,scan speed,hatch spacing,and layer thickness [170], based on the following formula:where Evis the energy density(J/mm3),P is laser power(W),v is scanning speed (mm/s), H is hatch spacing (mm), and T is layer thickness(mm).Manufacturing of denser parts can be achieved at reduced scanning speeds since it permits longer interaction time between powder and laser beam, which increases energy delivery rate to the powder bed [170]. At high scanning speeds less energy is transferred to the powder that may cause incomplete melting of particles and formation of pores inside the struts. Delivering sufficien energy to the powder bed enables adequate melting of particles to fully infiltrat into the voids to form a highly dense structure.Optimizing the process parameters is conducive to enhanced melt pools and favorable material densification Energy density is an essential parameter in controlling defects and improving mechanical properties [171]. In the literature, various studies on the optimization of process parameters for AM of Mg have been reported [172,173]. Niu et al. [128] identifie that the laser-introduced defects in SLM processed Mg initiated localized corrosion and reduced the overall corrosion resistance. The study suggested that these volume defects and the subsequent corrosion can be reduced by selecting suitable combination of the process parameters. Ng et al. [125] investigated the interaction between the laser source of SLM and the Mg powders by varying the laser power and scan speed under continuous and pulse wave irradiation modes. The required energy density range for continuous and pulse wave irradiation was determined as 1.27 ×109- 7.84 ×1012J/m2and 1.13 ×1012- 9.8 ×1012J/m2, respectively. Higher peak power under pulse wave irradiation required higher energy density in comparison to continuous wave irradiation. The surface morphology and dimension characteristics of the Mg specimens varied under different laser irradiation modes. Under continuous wave irradiation,disrupted surface and smooth regular beads were formed. Though, under pulsed wave irradiation relatively smooth and fla surface morphology was observed [143]. Yang et al. [68] also studied the influenc of laser energy density on the formation quality of Mg parts.They achieved continuous smooth Mg layers, without the presence of formation pores and cracks at input laser power of 50-80W, scanning speed of 300-700mm/min, and a corresponding laser energy density of 6.0-12.0J/mm. Sufficien energy density permitted total melting of solid Mg particles into liquid phase. Rapid solidificatio of the Mg melt lead to fine grains and formation of continuous smooth layers. Shuai et al. [130] studied the effect of SLM process parameters on the properties of as-built ZK60 alloys.Alloy samples with relative density of 97.3% was obtained at an energy density of 600J/mm3. High scanning speed and low energy density resulted in formation pores due to incomplete melting and high liquid viscosity, while low scanning speed caused formation of micro cracks, due to severe thermal stresses.
He et al. [76] achieved fabrication of AZ61 alloy with a relative density of 98%. Input laser power of 60-90W was identifie as the forming zone of AZ61. An increase of the laser power was found useful for enhancing degradation resistance and microhardness due to the improved densificatio and formation of uniformed equi-axed grains. However, excessive laser power lead to an increased mass loss and reduced microhardness due to coarsening of grains and a reduction in the solid solution of Al in the Mg matrix. Liu et al. [145] investigated the effect of process parameters on the properties of AZ61 manufactured by SLM. According to the results, applied laser energy density significantl affected the relative density, surface topography, and microstructure of the as-built samples. The relative density reached maximum 99.4% at a laser energy density 156.3J/ mm3and scanning speed 400mm/s. In addition, the optimal hatch spacing for highest relative density and surface quality was determined to be 0.06mm. Pawlak et al. [174] adopted the design of experiments approach to study the influenc of process parameters and their correlations, as well as the minimization of processing pores on SLM processed AZ31 through optimization.They developed a mathematical model,which provided a very good estimate of porosity values. Optimization allowed to fabricate AZ31 alloys with relative density above 99.5%using SLM process.
Ji et al. [175] developed an analytical model to predict the post-printing grain size in metal AM. According to the study, the temperature at top surface of the build part is not affected by the scan speed, while both the affected zone and peak value of the temperature beneath the surface of the build part increase with the increase of the laser power. The average grain size decreases with the increase of the laser power,while the average grain size decreases with the increase of the scan speed and then increases due to the incomplete melting effect. Thus, the study concluded that the selection of proper laser parameters is very important in controlling the post-printing grain size, so the mechanical properties of the build part. Yang et al. [139] incorporated mesoporous silica(MS) with ZK 60 alloy by SLM with optimal processing parameters; laser spot size 50μm, input power 120W, scanning speed 20 mm·s-1, hatch spacing 40μm, and layer thickness 100μm. A high densificatio 98.3±0.8% was achieved with ZK60, which was slightly reduced with the addition of mesoporous silica; 97.2±1.4% for ZK60/4MS and 96.4±2.4%for ZK60/8MS. Reduced densificatio was attributed to the high melting point of silica, which remained un-melted while ZK60 fully melted into liquid phase and infiltrate most voids that lead to high densification
The literature on the applied process parameters for AM of biodegradable Mg is summarized in Table 6. The laser power is the mostly investigated input parameter, which ranged from 20 to 200W. A broad range of laser scanning speeds was adopted for manufacturing of Mg-based biodegradable parts.Other parameters such as laser spot diameter, powder layer thickness, and hatch spacing was in a range of 50 - 150μm,20 - 200μm, and 50 - 250μm, respectively.
3.7. Powder properties
In addition to the process parameters, powder properties such as particle type, morphology, size and size distribution have significan influenc on the properties of the additively manufactured Mg implants and scaffolds [152,164,165].Flowability of the powder greatly depends on the size and size distribution, and morphology of particles. Spherical particles with narrow size distribution allow for enhanced powder fl w that in turn improves packing of powders and eliminate formation defects. Thus, powders with suitable properties should be selected/prepared to obtain products of high relative density [58,134] and favorable mechanical and biological properties. In this context, the properties of the powder materials used in different researches for AM of Mgbased biomaterials are summarized in Table 7. Researchers mostly employed particles with spherical morphology and size smaller than 100μm. Clinically validated WE43 powder is the mostly investigated Mg alloy, which was followed by ZK and AZ series Mg alloys.

Table 6 Process parameters for the additive manufacturing of Mg.
3.8. Post-processing
Post-treatment of additively manufactured Mg implants and scaffolds is important to enhance the mechanical performance,to better mimic human tissue, and satisfy desired biologic functions. The microstructure of additively manufactured materials differs from that of the materials manufactured by subtractive methods[20,176].The layer-by-layer build-up process of AM with high cooling rates results in significan internal stresses in the built structure [20]. The undesired instabilities of the melt pool during PBF process may lead to increased porosity and surface roughness. Thus, post-processing is routinely applied to reduce the thermal stresses and to enhance the microstructure in order to meet the product requirements.The post-treatment technologies can be classifie into two as heat treatment and surface treatment.
Heat treatment is an effective method to enhance the performance of an additively manufactured part by modifying its microstructure. Heat treatment also reduces the microstructural defects and residual thermal stress. In SLM, the powders fuse and form a molten pool under the scanning of high-energy laser, thus completing the fully melting and resolidificatio mechanism. However, the temperature distribution of the molten pool significantl varies at the inner and outer regions, resulting in different temperature gradients in different directions. As a result, the cooling rate in the molten pool differs in each direction, which leads to anisotropic microstructure in the built part. Also, there are re-melting areas present at each layer, which exhibits different microstructure in comparison to the regions without re-melting.SLM also involves very high cooling rates exceeding 105K/s [68], which causes residual stress in the as-built parts. Residual stress can significantl reduce the ductility of scaffolds.Thus,heat treatment is necessary to reduce the microstructural anisotropy and to relieve the residual stress existing in the SLM processed materials [177].
Porous magnesium scaffold possesses a high surface area,which stresses the vast degradation and early loss of mechanical integrity of the scaffold and tissue, unless prevented by suitable post-processing methods. Kopp et al. [178] manufactured Mg scaffolds using SLM method and studied the influ ence of two different post-processing methods; heat treatment and plasma electrolytic oxidation (PEO) on the degradation behavior of the scaffold in vitro. PEO treatment reduced the degradation rate of magnesium scaffold,and enhanced its mechanical performance, whereas heat treatment showed a detrimental effect. Heat-treated samples revealed an increased precipitation of alloying elements at the grain boundaries, which resulted in a more selective corrosion attack of especially single grains. The heat treated sample exhibited higher corrosion penetration depths due to high amount of grain border excretions, which caused increased corrosion rates. Gangireddy et al. [37] manufactured WE43 Mg alloy using SLM process and applied HIP as a post-treatment. Although, HIP treatment effectively densifie the samples of high initial porosities, its effect was insignifican at small porosity level due to the enclosed nature of the pores. Mechanical strength was highlydependent on the inherent porosity of the samples,which substantially reduced with increasing porosity.
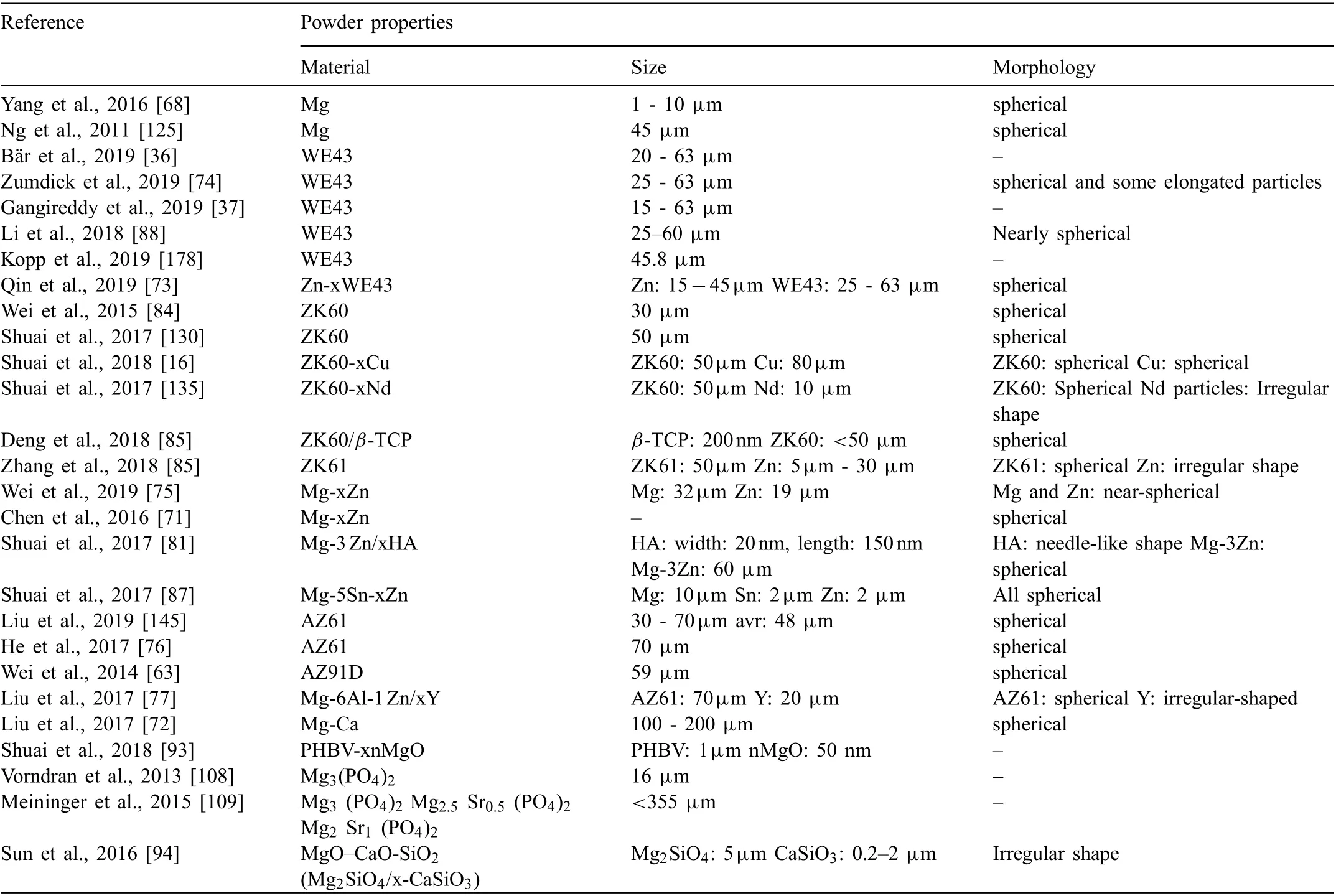
Table 7 Properties of powders used in additive manufacturing of biodegradable Mg.
A smooth surface is critical to enhance corrosion resistance and prevent fatigue failure [28]. However, due to the attachment of partially melt particles on the surface of asbuilt Mg parts during SLM process, surface modificatio is required [28]. Surface roughness plays an important role in the interaction between scaffold and cells [28]. Thus, biological performance of additively manufactured scaffolds, particularly bioactivity and biocompatibility can be significantl enhanced by surface modificatio [20].
Surface modificatio can be achieved by various techniques such as plasma spray, physical or chemical vapor deposition, ion implantation, electrochemical oxidation, acidic or alkali etching, sol-gel, heat-treatment, and surface machining or grinding. For porous metallic structures, there are two main approaches, based on surface coating and surface corrosion[20]. Corrosion-based surface treatment involves interfacial chemical reactions of structures in corrosive solution. Such chemical processes include chemical etching and anodization treatments. On the other hand, coating bioactive ceramics on scaffold surface is considered an effective way to improve the surface bioactivity of scaffolds [177].
4. Challenges of additive manufacturing of biodegradable Mg
The Powder Bed Fusion (PBF) of Mg-based metal powders holds two major challenges; vigorous evaporation and high chemical reactivity [37,74,84,179]. The vigorous evaporation of Mg powders is due to its low boiling point. The temperature gap between the melting and boiling point of Fe and Ti is about 1300K and 1600K, respectively, whereas this gap is less than 500K for Mg [28,64]. Therefore, the evaporation tendency of Mg is very high during SLM process [180,181]. Wei et al. [84] studied evaporation of ZK60 during SLM process. Both Mg and Zn are volatile elements,and diffusivity of the vaporized Zn is higher than vaporized Mg in argon atmosphere. Thus, higher Mg:Zn ratio and a lower content of Mg and Zn than the raw material was reported with a refine microstructure and reduced Mg-Zn precipitates. The relative density was reported to be limited to 94%. Negative effects of evaporation during SLM processing of Zn-WE43 was eliminated by Qin et al. [73]. They adopted a customized gas circulation system and optimized the laser energy input. They achieved above 99% relative density.
Mg is very reactive to oxygen even in its bulk form. The powder form of Mg used in SLM greatly increases the surface area,which can easily cause a strong exothermic reaction and serious dust explosions [28,74,88,182]. Therefore, most laboratories avoid AM of Mg due to safety concerns [183].Thus, it is recommended to passivate Mg powders by oxidation during atomization to ensure safe handling. Sometimes,Mg powders are even atomized under argon atmosphere in mixture with 1-2% oxygen, which forms a relatively stable oxide layer on Mg powders.However,oxygen in the Mg powder has a negative effect on SLM process [28]. The melting point of MgO is 2852°C, which is significantl higher than that of Mg and its alloys. More laser energy is required to melt the oxide layer, which in turn worsens evaporation due to the increased temperature in the molten pool. As a consequence, the formation quality deteriorates with increased processing pores and inclusions [28,184]. Also, the presence of a surface oxide fil on the preceding layer inhibits interlayer bonding due to the poor wettability of oxide film by liquid metals, which results in balling [17,64]. Occurrence of balling region is characterized by the agglomeration of a series of ball like particles, where the liquid phase splits into a row of spheres to reduce surface energy to form big melt pools [170]. Incidence of balling lead to deterioration of the surface features resulting from the combination of thermal stresses and weak interlayer bonding between grains and layers [17].
Despite the extremely low oxygen content in the high purity shielding Ar gas, Mg powder oxidizes during SLM process. Salehi et al. [64] investigated interaction of Mg powder with an ultra-high purity (99.9999%) Ar gas using the thermogravimetric analysis (TGA) technique at different heating rates (5, 10, 15, 20, 22.5, 25, and 28°C/min). The results showed that Mg powder reacts with even a very low oxygen content present as impurity in the Ar gas to form MgO at temperatures higher than 400°C. Thus, high purity Ar gas only provides inert atmosphere for Mg powder below 400°C.For temperatures exceeding 400°C, Mg powder significantl reacts with oxygen present as an impurity in ultra-high purity Ar gas to form MgO. With increasing heating rate, the onset of oxidation progressively shifted to higher temperatures.Oxidation reactions of Mg powder under Ar atmosphere was described by two different oxidation mechanisms, namely, the oxide thickening and sublimationvaporization of Mg.
During SLM process, due to the low absorption of magnesium, major portion of the laser energy is reflected Research should be conducted to assure that majority of energy is delivered to the material to achieve higher efficiencies Another important consideration is that the evaporation fume emitted during SLM of Mg should be carefully blown off through a continuous gas fl w. Also, a special care should be taken to prevent the disturbance of the very light-weight Mg powder bed from the continuous fl w of the shielding gas [28].
It should also be noted that solidificatio cracks may occur during SLM processing of base materials of brittle nature. Rapid heating and cooling of brittle metal powder in SLM process will lead to residual stress that will result in cracks in the as-built parts. Therefore, brittle powders should be avoided for SLM fabrication. In addition, SLM-built parts usually exhibit a rough surface due to the attachment of the partially melted particles during SLM process that can deteriorate the mechanical properties such as the fatigue strength.Thus, post treatments are necessary to improve the surface quality of the SLM-produced Mg parts [140].
5. Concluding remarks and future research
AM is the right method to produce Mg-based implants and porous scaffolds with customized structures based on the patient specifi anatomic data. Among the AM processes, Powder Bed Fusion (PBF) is the most suitable one, which allows for a precise control over the topology. The processes other than PBF employ sacrificia materials, such as binders for binder jetting and polymeric templates for infiltratio (indirect AM), which cause the waste of additional materials and lead to poor densification Though recent studies suggest the possibility of eliminating the sacrificia materials, as-built Mg parts still possess insufficien formation quality. EBM is considered not a suitable process since the severe evaporation of Mg affects the propagation of electron beam in vacuum inside the build chamber. SLM outperforms SLS process owing to the complete melting and effective infiltratio of Mg,which leaves no voids in the bulk material/struts, leading to high densification The high cooling rates and rapid solidificatio of SLM favors improved microstructure with refine grains, homogenized phase distribution, and enhanced solid solution. Thus, mechanical and corrosive performance of SLM produced Mg outperform that of traditionally produced counterparts. Powder properties and processing conditions significantl affect the formation quality, dimensional accuracy, microstructure, biological and mechanical functions of the as-built Mg implants and scaffolds.The optimal powder properties and processing conditions permit to achieve higher material densificatio and energy efficien y.In this regard,literature lacks studies investigating the effect of varying powder properties and process conditions on the energy efficien y of SLM process for the fabrication of Mg parts. Attachment,proliferation, and differentiation of new cells are significantl influence by the topology design of the scaffolds. Limited effort has been put forth to investigate the AM of Mg with different lattice structures.Hence,more research is required to comparatively investigate the effect of different lattice structures,porosity,and pore topology in order to identify the ideal scaffold design for best performance. Biological performance of the as-built Mg may differ in vitro and in vivo. Despite the availability of in vitro studies,literature lacks investigation of the biodegradation performance of the additively manufactured Mg in vivo.Thus,researchers are encouraged to conduct in vivo studies on the biodegradation performance of the additively manufactured Mg implants and scaffolds. Finally, considering the healing period of the defected tissues, the influ ence of time(up to ~2 months)on the in vivo performance of the additively manufactured Mg implants and scaffolds should be studied as a fourth dimension for the ultimate success of the implants and scaffolds in the clinical applications.
杂志排行
Journal of Magnesium and Alloys的其它文章
- Mechanism of Mn on inhibiting Fe-caused magnesium corrosion
- An efficien and comparative adsorption of Congo red and Trypan blue dyes on MgO nanoparticles: Kinetics, thermodynamics and isotherm studies
- Twin recrystallization mechanisms in a high strain rate compressed Mg-Zn alloy
- Corrosion behaviour and cytocompatibility of selected binary magnesium-rare earth alloys
- Correlation between test temperature, applied load and wear transition of Mg97Zn1Y2 alloy
- Residual stress and precipitation of Mg-5Zn-3.5Sn-1Mn-0.5Ca-0.5Cu alloy with different quenching rates
